Abstract
With the goal of identifying molecular components of the low-water-potential response, we have carried out a two-part selection and screening strategy to identify new Arabidopsis mutants. Using a system of polyethylene glycol-infused agar plates to impose a constant low-water-potential stress, putative mutants impaired in low-water-potential induction of the tomato (Lycopersicon esculentum) le25 promoter were selected. These lines were then screened for altered accumulation of free Pro. The seedlings of 22 mutant lines had either higher or lower Pro content than wild type when exposed to low water potential. Two mutants, designated low-water-potential response1 (lwr1) and lwr2, were characterized in detail. In addition to higher Pro accumulation, lwr1 seedlings had higher total solute content, greater osmotic adjustment at low water potential, altered abscisic acid content, and increased sensitivity to applied abscisic acid with respect to Pro content. lwr1 also had altered growth and morphology. lwr2, in contrast, had lower Pro content and less osmotic adjustment leading to greater water loss at low water potential. Both lwr1 and lwr2 also had altered leaf solute content and water relations in unstressed soil-grown plants. In both mutants, the effects on solute content were too large to be explained by the changes in Pro content alone, indicating that LWR1 and LWR2 affect multiple aspects of cellular osmoregulation.
Drought exposes plants to a decrease in soil water content, quantified as a decrease in soil water potential (ψw), which decreases the ability of plants to absorb water from the soil (Boyer, 1982, 1985). Osmotic adjustment functions to avoid excessive dehydration through the accumulation of intercellular solutes, which lowers cellular ψw and maintains a favorable ψw gradient for water movement into the plant (Morgan, 1984; Zhang et al., 1999). Osmotic adjustment is defined as the amount of solute accumulated in response to low ψw excluding any increase in solute concentration that occurs solely because of cellular water loss (Morgan, 1984) and is a specific example of the more general process of osmoregulation, the mechanisms that control cellular solute and water content, and turgor. In plants at constant high ψw, solute content and turgor remain relatively stable (Silk et al., 1986; Kutschera, 1991). Net deposition of new solutes occurs mainly in growing tissue where it is needed to drive the uptake of water necessary for cell expansion (Silk et al., 1986). Thus, solute deposition is closely coordinated with growth and solute content subject to tight homeostatic regulation. Exposure to low ψw alters this regulation and leads to the accumulation of additional solutes throughout much of the plant. The increase in solute content involves many solute species including K+, sugars, and various types of compatible solutes (Morgan, 1984; Sharp et al., 1990; Zhang et al., 1999). The upstream sensing and signaling mechanisms involved in perceiving external ψw and controlling cellular solute content and turgor are unknown.
Accumulation of the compatible solute Pro is a highly regulated stress response. Low-ψw-induced Pro accumulation involves increased Pro synthesis and decreased Pro catabolism indicated by both biochemical (Rhodes et al., 1986) and gene expression studies (Delauney and Verma, 1993; Rentsch et al., 1996; Yoshiba et al., 1997). In specific tissues, changes in import or export of Pro (Girouse et al., 1996; Verslues and Sharp, 1999) may also be important. There is strong evidence that Pro accumulation is dependent on abscisic acid (ABA; Bray, 1993; Ober and Sharp, 1994; Strizhov et al., 1997), although ABA is likely not the only regulatory factor involved (Savoure et al., 1997). Understanding the regulation of Pro accumulation is likely to be useful in understanding other aspects of low-ψw responses, including both osmotic adjustment and ABA-dependent responses.
Despite the importance of forward genetic analysis in Arabidopsis, we are not aware of any screen that has used low-ψw response as the primary criteria for mutant isolation. The most closely related work, performed by Zhu and colleagues (Ishitani et al., 1997; Xiong and Zhu, 2002), has identified numerous mutants with altered responses to salt stress, low temperature, and exogenous ABA. While salt stress has an osmotic component, after the first few hours of exposure, ion toxicity, caused by excess Na+, is the main factor causing plant stress (Munns, 2002). A mutant screen that identifies loci that alter the response to low-ψw treatment has the potential to find new loci specifically involved in the low-ψw response. Using a system of polyethylene glycol (PEG)-infused agar plates to impose reproducible, constant low-ψw treatments (van der Weele et al., 2000), we combined negative selection based on expression of a stress-induced promoter with screening for altered Pro accumulation to isolate mutants in the low-ψw response.
Twenty-two lines were identified in which induction of a low-ψw-regulated promoter was decreased and low-ψw-induced Pro accumulation was either increased or decreased relative to wild type. Detailed characterization of two mutants found that total solute content and osmotic adjustment were also altered. This work provides a means to understand not only Pro accumulation and other low-ψw responses, but also the processes underlying osmoregulation and osmosensing in plants.
RESULTS
Low-ψw Responses of Arabidopsis Seedlings
PEG-infused agar plates (adapted from van der Weele et al., 2000) were used to impose a precisely defined, constant, low-ψw treatment to Arabidopsis seedlings. This allowed the response to a particular ψw treatment to fully develop over time and reach a steady-state condition. Use of PEG for these experiments also has several advantages. High Mr PEG, such as the PEG-8000 used in these experiments, mimics the effects of soil drying by causing cytorrhysis (withdrawal of water from both the cell wall and cytoplasm) instead of plasmolysis (withdrawal of water only from the cytoplasm leading to separation of the cell wall and cell membrane; Carpita et al., 1979; Oertli, 1985). In addition, PEG is not taken up by the plant (Hohl and Schopfer, 1991) and has no toxic effects as long as root damage is avoided (Lawlor, 1970; Verslues et al., 1998; van der Weele et al., 2000). All the agar plate experiments reported here were completed without the addition of sugar to the media to avoid the effects of high levels of external sugars on osmoregulation and ABA response (Arenas-Huertero et al., 2000; Laby et al., 2000).
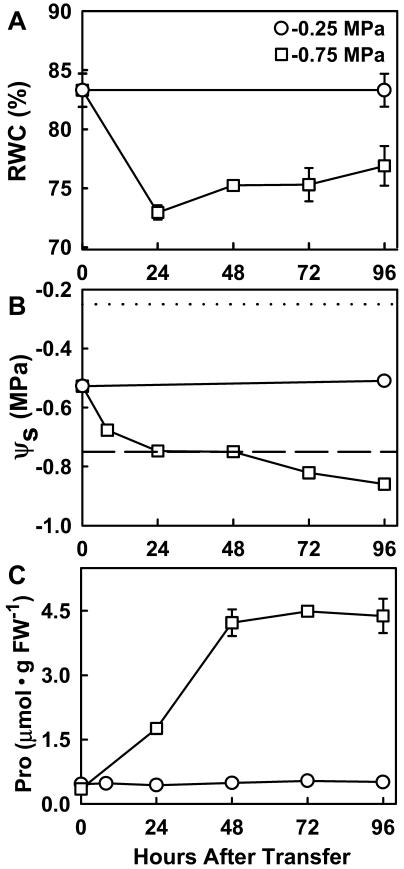
Three-day-old seedlings were transferred to PEG-infused plates for low-ψw treatment (−0.75 MPa) or to a new plate without PEG (−0.25 MPa) for the control treatment. Seedling relative water content (RWC) decreased in the first 24 h after transfer to low ψw and then partially recovered at later times (Fig. 1A). Likewise, seedling osmotic potential (ψs) decreased rapidly in the initial 24 h after transfer to low ψw, was equal to the agar ψw from 24 to 48 h, and then decreased further from 48 to 96 h (Fig. 1B). Pro content increased steadily until 48 h after transfer when Pro content stabilized (Fig. 1C). Even though Pro content increased approximately 10-fold, the total increase in Pro content could account for only a small portion (approximately 3%) of the total −0.33 MPa decrease in seedling ψs that occurred during the 96-h low-ψw treatment. Pro accumulation and osmotic adjustment occurred more slowly than other stress responses, such as gene expression, commonly studied in Arabidopsis. Thus, our subsequent experiments examining Pro accumulation and osmotic adjustment focused on the response 72 or 96 h after the start of low-ψw treatment.
Figure 1.
Wild-type response to low-ψw treatment. Three-day-old wild-type seedlings (le25:ADH Ben) were transferred to either −0.25 MPa (control) or −0.75 MPa PEG-infused agar plates, and low-ψw responses were measured over a 96-h period. A, Seedling RWC. Data are means ± se (n = 6). B, Seedling ψs. Dotted line shows the agar ψw of the −0.25 MPa treatment. Dashed line shows the agar ψw of the −0.75 MPa treatment. Data are means ± se (n = 3–4). C, Seedling Pro content. Data are means ± se (n = 4–6). Error bars are not shown when smaller than symbols.
Mutant Isolation
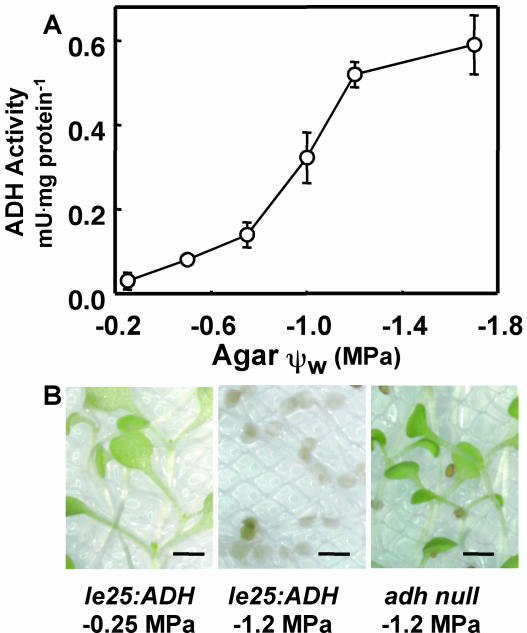
A negative selection scheme followed by a screen for altered Pro accumulation was used to isolate mutants affected in low-ψw responses. An adh− line of Arabidopsis (ecotype Bensheim) was transformed with a construct containing the ADH-coding region (Chory et al., 1995) controlled by the ABA- and stress-inducible le25 promoter of tomato (Lycopersicon esculentum). In le25:ADH transgenic Arabidopsis seedlings, ADH activity increased after seedlings were transferred to low ψw and reached a maximal level at approximately 48 h after transfer (data not shown), demonstrating that the le25 promoter was active in Arabidopsis. le25-driven ADH activity was measured 48 h after seedlings were transferred to plates of varying severities of low ψw. The highest ADH activity was measured at −1.7 MPa and was 10-fold greater than the activity of seedlings exposed to −0.25 MPa (Fig. 2A). Untransformed adh− plants had no detectable ADH activity at any ψw (data not shown). le25:ADH seedlings exposed to −1.2 MPa were sensitive to 1.2 mm allyl alcohol (Fig. 2B) because ADH metabolizes allyl alcohol to acrolein, a phytotoxic compound (Chory et al., 1995). The untransformed adh− or le25:ADH seedlings exposed to control media (−0.25 MPa) were not killed by this treatment (Fig. 2B). This le25:ADH line is hereafter referred to as wild type or Ben.
Figure 2.
ADH activity and allyl alcohol responses of le25:ADH Ben seedlings. A, Induction of ADH activity by low ψw. ADH activity was assayed 48 h after seedlings were transferred to a range of agar ψw. Data are means ± se (n = 5–7). B, Appearance of le25:adh and Ben adh null seedlings 7 d after a 2-h, 1.2 mm allyl alcohol treatment. Prior to the allyl alcohol treatment, 3-d-old seedlings were exposed to either control (−0.25 MPa) or low-water-potential stress (−1.2 MPa) for 2 d. After the allyl alcohol treatment, seedlings were transferred to control plates. Scale bars in pictures indicate 1 mm. The nylon mesh used to transfer seedlings between plates can be seen in the background.
Seeds of the wild-type line were ethyl methanesulfonate mutagenized and M1 seed collected in pools of 35 to 40 plants. Approximately 600 seeds from each of more than 300 pools were germinated, the seedlings transferred to low-ψw agar plates (−1.2 MPa) for 48 h, treated with 1.2 mm allyl alcohol for 2 h, and transferred to high-ψw plates for 7 d. Of the seedlings surviving the allyl alcohol treatment, approximately 1,000 were chosen and transferred to soil. M2 seed was harvested from approximately 600 individual plants. A total of 135 of these lines having either higher or lower seedling Pro content than wild type after a 72 h −1.2 MPa treatment were planted in soil. M3 seed was collected from each line and rescreened for altered Pro accumulation. Thirty-five lines, which showed substantial differences in Pro accumulation relative to wild type in both the M2 and M3 generations, were selected for further analyses. These lines were twice backcrossed to wild type, twice selfed, and allyl alcohol resistance and Pro accumulation at −1.2 MPa was quantified in seedlings from each of the backcrossed lines.
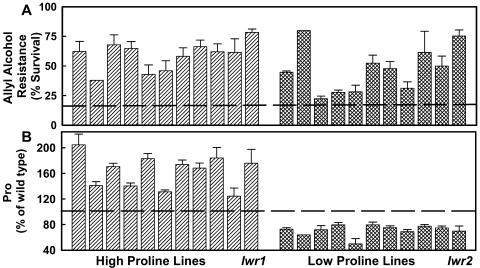
Twenty-two lines with increased allyl alcohol resistance (24% to 78% seedling survival after allyl alcohol treatment compared to 21% for Ben wild type) were chosen for further study (Fig. 3A). Of these lines, one-half had increased Pro accumulation (125% to 205% of the Ben wild type) and one-half had decreased Pro accumulation (50% to 80% of Ben wild type) in response to media of −1.2 MPa (Fig. 3B). The remainder of this report focuses on two mutants, designated as low-water-potential response1 (lwr1) and lwr2. lwr1 and lwr2 are recessive, nonallelic mutants (Table I).
Figure 3.
Allyl alcohol resistance and Pro content of 22 mutant lines. A, Percent survival of each mutant line to allyl alcohol treatment. Allyl alcohol selection was performed as in Figure 2 except that 3.0-mm allyl alcohol was used. Dashed line across the bottom of the figure indicates the wild type (unmutagenized le25:ADH Ben) response (21% survival). B, Pro content of each mutant line. Three-day-old seedlings were transferred to −1.2 MPa PEG-infused plates for 3 d. Pro content was determined and expressed relative to the wild-type control. The dashed line indicates the wild-type level (100%, which in these experiments was 6.42 ± 0.35 μmol g FW−1). The same mutant lines are depicted in the same order in both panels. Therefore, the allyl alcohol resistance and Pro content for the same line can be compared by looking at the bars in the same horizontal position in the two sections. The lwr1 and lwr2 mutants are individually labeled. Data are means ± se (n = 3–6) in both A and B.
Table I.
Genetic analysis of lwr1 and lwr2
| Genotype | Allyl Alcohol Resistance (% Seedling Survival) |
|---|---|
| Ben | 35 ± 6 |
| lwr1 | 66 ± 9a |
| lwr2 | 75 ± 5a |
| Ben × lwr1 | 6 ± 2 |
| Ben × lwr2 | 17 ± 4 |
| lwr1 × lwr2 | 23 ± 7 |
Percent survival of wild type (Ben; unmutagenized le25:ADH), lwr1, lwr2, and F1 seedlings from crosses of these three genotypes after a 2-h allyl alcohol treatment. Seedlings were transferred to control media after the allyl alcohol treatment (2 mm), and survival was measured 7 d after transfer.
Significantly greater than Ben at P ≤ 0.01.
Altered Pro Content of lwr1 and lwr2
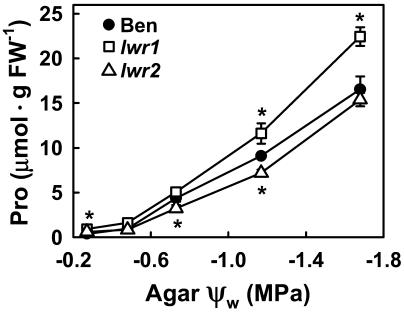
Steady-state Pro accumulation of both mutants and wild type was further characterized by quantifying Pro content 96 h after transfer of seedlings to a range of ψw treatments (Fig. 4). In wild type, Pro content increased in an approximately linear manner with decreasing ψw and was 44-fold greater at −1.7 MPa than at −0.25 MPa. The Pro content of lwr1 seedlings was 1.4-fold greater than that of wild type when exposed to −1.2 and −1.7 MPa. The Pro content of lwr2 seedlings was approximately 75% of the wild type at −0.75 and −1.2 MPa. Both lwr1 and lwr2 had significantly higher Pro content than wild type in the unstressed (−0.25 MPa) treatment.
Figure 4.
Pro contents of wild type (Ben), lwr1, and lwr2 in response to a range of agar ψw. Pro content was assayed 96 h after transfer of 3-d-old seedlings to the indicated ψw. Data are means ± se (n = 5–8). Significant differences between mutant and wild type (P ≤ 0.05) are indicated by asterisks in the figure.
Altered Solute Content and Osmotic Adjustment of lwr1 and lwr2
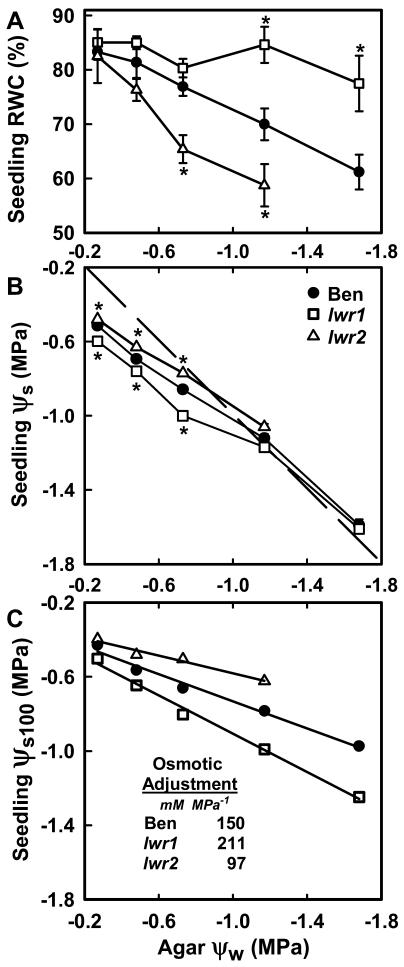
To determine the extent of water loss, solute accumulation, and osmotic adjustment in the three genotypes, the RWC and ψs of seedlings was quantified after 96 h of exposure to a range of ψw treatments. RWC of wild-type seedlings steadily decreased in response to increasing severities of low ψw agar (Fig. 5A). As would be expected for turgid tissue, seedling ψs was lower than agar ψw from −0.2 to −0.75 MPa (Fig. 5B). At −1.2 and below, seedling ψs and agar ψw were equivalent (the dashed line in Fig. 5B indicates the points where seedling ψs equals agar ψw), indicating that seedling turgor was near zero.
Figure 5.
Analysis of osmotic adjustment of wild type (Ben), lwr1, and lwr2. A, RWC of mutants and wild type after 96 h of exposure to a range of agar ψw. Data are means ± se (n = 4–10). Significant differences between mutant and wild type are indicated by an asterisk in the figure (P < 0.05). B, ψs of wild-type and mutant seedlings measured 96 h after transfer to a range of agar ψw. The dashed diagonal line indicates where seedling ψs equals ψw of the agar plate. Data are means ± se (n = 4–10). Error bars are smaller than symbols for all data points. Significant differences between mutant and wild type are indicated by an asterisk in the figure (P < 0.001). C, ψs at 100% RWC (ψs100) of mutant and wild-type seedlings calculated from data in A and B. Lines are regression lines fitted to the data for each genotype (r2 > 0.97 in all cases). The regression lines of both lwr1 and lwr2 were significantly different from that of wild type (F test; P = 0.0003 for lwr1 and wild type and P = 0.003 for lwr2 and wild type). Inset, Osmotic adjustment (slope of each regression line converted to millimolar solute concentration) per MPa decrease in ψw.
To quantify seedling osmotic adjustment, we used the data in Figure 5, A and B to calculate ψs at 100% RWC (ψs100; Wilson et al., 1979; Babu et al., 1999) for each ψw treatment. Calculating ψs100 removes any decrease in ψs caused solely by dehydration-induced increase in concentration of existing solutes. We then used regression analysis to quantify the extent that ψs100 decreased in response to decreased agar ψw. The slope of the line when seedling ψs100 is plotted against agar ψw is independent of the basal solute content in the unstressed (−0.25 MPa) treatment and thus provides an accurate account of low ψw-induced osmotic adjustment. When this analysis was performed for wild-type seedlings there was a linear relationship between ψs100 and agar ψw (ψs100 = 0.37ψw − 0.37; r2 = 0.98). The slope of this line indicated that wild-type seedlings had just 37% (150 mm of solutes per MPa decrease in agar ψw; Fig. 5C inset) of the solute accumulation needed to fully osmotically adjust to decreased agar ψw and avoid water loss.
For lwr1 seedlings, RWC was not significantly different from wild type from −0.25 to −0.75 MPa (Fig. 5A). In the more severe stress treatments of −1.2 and −1.7 MPa, the RWC of lwr1 seedlings was significantly higher than wild type (Fig. 5A). The ψs of lwr1 seedlings was lower than wild type above −1.2 MPa (Fig. 5B). At −0.75 MPa, for example, the solute concentration in lwr1 seedlings was 58 mm higher than wild type. For lwr1, the regression line of ψs100 versus agar ψw (ψs100 = 0.51ψw − 0.39; r2 = 0.99) was significantly different than wild type (F test, P = 0.0003). The difference in the slopes of the regression lines for wild type and lwr1 (211 mm MPa−1; Fig. 5C inset) indicated that lwr1 had one-third greater osmotic adjustment than wild type.
In contrast, lwr2 had reduced RWC at −0.75 and −1.2 MPa, indicating a lack of solute accumulation that would allow the seedlings to retain water. Consistent with this, the ψs of lwr2 seedlings was significantly higher than wild type at −0.25, −0.5, and −0.75 MPa (P ≤ 0.001; Fig. 5B). The regression line for ψs100 plotted against agar ψw (ψs100 = 0.24ψw − 0.34; r2 = 0.97) differed from that of wild type (F test, P = 0.003), and the difference in slope of these lines demonstrated that osmotic adjustment of lwr2 (97 mm MPa−1; Fig. 5C inset) was one-third less than that of wild type. At −1.7 MPa, lwr2 seedlings were visibly more dehydrated than wild-type seedlings, yet they increased in fresh weight (FW) only slightly when rehydrated indicating that the seedlings were damaged by severe water loss at this ψw. Thus, data for lwr2 at −1.7 MPa were not included in the above analysis of osmotic adjustment.
The increased solute content and osmotic adjustment of lwr1 was unique in our mutant collection. However, we have found six other low-Pro lines that were similarly reduced in osmotic adjustment as lwr2 (P.E. Verslues and E.A. Bray, unpublished data). Complementation testing between these other six lines and lwr2 is currently under way. None of the 14 other mutant lines had substantial changes in osmotic adjustment despite having, in many cases, even larger changes in Pro accumulation than lwr1 or lwr2.
K+ and Other Solutes Contribute to Changes in Osmotic Content of lwr1 and lwr2
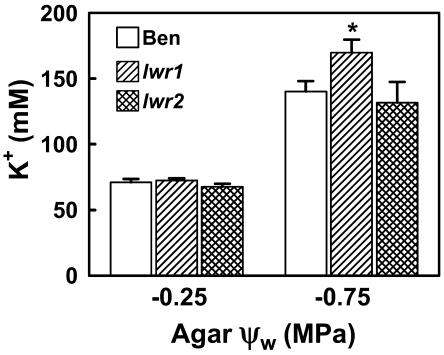
The changes in solute content and osmotic adjustment of lwr1 and lwr2 were too large to be explained solely by the change in Pro content of the mutants. To begin to determine the types of metabolic alterations caused by the mutations in lwr1 and lwr2, we determined the levels of K+ in mutant and wild-type seedlings (Fig. 6). K+ is present at high concentrations in plant cells and can have a large impact on overall cellular solute content and osmoregulation (Sharp et al., 1990). At −0.25 MPa, the K+ concentration of both mutants and wild type were similar. When seedlings were transferred to −0.75 MPa, there was a 2-fold increase in K+ concentration for Ben and lwr2 but a significantly greater (2.4-fold) increase for lwr1.
Figure 6.
Concentration of K+ in unmutagenized le25:ADH (Ben), lwr1, and lwr2 seedlings at −0.25 and −0.75 MPa. Data are means ± se (n = 4–8). lwr1 was significantly different from wild type (P = 0.05) at −0.75 MPa (indicated by an asterisk).
The total difference in solute content between mutant and wild-type seedlings was calculated from the ψs100 data in Figure 5C and the concentrations of K+ and Pro at 100% RWC then used to calculate the contribution of each solute to the total change in solute content of each mutant compared to the wild type (Table II). At −0.75 MPa, one-half of the difference in solute content between wild type and lwr1 can be explained by the higher K+ content of the mutant (Table II). Since the anions needed to balance the charge of the K+ are likely to be present at concentrations similar to that of K+ itself, K+ and its associated anions can account for nearly all of the increased solute content of lwr1 at −0.75 MPa. The contribution of Pro to the increased solute content of lwr1 was only about 1% of the total (Table II). In unstressed lwr1 seedlings (−0.25 MPa), the solute content was 30 mm higher than wild type with Pro contributing about 2% of the difference. Unlike the situation with moderate stress, K+ only accounted for approximately 7% of the increased solute content. The identity of the solutes accumulating in unstressed lwr1 seedlings is unknown.
Table II.
Contribution of Pro and K+ to the altered solute content of lwr1 and lwr2
| Agar
|
||||||
|---|---|---|---|---|---|---|
| Ben
|
lwr1 ψw
|
lwr2
|
||||
| −0.25 | −0.75 | −0.25 | −0.75 | −0.25 | −0.75 | |
| MPa | ||||||
| ψs100 (MPa) | −0.428 | −0.661 | −0.502 | −0.803 | −0.396 | −0.503 |
| Solute content (mm)a | 175 | 270 | 205 | 328 | 162 | 206 |
| Solute difference (mm)b | 30 | 58 | −13 | −64 | ||
| Pro100 (mm)c | 0.31 | 3.20 | 0.76 | 3.86 | 0.49 | 2.03 |
| Pro difference (mm) | 0.45 | 0.66 | 0.18 | −1.17 | ||
| % Solute differenced | 2 | 1 | 0 | 2 | ||
| K100+, (mm)e | 59 | 108 | 61 | 136 | 55 | 86 |
| K+ difference (mm) | 2 | 29 | −4 | −22 | ||
| % Solute differencef | 7 | 50 | 26 | 34 | ||
ψs100 (Fig. 5) was converted to solute concentration (mm) and used to calculate the difference in solute concentration between mutants and wild type at −0.25 and −0.75 MPa. Pro (Fig. 4) and K+ (Fig. 6) contents were adjusted to 100% RWC and used to calculate the difference in content of Pro and K+ relative to wild type. These values were then compared to the total difference in solute content between mutant and wild type to estimate the contributions of Pro and K+ to the altered solute content of the mutants.
Total solute content at 100% RWC calculated using the equation: ψs = −RTC.
Difference in total solute content at 100% RWC between the mutant and wild type (Ben); negative values for lwr2 indicate that wild-type solute content was greater than that of lwr2.
Pro content at 100% RWC calculated from data in Figures 4 and 5; a correction for percent dry weight (≤4%) was applied to the Pro data.
Percent of the total difference in solute content at 100% RWC that can be accounted for by altered Pro content.
Percent of the total difference in solute content at 100% RWC that can be accounted for by altered K+ content.
For lwr2 at −0.75 MPa, total solute content at 100% RWC was 64 mm less than wild type (Table II). K+ content at 100% RWC was 22 mm less in lwr2 than wild-type seedlings, indicating that accumulation of K+ was reduced in lwr2 compared to wild type. In terms of total solute content, reduced K+ content accounted for one-third of the difference between mutant and wild type at both −0.25 and −0.75 MPa. Pro made a small (approximately 2% of the total) contribution to the altered solute content of lwr2 mutant.
These calculations showed that the lwr1 and lwr2 mutations affected the accumulation of more than one solute species. Although differences in subcellular compartmentation and tissue distribution could not be quantified in the above calculations, the relative contributions of Pro and K+ to the overall difference in solute content was consistent with their expected compartmentation within the cell. Pro is accumulated primarily in the relatively small volume of the cytoplasm and is an osmotically significant solute in this compartment (Leigh et al., 1981; Voetberg and Sharp, 1991; Hare et al., 1998). K+ accumulates in the much larger vacuolar volume and hence makes a larger contribution to the bulk tissue solute content.
Carbohydrates can also be osmotically important solutes (Sharp et al., 1990). We assayed Glc content of wild type and both mutants and found that Glc content was low (2 to 4 mm) and did not change in response to low ψw. This provides an indication that altered sugar content is not a factor in the altered solute content of lwr1 and lwr2.
lwr1 and lwr2 Have Altered ABA Accumulation or Response
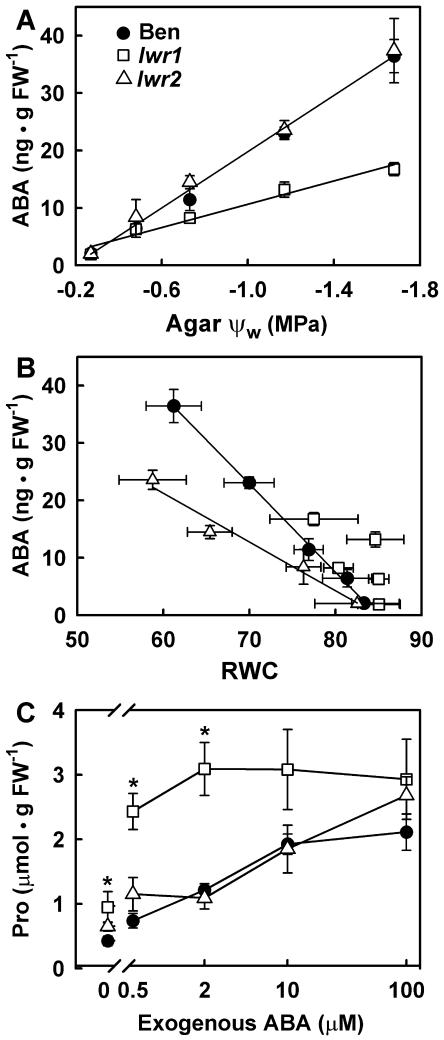
In our PEG-infused plate system, transfer of seedlings to low ψw elicited a rapid increase in ABA content, which peaked approximately 8 h after transfer to low ψw and then declined to a steady-state value that was up to 18-fold higher than the unstressed level (P.E. Verslues and A.E. Bray, unpublished data). The steady-state ABA content of lwr1, lwr2, and wild-type seedlings, assayed 96 h after transfer to a range of ψw treatments, linearly increased in response to decreasing external ψw (Fig. 7A). However, the increase was less for lwr1 than wild type. In contrast, the ABA content of lwr2 did not differ from wild type when expressed as a function of ψw (Fig. 7A). The peak ABA content at 8 h after transfer to −1.2 MPa was also reduced in lwr1 but unaffected in lwr2 (data not shown).
Figure 7.
ABA content and response of wild type (Ben), lwr1, and lwr2 seedlings to applied S(+)-ABA. A, ABA content of 3-d-old seedlings after 96 h at the indicated ψw. Data are means ± se (n = 4–9). Regression lines were fitted to the combined data of wild type and lwr2 (r2 = 0.99) and lwr1 (r2 = 0.97). The two regression lines were significantly different (F test, P = 5 × 10−12). B, ABA content expressed as a function of RWC. Regression lines were fitted for each genotype (Ben, r2 = 0.99; lwr1, r2 = 0.44 [this line is not shown in the figure]; lwr2, r2 = 0.97). The wild-type regression line was significantly different from that of lwr2 (F test, P = 0.0003) C, Pro content 96 h after transfer to plates containing the indicated concentrations of S(+)-ABA. Data are means ± se (n = 7). Significant differences between mutant and wild type are indicated by an asterisk in the figure (P < 0.002).
It has been suggested that the factor that induces ABA accumulation is loss of water and accompanying changes in turgor (Pierce and Rashke, 1980; Creelman and Zeevaart, 1985). Because lwr1 and lwr2 differ from wild type in RWC at low ψw, it is also of interest to examine the relationship between RWC and ABA content (Fig. 7B). Steady-state seedling ABA content was linearly related to decreasing RWC in wild type (r2 = 0.99). In lwr1 seedlings, RWC and ABA content were not well correlated, primarily because there was little change in the RWC of lwr1 with decreasing external ψw. The decreased ABA content of lwr1 compared to wild type at the same ψw may be an indirect effect of the increased RWC of lwr1 seedlings. The ABA content of lwr2 seedlings was also linearly related to RWC (r2 = 0.97), but the slope of the line was less than wild type. Thus, lwr2 seedlings were less sensitive to water loss than wild type with respect to ABA accumulation.
A change in responsiveness to ABA could also contribute to the phenotypes of lwr1 and lwr2. To test this possibility, we quantified the Pro content of mutant and wild-type seedlings after 96 h of exposure to a range of ABA concentrations applied at −0.25 MPa. In the wild type, Pro accumulation saturated at approximately 10 μm exogenous ABA in which a more than 4-fold increase in Pro content was observed (Fig. 7C). In lwr1, the maximum increase in Pro content was similar to wild type but saturated between 0.5 and 2 μm exogenous ABA. Internal ABA content was the same for all three genotypes and increased dramatically in response to exogenous ABA (18 ng g FW−1 at 0.5 μm exogenous ABA, 75 ng g FW−1 at 2.0 μm ABA, 245 ng g FW−1 at 10 μm ABA, and 3,058 ng g FW−1 at 100 μm ABA). The increased Pro levels in response to ABA application of lwr1 indicated an increased responsiveness to ABA in this mutant. In contrast, the Pro accumulation response of lwr2 to exogenous ABA did not differ from wild type (Fig. 7C), raising the possibility that lwr2 may affect low-ψw responses independently of ABA or may act upstream of ABA.
lwr1 and lwr2 Affect Leaf Water Relations of Well-Watered Mature Plants
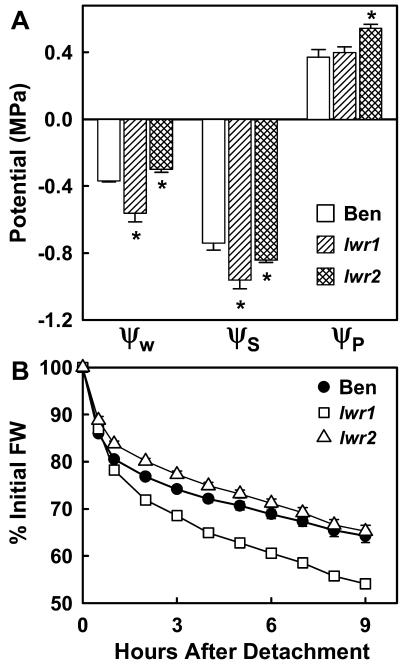
To determine whether the differences in solute content observed in seedlings were also present in adult plants, we measured water relations parameters of fully expanded leaves from mutant and wild-type plants grown under well-watered conditions (Fig. 8A). The soil ψw was −0.24 MPa and did not vary significantly between the three genotypes. The ψs of lwr1 leaves was −0.96 MPa, while the ψs of wild-type leaves was −0.74 MPa. The leaf ψw of lwr1 was less than wild type while the calculated ψp was similar to wild type. The decreased leaf ψw of lwr1 plants could indicate either an increased resistance to water flow through the plant or an increased rate of leaf water loss (Boyer, 1985). Consistent with the later possibility, detached leaves of lwr1 lost water more quickly than wild type (Fig. 8B). The difference in water loss was not caused by a difference in initial leaf hydration as leaves of all three genotypes were fully hydrated at the time of detachment (RWC > 97%). Although increased leaf water loss can explain the decreased leaf ψw of lwr1, it cannot explain the increased solute content and osmotic adjustment in seedlings grown under conditions of minimal transpiration (Fig. 5).
Figure 8.
Leaf water relations of wild type (Ben), lwr1, and lwr2 under well-watered conditions (soil ψw = −0.24 MPa). A, Leaf ψw, ψs, and calculated ψp. Data are means ± se (n = 4−5). Significant differences (P < 0.05) of mutants versus wild type are marked with an asterisk. B, Water loss of detached leaves. Data are means ± se (n = 14). Error bars are not shown when smaller than symbols.
There were also alterations in leaf water relation parameters of lwr2 plants grown at high ψw. Interestingly, the ψs of lwr2 leaves was −0.1 MPa (40 mm) greater than wild type (Fig. 8A) and calculated leaf turgor was 0.17 MPa higher than wild type.
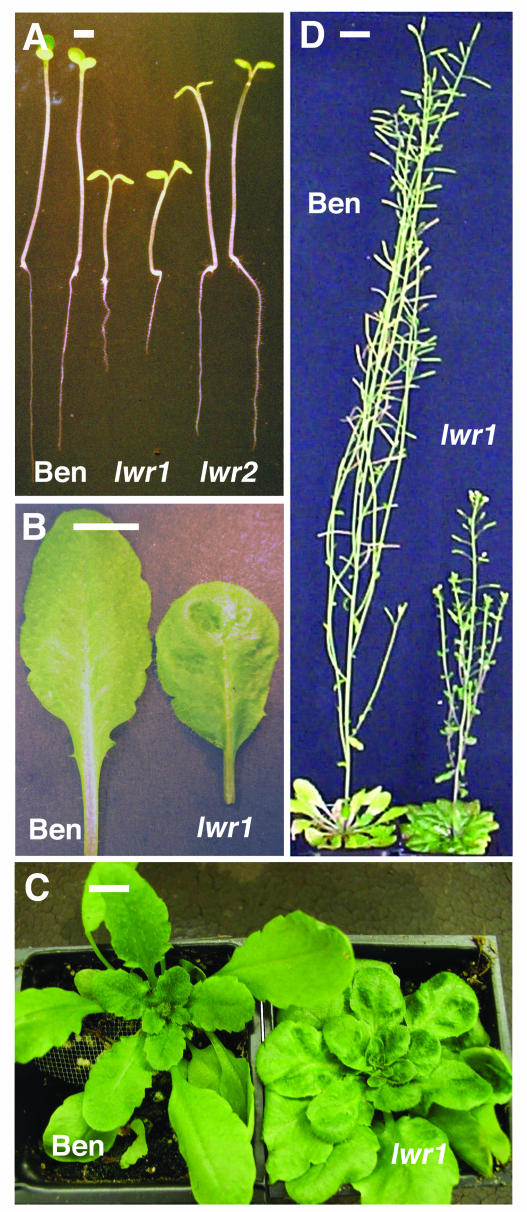
lwr1 Plants Have Altered Growth and Morphology
lwr1 plants exhibited several conspicuous alterations in growth and morphology. Unstressed lwr1 seedlings had reduced elongation of the hypocotyl and root (Fig. 9A). Well-watered soil-grown plants had altered leaf shape and appearance including a crinkled, uneven leaf surface and shorter petiole (Fig. 9B), and the production of additional rosette leaves compared to wild type (Fig. 9C). lwr1 plants also bolted later than wild type and were reduced in stature after bolting (Fig. 9D). Analysis of F3 seedlings collected from more than 100 plants in a segregating F2 population indicated that these changes in leaf morphology cosegregated with the increased seedling solute content described above (data not shown). We have not observed any changes in morphology or development in the lwr2 mutant.
Figure 9.
Morphological differences of lwr1 compared to wild type (Ben). A, Five-day-old seedlings of Ben, lwr1, and lwr2 at −0.25 MPa. Scale bar indicates 1 mm. B, Fully expanded rosette leaves of Ben and lwr1. Scale bar indicates 1 cm. C, Rosette stage plants of Ben and lwr1. Scale bar indicates 1 cm. D, Flowering plants of Ben and lwr1. Scale bar indicates 2 cm.
DISCUSSION
The isolation of mutants with altered low-ψw responses is a key step in understanding perception of water status and the mechanisms of adaptation to suboptimal water availability. In this study, several critical factors allowed us to isolate a group of mutant lines with altered low-ψw responses. Our experimental system permitted a steady low-ψw treatment to be applied over a sufficient period of time for Pro accumulation to reach steady-state levels, allowing this trait to be used for isolating and characterizing new mutant lines. Also, combining allyl alcohol selection with a screen for altered Pro accumulation allowed us to rapidly generate a population enriched for mutations affecting low-ψw responses, making the relatively laborious Pro screen feasible. Using two low-ψw-regulated traits together favored the isolation of mutants, such as lwr1 and lwr2, which are altered in multiple aspects of the low-ψw response.
Physiology and Genetics of Osmoregulation
The observations that content of solutes other than Pro is altered in lwr1 and lwr2 and that many of our other mutants did not have changes in osmotic adjustment despite having similar changes in Pro accumulation support the conclusion that the osmotic adjustment phenotypes observed in lwr1 and lwr2 are specific effects of these mutations and not simply a consequence of the altered Pro accumulation. Our calculated values of osmotic adjustment are consistent with values reported for several plant species using several methods of calculation (Zhang et al., 1999). In particular, our data of seedling ψs versus agar ψw follow the same pattern as previous data of ψs versus tissue ψw (Morgan, 1991).
Solute deposition is normally well coordinated with growth (Kutschera, 1991) and in both growing and nongrowing tissues the constancy of solute content over time indicates the existence of solute homeostatic regulation. lwr1 exhibits increased solute content in both nongrowing, unstressed leaves and seedlings at high and low ψw. The altered solute content in nongrowing leaves strongly suggests that the altered solute content of lwr1 is not solely an effect of altered growth. In unstressed seedlings where substantial growth is occurring, the increased solute content of lwr1 suggests that the normally rigid homeostatic mechanisms that coordinate growth and solute deposition have been altered. Like lwr1, the freezing-tolerant mutant esk1 has decreased leaf ψs, elevated Pro at high ψw, and reduced growth compared to wild type (Xin and Browse, 1998), although it does not appear to have the other morphological changes present in lwr1. When the water-stress- and ABA-inducible homeobox gene ATHB7 is constitutively overexpressed the plants are morphologically similar to lwr1, although it is not known if these plants have increased solute content (Hjellstrom et al., 2003). Future experiments will need to address the question of whether both the altered growth and increased solute content are direct effects of the lwr1 mutation. In either case, identification and further characterization of lwr1 will yield information on how solute deposition is regulated and coordinated with growth.
Because of the possibility of manipulating osmotic adjustment to increase productivity during drought stress, osmotic adjustment has been mainly studied in crop species where a number of studies have identified loci controlling osmotic adjustment (Morgan, 1991; Lilley et al., 1996; Teulat et al., 1998). While these studies have not conclusively shown that enhanced osmotic adjustment is useful in conferring stress resistance (Munns, 1988; Serraj and Sinclair, 2002), they have established that a relatively small number of loci explain most of the variation in osmotic adjustment. Grumet and Hanson (1986) selected barley (Hordeum vulgare) isolines based on differences in Gly betaine content. These barley isolines were found to also have alterations in ψs100, which could not be explained by the difference in Gly betaine content alone, similar to the altered ψs100 of lwr1 and lwr2. Grumet and Hanson (1986) concluded that Gly betaine content is controlled at least in part by osmoregulatory genes, which determine the overall cellular solute content. Our experiments suggest that Pro accumulation in Arabidopsis may also be controlled in part by osmoregulatory genes such as those proposed by Grumet and Hanson (1986). We now have the advantage that the well-developed molecular genetic resources available in Arabidopsis will allow the LWR1 and LWR2 gene products to be identified. Toward this goal, we have generated mapping populations by crossing lwr1 and lwr2 in the Ben ecotype to the Columbia ecotype, and scoring of these populations with appropriate molecular markers is proceeding.
Mechanisms of Osmosensing
In plant low ψw responses, the type of stimulus that is sensed and the type of sensors and signal transduction components involved remain to be identified (Wood, 1999). Evidence that loss of turgor is well correlated with ABA accumulation (Pierce and Rashke, 1980; Creelman and Zeevaart, 1985) and the fact that other possible stimuli such as water activity and structure change little over the range of ψw sensed by plants (Hsiao, 1973) have led to the idea that a change in turgor is the primary stimuli that elicits ABA accumulation and other low ψw responses. The hypothesis that lwr2 was either less able to sense water loss or turgor or less able to transmit the stimulus and activate downstream responses offers a compelling explanation for the observed phenotypes. A reduced ability to respond to low ψw-induced changes in water content or turgor could explain why lwr2 seedlings accumulated less ABA than wild type at a given RWC as well as the reduced solute content and osmotic adjustment of lwr2 across a range of ψw. In lwr2 source leaves at high ψw, disrupted sensing of turgor or water content may lead to the build up of solutes, either produced by photosynthesis or arriving in the transpiration stream, which otherwise would be exported or metabolized. This in turn would lead to higher leaf turgor in the mutant compared to wild type. The fact that lwr2 did not show altered response to exogenous ABA suggests the intriguing possibility that lwr2 acts upstream of ABA action or acts independently of ABA. Also, the observation that lwr2 did not have increased leaf water loss shows that the reduced solute content and osmotic adjustment of lwr2 are regulated independently of leaf water loss. This is consistent with previous observations that regulation of stomatal conductance is not dependent on a change in leaf water status (Wilkinson and Davies, 2002) and that ABA-induced stomatal closure is independent of ABA-induced Pro accumulation (Maggio et al., 2002).
In the case of lwr1, increased Pro and solute content could be caused by an overactivation of the osmosensing or osmoregulatory mechanism. The reduced expression from the le25 promoter can be explained by the reduced ABA content of the mutant at −1.2 MPa. The decreased ABA content may have been caused by the higher RWC of lwr1. Thus, the reduced ABA accumulation in lwr1 could be an indirect effect of the increased solute content resulting in reduced loss of water in the mutant compared to the wild type instead of a direct regulatory effect of lwr1 on ABA content.
MATERIALS AND METHODS
Plant Growth Conditions and Low ψw, Allyl Alcohol, and ABA Treatment
The PEG-infused plate system was a modification of that described by van der Weele et al. (2000). All experiments were done without the addition of any carbon source to the media. PEG-infused plates were made by dissolving solid PEG-8000 (Sigma, St. Louis) in a sterilized solution of basal media (half-strength Murashige and Skoog salts with 2 mm MES buffer) and adjusting the pH to 5.7. This PEG solution was then overlaid on agar-solidified (15 g L−1 Bacto agar) basal media (3:2, v/v). The agar media and PEG solution were then allowed to equilibrate for at least 12 h before the excess PEG solution was removed. ψw of the agar was verified using a vapor pressure osmometer as described below.
Seeds were surface sterilized and spread on basal agar media that had previously been overlaid with a nylon mesh to facilitate transfer of seedlings between plates. After stratification for 3 d at 4°C in low light, plates were moved to a growth room at 23°C having a 16-h light period (light intensity of 150–180 μE cm−2 min−1) and kept vertically so that seedlings grew along the surface of the agar. Plates were kept within a Plexiglas enclosure lined with wet paper towels. After 3 d of growth, seedlings were transferred on the nylon mesh to either fresh agar-solidified basal media (unstressed control) or PEG-infused agar.
Allyl alcohol treatment was performed 48 h after transfer of seedlings to a −1.2 MPa PEG-infused agar plate. At this time, plates were removed from the growth room and a solution of allyl alcohol dissolved in basal media was pipetted onto the top of the agar, covering the seedlings. After a 2-h incubation, seedlings were rinsed three times with sterile water, transferred to a fresh plate of agar-solidified basal media, and returned to the growth room. The number of surviving seedlings was scored 7 to 9 d later.
ABA treatments were performed by adding the indicated concentrations of S (+)-ABA (Lomon Bio Technology, Sichuan, China) dissolved in a small volume of ethanol to the basal growth media. Seedlings were transferred to the ABA-containing plates after 3 d of growth on basal media. Controls with ethanol only added to the basal media showed no effect on seedling Pro or ABA content.
Soil-grown plants were kept under long-day conditions (23°C, 16-h photoperiod).
Construction and Mutagenesis of le25:ADH Transgenic Arabidopsis
le25, which encodes a late-embryogenesis abundant protein, is induced by low-ψw treatment only under conditions of ABA accumulation (Cohen et al., 1991, 1999; Imai et al., 1995) and is partially induced by ABA application at high ψw (Imai et al., 1995). Deletion analysis and promoter mutagenesis showed that the le25 promoter contains a functional ABA-response element as well as at least one other cis-acting element required for full stress induction. The 392-bp fragment from the 3′ end of the le25 promoter, which was shown to confer full transcriptional activity in transgenic tomato plants (Lycopersicon esculentum; Shih, 1998), was fused to the ADH coding region (from plasmid pMY425 provided by the laboratory of M. Yanofsky). The fusion construct was ligated into pBI-101 and was used to transform Agrobacterium tumefaciens strain EHA105. The T-DNA fragment also contained a fusion of the Leu amino peptidase A promoter from tomato to the green fluorescent protein-coding region, which was not used in this study. An adh− line of Arabidopsis (Bensheim R002 provided by J. Chory) was transformed by root cocultivation. One transgenic line exhibiting stress induction of the ADH transgene was chosen and confirmed to have a single T-DNA insertion by DNA blotting of restriction enzyme digested genomic DNA using a probe to the ADH-coding region and by single copy reconstruction (data not shown).
Seeds of this le25:ADH transgenic line were ethane methylsulfonic acid mutagenized and M0 seed planted to soil. M1 seed was collected as pools each containing seed from 20 to 35 M0 plants. An aliquot (approximately 600 seeds) from each pool was plated and used for the initial allyl alcohol resistance selection.
Analysis of Plant Water Relations
Measurements of ψw and ψs were carried out using a vapor pressure osmometer (Model 5100C; Wescor, Logan, UT). ψs of plant tissue (approximately 20–50 seedlings or 3 10-mm-diameter leaf discs) was determined by twice freezing and thawing the tissue, grinding and briefly centrifuging to remove insoluble material, and measuring the osmolarity of the cell sap. For ψw measurements of soil or leaf tissue, a larger size sample chamber was used. Leaf ψw was measured on fully expanded leaves collected during the middle of the dark period. Leaf discs (4 discs of 10-mm diameter) were collected, immediately transferred to the sample chamber, and allowed to equilibrate in the instrument for 3 h. A stable instrument reading was obtained at that time. Solute concentration was converted to ψs using the van't Hoff equation ψs = −RTC, where R is the gas constant, T is absolute temperature, and C is the molar solute concentration. Turgor (ψP) was calculated using the equation ψw = ψs + ψP.
To quantify RWC, seedlings were removed from the agar plate using the nylon mesh, gently blotted, weighed, and placed in ice-cold water to rehydrate for 2 to 3 h. Seedlings, still on the nylon mesh, were then blotted and reweighed and dried at 65°C for 12 h. After obtaining the total dry weight, the weight of the nylon mesh without seedlings was measured and subtracted from the other weights before calculating RWC. RWC was multiplied by ψs or Pro or K+ concentration to determine the ψs100 or Pro or K+ concentration at 100% RWC. Because Pro was routinely expressed on a fresh-weight basis, a correction was applied for the percent dry weight (as determined during the RWC measurements) of the seedlings. The correction was small (less than 4%) in all cases and did not affect the interpretation of the data.
To determine leaf water loss, individual rosette leaves were weighed immediately after detachment and then at 30 min or 1 h intervals thereafter. In between measurements, leaves were kept on the laboratory bench. Leaves were collected near the end of the light period and were fully hydrated (RWC > 97%) at the beginning of each experiment.
Metabolite, ADH, and ABA Analysis
Pro was assayed on water-extracted seedling samples (Bates et al., 1973). K+ was assayed by atomic adsorption spectrometry. ADH activity was assayed by the ethanol-dependent reduction of NAD+ (Bailey-Serres and Dawe, 1996). ABA was assayed by radioimmunoassay (Bray and Beachy, 1985). For ABA quantification, seedlings were removed from the agar plate using the nylon mesh and twice rinsed (each rinse < 10 s) with a NaCl solution of the same ψw as the agar to remove any PEG or exogenous ABA, which may interfere with the assay of seedling internal ABA. Seedlings were then blotted dry and weighed.
Data reported for these assays represent the combined mean of at least 3 independent experiments with two or three samples collected in each experiment. Seedling samples typically consisted of 20 to 100 seedlings (15–200 mg of tissue) depending on assay and experimental treatment. Significant statistical differences were determined by standard two-tailed T-test with P values as noted in text or figures. Significant difference of regression lines was determined by F test (www.graphpad.com/curvefit).
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Acknowledgments
We thank Dr. Kelly Hershey for generating the le25:adh transgenic line used in this study and completing the mutagenesis; Dr. David Parker for use of equipment and assistance with the K+ analysis; Drs. Linda Walling, Patricia Springer, and Christina Walters for useful advice and discussion; and Rui Yu, Mayuki Tanaka, Suzie Kim, and Ramon Barajas for assistance in the laboratory.
This work was supported by the National Science Foundation (grant no. GE–9355042 to E.A.B.), and by the Graduate Division and the Department of Botany and Plant Sciences, University of California, Riverside (P.E.V.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.045856.
References
- Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, Leon P (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14: 2085–2096 [PMC free article] [PubMed] [Google Scholar]
- Babu RC, Pathan MS, Blum A, Nguyen HT (1999) Comparison of measurement methods of osmotic adjustment in rice cultivars. Crop Sci 39: 150–158 [Google Scholar]
- Bailey-Serres J, Dawe RK (1996) Both 5′ and 3′ sequences of maize adh1 mRNA are required for enhanced translation under low-oxygen conditions. Plant Physiol 112: 685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline in water-stress studies. Plant Soil 39: 205–207 [Google Scholar]
- Boyer JS (1982) Plant productivity and environment. Science 218: 443–448 [DOI] [PubMed] [Google Scholar]
- Boyer JS (1985) Water transport. Annu Rev Plant Physiol 36: 473–516 [Google Scholar]
- Bray EA (1993) Molecular responses to water deficit. Plant Physiol 103: 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray EA, Beachy RN (1985) Regulation by ABA of β-conglycinin expression in cultured developing soybean cotyledons. Plant Physiol 79: 746–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N, Sabularse D, Montezinos D, Delmer DP (1979) Determination of the pore size of cell walls of living plant cells. Science 205: 1144–1147 [DOI] [PubMed] [Google Scholar]
- Chory J, Li H-M, Mochizuki N (1995) Molecular methods for isolation of signal transduction pathway mutants. In DW Gailbraith, HJ Bohnert, DP Bourque, eds, Methods in Cell Biology, Vol 49. Academic Press, San Diego, pp 441–454 [DOI] [PubMed]
- Cohen A, Moses MS, Plant ÁL, Bray EA (1999) Multiple mechanisms control the expression of abscisic acid ABA-requiring genes in tomato plants exposed to soil water deficit. Plant Cell Environ 25: 989–998 [Google Scholar]
- Cohen A, Plant ÁL, Moses MS, Bray EA (1991) Organ-specific and environmentally regulated expression of two abscisic acid-induced genes of tomato. Plant Physiol 97: 1367–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Zeevaart JAD (1985) Abscisic acid accumulation in spinach leaf slices in the presence of penetrating and nonpenetrating solutes. Plant Physiol 77: 25–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4: 215–223 [Google Scholar]
- Girouse C, Bournoville R, Bonnemain J-L (1996) Water deficit-induced changes in concentration in proline and some other amino acids in the phloem sap of alfalfa. Plant Physiol 111: 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet R, Hanson AD (1986) Genetic evidence for an osmoregulatory function of glycine betaine accumulation in barley. Aust J Plant Physiol 13: 353–364 [Google Scholar]
- Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21: 535–553 [Google Scholar]
- Hjellstrom M, Olsson ASB, Engstrom P, Soderman EM (2003) Constitutive expression of the water deficit-inducible homeobox gene ATHB7 in transgenic Arabidopsis causes a suppression of stem elongation growth. Plant Cell Environ 26: 1127–1136 [Google Scholar]
- Hohl M, Schopfer P (1991) Water relations of growing maize coleoptiles. Comparison between mannitol and polyethylene glycol 6000 as external osmotica for adjusting turgor pressure. Plant Physiol 95: 716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao TC (1973) Plant responses to water stress. Annu Rev Plant Physiol 24: 519–570 [Google Scholar]
- Imai R, Moses MS, Bray EA (1995) Expression of an ABA-induced gene of tomato in transgenic tobacco during periods of water deficit. J Exp Bot 46: 1077–1084 [Google Scholar]
- Ishitani M, Xiong L, Stevenson B, Zhu JK (1997) Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell 9: 1935–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U (1991) Osmotic relations during elongation growth in hypocotyls of Helianthus annus L. Planta 184: 61–66 [DOI] [PubMed] [Google Scholar]
- Laby RJ, Kincaid MS, Kim DG, Gibson SI (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23: 587–596 [DOI] [PubMed] [Google Scholar]
- Lawlor DW (1970) Absorption of polyethylene glycols by plants and their effects on plant growth. New Phytol 69: 501–513 [Google Scholar]
- Leigh RA, Ahmad N, Wyn Jones RG (1981) Assessment of glycinebetaine and proline compartmentation by analysis of isolated beet vacuoles. Planta 153: 34–41 [DOI] [PubMed] [Google Scholar]
- Lilley JM, Ludlow MM, McCouch SR, O'Toole JC (1996) Locating QTL for osmotic adjustment and dehydration tolerance in rice. J Exp Bot 47: 1427–1436 [Google Scholar]
- Maggio A, McCully MG, Kerdnaimongkol K, Bressan RA, Hasegawa PM, Joly RJ (2002) The ascorbic acid cycle mediates signal transduction leading to stress-induced stomatal closure. Funct Plant Biol 29: 845–852 [DOI] [PubMed] [Google Scholar]
- Morgan JM (1984) Osmoregulation and water stress in higher plants. Annu Rev Plant Physiol 35: 299–319 [Google Scholar]
- Morgan JM (1991) A gene controlling differences in osmoregulation in wheat. Aust J Plant Physiol 18: 249–257 [Google Scholar]
- Munns R (1988) Why measure osmotic adjustment? Aust J Plant Physiol 15: 717–726 [Google Scholar]
- Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25: 239–250 [DOI] [PubMed] [Google Scholar]
- Ober ES, Sharp RE (1994) Proline accumulation in maize (Zea mays L.) primary roots at low water potentials I: requirement for increased levels of abscisic acid. Plant Physiol 105: 981–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertli JJ (1985) The response of plant cells to different forms of moisture stress. J Plant Physiol 121: 295–300 [Google Scholar]
- Pierce M, Rashke K (1980) Correlation between loss of turgor and accumulation of abscisic acid in detached leaves. Planta 148: 174–182 [DOI] [PubMed] [Google Scholar]
- Rentsch D, Hirner B, Schmelzer E, Frommer WB (1996) Salt stress-induced proline transporters and salt stress-repressed broad specificity amino acid permeases identified by suppression of a yeast amino acid permease-targeting mutant. Plant Cell 8: 1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D, Handa S, Bressan RA (1986) Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol 82: 890–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoure A, Hua X-J, Bertauche N, Van Montagu M, Verbruggen N (1997) Abscisic acid-independent and abscisic acid-dependent regulation of proline biosynthesis following cold and osmotic stresses in Arabidopsis thaliana. Mol Gen Genet 254: 104–109 [DOI] [PubMed] [Google Scholar]
- Serraj R, Sinclair TR (2002) Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ 25: 333–341 [DOI] [PubMed] [Google Scholar]
- Sharp RE, Hsiao TC, Silk WK (1990) Growth of the maize primary root at low water potentials II: role of growth and deposition of hexose and potassium in osmotic adjustment. Plant Physiol 93: 1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T-Y (1998) Characterization of the ABA- and Drought-Responsive Genes H1-S and le25 of Tomato. PhD Thesis, University of California, Riverside, CA
- Silk WK, Hsiao TC, Diedenhofen U, Matson C (1986) Spatial distribution of potassium, solutes, and their deposition rates in the growth zone of the primary corn root. Plant Physiol 82: 853–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizhov N, Abraham E, Okresz L, Blickling S, Zilberstein A, Schell J, Koncz C, Szabados L (1997) Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J 12: 557–569 [DOI] [PubMed] [Google Scholar]
- Teulat B, This D, Khairallah M, Borries C, Ragot C, Sourdille P, Leroy P, Monneveux P, Charrier A (1998) Several QTLs involved in osmotic-adjustment trait variation in barley (Hordeum vulgare L.). Theor Appl Genet 96: 688–698 [Google Scholar]
- van der Weele CM, Spollen WG, Sharp RE, Baskin TI (2000) Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J Exp Bot 51: 1555–1562 [DOI] [PubMed] [Google Scholar]
- Verslues PE, Ober ES, Sharp RE (1998) Root growth and oxygen relations at low water potentials: impact of oxygen availability in polyethylene glycol solutions. Plant Physiol 116: 1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Sharp RE (1999) Proline accumulation in maize (Zea mays L.) primary roots at low water potentials. II; metabolic source of increased proline deposition in the elongation zone. Plant Physiol 119: 1349–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voetberg GS, Sharp RE (1991) Growth of the maize primary root at low water potentials III: role of increased proline deposition in osmotic adjustment. Plant Physiol 96: 1125–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ (2002) ABA-based chemical signaling: the co-ordination of responses to stress in plants. Plant Cell Environ 25: 195–210 [DOI] [PubMed] [Google Scholar]
- Wilson JR, Fisher MJ, Schulze ED, Dolby GR, Lullow MM (1979) Comparison between pressure-volume and dew point-hygrometry techniques for determining the water relations characteristics of grass and legume leaves. Oecologia 41: 77–88 [DOI] [PubMed] [Google Scholar]
- Wood JM (1999) Osmosensing by bacteria: signals and membrane-based sensors. Microbiol Mol Biol Rev 63: 230–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Browse J (1998) eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc Natl Acad Sci USA 95: 7799–7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ 25: 131–139 [DOI] [PubMed] [Google Scholar]
- Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K (1997) Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol 38: 1095–1102 [DOI] [PubMed] [Google Scholar]
- Zhang J, Nguyen HT, Blum A (1999) Genetic analysis of osmotic adjustment in crop plants. J Exp Bot 50: 292–302 [Google Scholar]