Abstract
A growing body of evidence sheds light on the neurodevelopmental nature of schizophrenia with symptoms typically emerging during late adolescence or young adulthood. We compared the pre-symptomatic adolescence period with the full symptomatic period of adulthood at the behavioral and neurobiological level in the poly I:C maternal immune stimulation (MIS) rat model of schizophrenia. We found that in MIS-rats impaired sensorimotor gating, as reflected in disrupted prepusle inhibition (PPI), emerged post-pubertally, with behavioral deficits being only recorded in adulthood but not during adolescence. Using post mortem HPLC we found that MIS-rats show distinct dopamine and serotonin changes in the medial prefrontal cortex (mPFC), nucleus accumbens (Nacc), caudate putamen, globus pallidus, and hippocampus. Further, FDG-PET has shown that these animals had lower glucose uptake in the ventral hippocampus and PFC and a higher metabolism in the amygdala and Nacc when compared to controls. Changes in neurotransmission and metabolic activity varied across brain structures with respect to first appearance and further development. In the mPFC and Hipp, MIS-rats showed abnormal neurochemical and metabolic activity prior to and with the development of behavioral deficits in both adolescent and adult states, reflecting an early impairment of these regions. In contrast, biochemical alteration in the Nacc and globus pallidus developed as a matter of age. Our findings suggest that MIS-induced neurochemical and metabolic changes are neurodevelopmental in nature and either progressive or non-progressive and that the behavioral deficits manifest as these abnormalities increase.
Keywords: Poly I:C, Schizophrenia, Sensorimotor deficits, FDG-PET, Neurotransmission, Neurobiological trajectories
1. Introduction
Converging evidence from epidemiology, neuroimaging and postmortem studies suggests that schizophrenia is a neurodevelopmental disorder with disruptions to early brain development interacting with peri-adolescent brain maturation and leading to aberrant behavior, typically emerging during late adolescence or young adulthood. Although this postnatal delay is a characteristic feature of schizophrenia, the exact course and neurobiological level of the maldevelopment are not fully understood. Animal models serve as important tools for identifying and studying neurobiological alterations from early age to full symptom manifestation. However, only few experimental preclinical approaches consider developmental aspects.
Based on the observations that prenatal exposure to infection constitutes a risk factor for schizophrenia, animal models implicating maternal immune stimulation (MIS) have been established. Exposing pregnant rodents to the viral mimic polyriboinosinic-polyribocytidilic acid (poly I:C) is a commonly used neurodevelopmental approach to model schizophrenia. In this model, MIS results in the emergence of myriad of behavioral, neurochemical and brain structural abnormalities in the offspring, all related to schizophrenia (Meyer and Feldon, 2012). Previous studies (Piontkewitz et al., 2012) have demonstrated that the behavioral abnormalities induced by poly I:C first emerge in adulthood, resembling the developmental delay of symptom manifestation observed in the clinic. In contrast, neuropathological alterations have been detected at different time points. While some are seen during adolescence and predate behavioral deficits (i.e. reduced hippocampal volumes and neurogenesis), others are first observed in adulthood (i.e. enlarged lateral ventricles and reduced prefrontal cortex volumes) (Piontkewitz et al., 2009). It was this progressive nature of brain abnormalities that led clinicians and scientists to administer atypical antipsychotic drugs prior to the full manifestation of symptoms in an attempt to halt disease progression (McGlashan et al., 2006; Piontkewitz et al., 2009).
In this study, we were interested in comparing behavioral and neurobiological characteristics of pre-symptomatic adolescents with the full symptomatic period of adulthood using offspring of MIS rats. More specifically, we sought to study the protracted emergence of a schizophrenia related behavior (i.e. deficits in sensorimotor gating as reflected in disrupted prepulse inhibition), along with the development of abnormal brain activity patterns using 18 fluoro desoxyglucose positron emission tomography (FDG-PET) and changes in neurotransmitter levels using post mortem HPLC.
2. Methods and materials
2.1. Animals
Adolescent (post natal day (PND) 35 and 60) and adult (PND100) male Wistar rats were housed 2–4/cage in a temperature and humidity controlled vivarium with a 12-h light–dark cycle and with ad lib food and water. Experiments were performed during day time, according to the guidelines of the European Union Council Directive 2010/63/EU for care of laboratory animals and were approved by the local ethic committee (Regierungspräsidium Dresden, Germany for behavioral and biochemical studies and Ethics Committee for Animal Experimentation of Hospital Gregorio Marañón Madrid, Spain for FDG-PET studies).
2.2. Prenatal Poly I:C treatment and allocation of animals to experimental groups
On gestation day 15, pregnant dams (Harlan Laboratories, Germany and Spain) were given a single i.v. injection to the tail vein of either poly I:C (4 mg/kg; SIGMA, Germany) dissolved in saline, or saline alone (Zuckerman et al., 2003; Klein et al., 2013). Behavioral phenotyping was performed on 35, 60 and 100 day old offspring (n = 10 in both poly I:C and saline groups). Biochemical analysis was performed on brains of 35 and 100 day old offspring (PND35: n = 10 poly I:C and n = 10 saline, PND100: n = 16 poly I:C and n = 21 saline offspring). FDG-PET analysis was carried-out in two imaging sessions at PND35 and 100 (n = 15 poly I:C and n = 10 saline offspring). Each experimental group consisted of only male offspring derived from multiple independent litters.
2.3. Behavioral phenotyping
Prepulse inhibition (PPI) of the acoustic startle response (ASR) was measured in a sound-attenuated chamber using a movement-sensitive piezoelectric measuring platform (Startle Response System, TSE, Germany). Test sessions consisted of seven different trial types delivered in pseudorandom order: 1) pulse alone (100 dB sound pressure level (SPL), white noise, 20 ms); 2) control (no stimulus); 3e4) prepulse alone (72 or 68 dB, pure tone, 10 kHz, 20 ms); 5e7) prepulse (72, 68, or 64 dB) each followed by a pulse with an interstimulus interval of 100 ms. A total of 10 presentations of each type was given with 20–30 s inter-trial intervals. Background noise intensity during the whole experiment was 60 dB SPL, white noise. The average PPI over the three prepulse intensities was calculated (Klein et al., 2013; Mattei et al., 2014).
2.4. Post-mortem neurochemical analyses
Rats were decapitated and micropunches were taken from 0.5–1 mm thick brain slices from the medial prefrontal cortex (mPFC), nucleus accumbens (Nacc), caudate-putamen (CPu), hippocampus (Hipp), globus pallidus (GP), thalamus (Thal) and ventral tegmental area (VTA). Monoamines (dopamine (DA), 5-HT) and their metabolites (DOPAC, HVA, 5-HIAA) were separated on a column (ProntoSil 120-3-C18-SH; Bischoff Analysentechnik und -geräte GmbH, Germany) and electrochemically detected (41,000, Chromsystems Instruments & Chemicals GmbH, Germany). Glutamate and GABA were precolumn-derivatized with o-phthalaldehyde-2-mercaptoethanol, separated on a column (ProntoSil C18 ace-EPS) and detected by their fluorescence at 450 nm after excitation at 330 nm (Winter et al., 2009).
2.5. Imaging
[18F]FDG was injected into the tail vein and, after a 45 min uptake period, animals were scanned for 45 min under isoflurane anesthesia (3% induction and 1.5% maintenance in 100% O2) using a small-animal PET/CT scanner (ARGUS PET/CT, SEDECAL, Madrid). Images were reconstructed using a 2D OSEM (ordered subset expectation maximization algorithm) with a spatial resolution of 1.45 mm FWHM (full width at half maximum), a voxel size of 0.3875 × 0.3875 × 0.7750 mm and an energy window of 400–700 keV. CT studies were acquired with the following parameters: 320 mA, 45 KV, 360 projections, 8 shots, and 200 μm of resolution and reconstructed using a Feldkamp algorithm (isotropic voxel size: 0.121 mm). One rat in each group (PND35 and PND100) was additionally scanned using a 7-T Biospec 70/20 MRI scanner (Bruker, Germany) under sevoflurane anaesthesia (4.5% induction and 2.5% maintenance in 100% O2) to provide anatomical templates for analysis of PET images. A T2-weighted spin echo sequence was acquired (TE = 33 ms, TR = 3732 ms, 34 slices of 0.8 mm, matrix size: 256 × 256 pixels at an FOV of 3.5 × 3.5 cm2).
PET images were co-registered in order to perform voxel-by-voxel comparisons and obtain statistical parametric maps. A random reference CT scan was selected for each group (CTref35 for PND35 and CTref100 for PND100), and all CT studies were co-registered with the respective reference CT scan. A non-rigid registration transformation was calculated in order to align CTref35 with CTref100 and applied to CT studies in group PND35 already aligned with CTref35. Final CT images were aligned with CTref100. The spatial transformation obtained for each CT was then applied to the corresponding PET image. MRI studies were spatially co-registered to the reference CT scan. A brain mask was segmented and applied to PET images. Resulting images were smoothed and voxel values were normalized to the average brain intensity (Pascau et al., 2009).
2.6. Statistical analysis
One way ANOVA and one-sample t-test were used for behavioral analysis. Two way ANOVAs with the factors MIS and age were used for biochemical analysis. When appropriate, ANOVAS were followed by Holm Sidak post hoc test. PET data was analyzed using SPM5 software package (Wellcome Trust Centre for Neuroimaging, UK). Groups were compared using a Flexible Factorial test, uncorrected for multiple comparisons. To reduce type I error, a 50-voxel clustering threshold was applied. A p-value of 0.05 was considered statistically significant.
3. Results
3.1. PPI
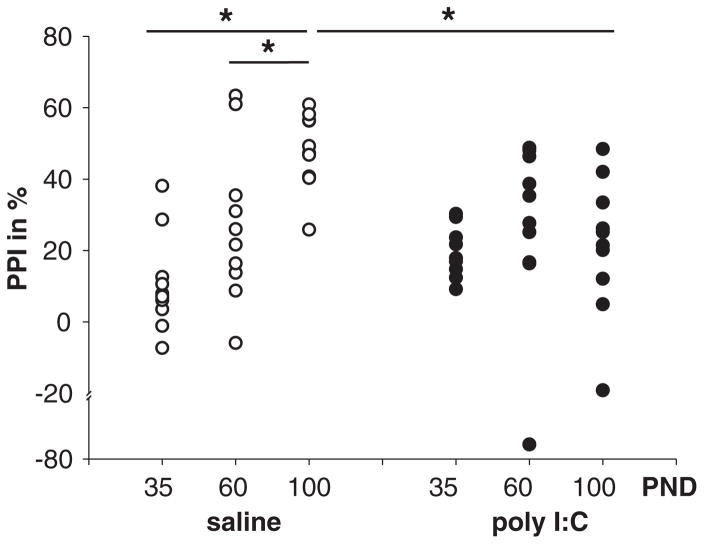
A significant difference was found in saline offspring (F(2,29) = 13.794, p < 0.001) but not in poly I:C offspring (F(2,29) = 0.059, p = 0.943), such that higher levels of PPI were apparent at PND100 when compared to PND60 and 35. Furthermore, a significant difference was found between poly I:C and saline offspring at PND100 (t(3.84), p = 0.001) but not at PND35 (t(−1.76), p = 0.09) or PND60 (t(0.325), p = 0.74). At PND100, poly I:C offspring exhibited lower levels of PPI when compared to saline offspring (Fig. 1).
Fig. 1.
The developmental course of PPI in saline and poly I:C offspring. Offspring of saline and poly I:C treated dams were tested for PPI at PND35, PND60 and PND100. Vertical point plots depict the percentage of PPI inhibition (%PPI) in each animal for each condition. In saline rats (left) PPI develops over time: a significant difference between PND35 and PND100 as well as between PND60 and PND100 was found. In poly I:C rats (right) no significant differences were found over all time-course comparisons. Comparisons between saline and poly I:C offspring revealed a significant difference at PND100 but not between PND30 or PND60. Significant differences indicated by asterisks (*); p < 0.05.
3.2. Neurochemistry
3.2.1. Age-related effects (Table 1 and Fig. 2)
Table 1.
Effects of maternal immune stimulation and age on neurotransmitter levels. Neurochemical contents were examined in adolescent (35) and adult (100), control and MIS (poly-I:C) offspring. Neurotransmitters (DA, DOPAC, HVA, dopamine turnover (DOPAC + HVA/dopamine) were measured in the mPFC, Hipp, Thal, Nacc, CPU, GP, and VTA and are expressed as mean values ± S.E.M. of μM per g protein. The presence of age- and MIS-dependent effects are depicted by age, MIS, and interaction, degrees of freedom (DF), F-values and statistical p-values based on two-way ANOVA of the neurochemical content in the corresponding brain area.
| Transmitter | Region | MIS | PND | Content in μM/g protein | Two-way ANOVA effects | DF | F-value | p-Value |
|---|---|---|---|---|---|---|---|---|
| DA | mPFC | control | 35 | 7.21 ± 0.73 | MIS | 1,41 | 0.266 | 0.609 |
| 100 | 9.28 ± 1.13 | age | 1,41 | 4.403 | 0.042 | |||
| poly I:C | 35 | 6.58 ± 0.77 | interaction | 1,41 | 0.008 | 0.093 | ||
| 100 | 8.84 ± 1.15 | |||||||
| Hipp | control | 35 | 3.78 ± 0.74 | MIS | 1,48 | 1.666 | 0.203 | |
| 100 | 6.58 ± 0.78 | age | 1,48 | 2.569 | 0.116 | |||
| poly I:C | 35 | 4.28 ± 0.66 | interaction | 1,48 | 3.670 | 0.061 | ||
| 100 | 4.03 ± 0.66 | |||||||
| Thal | control | 35 | 6.68 ± 0.62 | MIS | 1,47 | 1.603 | 0.211 | |
| 100 | 11.36 ± 1.40 | age | 1,47 | 8.154 | 0.006 | |||
| poly I:C | 35 | 8.20 ± 1.03 | interaction | 1.47 | 0.256 | 0.616 | ||
| 100 | 14.88 ± 2.83 | |||||||
| Nacc | control | 35 | 224.15 ± 30.02 | MIS | 1,40 | 7.529 | 0.009 | |
| 100 | 291.67 ± 36.02 | age | 1,40 | 4.971 | 0.032 | |||
| poly I:C | 35 | 309.24 ± 28.36 | interaction | 1,40 | 0.064 | 0.802 | ||
| 100 | 394.00 ± 30.58 | |||||||
| CPu | control | 35 | 458.38 ± 29.87 | MIS | 1,47 | 0.015 | 0.903 | |
| 100 | 548.46 ± 9.86 | age | 1,47 | 36.817 | 0.000 | |||
| poly I:C | 35 | 426.49 ± 12.55 | interaction | 1,47 | 2.237 | 0.141 | ||
| 100 | 575.52 ± 24.70 | |||||||
| GP | control | 35 | 42.10 ± 5.45 | MIS | 1,41 | 1.101 | 0.300 | |
| 100 | 38.58 ± 3.10 | age | 1,41 | 1.687 | 0.201 | |||
| poly I:C | 35 | 37.32 ± 4.20 | interaction | 1,41 | 3.973 | 0.053 | ||
| 100 | 54.00 ± 7.46 | |||||||
| VTA | control | 35 | 135.70 ± 5.59 | MIS | 1,48 | 0.423 | 0.519 | |
| 100 | 98.77 ± 9.84 | age | 1,48 | 0.964 | 0.331 | |||
| poly I:C | 35 | 102.49 ± 16.47 | interaction | 1,48 | 5.334 | 0.025 | ||
| 100 | 117.38 ± 7.79 | |||||||
| DOPAC | mPFC | control | 35 | 5.75 ± 0.81 | MIS | 1,40 | 6.209 | 0.017 |
| 100 | 5.83 ± 0.77 | age | 1,40 | 0.851 | 0.362 | |||
| poly I:C | 35 | 4.63 ± 0.70 | interaction | 1,40 | 1.040 | 0.314 | ||
| 100 | 3.14 ± 0.66 | |||||||
| Hipp | control | 35 | 1.58 ± 0.19 | MIS | 1,38 | 0.003 | 0.858 | |
| 100 | 3.37 ± 0.74 | age | 1,38 | 0.230 | 0.634 | |||
| poly I:C | 35 | 2.86 ± 0.51 | interaction | 1,38 | 3.284 | 0.078 | ||
| 100 | 1.81 ± 0.63 | |||||||
| Thal | control | 35 | 3.05 ± 0.73 | MIS | 1,44 | 0.048 | 0.828 | |
| 100 | 5.17 ± 0.98 | age | 1,44 | 1.676 | 0.202 | |||
| poly I:C | 35 | 3.94 ± 0.63 | interaction | 1,44 | 0.326 | 0.571 | ||
| 100 | 4.77 ± 1.38 | |||||||
| Nacc | control | 35 | 40.61 ± 7.46 | MIS | 1,40 | 0.550 | 0.463 | |
| 100 | 58.97 ± 8.99 | age | 1,40 | 5.357 | 0.026 | |||
| poly I:C | 35 | 46.48 ± 5.18 | interaction | 1,40 | 0.000 | 1,000 | ||
| 100 | 64.84 ± 5.99 | |||||||
| CPu | control | 35 | 60.77 ± 3.45 | MIS | 1,47 | 3.477 | 0.069 | |
| 100 | 67.27 ± 5.83 | age | 1,47 | 0.504 | 0.481 | |||
| poly I:C | 35 | 54.52 ± 2.45 | interaction | 1,47 | 0.362 | 0.550 | ||
| 100 | 55.05 ± 3.42 | |||||||
| GP | control | 35 | 12.95 ± 0.70 | MIS | 1,42 | 0.000 | 0.994 | |
| 100 | 12.27 ± 1.03 | age | 1,42 | 0.229 | 0.635 | |||
| poly I:C | 35 | 13.01 ± 0.63 | interaction | 1,42 | 0.001 | 0.973 | ||
| 100 | 12.24 ± 2.47 | |||||||
| VTA | control | 35 | 30.32 ± 1.67 | MIS | 1,47 | 0.000 | 0.989 | |
| 100 | 18.04 ± 1.83 | age | 1,47 | 8.339 | 0.006 | |||
| poly I:C | 35 | 25.72 ± 4.28 | interaction | 1,47 | 3.077 | 0.086 | ||
| 100 | 22.72 ± 2.12 | |||||||
| HVA | mPFC | control | 35 | 7.64 ± 1.14 | MIS | 1,41 | 0.390 | 0.536 |
| 100 | 5.18 ± 0.95 | age | 1,41 | 1.418 | 0.241 | |||
| poly I:C | 35 | 7.34 ± 0.89 | interaction | 1,41 | 0.765 | 0.387 | ||
| 100 | 6.96 ± 1.61 | |||||||
| Hipp | control | 35 | 1.93 ± 1.29 | MIS | 1,47 | 0.760 | 0.388 | |
| 100 | 1.07 ± 0.26 | age | 1,47 | 0.279 | 0.560 | |||
| poly I:C | 35 | 2.28 ± 1.04 | interaction | 1,47 | 0.216 | 0.644 | ||
| 100 | 2.23 ± 0.98 | |||||||
| Thal | control | 35 | 2.83 ± 1.07 | MIS | 1,48 | 1.195 | 0.280 | |
| 100 | 2.24 ± 0.66 | age | 1,48 | 0.552 | 0.461 | |||
| poly I:C | 35 | 1.99 ± 0.56 | interaction | 1,48 | 0.006 | 0.940 | ||
| 100 | 1.51 ± 0.33 | |||||||
| Nacc | control | 35 | 20.70 ± 4.49 | MIS | 1,40 | 0.181 | 0.673 | |
| 100 | 22.63 ± 3.46 | age | 1,40 | 1.585 | 0.215 | |||
| poly I:C | 35 | 19.69 ± 2.32 | interaction | 1,40 | 0.506 | 0.481 | ||
| 100 | 26.62 ± 2.94 | |||||||
| CPu | control | 35 | 46.33 ± 3.51 | MIS | 1,47 | 1.304 | 0.259 | |
| 100 | 36.74 ± 1.86 | age | 1,47 | 5.990 | 0.018 | |||
| poly I:C | 35 | 40.24 ± 2.03 | interaction | 1,47 | 1.282 | 0.263 | ||
| 100 | 36.72 ± 3.11 | |||||||
| GP | control | 35 | 13.71 ± 1.00 | MIS | 1,43 | 0.586 | 0.448 | |
| 100 | 11.42 ± 1.53 | age | 1,43 | 2.879 | 0.097 | |||
| poly I:C | 35 | 15.39 ± 1.24 | interaction | 1,43 | 0.076 | 0.784 | ||
| 100 | 12.21 ± 1.90 | |||||||
| VTA | control | 35 | 15.00 ± 2.38 | MIS | 1,47 | 0.725 | 0.399 | |
| 100 | 10.96 ± 1.71 | age | 1,47 | 7.511 | 0.009 | |||
| poly I:C | 35 | 17.54 ± 2.25 | interaction | 1,47 | 0.285 | 0.595 | ||
| 100 | 11.54 ± 0.78 | |||||||
| 5-HT | mPFC | control | 35 | 9.66 ± 0.54 | MIS | 1,41 | 1.021 | 0.318 |
| 100 | 10.54 ± 2.07 | age | 1,41 | 0.029 | 0.867 | |||
| poly I:C | 35 | 8.79 ± 0.80 | interaction | 1,41 | 0.181 | 0.673 | ||
| 100 | 8.41 ± 1.05 | |||||||
| Hipp | control | 35 | 6.49 ± 0.70 | MIS | 1,47 | 1.997 | 0.164 | |
| 100 | 9.89 ± 1.37 | age | 1,47 | 1.860 | 0.179 | |||
| poly I:C | 35 | 6.46 ± 0.96 | interaction | 1,47 | 1.927 | 0.172 | ||
| 100 | 6.43 ± 0.74 | |||||||
| Thal | control | 35 | 8.48 ± 1.68 | MIS | 1,49 | 0.111 | 0.741 | |
| 100 | 11.01 ± 2.00 | age | 1,49 | 3.393 | 0.072 | |||
| poly I:C | 35 | 6.94 ± 1.05 | interaction | 1,49 | 0.241 | 0.626 | ||
| 100 | 11.31 ± 1.45 | |||||||
| Nacc | control | 35 | 24.96 ± 1.23 | MIS | 1,40 | 0.005 | 0.946 | |
| 100 | 23.71 ± 2.85 | age | 1,40 | 1.623 | 0.210 | |||
| poly I:C | 35 | 27.24 ± 2.36 | interaction | 1,40 | 0.637 | 0.430 | ||
| 100 | 21.79 ± 2.62 | |||||||
| CPu | control | 35 | 7.19 ± 1.04 | MIS | 1,46 | 4.884 | 0.032 | |
| 100 | 9.44 ± 1.56 | age | 1,46 | 3.049 | 0.088 | |||
| poly I:C | 35 | 4.20 ± 0.54 | interaction | 1,46 | 0.002 | 0.960 | ||
| 100 | 6.58 ± 1.03 | |||||||
| GP | control | 35 | 13.86 ± 0.96 | MIS | 1,42 | 1.987 | 0.166 | |
| 100 | 10.06 ± 1.58 | age | 1,42 | 26.424 | 0.000 | |||
| poly I:C | 35 | 15.08 ± 0.67 | interaction | 1.42 | 5.376 | 0.025 | ||
| 100 | 5.04 ± 0.49 | |||||||
| VTA | control | 35 | 27.66 ± 2.51 | MIS | 1,48 | 0.407 | 0.527 | |
| 100 | 20.31 ± 2.42 | age | 1,48 | 0.443 | 0.509 | |||
| poly I:C | 35 | 20.21 ± 2.27 | interaction | 1,48 | 7.335 | 0.009 | ||
| 100 | 32.35 ± 4.39 | |||||||
| 5-HIAA | mPFC | control | 35 | 7.97 ± 0.71 | MIS | 1,41 | 4.653 | 0.037 |
| 100 | 6.00 ± 0.70 | age | 1,41 | 12.320 | 0.001 | |||
| poly I:C | 35 | 6.86 ± 0.32 | interaction | 1,41 | 0.180 | 0.674 | ||
| 100 | 4.35 ± 0.59 | |||||||
| Hipp | control | 35 | 6.95 ± 0.53 | MIS | 1,47 | 6.323 | 0.015 | |
| 100 | 9.26 ± 0.84 | age | 1,47 | 0.183 | 0.671 | |||
| poly I:C | 35 | 7.07 ± 0.36 | interaction | 1,47 | 7.171 | 0.010 | ||
| 100 | 5.39 ± 0.42 | |||||||
| Thal | control | 35 | 9.29 ± 0.93 | MIS | 1,49 | 0.836 | 0.365 | |
| 100 | 6.67 ± 1.04 | age | 1,49 | 9.405 | 0.003 | |||
| poly I:C | 35 | 8.67 ± 0.60 | interaction | 1,49 | 0.066 | 0.798 | ||
| 100 | 5.58 ± 0.45 | |||||||
| Nacc | control | 35 | 15.04 ± 1.16 | MIS | 1,40 | 0.461 | 0.501 | |
| 100 | 11.93 ± 1.44 | age | 1,40 | 3.781 | 0.059 | |||
| poly I:C | 35 | 13.74 ± 0.77 | interaction | 1,40 | 0.054 | 0.817 | ||
| 100 | 11.29 ± 1.73 | |||||||
| CPu | control | 35 | 10.96 ± 0.99 | MIS | 1,47 | 3.294 | 0.076 | |
| 100 | 9.64 ± 2.41 | age | 1,47 | 0.422 | 0.519 | |||
| poly I:C | 35 | 7.44 ± 0.82 | interaction | 1,47 | 0.002 | 0.959 | ||
| 100 | 6.31 ± 0.71 | |||||||
| GP | control | 35 | 14.16 ± 1.29 | MIS | 1,43 | 0.052 | 0.821 | |
| 100 | 8.07 ± 1.10 | age | 1,43 | 17.963 | 0.000 | |||
| poly I:C | 35 | 14.20 ± 1.35 | interaction | 1,43 | 0.067 | 0.798 | ||
| 100 | 7.33 ± 2.12 | |||||||
| VTA | control | 35 | 18.08 ± 1.32 | MIS | 1,48 | 0.274 | 0.603 | |
| 100 | 10.55 ± 1.15 | age | 1,48 | 4.552 | 0.038 | |||
| poly I:C | 35 | 15.46 ± 2.24 | interaction | 1,48 | 3.772 | 0.058 | ||
| 100 | 15.10 ± 1.99 | |||||||
| Gutamate | mPFC | control | 35 | 100.73 ± 1.82 | MIS | 1,45 | 0.591 | 0.446 |
| 100 | 105.43 ± 3.11 | age | 1,45 | 2.136 | 0.151 | |||
| poly I:C | 35 | 98.14 ± 2.43 | interaction | 1,45 | 1.030 | 0.316 | ||
| 100 | 124.19 ± 20.35 | |||||||
| Hipp | control | 35 | 73.85 ± 1.59 | MIS | 1.50 | 0.226 | 0.637 | |
| 100 | 86.40 ± 3.83 | age | 1,50 | 13.631 | 0.001 | |||
| poly I:C | 35 | 74.22 ± 2.62 | interaction | 1,50 | 0.142 | 0.708 | ||
| 100 | 89.62 ± 3.40 | |||||||
| Thal | control | 35 | 88.19 ± 3.17 | MIS | 1,50 | 0.506 | 0.480 | |
| 100 | 88.6 ± 4.90 | age | 1,50 | 0.431 | 0.515 | |||
| poly I:C | 35 | 88.33 ± 2.72 | interaction | 1,50 | 0.546 | 0.464 | ||
| 100 | 81.00 ± 5.86 | |||||||
| Nacc | control | 35 | 65.12 ± 2.60 | MIS | 1,47 | 0.644 | 0.426 | |
| 100 | 71.38 ± 3.52 | age | 1,47 | 9.387 | 0.003 | |||
| poly I:C | 35 | 64.31 ± 1.95 | interaction | 1,47 | 1.123 | 0.294 | ||
| 100 | 77.21 ± 2.61 | |||||||
| CPu | control | 35 | 74.17 ± 2.45 | MIS | 1,48 | 0.137 | 0.712 | |
| 100 | 66.71 ± 2.92 | age | 1,48 | 8.458 | 0.006 | |||
| poly I:C | 35 | 76.43 ± 1.90 | interaction | 1,48 | 0.924 | 0.341 | ||
| 100 | 61.60 ± 5.31 | |||||||
| GP | control | 35 | 37.73 ± 1.78 | MIS | 1,49 | 0.248 | 0.621 | |
| 100 | 47.62 ± 4.16 | age | 1,49 | 7.735 | 0.008 | |||
| poly I:C | 35 | 37.90 ± 0.96 | interaction | 1,49 | 0.210 | 0.649 | ||
| 100 | 51.69 ± 4.61 | |||||||
| VTA | control | 35 | 49.99 ± 1.69 | MIS | 1,48 | 0.027 | 0.870 | |
| 100 | 69.76 ± 10.88 | age | 1,48 | 4.688 | 0.035 | |||
| poly I:C | 35 | 45.73 ± 2.69 | interaction | 1,48 | 0.062 | 0.804 | ||
| 100 | 70.63 ± 9.68 | |||||||
| GABA | mPFC | control | 35 | 17.34 ± 0.41 | MIS | 1,45 | 0.838 | 0.365 |
| 100 | 21.31 ± 0.81 | age | 1,45 | 5.664 | 0.022 | |||
| poly I:C | 35 | 17.82 ± 0.57 | interaction | 1,45 | 0.507 | 0.480 | ||
| 100 | 25.19 ± 4.57 | |||||||
| Hipp | control | 35 | 22.94 ± 1.18 | MIS | 1,50 | 0.024 | 0.877 | |
| 100 | 26.93 ± 1.06 | age | 1,50 | 15.156 | 0.000 | |||
| poly I:C | 35 | 22.15 ± 0.90 | interaction | 1,50 | 0.260 | 0.613 | ||
| 100 | 27.35 ± 1.09 | |||||||
| Thal | control | 35 | 22.30 ± 1.66 | MIS | 1,51 | 0.000 | 0.977 | |
| 100 | 27.28 ± 1.47 | age | 1,51 | 11.343 | 0.001 | |||
| poly I:C | 35 | 20.97 ± 0.65 | interaction | 1,51 | 0.468 | 0.497 | ||
| 100 | 28.50 ± 2.33 | |||||||
| Nacc | control | 35 | 58.68 ± 3.60 | MIS | 1,47 | 0.494 | 0.485 | |
| 100 | 45.59 ± 4.31 | age | 1,47 | 5.353 | 0.025 | |||
| poly I:C | 35 | 53.39 ± 4.64 | interaction | 1,47 | 0.152 | 0.698 | ||
| 100 | 44.08 ± 5.33 | |||||||
| CPu | control | 35 | 16.75 ± 0.88 | MIS | 1,48 | 0.026 | 0.871 | |
| 100 | 32.43 ± 6.00 | age | 1,48 | 7.95 | 0.007 | |||
| poly I:C | 35 | 18.41 ± 0.53 | interaction | 0.023 | 0.879 | |||
| 100 | 32.48 ± 5.35 | |||||||
| GP | control | 35 | 55.16 ± 4.17 | MIS | 1,49 | 0.292 | 0.592 | |
| 100 | 51.02 ± 4.27 | age | 1,49 | 9.930 | 0.003 | |||
| poly I:C | 35 | 62.36 ± 3.27 | interaction | 1,49 | 4.831 | 0.033 | ||
| 100 | 39.13 ± 3.51 | |||||||
| VTA | control | 35 | 51.86 ± 3.57 | MIS | 1,48 | 0.006 | 0.940 | |
| 100 | 46.77 ± 4.04 | age | 1,48 | 0.652 | 0.423 | |||
| poly I:C | 35 | 50.28 ± 4.80 | interaction | 1,48 | 0.064 | 0.801 | ||
| 100 | 47.62 ± 4.61 |
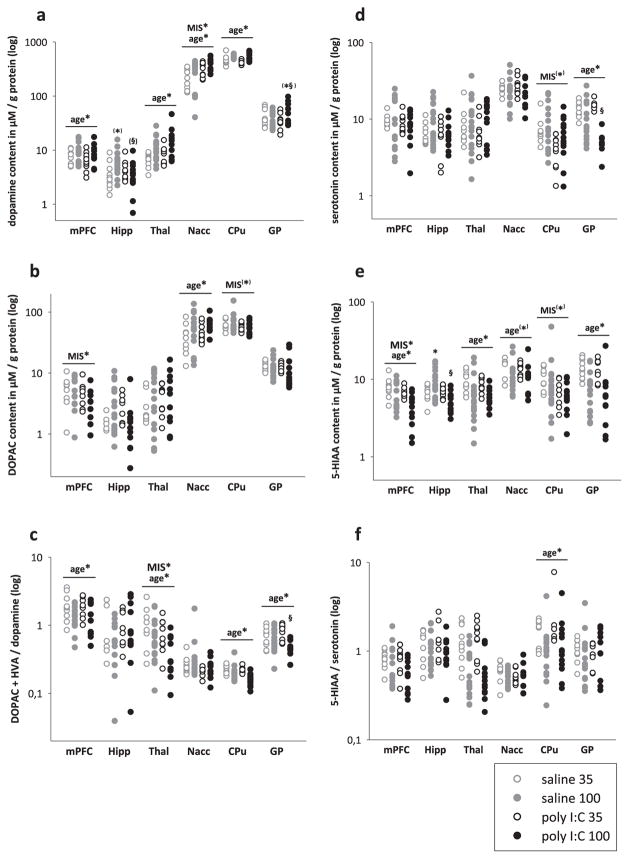
Fig. 2.
Effects of maternal immune stimulation and age on monoamine levels (dopamine, serotonin) and their metabolites (DOPAC, HVA, 5-HIAA). Neurochemical contents were examined in adolescent (35) and adult (100), control (saline) or MIS (poly-I:C) offspring. Vertical point plots depict the contents of monoamines and their metabolites measured in the mPFC, Hipp, Thal, Nacc, CPU and GP and are expressed in μM per g protein. The presence of age- and MIS-dependent effects are depicted by age* and MIS*, respectively (*p < 0.05), based on two-way ANOVA of the neurochemical content in the corresponding brain area. Significant differences of post hoc comparisons are indicated by asterisks (*, versus respective juvenile group) and paragraph (§, versus age-matched control group). a) Results for dopamine. b) Results for DOPAC. c). Results for dopamine turnover (DOPAC + HVA)/dopamine. d) Results for serotonin. e) Results for 5-HIAA. f) Results for serotonin turnover (5-HIAA/serotonin).
In the mPFC, contents of DA and GABA increased while 5-HIAA and DA-turnover decreased with age. In the Hipp, age-related effects were found for glutamate and GABA with higher levels being detected at PND100 as compared to PND35. In the Thal, contents of DA increased, whereas those of 5-HIAA and DA-turnover decreased with age. In the Nacc PND100 rats displayed increased levels of DA, DOPAC, and glutamate as well as decreased levels of 5-HIAA and GABA when compared to PND35 rats. In the CPU, contents of GABA increased whereas those of glutamate, DA and 5-HT-turnover decreased with age. In the GP, levels of GABA increased whereas 5-HT, 5-HIAA, glutamate and DA-turnover decreased over time. Finally, in the VTA, 5-HIAA and DOPAC decreased whereas glutamate was increased when PND100 were compared to PND35 rats.
3.2.2. MIS-related effects (Table 1 and Fig. 2)
In the mPFC MIS led to significantly lower contents in DOPAC and 5-HIAA. In the Hipp, it led to significantly lower 5-HIAA-content and a significant MIS × age interaction for this metabolite. Post-hoc pairwise comparisons confirmed a significant increase of 5-HIAA with age in saline offspring (p < 0.05) and significant higher 5-HIAA-levels in PND100 saline offspring when compared to poly I:C (p < 0.05). A trend towards a significant interaction between MIS and age was found for DA. In the Thal no MIS-related effects were seen. In the Nacc, maternal exposure to poly I:C increased DA-contents, whereas in the CPU it reduced 5-HT-contents. In the GP, we found a significant MIS × age interaction for 5-HT with post-hoc pairwise comparisons confirming an effect of age in both poly I:C (p < 0.05) and saline offspring (p < 0.05) as well as significantly reduced levels of 5-HT in adult poly I:C as compared to adult saline offspring (p < 0.05). Further, a significant MIS × age interaction was found for GABA with post hoc pairwise comparisons confirming significantly lower levels of GABA in adults as compared to adolescent poly I:C offspring (p < 0.05) as well as in adult poly I:C as compared to adult saline offspring (p < 0.05). A trend to significant MIS × age interaction was found for DA. In the VTA, a significant MIS × age interaction was observed for DA and 5-HT. For DA, post-hoc pairwise comparisons confirmed a significant reduction of DA-content with ageing in saline offspring (p < 0.05). For 5-HT, it revealed a significant increment of 5-HT with ageing in poly I:C offspring (p < 0.05) and a significant higher 5-HT-content in poly I:C than in saline offspring at adulthood (p < 0.05).
3.3. Imaging
3.3.1. Age-related effects (Fig. 3)
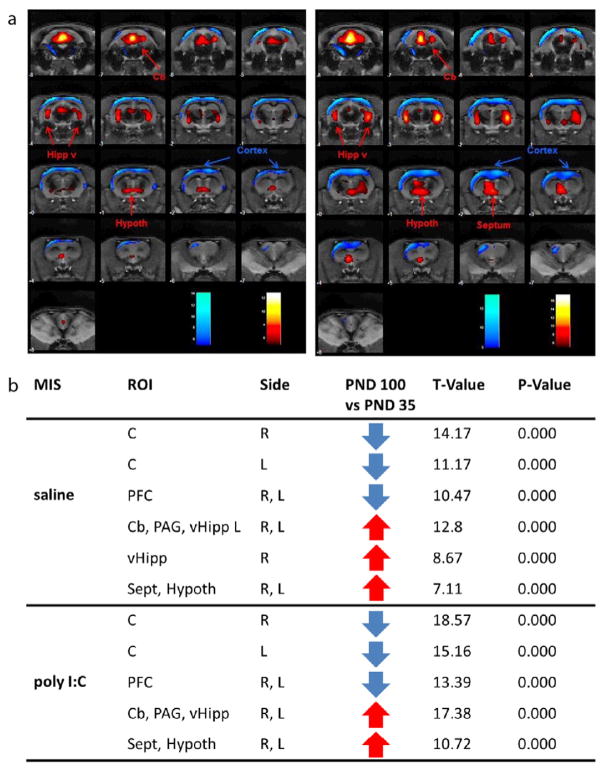
Fig. 3.
Age-related effects on glucose metabolism measured via FDG-PET. a) Colored PET overlays on the MR reference indicate increased [18F]FDG uptake (hot colors) or decreased [18F]FDG uptake (cold colors) following maturation in saline offspring (left panel) and MIS offspring (right panel). b) saline and poly I:C offspring (MIS), region of interest (ROI), side left and right, T-value, glucose metabolism (increase ↑ or decrease ↓), statistical p-value for cortex (C), prefrontal cortex (PFC), cerebellum (Cb), periaqueductal gray matter (PAG), ventral Hipp (vHipp), septum (Sep) and hypothalamus (hypoth).
PND100 saline offspring as well as PND100 poly I:C offspring showed lower glucose metabolism in the cortex in both hemispheres and prefrontal cortex as well as higher glucose metabolism in the ventral Hipp, cerebellum and periaqueductal gray matter (PAG), hypothalamus and septum when compared to respective PND35 animals.
3.3.2. MIS-related effects (Fig. 4)
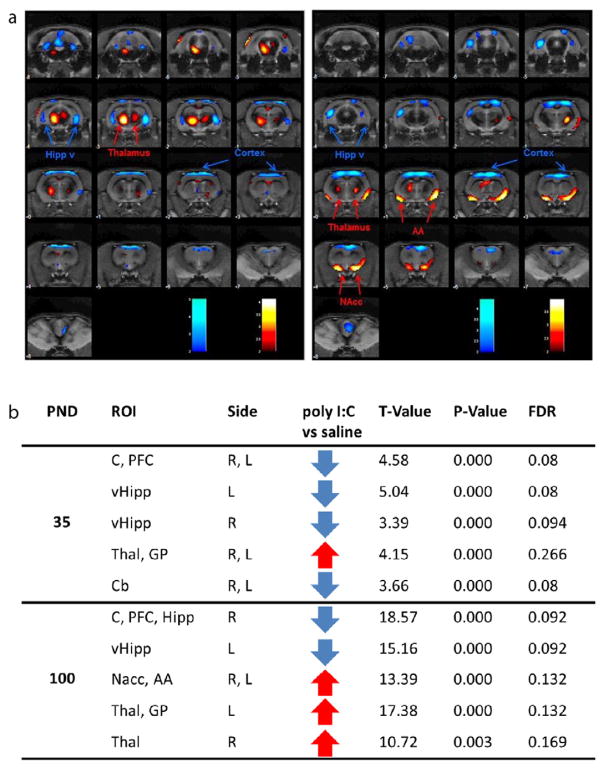
Fig. 4.
Age-related effects on glucose metabolism measured via FDG-PET. a) Colored PET overlays on the MR reference indicate increased [18F]FDG uptake (hot colors) or decreased [18F]FDG uptake (cold colors) following maturation in saline offspring (left panel) and MIS offspring (right panel). b) post natal day (PND) 35 and 100, region of interest (ROI), side left and right, T-value, glucose metabolism (increase ↑ or decrease ↓), statistical p value for cortex (C), prefrontal cortex (PFC), cerebellum (Cb), periaqueductal gray matter (PAG), ventral Hipp (vHipp), septum (Sep) and hypothalamus (hypoth).
At PND35 poly I:C offspring showed lower glucose metabolism in the ventral Hipp in both hemispheres, in the cortex/PFC and in the cerebellum as well as higher glucose metabolism in the Thal and GP when compared to saline offspring. Most of these MIS-dependent differences seen in adolescence were maintained in adulthood. At PND100, poly I:C offspring showed lower glucose metabolism in the ventral Hipp in both hemispheres and cortex/PFC as well as higher glucose metabolism in Thal when compared to controls. In contrast to adolescent, adult poly I:C offspring additionally showed higher glucose metabolism in the amygdala and Nacc in both hemispheres when compared to saline offspring but no difference in glucose metabolism in the cerebellum anymore.
4. Discussion
In recent years there has been a growing body of evidence pointing to neurotransmission and network abnormalities in schizophrenia. However, most of the data derived from patients is confounded by heterogeneous symptom profile, medication status and disease history. Animal models may overcome this limitation. In line with previous demonstrations of abnormal brain volumetric trajectories underlying delayed symptom manifestation in poly I:C offspring, we were now able to demonstrate that MIS affects metabolic brain activity, neurotransmission and sensorimotor gating, in a maturation-dependent manner.
4.1. Behavioral trajectories
Sensory gating deficits as reflected in impaired PPI constitute one of the core features of schizophrenia (Braff et al., 2001). Being a cross species phenomenon (Swerdlow et al., 2008), PPI has been previously shown to be disrupted in adult poly-I:C offspring (Ozawa et al., 2006; Meyer and Feldon, 2010; Mattei et al., 2014) and here we replicated this finding. Furthermore, our longitudinal assessment revealed that the PPI deficit emerged post-pubertally so that there were no differences in PPI levels between poly I:C and controls on PND35 and PND60 but poly-I:C offspring had lower PPI levels than controls on PND100. It could be argued that time-dependent emergence of PPI disruption in poly-I:C exposed offspring is due to low levels of PPI in controls, which make any decrease in PPI difficult to demonstrate due to a potential “floor” effect. However, a different interpretation is possible when comparing the developmental trajectories of PPI in controls and poly-I:C offspring: While in control animals PPI increased in magnitude from 35 to 100 days of age, such maturation-dependent increase in sensorimotor gating was prevented/attenuated in the offspring of dams exposed to poly-IC, leading to an adult deficit in this behavioral phenomenon. Very similar results and interpretation have been presented by Romero et al. (2010) on the longitudinal effects of prenatal administration of the MIS-inducing agent bacterial endotoxin lipopolysaccharide (LPS) on PPI. Prenatal LPS treatment also led to the induction of a behavioral deficit, which emerged first at puberty and persisted throughout adulthood (Romero et al., 2010). The present results are in agreement with the impaired developmental trajectory of PPI observed after prenatal administration of the MIS-inducing agent bacterial endotoxin lipopolysaccharide (Romero et al., 2010). Altogether, our data are consistent with the well-documented post-pubertal onset of psychotic behavior in the majority schizophrenic patients.
4.2. Neurobiological trajectories
We found age-dependent changes in all neurotransmission systems as well as brain structures examined regardless of the MIS status and a high overlap between age-related brain activity changes in MIS and control offspring. Neurobiological changes that develop over time regardless of the maternal immune status most likely reflect normal neurobiological maturation processes whereas those that develop against the maternal immune status indicate abnormal maturation processes and are likely to contribute to the delayed manifestation of behavioral deficits seen in poly I:C offspring (Piontkewitz et al., 2012).
Adding on to previous data (Winter et al., 2009; Giovanoli et al., 2013) on a neurochemical level, probably our most exciting MIS-related effects refer to the DA-system such that poly I:C offspring show higher levels of DA in the Nacc and lower levels of DOPAC in the mPFC when compared to saline offspring. Compelling evidence points to the participation of abnormally enhanced DA-activity in the mesolimbic system, especially the Nacc, in the disruption of sensorimotor gating (Geyer et al., 2001). Moreover, alterations in the DA-system in the form of imbalances between subcortical and cortical DA are well documented in schizophrenia. Specifically, it has been suggested that in line with the data of the present study enhanced activity in the mesolimbic DA-system together with hypoactive mesocortical DA-projections to the PFC contribute to the pathophysiology of schizophrenia (Abi-Dargham and Moore, 2003; Winterer and Weinberger, 2004; Giulivi et al., 2013). As abnormal glucose metabolism is thought to be an indicator of an underlying pathology, our FDG-PET data further strengthen this notion demonstrating that in comparison to controls poly I:C offspring exhibit lower glucose metabolism in the ventral Hipp, cortex and PFC and higher glucose metabolism in the amygdala and Nacc.
Interestingly, DAergic dysregulations as well as alterations in metabolic activity pattern differed across mPFC and Nacc with respect to first appearance and further development. In the mPFC, poly I:C offspring showed reduced levels of DOPAC as well as metabolic activity prior to and with manifestation of behavioral deficits in adolescent and adult states as compared to age-matched controls. Together with an early reduction in 5-HIAA, this finding reflects an early impairment of the mPFC in the poly I:C model of schizophrenia. This is of special interest as cognitive processes that are supported by the integrity of the PFC are among the first to occur in schizophrenia patients, presenting much before the onset of psychotic symptoms (Volk and Lewis, 2014). In contrast, DA-contents in the Nacc increased with age in both, saline and poly I:C offspring with the latter presenting further increments as compared to controls only during adulthood. Likewise, adult but not adolescent poly I:C offspring displayed increased Nacc metabolic activity as compared to saline offspring. A recent study by Alam et al. (2015) demonstrated reduced firing rates and burst behavior of mPFC neurons along with increased firing rates of Nacc neurons to subserve impaired sensorimotor gating in rats. This data suggests that only the combined deficit in mPFC and Nacc functioning as found here in adult but not adolescent poly I:C offspring is capable of inducing PPI deficits in rats.
Schizophrenia has further been associated with hippocampal abnormalities. Structural imaging and post mortem studies consistently report decreased hippocampal volumes in schizophrenia patients and functional imaging studies demonstrate elevated basal cerebral perfusion and point to a failure in recruiting the Hipp during memory tasks (Heckers et al., 1998; Medoff et al., 2001; Weiss et al., 2003; Schobel et al., 2009; Tamminga et al., 2010). Not much is known about the onset of hippocampal abnormalities in relation to symptom manifestation. Piontkewitz and colleagues (Piontkewitz et al., 2011) found in the poly I:C model of schizophrenia reduced hippocampal volumes at PND46, still prior to behavioral deficit manifestation. Poly I:C offspring has further been shown to exhibit down-regulated hippocampal neurogenesis (Meyer et al., 2010; Wolf et al., 2011; Mattei et al., 2014) that likewise precedes behavioral deficits manifestation (Piontkewitz et al., 2012) and probably associates to hippocampal volume reductions since normal adult hippocampal neurogenesis results in a pool of new neurons and volume gain (Ming and Song, 2005). Here, we report that hippocampus-related functional alteration may also occur prior to symptom manifestation. As such we found previously reported abnormalities in the serotonergic system (Winter et al., 2009) and decreased metabolic activity in the hippocampus at both adolescent and adult stages.
4.3. Conclusion
Taken together, to the best of our knowledge this is the first report of FDG-PET in parallel with neurochemical investigations to study and identify brain networks associated to the delayed emergence of schizophrenia-related behavioral deficits in an animal model of this affliction. Our finding suggest that MIS-induced metabolic and neurochemical changes are neurodevelopmental in nature and either progressive or non-progressive and that the behavioral deficits manifest as these abnormalities increase.
Acknowledgments
Role of the Funding Source
The Funding Source had no role in study design, in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
This research was conducted under the EraNet Neuron framework (DBS_F20rat) and supported by the Federal Ministry of Education and Research, Germany (BMBF 01EW1103), Ministerio de Ciencia e Innovación (PI11/00616, PI10/02986, PI14/00860 TEC2010-21619-C04-01), Comunidad de Madrid, Fundación Mapfre, and the Canadian Institutes of Health Research. RH and FW are financed by the German Research Foundation (DFG PAK 591 (WI 2140/2-1) and DFG KFO 247 (WI 2140/1-1+2)).
Footnotes
Contributors
RH: Conducted and analyzed behavioral and biochemical studies, wrote ms.
MSM: Conducted FDG-PET studies, contributed to discuss data for ms.
TG: Contributed to conducting behavioral studies.
FW and RS: Conducted biochemical analysis.
CH and IW: Contributed to design of experiments and writing ms.
MD: Contributed to design and analysis of FDG-PET study.
JP: Designed and analyzed FDG-PET study.
CW: Designed study, wrote ms, analyzed behavioral and biochemical analysis.
Conflict of Interest
The authors declare no conflict of interest.
References
- Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9(5):404–416. doi: 10.1177/1073858403252674. [DOI] [PubMed] [Google Scholar]
- Alam M, Angelov S, Stemmler M, von Wrangel C, Krauss JK, Schwabe K. Neuronal activity of the prefrontal cortex is reduced in rats selectively bred for deficient sensorimotor gating. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;56:174–184. doi: 10.1016/j.pnpbp.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156(2–3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156(2–3):117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Feldon J, Schedlowski M, Meyer U. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science (New York, NY) 2013;339(6123):1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- Giulivi C, Napoli E, Schwartzer J, Careaga M, Ashwood P. Gestational exposure to a viral mimetic poly(i:C) results in long-lasting changes in mitochondrial function by leucocytes in the adult offspring. Mediat Inflamm. 2013;2013:609602. doi: 10.1155/2013/609602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Klein J, Hadar R, Gotz T, Manner A, Eberhardt C, Baldassarri J, Schmidt TT, Kupsch A, Heinz A, Morgenstern R, Schneider M, Weiner I, Winter C. Mapping brain regions in which deep brain stimulation affects schizophrenia-like behavior in two rat models of schizophrenia. Brain Stimul. 2013;6(4):490–499. doi: 10.1016/j.brs.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Mattei D, Djodari-Irani A, Hadar R, Pelz A, de Cossio LF, Goetz T, Matyash M, Kettenmann H, Winter C, Wolf SA. Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun. 2014;38:175–184. doi: 10.1016/j.bbi.2014.01.019. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW, Hawkins KA, Hoffman RE, Preda A, Epstein I, Addington D, Lindborg S, Trzaskoma Q, Tohen M, Breier A. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163(5):790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11(5):543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90(3):285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. To poly(I:C) or not to poly(I:C): advancing preclinical schizophrenia research through the use of prenatal immune activation models. Neuropharmacology. 2012;62(3):1308–1321. doi: 10.1016/j.neuropharm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Meyer U, Knuesel I, Nyffeler M, Feldon J. Chronic clozapine treatment improves prenatal infection-induced working memory deficits without influencing adult hippocampal neurogenesis. Psychopharmacology. 2010;208(4):531–543. doi: 10.1007/s00213-009-1754-6. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59(6):546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Pascau J, Gispert JD, Michaelides M, Thanos PK, Volkow ND, Vaquero JJ, Soto-Montenegro ML, Desco M. Automated method for small-animal PET image registration with intrinsic validation. Mol Imaging Biol. 2009;11(2):107–113. doi: 10.1007/s11307-008-0166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry. 2009;66(11):1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Abnormal trajectories of neurodevelopment and behavior following in utero insult in the rat. Biol Psychiatry. 2011;70(9):842–851. doi: 10.1016/j.biopsych.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Tracing the development of psychosis and its prevention: what can be learned from animal models. Neuropharmacology. 2012;62(3):1273–1289. doi: 10.1016/j.neuropharm.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol Psychiatry. 2010;15(4):372–383. doi: 10.1038/mp.2008.44. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199(3):331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167(10):1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Early developmental disturbances of cortical inhibitory neurons: contribution to cognitive deficits in schizophrenia. Schizophr Bull. 2014;40(5):952–957. doi: 10.1093/schbul/sbu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AP, Schacter DL, Goff DC, Rauch SL, Alpert NM, Fischman AJ, Heckers S. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol Psychiatry. 2003;53(1):48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- Winter C, Djodari-Irani A, Sohr R, Morgenstern R, Feldon J, Juckel G, Meyer U. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int J Neuropsychopharmacol. 2009;12(4):513–524. doi: 10.1017/S1461145708009206. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27(11):683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Melnik A, Kempermann G. Physical exercise increases adult neurogenesis and telomerase activity, and improves behavioral deficits in a mouse model of schizophrenia. Brain Behav Immun. 2011;25(5):971–980. doi: 10.1016/j.bbi.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Psychopharmacology. 2003;28(10):1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]