Summary
Promoting neurogenesis is a promising strategy for the treatment of cognition impairment associated with Alzheimer's disease (AD). Ganoderma lucidum is a revered medicinal mushroom for health-promoting benefits in the Orient. Here, we found that oral administration of the polysaccharides and water extract from G. lucidum promoted neural progenitor cell (NPC) proliferation to enhance neurogenesis and alleviated cognitive deficits in transgenic AD mice. G. lucidum polysaccharides (GLP) also promoted self-renewal of NPC in cell culture. Further mechanistic study revealed that GLP potentiated activation of fibroblast growth factor receptor 1 (FGFR1) and downstream extracellular signal-regulated kinase (ERK) and AKT cascades. Consistently, inhibition of FGFR1 effectively blocked the GLP-promoted NPC proliferation and activation of the downstream cascades. Our findings suggest that GLP could serve as a regenerative therapeutic agent for the treatment of cognitive decline associated with neurodegenerative diseases.
Keywords: Ganoderma lucidum, Alzheimer's disease, cognition, adult neurogenesis, fibroblast growth factor receptor 1
Highlights
-
•
G. lucidum polysaccharides (GLP) improve cognition in transgenic AD mice
-
•
GLP promote neural progenitor proliferation and self-renewal to enhance neurogenesis
-
•
GLP potentiate FGFR signaling
G. lucidum is a revered medicinal herb for promoting health. Pei and colleagues found that the polysaccharides from G. lucidum (GLP) promoted neural progenitor proliferation to enhance neurogenesis and ameliorated cognition deficits in transgenic Alzheimer's disease model mice. These findings suggest that GLP could serve as a regenerative therapeutic agent for the treatment of cognitive decline associated with neurodegenerative diseases.
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disease that lays a heavy burden on society. AD is characterized by continuous cognitive decline and worsening of daily living performance, which results from loss of functional neurons and synapses (Hardy and Selkoe, 2002). Despite progress in understanding the pathophysiology of AD, effective treatments remain to be explored.
One potential therapeutic avenue is to promote neurogenesis by mobilizing endogenous neural progenitor cells (NPC) (Felsenstein et al., 2014, Lie et al., 2004, Miller and Kaplan, 2012). Neurogenesis in adult mammalian brains is essential for maintaining brain functions such as cognition and mood regulation (Zhao et al., 2008). Moreover, in response to brain pathology, NPC readily proliferate, redirect to the area of neurodegeneration, and lead to neurogenesis involved in the repair of neural damage (Erlandsson et al., 2011, Kolb et al., 2007). In AD patients and animal models, the proliferation and self-renewal of NPC is dysregulated and results in aberrant neurogenesis (Jin et al., 2004a, Jin et al., 2004b, Niidome et al., 2008, Rodriguez et al., 2008). Growing evidence has shown that pharmaceutical approaches that promote NPC proliferation alleviate AD-related cognitive decline, which are therefore considered as a feasible therapeutic strategy for AD (Fiorentini et al., 2010, Jin et al., 2006, Wang et al., 2010).
Ganoderma lucidum, an edible medicinal mushroom, has been used to promote health and longevity for centuries in the Orient (Sanodiya et al., 2009). G. lucidum has diverse bioactivities including anti-diabetes, anti-tumor, and immunomodulation (Sanodiya et al., 2009). Recent studies have revealed its benefits to the brain; for example, the water extract of G. lucidum (WGL) induces neuronal differentiation and neurite outgrowth of PC12 cells and has hypnotic and antidepressant effects in vivo (Cheung et al., 2000, Chu et al., 2007, Matsuzaki et al., 2013). G. lucidum polysaccharides (GLP), one of the major active components in G. lucidum, also protect neurons from hypoxia/reoxygenation injury in vitro (Zhao et al., 2004). In addition, oil from G. lucidum spores protects dopaminergic neurons and ameliorates behavioral deficits in a Parkinson's disease rat model (Ding et al., 2010). However, it is still not clear whether and how G. lucidum has beneficial potential in the treatment of AD.
In this study, we report that GLP as well as WGL alleviated cognition deficits and promoted hippocampal neurogenesis in transgenic AD mice. Proliferation and self-renewal of NPC was enhanced by GLP treatment. We further showed that treatment of GLP potentiated the activation of fibroblast growth factor receptor 1 (FGFR1). Our results suggest that GLP could serve as a regenerative therapeutic agent against cognitive decline associated with neurodegenerative diseases.
Results
GLP Improve Cognitive Function in Transgenic AD Mice
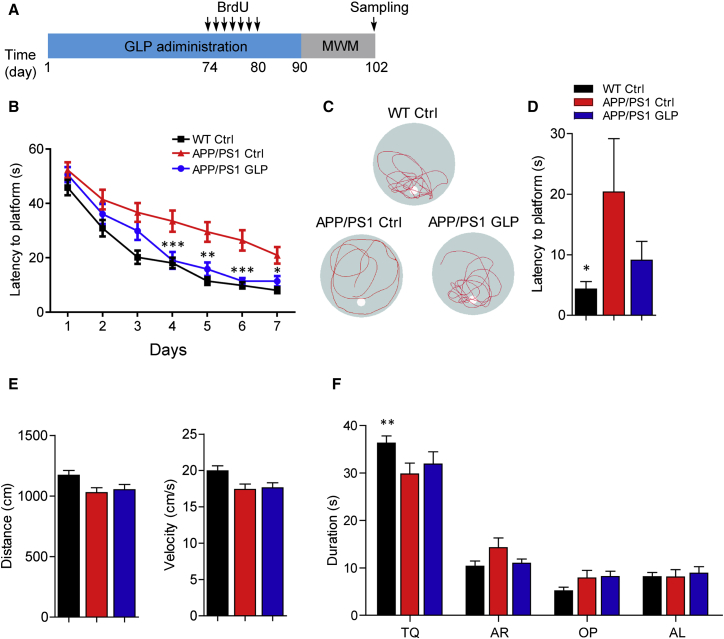
To investigate whether G. lucidum has therapeutic benefits for AD, we first chronically treated 6-month-old transgenic APP/PS1 mice and their wild-type littermates with GLP or vehicle by oral gavage for 90 days. There were no obvious adverse effects or body weight loss following GLP treatment (data not shown). Morris water maze (MWM) analysis was performed at the end of drug administration to evaluate learning and memory in these mice (Figure 1A). There was no obvious difference in the swimming velocity and distance among the groups of animals, indicating that GLP treatment did not affect locomotor activity (Figure 1E). However, compared with the wild-type littermates, APP/PS1 mice spent more time in locating the hidden platform, reflecting impairment of spatial memory in these mice (Figure 1B). Interestingly, APP/PS1 mice treated with GLP showed improved performance, indicating that deficits in spatial memory were ameliorated by chronic treatment of GLP (Figure 1B). Moreover, during the probe trial on day 7, GLP-treated APP/PS1 mice took less time to reach the position of the platform (p = 0.1906, APP/PS1 Ctrl mice versus APP/PS1 GLP mice, Figure 1D), spent slightly more time in the target quadrant (p = 0.7211, APP/PS1 Ctrl mice versus APP/PS1 GLP mice, Figure 1F), and crossed more frequently within the platform area than vehicle-treated mice (Figure 1C). These results indicate that treatment with GLP alleviates the deficits in spatial learning and memory in APP/PS1 mice. Similar to GLP, WGL ameliorated cognitive decline in transgenic AD mice (Figures S1A–S1E). WGL also improved locomotor functions and prolonged the life span of AD transgenic Drosophila which expressed amyloid-β1–42 (Aβ42) (Figures S1F–S1H).
Figure 1.
Ganoderma lucidum Polysaccharides Reduce Cognition Deficits in Transgenic AD Mice
(A) Diagram depicting the experimental design employed for neurogenesis and Morris water maze (MWM) analysis.
(B) MWM test for GLP and vehicle (Ctrl)-treated APP/PS1 and wild-type (WT) mice (n = 8–14 per group).
(C) Representative tracks of each group of mice in probe trial test at day 7.
(D) Latency to platform for each group of mice in probe trial (n = 8–14 per group).
(E) Swimming distance and velocity in the probe trial (n = 8–14 per group).
(F) Time spent by mice in the target quadrant (n = 8–14 per group). TQ, target quadrant; AR, adjacent right; OP, opposite; AL, adjacent left.
Quantifications are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, analyzed by two-way ANOVA (B, F) or one-way ANOVA (D, E) followed by Bonferroni test. See also Figure S1.
GLP Promote Neurogenesis in Transgenic AD Mice
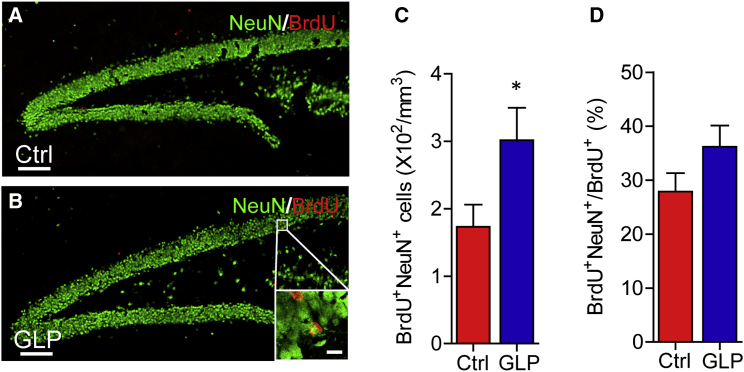
Aberrant neurogenesis is associated with cognitive decline in AD. We asked whether neurogenesis in APP/PS1 mice was affected along with the improvement in cognition by GLP treatment. To address this question, we injected the APP/PS1 mice with bromodeoxyuridine (BrdU) from the 74th to 80th day of drug administration and euthanized the mice 28 days later. Compared with that in vehicle-treated mice, the number of BrdU/NeuN double-positive cells in the hippocampus of APP/PS1 mice was markedly increased following treatment of GLP (Figures 2A–2C). However, there was no significant change in the proportion of BrdU/NeuN double-positive cells in the BrdU retaining cells, indicating that the neuronal lineage commitment was not affected by GLP treatment (Figure 2D). These data indicate that treatment of GLP enhances neurogenesis along with reducing cognitive deficits in transgenic AD mice. Since amyloid-β (Aβ) plays an important role in AD pathogenesis (Gimbel et al., 2010, Hardy and Selkoe, 2002, Kaufman et al., 2015, Palop and Mucke, 2010), we examined the effect of GLP on amyloid deposits and found that 6E10-positive Aβ area was significantly reduced in cortex of GLP-treated APP/PS1 mice compared with that in vehicle-treated mice, indicating that GLP reduced amyloid deposits (Figures S2A–S2C). Consistently, enhanced neurogenesis was also observed in WGL-treated transgenic AD mice (Figures S2D–S2G).
Figure 2.
G. lucidum Polysaccharides Enhance Neurogenesis in Transgenic AD Mice
(A and B) BrdU (red) and NeuN (green) staining of dentate gyrus (DG) sections from mice treated with vehicle (Ctrl, A) and GLP (B).
(C) Quantification of BrdU+NeuN+ cells from sections as in (A) and (B). n = 9 per group.
(D) Proportion of BrdU+NeuN+ cells in BrdU+ cells (n = 9 per group).
Quantifications are presented as mean ± SEM. ∗p < 0.05, analyzed by two-tailed t test (C, D) compared with APP/PS1 Ctrl group. Scale bars in (A) and (B), 100 μm; inset is image of high magnification with scale bar representing 10 μm. See also Figure S2.
GLP Promote NPC Proliferation in Transgenic AD and Young Adult Mice
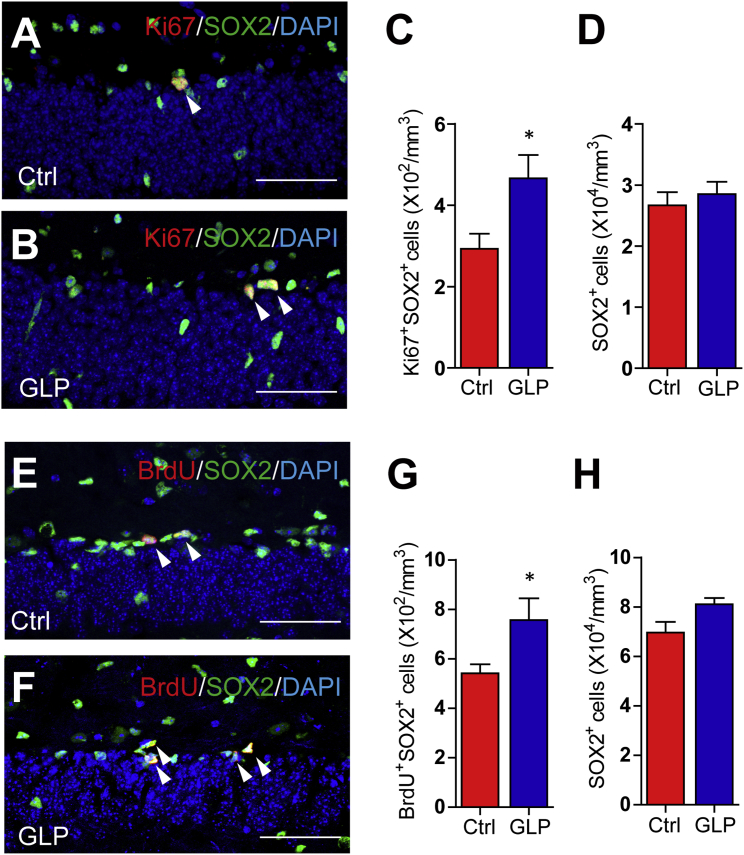
NPC proliferation, differentiation, and survival of newborn neurons are critical stages in neurogenesis. We observed that GLP treatment did not alter the proportion of neuronal progeny in APP/PS1 mice. Therefore, it is likely that GLP treatment enhanced NPC proliferation to promote neurogenesis. To test this hypothesis, we monitored the expression of a proliferation marker Ki67 in SOX2-positive NPC. Compared with the vehicle-treated APP/PS1 mice, the number of Ki67 and SOX2 double-positive proliferating NPC was increased in the GLP-treated mice (Figures 3A–3C). However, the number of SOX2-positive cells was not changed, indicating GLP did not affect NPC pool in APP/PS1 mice (Figure 3D). To further investigate whether GLP also promote NPC proliferation in normal mice, we treated 8-week-old C57BL/6 mice with GLP or vehicle for 14 days and mitotic cells were labeled by three BrdU injections on the last day. Histological analysis revealed that there were more BrdU and SOX2 double-positive proliferating NPC in the subgranular zone (SGZ) of GLP-treated mice than in vehicle-treated mice (Figures 3E–3G). However, the number of SOX2-positive cells in the SGZ was not changed, indicating that the NPC pool was not altered by GLP (Figure 3H). These results indicate that GLP treatment has general effects of promoting NPC proliferation in both neurodegenerating and normal brains.
Figure 3.
G. lucidum Polysaccharides Enhance Hippocampal NPC Proliferation
(A and B) Immunostaining for Ki67 (red), SOX2 (green), and DAPI (blue) counterstain in coronal hippocampal DG sections from APP/PS1 mice treated with vehicle (Ctrl, A) and GLP (B). Arrowheads indicate Ki67+SOX2+ cells.
(C) Quantification of Ki67+SOX2+ cells from sections as in (A) and (B). n = 8–11 per group.
(D) Quantification of SOX2+ cells (n = 8–11 per group).
(E and F) Eight-week-old C57BL/6 mice were treated with vehicle (Ctrl, E) and GLP (F) for 14 days followed by BrdU injections. Representative images of BrdU (red), SOX2 (green), and DAPI (blue) staining in DG sections are shown. Arrowheads indicate BrdU+SOX2+ cells.
(G) Quantification of BrdU+SOX2+ cells from sections as in (E) and (F). n = 5–6 per group.
(H) Quantification of SOX2+ cells (n = 5–6 per group).
Quantifications are presented as mean ± SEM. ∗p < 0.05, analyzed by two-tailed t test. Scale bars, 50 μm.
GLP Enhance NPC Proliferation and Self-Renewal In Vitro
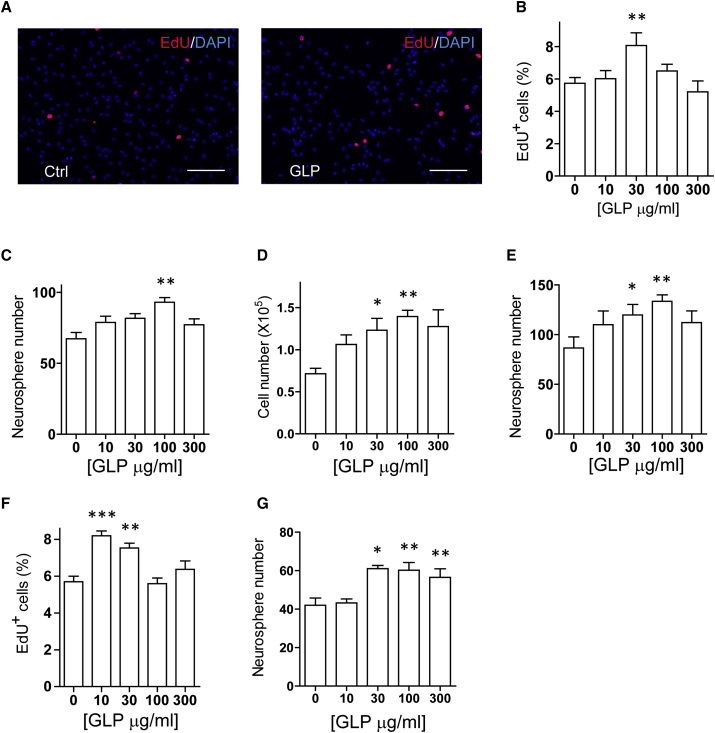
To assess the direct effects of GLP on NPC, we derived NPC from adult mouse hippocampus and embryonic mouse cortex. The NPC cultures were maintained regularly in the standard culture medium containing 20 ng/mL epidermal growth factor (EGF) and 10 ng/mL basic FGF (bFGF). According to a previously described culture strategy (Mao et al., 2015), we reduced the growth factors in the culture medium to mimic pathological conditions and examined the effects of GLP by ethynyldeoxyuridine (EdU) incorporation. In both the adherent adult hippocampal NPC culture and the embryonic neural precursor culture, GLP treatment resulted in a dose-dependent increase of EdU incorporation (Figures 4A, 4B, and S3A). To assess the effects of GLP on neurosphere formation, we seeded NPC at low cell density and maintained the suspension culture in the presence or absence of GLP. Compared with vehicle-treated culture, both neurosphere and cell number were increased in a dose-dependent manner by GLP treatment (Figures 4C, 4D, S3B, and S3C). To assess self-renewal capacity of the NPC, we dissociated cells from these neurospheres and replated them under identical neurosphere-forming conditions. The cells from GLP-treated neurospheres generated more neurospheres than those of vehicle treatment (Figures 4E and S3D). We also derived NPC from hippocampus of adult APP/PS1 mouse to assess the effects of GLP on proliferation of these cells. A similar increase of EdU incorporation was observed after GLP treatment (Figure 4F). These data indicate that GLP treatment enhances proliferation and self-renewal of mouse NPC.
Figure 4.
G. lucidum Polysaccharides Increase NPC Proliferation and Self-Renewal In Vitro
(A and B) Monolayer adult hippocampal NPC cultures were treated with GLP of different concentrations for 24 hr in culture medium containing 1 ng/mL EGF and 1 ng/mL bFGF. EdU was added 2 hr prior to fixation. Representative images of EdU (red) and DAPI (blue) staining in the culture treated with (A, left) vehicle (Ctrl) or (A, right) 30 μg/mL GLP are shown. The percentage of EdU+ cells among total cells in the culture was determined (B). n = 3 independent experiments.
(C–E) Adult hippocampal NPC were cultured in neurosphere-forming conditions in the presence of absence of GLP. Six days later, the number of neurospheres (C) and cells (D) were quantified for each condition. All neurospheres from each condition were collected, dissociated, and replated in the untreated culture medium. Six days later, the number of neurospheres was determined (E). n = 4 independent experiments.
(F) Adult hippocampal NPC from APP/PS1 mice were cultured and treated the same as in (B), and the percentage of EdU+ cells among total cells in the culture determined. n = 3 independent experiments.
(G) Human iPSC-derived NPC cultures were treated with GLP of different concentration for 6 days in neurosphere-forming conditions. Neurospheres were quantified (n = 4 independent experiments).
Quantifications are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 analyzed by one-way ANOVA followed by Fisher's protected least significant difference test. Scale bars, 100 μm. See also Figure S3.
To further investigate the therapeutic implication of GLP, we cultured human induced pluripotent stem cell (iPSC)-derived NPC and examined the effects of GLP on NPC proliferation by EdU incorporation. As shown in Figure 4G, GLP significantly increased EdU incorporation in a dose-dependent manner. Therefore, GLP promote proliferation of both rodent and human NPC culture.
GLP Potentiate FGFR1 Activation to Promote NPC Proliferation
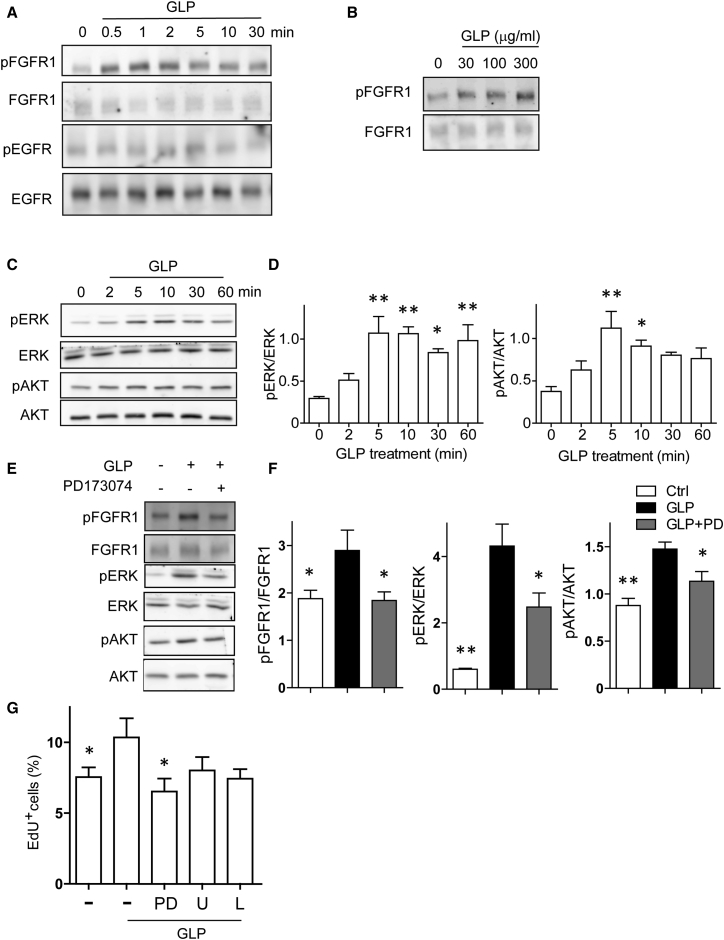
Recent reports have shown that membrane receptors such as TLR4, CR3, and FGFR1 mediate the effects of polysaccharides (Chen et al., 2009, Makani et al., 2016). Therefore, we hypothesized that membrane proteins associated with NPC proliferation might act as a potential target for GLP. FGFR1 and EGF receptor (EGFR) are receptors for bFGF and EGF, two critical growth factors for NPC proliferation and self-renewal in the hippocampus (Kuhn et al., 1997). Thus, we monitored the activity of these receptors in the NPC cultured in the reduced-growth-factor medium on immunoblots. As shown in Figure 5A, following GLP treatment FGFR1 phosphorylation increased, peaked at 1–2 min, then declined slowly. GLP treatment increased FGFR1 phosphorylation in a dose-dependent manner (Figure 5B). However, we did not observe any significant alteration on the EGFR phosphorylation levels (Figure 5A). In addition, because brain-derived neurotrophic factor (BDNF) is recognized as an important modulator of AD pathogenesis and neurogenesis (Elliott et al., 2005, Kazim et al., 2014, Li et al., 2008, Lu et al., 2013, Taliaz et al., 2010), we analyzed BDNF expression in brain tissues from APP/PS1 mice by immunoblotting and ELISA. As shown in Figures S4A–S4C, no significant difference in BDNF expression was observed between vehicle- and GLP-treated mice. MEK/ERK and phosphatidylinositol-3 kinase (PI3K)/AKT cascades are two downstream signaling pathways responsible for NPC proliferation following FGFR1 activation. Cellular ERK phosphorylation and AKT phosphorylation were also increased following GLP treatment and reached peaks at 5–10 min (Figures 5C and 5D). Interestingly, in the no-growth-factor condition, GLP did not activate FGFR signaling in the NPC (Figure S4D). However, simultaneous treatment of GLP and bFGF had synergistic effects on FGFR1 phosphorylation compared with bFGF treatment alone (Figure S4E), indicating that GLP potentiated FGFR activation. Further, the activation of FGFR1, ERK, and AKT by GLP could be blocked by the FGFR1 inhibitor PD173074 (Figures 5E and 5F). To investigate whether promotion of NPC proliferation by GLP depended on FGFR1 activation, we treated NPC with GLP in the presence of PD173074 and MEK inhibitor U0126 or PI3K inhibitor LY294002. As shown in Figure 5G, GLP-promoted NPC proliferation was largely blocked by PD173074, and U0126 and LY294002 also had moderate blockade effects, while treatment with tropomyosin-related kinase inhibitor K252a had little effect on GLP-promoted NPC proliferation (Figure S4F), suggesting that activation of FGFR signaling was required for promoting NPC proliferation by GLP treatment.
Figure 5.
G. lucidum Polysaccharides Strengthen FGFR Signaling to Promote NPC Proliferation
NPC were cultured in medium containing 1 ng/mL EGF and 1 ng/mL bFGF for 24 hr before any treatment.
(A) Cells were treated with 300 μg/mL GLP for the indicated time. Protein samples were analyzed on western blots using antibodies against the phosphorylated FGFR1 and EGFR. Blots were probed for total FGFR1 and EGFR as loading controls. Three independent experiments showed similar results.
(B) NPC were treated with GLP of different concentration for 2 min. Blots of the phosphorylated FGFR1 and total FGFR1 are shown. Three independent experiments showed similar results.
(C and D) NPC were treated with 300 μg/mL GLP for the indicated time. Blots of the phosphorylated ERK1/2, total ERK, phosphorylated AKT, and AKT are shown. n = 3 independent experiments.
(E and F) FGFR1 inhibitor PD173074 (1 μM) was added to the NPC culture. After 30 min, cells were treated with 300 μg/mL GLP or vehicle for 2 min to analyze FGFR1 phosphorylation (upper two panels) or 5 min to analyze ERK and AKT phosphorylation (lower four panels). Protein samples were analyzed on western blots using antibodies against the phosphorylated FGFR1, total FGFR1, phosphorylated ERK, total ERK, phosphorylated AKT, and total AKT. n = 3 independent experiments.
(G) NPC were pre-treated with FGFR1 inhibitor PD173074 (PD, 1 μM), MEK inhibitor U0126 (U, 0.3 μM), or PI3K inhibitor LY294002 (L, 3 μM) for 30 min. GLP (30 μg/mL) were then added and the cells were incubated for an additional 24 hr. EdU incorporation in the last 2 hr was visualized with staining and quantified. All groups were compared with the cell culture treated with GLP alone. n = 3 independent experiments.
Quantifications are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, analyzed by one-way ANOVA followed by Bonferroni test. See also Figure S4.
Discussion
AD is a devastating neurodegenerative disease with no effective treatment as yet. Current US Food and Drug Administration-approved AD drugs such as acetylcholinesterase inhibitors and NMDA receptor antagonists only alleviate disease symptoms in about half of the patients for approximately 6–12 months (Winslow et al., 2011). Recent advances in regenerative medicine, which includes stem cell-based therapy and modulation of endogenous neurogenesis, offer a novel therapeutic avenue (Lie et al., 2004). While both of the strategies proved to be feasible and effective in AD animal models, augmenting endogenous neurogenesis by pharmaceutical approaches seems to be more acceptable for patients because of its easy delivery (Miller and Kaplan, 2012). Here, we demonstrate that GLP and WGL ameliorated cognitive dysfunction and promoted NPC proliferation in transgenic AD model mice. Moreover, in an AD Drosophila model, WGL extended the life span and promoted locomotor function, indicating that the benefits of WGL on AD are conservative across model animals of different species. Our results indicate that GLP and WGL have beneficial potential as both preventives and therapeutics for neurodegenerative diseases.
G. lucidum is a medicinal mushroom well known for its legendary anti-aging benefits. Our findings that GLP not only promote NPC proliferation but also reduce amyloid deposits provide potential underlying mechanisms of its anti-aging effects. Extract of G. lucidum has also been reported to inhibit the lipopolysaccharide-induced inflammatory cytokines by activated microglia and thus to protect dopaminergic neurons (Ding et al., 2010). Moreover, WGL attenuates Aβ-induced synaptotoxicity and apoptosis by preserving the synaptic density protein synaptophysin (Lai et al., 2008). These reports, together with our findings, suggest that the multi-target effects of GLP and WGL may have desirable advantages for the treatment of multi-factorial neurodegenerative diseases such as AD.
Despite progress in revealing therapeutic potentials of G. lucidum, the molecular targets of GLP or WGL are still not clear. bFGF and EGF are two critical growth factors that regulate NPC proliferation, survival, and differentiation in the neurogenic region. In this study, we found that GLP activated FGFR1 signaling but not EGFR signaling in a growth-factor-deficient condition. In line with this, inhibition of FGFR1 blocked GLP-promoted NPC proliferation, while inhibition of either downstream ERK or AKT only had moderate blockade effects. Interestingly, GLP had differential effects on FGFR1 activation in the presence or absence of low concentration of bFGF, suggesting that GLP potentiated the response of FGFR1 to bFGF rather than activating FGFR1 by itself. Our results indicate that GLP could potentiate FGFR signaling to promote neurogenesis upon growth factor deficiency, and may serve as a preventive and therapeutic agent against neurodegenerative diseases.
Experimental Procedures
Animals
APP/PS1 transgenic mice were obtained from The Jackson Laboratory (stock no. 004462) and express a chimeric mouse/human amyloid precursor protein containing the K595N/M596L Swedish mutations (APPswe) and a human presenilin 1 with a deletion of exon 9. Heterozygous mice were maintained by crossing with C57BL/6 mice. C57BL/6 mice were obtained from Shanghai Laboratory Animal Center (Chinese Academy of Sciences). The experimental procedures for the use and care of the animals were approved by the Ethics Committees of the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. All mice were given ad libitum access to food and water.
Extraction and Isolation of Polysaccharides from G. lucidum
Extraction of crude polysaccharides was performed by a previously described procedure (Wang et al., 2014). In brief, the dried conidial powder of G. lucidum (1.35 kg) was defatted with 95% EtOH for 1 week and then extracted with boiling water six times, 5 hr each time. The combined supernatant was concentrated and centrifuged, then three volumes of 95% EtOH were added to the concentrated supernatant to precipitate the crude polysaccharide, CPW (28.2 g, 2%). CPW (7 g) was fractionated on a diethylaminoethyl-cellulose column (Cl–, 120 × 6 cm) and eluted with distilled water to obtain the fraction GPWA (3.25 g). The polysaccharide GPWA was then further purified on a Sephacryl S-300 column (2.6 × 100 cm), eluted with 0.2 M NaCl, to obtain GLP. The eluate was monitored with a phenol-sulfuric acid method. The relative molecular weight of GLP was estimated to be 15.0 kDa by the HPGPC method. It contained no protein and uronic acid after measurement by the Lowry method and m-hydroxydiphenyl method, respectively.
Drug Administration and BrdU Injections
WGL (Alpha Bio-technology, Fujian) and GLP were dissolved in water. BrdU (Sigma-Aldrich) intraperitoneal injections were applied as described by Encinas et al. (2011). As illustrated in Figure 1A, 5-to 6-month-old APP/PS1 mice were treated with 200 mg/kg (body weight) WGL, 30 mg/kg GLP, or water (vehicle) by gavage once per day for 90 days followed by MWM tests. For evaluation of neurogenesis, these mice were injected with BrdU (50 mg/kg) once per day from days 74 to 80 of drug administration. On day 102 at the end of behavioral tests, the animals were transcardially perfused successively with cold PBS and 4% paraformaldehyde (PFA).
For monitoring NPC proliferation, 8-week-old C57BL/6 mice were treated with 30 mg/kg GLP or water (vehicle) by gavage once per day for 14 days. On the day following the last drug administration, 150 mg/kg BrdU was injected three times separated by 3-hr intervals. Two hours later the animals were perfused successively with PBS and PFA.
Morris Water Maze Test
The MWM test was performed as described previously (Morris, 1984, Teng et al., 2010) with modifications. The apparatus was a circular pool of 120 cm diameter filled with water containing small white plastic balls. The water temperature was maintained at 23.0°C ± 0.5°C. Animals were brought to the behavior room, acclimatized, and trained. During training, a transparent platform of 11 cm diameter was placed 1 cm below the water surface at a fixed point of one quadrant. The training consisted of 7 consecutive days, with four trials per day. On days 4 and 7, a probe trial was performed. Swim paths were monitored using an automated tracking system (Ethovision XT software).
Cell Culture
Adult hippocampal NPC were derived from hippocampi of 8- to 10-week-old C57BL/6 and APP/PS1 mice and maintained as neurospheres in NeuroCult NSC basal medium with proliferation supplement (STEMCELL Technologies), 20 ng/mL EGF, 20 ng/mL bFGF (Gibco), and 5 μg/mL heparin (Sigma-Aldrich) as previously described (Brewer and Torricelli, 2007). Embryonic cortical precursors were isolated from cortices of E12.5 C57BL/6 mouse embryos and cultured as neurospheres in DMEM/F-12 (1:1) medium supplemented with B27 supplement (Gibco), 20 ng/mL EGF, and 10 ng/mL bFGF as described by Ebert et al. (2008). Human iPSC-derived NPC were provided by IxCell Biotechnology and the monolayer cell culture was maintained in neural stem cell culturing medium (IxCell) in Matrigel (Corning)-coated dishes.
In Vitro EdU Incorporation Assay
Neurospheres were dissociated with Accutase (Sigma-Aldrich) and cells were seeded at a density of 4 × 104 cells/cm2 onto poly-D-lysine (PDL) and laminin-coated 96-well plates in NeuroCult NSC proliferation medium (for adult hippocampal NPC) or B27-supplemented DMEM/F-12 medium (for embryonic neural precursors) containing 1 ng/mL EGF and 1 ng/mL bFGF. On the following day, the cells were pre-treated with or without PD173074 (1 μM; Selleck), U0126 (0.3 μM; Sigma-Aldrich), LY294002 (3 μM; Sigma-Aldrich), and K252a (200 nM, Sigma-Aldrich) for 30 min before GLP was added. Cells were incubated for 24 hr and EdU (10 μM; Sigma-Aldrich) was added for the last 2 hr prior to fixation.
In Vitro Neurosphere Formation Assay
Adult mouse hippocampal NPC were seeded at density of 3 cells/μL in NeuroCult NSC proliferation medium containing 20 ng/mL EGF and 20 ng/mL bFGF. The cells were treated with different concentrations of GLP for 6 days. Neurospheres possessing a diameter ≥50 μm were quantified. Neurospheres from each condition were collected, dissociated, and replated at a density of 3 cells/μL in the same untreated culture medium. The number of neurospheres was quantified 6 days later.
Human iPSC-derived NPC were seeded at a density of 6 cells/μL in B27- and N2-supplemented DMEM/F-12 medium containing 20 ng/mL EGF, 20 ng/mL bFGF, and 10 ng/mL leukemia inhibitory factor in the presence or absence of GLP. Six days later, the neurospheres with diameter ≥50 μm from each condition were quantified, dissociated, and replated at density of 6 cells/μL in the same untreated culture medium. The number of neurospheres was quantified 6 days later.
Western Blotting
Embryonic neural precursors were seeded at 1.2 × 105 cells per well in a PDL/laminin-coated 12-well plate in B27-supplemented DMEM/F-12 medium containing 1 ng/mL EGF and 1 ng/mL bFGF. On the next day, cells were pre-treated with 1 μM PD173074 for 30 min before GLP was added. After treatment for the indicated time, cells were washed with cold PBS and lysed with Laemmli's sample buffer. For detection of signaling activation in the absence of growth factors, NPC were seeded and cultured in B27-supplemented DMEM/F-12 containing 10 ng/mL EGF and 10 ng/mL bFGF. On the following day, the culture medium was replaced with DMEM/F-12 for cell starvation and 3 hr later, NPC were treated with GLP for the indicated time, washed, and lysed. Proteins in the cell lysates were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. Proteins were labeled with primary antibodies as follows: rabbit monoclonal antibodies against phospo-FGFR1 (Tyr653/Tyr654, 06-1433, Millipore), EGFR (06-847, Millipore), FGFR1 (9740, Cell Signaling Technology), phospho-EGFR (Tyr1068, 2234, Cell Signaling), phospho-ERK (9101, Cell Signaling), ERK (9102, Cell Signaling), phospho-AKT (4060, Cell Signaling) or AKT (4691, Cell Signaling), BDNF (sc-546, Santa Cruz Biotechnology), and ACTIN (A2066, Sigma-Aldrich). Immunoreactive bands on the membrane were revealed by chemiluminescent detection (Bio-Rad) of peroxidase-conjugated, subtype-specific antibody (M21002, Abmart). For quantification of different phosphorylated proteins or BDNF, the intensity of each band was measured with ImageJ and normalized to its corresponding band of total protein or ACTIN.
Immunostaining
The brain tissues collected were post-fixed with 4% PFA for 24 hr and allowed to settle in a 30% sucrose solution for 72 hr. The entire rostrocaudal length of the dentate gyrus (DG) was sectioned at 30 μm thickness, numbered, and every eighth section evenly distributed was processed for immunostaining. Coronal, cryostat sections including the DG area were permeabilized and blocked with PBS containing 10% donkey serum and 0.3% Triton X-100 for 45 min at room temperature. Afterward, samples were incubated with primary antibodies at 4°C overnight and then with appropriated fluorescent probe-conjugated secondary antibodies for 1 hr at room temperature. Nuclei were counterstained with DAPI. The entire DG was scanned using an Olympus FV100i confocal microscope for evaluating neurogenesis and proliferation. The number of single- or double-stained cells was counted using Image Pro-Plus software. For BrdU staining, sections were treated in 2 M HCl at 37°C for 20 min and rinsed in 0.1 M borate buffer (pH 8.5) before blocking. For NeuN, SOX2, and Ki67 staining, antigens were retrieved with citrate buffer (10 mM [pH 6.5]) for 20 min at 95°C before blocking. Specific primary antibodies used included rat anti-BrdU (1:2,000, OBT0030G, AbD Serotec), rabbit anti-NeuN (1:200, MAB377, Millipore), Ki67 (1:100, ab15580, Abcam), goat anti-SOX2 (1:100, AF2018, R&D Systems), and mouse anti-β-amyloid (6E10, 1:1,000, SIG-39300, Covance) antibodies.
For quantitative image data analysis, overlapping images of the entire DG on each section were captured by confocal microscopy in z stack covered from the top to the bottom layers of cells (usually 12–13 stacks with a 2-μm step). All positive-staining cells instead of partial cells within the SGZ or granule cell layer on each section were counted. For counting of double-stained cells, e.g., NeuN+BrdU+, Ki67+SOX2+, and BrdU+SOX2+, the co-localization of different staining in each cells was carefully examined across the z stack. For estimation of cell densities, labeled cells were divided by the area and 30 μm thickness. For quantification of amyloid deposits, the whole brain sections were scanned with a Zeiss Z1 microscope. Image Pro-Plus software was applied to annotate and analyze the area of hippocampus or cortex and 6E10-positive area in these regions. All assessments were performed in a blinded manner.
NPC were fixed in 4% PFA for 15 min and permeabilized with 0.1% Triton X-100 in PBS for further staining. EdU staining was performed by following the manufacturer's protocol (Click-iT, Invitrogen). Specific primary antibodies used included mouse anti-TUJ1 (1:1,000, MMS-435P, Convance) and rabbit anti-GFAP (1:1,000, 20334, Dako) antibodies. Stained cells were scanned and counted using a Cellomics ArrayScan VTI 700 (Thermo Scientific).
Statistical Analysis
All quantified data are presented as mean ± SEM. Results were analyzed by two-tailed t test to determine statistical significance of treatment sets. For multiple comparisons, results were analyzed by one-way ANOVA or two-way ANOVA followed by Bonferroni test when appropriate with GraphPad Prism 6 Software. p Values less than 0.05 are considered indicative of significance.
Author Contributions
G.P. substantially controlled study conception and design, interpretation of data, and revision of the manuscripts critically for important intellectual content. S.H. designed and performed NPC functional assays. J.M. designed and performed the mechanistic study and drafted the manuscript. S.H. and J.M. designed and performed in vivo NPC experiments. W.Y., Y.Z., and X.Z. performed MWM tests. C.Z. performed Drosophila survival and behavior tests. K.D., P.W., and J.Y. isolated polysaccharides from G. lucidum and performed analysis. S.H., J.M., W.Y., C.Z., P.W., and J.Y. collected, analyzed, and interpreted data. P.X. reviewed and edited the manuscript. All authors contributed to the data analysis, manuscript preparation, and final approval of the version to be submitted.
Acknowledgments
We are grateful to Shunmei Xin for technical assistance. The work using Cellomics ArrayScan VTI 700 was performed at the National Center for Protein Science, Shanghai, and we are grateful to Shufang He for her help. We thank all members of the laboratory for sharing reagents and advice. This study was supported by Science and Technology Commission of Shanghai Municipality (15JC1400202), the National Key Research and Development Program of China Stem Cell and Translational Research (2016YFA0101200, 2016YFA0101202), the Ministry of Science and Technology of China (2014CB965002), and National Natural Science Foundation of China (31400690).
Published: January 10, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.12.007.
Supplemental Information
References
- Brewer G.J., Torricelli J.R. Isolation and culture of adult neurons and neurospheres. Nat. Protoc. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- Chen S., Yin D.K., Yao W.B., Wang Y.D., Zhang Y.R., Gao X.D. Macrophage receptors of polysaccharide isolated from a marine filamentous fungus Phoma herbarum YS4108. Acta Pharmacol. Sin. 2009;30:1008–1014. doi: 10.1038/aps.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W.M., Hui W.S., Chu P.W., Chiu S.W., Ip N.Y. Ganoderma extract activates MAP kinases and induces the neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS Lett. 2000;486:291–296. doi: 10.1016/s0014-5793(00)02317-6. [DOI] [PubMed] [Google Scholar]
- Chu Q.P., Wang L.E., Cui X.Y., Fu H.Z., Lin Z.B., Lin S.Q., Zhang Y.H. Extract of Ganoderma lucidum potentiates pentobarbital-induced sleep via a GABAergic mechanism. Pharmacol. Biochem. Behav. 2007;86:693–698. doi: 10.1016/j.pbb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Ding H., Zhou M., Zhang R.P., Xu S.L. Ganoderma lucidum extract protects dopaminergic neurons through inhibiting the production of inflammatory mediators by activated microglia. Sheng Li Xue Bao. 2010;62:547–554. [PubMed] [Google Scholar]
- Ebert A.D., McMillan E.L., Svendsen C.N. Isolating, expanding, and infecting human and rodent fetal neural progenitor cells. Curr. Protoc. Stem Cell Biol. 2008;Chapter 2 doi: 10.1002/9780470151808.sc02d02s6. Unit 2D 2. [DOI] [PubMed] [Google Scholar]
- Elliott E., Atlas R., Lange A., Ginzburg I. Brain-derived neurotrophic factor induces a rapid dephosphorylation of tau protein through a PI-3 Kinase signalling mechanism. Eur. J. Neurosci. 2005;22:1081–1089. doi: 10.1111/j.1460-9568.2005.04290.x. [DOI] [PubMed] [Google Scholar]
- Encinas J.M., Michurina T.V., Peunova N., Park J.H., Tordo J., Peterson D.A., Fishell G., Koulakov A., Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsson A., Lin C.H., Yu F., Morshead C.M. Immunosuppression promotes endogenous neural stem and progenitor cell migration and tissue regeneration after ischemic injury. Exp. Neurol. 2011;230:48–57. doi: 10.1016/j.expneurol.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Felsenstein K.M., Candelario K.M., Steindler D.A., Borchelt D.R. Regenerative medicine in Alzheimer's disease. Transl Res. 2014;163:432–438. doi: 10.1016/j.trsl.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini A., Rosi M.C., Grossi C., Luccarini I., Casamenti F. Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS One. 2010;5:e14382. doi: 10.1371/journal.pone.0014382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel D.A., Nygaard H.B., Coffey E.E., Gunther E.C., Lauren J., Gimbel Z.A., Strittmatter S.M. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J. Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Jin K., Galvan V., Xie L., Mao X.O., Gorostiza O.F., Bredesen D.E., Greenberg D.A. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proc. Natl. Acad. Sci. USA. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Peel A.L., Mao X.O., Xie L., Cottrell B.A., Henshall D.C., Greenberg D.A. Increased hippocampal neurogenesis in Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Xie L., Mao X.O., Greenberg D.A. Alzheimer's disease drugs promote neurogenesis. Brain Res. 2006;1085:183–188. doi: 10.1016/j.brainres.2006.02.081. [DOI] [PubMed] [Google Scholar]
- Kaufman A.C., Salazar S.V., Haas L.T., Yang J., Kostylev M.A., Jeng A.T., Robinson S.A., Gunther E.C., van Dyck C.H., Nygaard H.B. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Ann. Neurol. 2015;77:953–971. doi: 10.1002/ana.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazim S.F., Blanchard J., Dai C.L., Tung Y.C., LaFerla F.M., Iqbal I.G., Iqbal K. Disease modifying effect of chronic oral treatment with a neurotrophic peptidergic compound in a triple transgenic mouse model of Alzheimer's disease. Neurobiol. Dis. 2014;71:110–130. doi: 10.1016/j.nbd.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Kolb B., Morshead C., Gonzalez C., Kim M., Gregg C., Shingo T., Weiss S. Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J. Cereb. Blood Flow Metab. 2007;27:983–997. doi: 10.1038/sj.jcbfm.9600402. [DOI] [PubMed] [Google Scholar]
- Kuhn H.G., Winkler J., Kempermann G., Thal L.J., Gage F.H. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.S., Yu M.S., Yuen W.H., So K.F., Zee S.Y., Chang R.C. Antagonizing beta-amyloid peptide neurotoxicity of the anti-aging fungus Ganoderma lucidum. Brain Res. 2008;1190:215–224. doi: 10.1016/j.brainres.2007.10.103. [DOI] [PubMed] [Google Scholar]
- Li Y., Luikart B.W., Birnbaum S., Chen J., Kwon C.H., Kernie S.G., Bassel-Duby R., Parada L.F. TRKB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie D.C., Song H., Colamarino S.A., Ming G.L., Gage F.H. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu. Rev. Pharmacol. Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Lu B., Nagappan G., Guan X., Nathan P.J., Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- Makani V., Jang Y.G., Christopher K., Judy W., Eckstein J., Hensley K., Chiaia N., Kim D.S., Park J. BBB-permeable, neuroprotective, and neurotrophic polysaccharide, Midi-GAGR. PLoS One. 2016;11:e0149715. doi: 10.1371/journal.pone.0149715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J., Huang S., Liu S., Feng X.L., Yu M., Liu J., Sun Y.E., Chen G., Yu Y., Zhao J. A herbal medicine for Alzheimer's disease and its active constituents promote neural progenitor proliferation. Aging Cell. 2015;14:784–796. doi: 10.1111/acel.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki H., Shimizu Y., Iwata N., Kamiuchi S., Suzuki F., Iizuka H., Hibino Y., Okazaki M. Antidepressant-like effects of a water-soluble extract from the culture medium of Ganoderma lucidum mycelia in rats. BMC Complement Altern. Med. 2013;13:370. doi: 10.1186/1472-6882-13-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F.D., Kaplan D.R. Mobilizing endogenous stem cells for repair and regeneration: are we there yet? Cell Stem Cell. 2012;10:650–652. doi: 10.1016/j.stem.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Niidome T., Taniuchi N., Akaike A., Kihara T., Sugimoto H. Differential regulation of neurogenesis in two neurogenic regions of APPswe/PS1dE9 transgenic mice. Neuroreport. 2008;19:1361–1364. doi: 10.1097/WNR.0b013e32830e6dd6. [DOI] [PubMed] [Google Scholar]
- Palop J.J., Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat. Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J.J., Jones V.C., Tabuchi M., Allan S.M., Knight E.M., LaFerla F.M., Oddo S., Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS One. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanodiya B.S., Thakur G.S., Baghel R.K., Prasad G.B., Bisen P.S. Ganoderma lucidum: a potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009;10:717–742. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- Taliaz D., Stall N., Dar D.E., Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol. Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng L., Zhao J., Wang F., Ma L., Pei G. A GPCR/secretase complex regulates beta- and gamma-secretase specificity for Abeta production and contributes to AD pathogenesis. Cell Res. 2010;20:138–153. doi: 10.1038/cr.2010.3. [DOI] [PubMed] [Google Scholar]
- Wang J.M., Singh C., Liu L., Irwin R.W., Chen S., Chung E.J., Thompson R.F., Brinton R.D. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2010;107:6498–6503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Liao W., Fang J., Liu Q., Yao J., Hu M., Ding K. A glucan isolated from flowers of Lonicera japonica Thunb. inhibits aggregation and neurotoxicity of Abeta42. Carbohydr. Polym. 2014;110:142–147. doi: 10.1016/j.carbpol.2014.03.060. [DOI] [PubMed] [Google Scholar]
- Winslow B.T., Onysko M.K., Stob C.M., Hazlewood K.A. Treatment of Alzheimer disease. Am. Fam. Physician. 2011;83:1403–1412. [PubMed] [Google Scholar]
- Zhao H.B., Lin S.Q., Liu J.H., Lin Z.B. Polysaccharide extract isolated from Ganoderma lucidum protects rat cerebral cortical neurons from hypoxia/reoxygenation injury. J. Pharmacol. Sci. 2004;95:294–298. doi: 10.1254/jphs.sc0040011. [DOI] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.