Abstract
Purpose
Use of androgen deprivation therapy (ADT) may be associated with an increased risk of diabetes mellitus but the risk of both acute myocardial infarction (AMI) and cardiovascular mortality remain controversial because few outcomes and conflicting findings have been reported. We sought to clarify whether ADT is associated with these outcomes in a large, representative cohort.
Methods
Using linked administrative databases in Ontario, Canada, men age 66 years or older with prostate cancer given continuous ADT for at least 6 months or who underwent bilateral orchiectomy (n = 19,079) were matched with men with prostate cancer who had never received ADT. Treated and untreated groups were matched 1:1 (ie, hard-matched) on age, prior cancer treatment, and year of diagnosis and propensity-matched on comorbidities, medications, cardiovascular risk factors, prior fractures, and socioeconomic variables. Primary outcomes were development of AMI, sudden cardiac death, and diabetes. Fragility fracture was also examined.
Results
The cohort was observed for a mean of 6.47 years. In time-to-event analyses, ADT use was associated with an increased risk of diabetes (hazard ratio [HR], 1.16; 95% CI, 1.11 to 1.21) and fragility fracture (HR, 1.65; 95% CI, 1.53 to 1.77) but not with AMI (HR, 0.91; 95% CI, 0.84 to 1.00) or sudden cardiac death (HR, 0.96; 95% CI, 0.83 to 1.10). Increasing duration of ADT was associated with an excess risk of fragility fractures and diabetes but not cardiac outcomes.
Conclusion
Continuous ADT use for at least 6 months in older men is associated with an increased risk of diabetes and fragility fracture but not AMI or sudden cardiac death.
INTRODUCTION
Recent data suggest that one in two men with prostate cancer will receive androgen deprivation therapy (ADT) at some point after diagnosis.1 Most men starting ADT take it for a minimum of 2 to 3 years.2,3
ADT use is associated with numerous adverse effects, including worse quality of life, sexual dysfunction, fatigue, anemia, and loss of bone density.4–8 These adverse effects occur equally with luteinizing hormone releasing hormone (LHRH) agonists and orchiectomy.4–6 ADT use is also associated with an increased risk of fractures, as demonstrated in five retrospective cohort studies.9–13
Other potentially serious toxicities from ADT have been described recently, notably cardiovascular and endocrine complications. Keating et al14 first described an excess risk of myocardial infarction (MI), diabetes, and sudden cardiac death with ADT in a large cohort of men age 66 years or older using administrative data. D’Amico et al15 analyzed data on 1,372 men from three randomized trials of ADT and found an earlier onset of fatal MI among ADT users age 65 years or older compared with nonusers age 65 years or older. However, the total number of observed MIs was only 51.15 Saigal et al16 reported excess cardiovascular morbidity with ADT use among 4,810 men age 65 years or older compared with controls by using administrative data. The final article, by Tsai et al,17 identified 1,015 men given ADT in a clinical urologic database and demonstrated an increased risk of cardiovascular mortality with ADT use but not with diabetes or baseline heart disease. This study included only 61 deaths.
In contrast to the above findings, a recent updated analysis of RTOG (Radiation Therapy Oncology Group) 92-02 reported no increased cardiovascular mortality with 28 months versus 4 months of ADT.18 In RTOG 86-10, neoadjuvant short-term ADT was not associated with an increased risk of fatal cardiac events.19 In RTOG 85-31, adjuvant ADT was similarly not associated with an increased risk of cardiovascular mortality.20 Together, these observations challenge the interpretation of the cardiovascular findings. At the same time, a growing body of literature has shown that ADT increases fat mass, increases insulin resistance, and adversely affects arterial vasculature,5,21–24 which support an increased likelihood of developing diabetes and cardiovascular disease. As such, these results have collectively created tremendous uncertainty in the clinical community.
To better understand these issues, we undertook a propensity-matched analysis of 19,079 ADT users and nonusers to examine whether ADT use is associated with adverse cardiovascular and endocrine effects.
METHODS
Study Design
We performed a matched cohort study using linked administrative data at the Institute for Clinical Evaluative Sciences (ICES)25 in Ontario, Canada (population of approximately 11,000,000). Men with prostate cancer were identified using the Ontario Cancer Registry (OCR). The OCR is a comprehensive provincial registry that captures more than 95% of cancer cases.26 OCR records for each patient were linked by each patient’s unique health card number to other databases at ICES (Appendix, Databases Used in the Study, online only).
Study Cohort
The study cohort consisted of men age 66 years or older diagnosed with prostate cancer between January 1, 1995, and December 31, 2005. An age cutoff of 66 years or older was chosen to allow a 1-year look-back period for prior drug claims. Patients who received ADT were paired with those who did not receive ADT based on propensity-score matching.
Patients receiving at least 6 months of continuous medical ADT (either LHRH agonists, nonsteroidal antiandrogens, or steroidal antiandrogens, alone or in combination) or who underwent orchiectomy were classified as ADT users. First prescription of medical ADT (with a 1-year look-back period to ensure no prior use) was captured through unique drug information numbers for all available LHRH agonists, nonsteroidal antiandrogens, and steroidal antiandrogens. Date of prescription, dose, and duration of prescription were also available. Orchiectomy was identified using specific procedure codes from discharge abstract data.27
ADT nonusers were men with prostate cancer who had never received ADT between diagnosis and December 31, 2006, the final date for outcome ascertainment. ADT nonusers were hard-matched to ADT users in a 1:1 fashion on age at diagnosis and prior radical prostatectomy, and by using a propensity score–based technique that incorporated age, year of diagnosis, comorbidity, medication use, and socioeconomic factors (Appendix, Details of the Propensity Model). ADT nonusers had to be alive on the index date (the date that the matched ADT user initiated ADT). The propensity score model balances the distribution of possible confounders between patients receiving ADT versus those not receiving ADT, allowing an unbiased estimate of the average ADT effect on the risk of the outcome (eg, MI) to be obtained.28–30
Outcome Variables
Primary outcomes included development of MI, cardiovascular mortality, and diabetes. Outcomes were obtained from linked inpatient and outpatient databases using specific diagnostic codes and validated algorithms where possible (Appendix Table A1, online only).
Development of MI was based on the algorithm of the Ontario MI Database, which has been extensively validated and was shown to have a sensitivity of 95% and false-positive rate of < 5%31,32 (Appendix, Details of the Propensity Model).
Sudden cardiac death was based on cause of death information among deceased patients. Sudden cardiac death was defined as death due to atrial or ventricular arrhythmias or sudden cardiac death, using specific International Classification of Diseases, 9th Revision (ICD-9) and ICD-10 diagnostic codes in accordance with prior studies.14,16,32
Development of incident diabetes was obtained from the Ontario Diabetes Database, which has more than 85% accuracy for incident cases using defined algorithms.33 To ensure that only incident cases were captured, patients who had diabetes before or within 30 days of starting ADT were excluded from analyses of this outcome.
Secondary outcomes included fractures and vascular events. Fracture outcomes were captured using both inpatient hospital claims and outpatient claims from physician billings. Fractures were stratified into fragility and nonfragility types according to the methodology of Shahinian10 and using specific ICD-9 and ICD-10 diagnostic codes for each fracture location. Men with prior metastases (identified using specific ICD-9 and ICD-10 diagnostic codes) were excluded from fracture outcome analyses.
Vascular outcomes were hypothesized to be related to ADT, given their relationship with the same vascular risk factors that lead to cardiovascular disease. Secondary outcomes included acute stroke, congestive heart failure, use of diagnostic coronary angiography, and cardiovascular revascularization (angioplasty or cardiac bypass surgery). The former two outcomes were diagnosed on the basis of ICD-9 and ICD-10 diagnostic codes, whereas the latter two outcomes were based on hospital procedure codes and physician billings (Appendix Table A1) and have been shown to have high accuracy.28
Covariates
A comprehensive set of covariates was obtained for matching and risk adjustment, including comorbidity, prior radical prostatectomy, prior medication use, income quintile, a measure of rurality, and a measure of access to a regular primary care physician. Comorbidities were captured with a 2-year look-back period using the 11 collapsed ambulatory diagnostic groups of the Ambulatory Case Mix Groups system from Johns Hopkins.29,30 In addition, a prior diagnosis of fragility fracture, a prior bone mineral density test, and prior diagnosis or treatment of osteoporosis during the look-back period were recorded separately. Medications were grouped into clinically relevant analytic categories, including aspirin, bone-thinning medications (eg, phenytoin, glucocorticoids), bone-enhancing medications (eg, bisphosphonates), cholesterol agents, and diabetic medications. Patients were considered to have taken a medication as long as a single prescription was filled during the look-back period.
Statistical Analyses
Examining the effects of ADT
For each of the primary time-to-event outcomes (fracture, MI, sudden cardiac death, and diabetes), the Kaplan-Meier estimate of the survivor function was computed for both ADT users and nonusers. This approach provides estimates of the proportion of patients in each group who have experienced the outcome over time. Time zero was the index date. To estimate the effect of ADT after adjusting for other covariates, methods for the analysis of bivariate time-to-event data were implemented to account for correlation among the event times that may arise due to the matched-pairs design. In particular, a Cox proportional hazards model was used, incorporating pair-specific random effects.34 The proportional hazards assumption was assessed by examining residuals and with a log-log plot. Secondary outcomes were examined using the same methodology as for primary outcomes. Covariates in the Cox models included comorbidity, medication use, income quintile, rurality, and regular access to a primary care physician.
Relationship between duration of ADT use and outcomes
To examine whether duration of ADT use was associated with outcomes, we analyzed duration of ADT use as both a continuous and categoric variable among ADT users. For this analysis, we included all ADT users regardless of duration of ADT use. We defined intervals of ADT use a priori based on common clinically used durations—less than3 months, 3–6 months, 6–24 months, and more than 24 months. A Cox proportional hazards model was used for each of the primary outcomes and fragility fractures, adjusting for the same covariates as for the primary time-to-event outcomes.
Sensitivity analysis of cardiovascular outcomes
Given the controversies regarding ADT use and cardiovascular outcomes, we performed an additional analysis by including four classes of cardioprotective drugs—angiotensin antagonists (both angiotensin-converting enzyme inhibitors and angiotensin receptor blockers), beta-blockers, cholesterol-lowering agents, and aspirin—as time-varying covariates in our models of MI and sudden cardiac death.
RESULTS
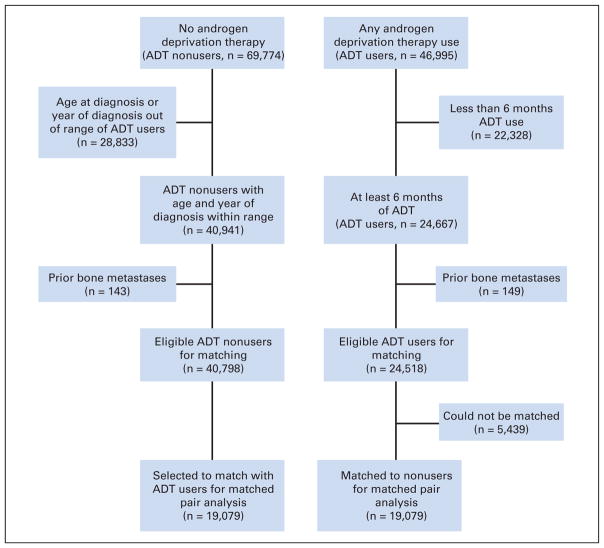
Figure 1 summarizes the selection process of ADT users and nonusers. During the study period, a total of 116,769 patients were diagnosed with prostate cancer according to the OCR. Linking the OCR with both the Ontario Drug Benefit Formulary and hospital discharge abstract data, 46,995 men received a form of ADT (medical, surgical, or both) during the study period. Of these men, 22,328 men received less than 6 months of ADT and were excluded from the matched analysis, leaving 24,667 men. From this group, 149 men were excluded because of a diagnosis of bone metastases before ADT use, leaving 24,518 eligible ADT users.
Fig 1.
Diagram of patient flow for matched cohort study of androgen deprivation therapy (ADT) users and nonusers.
There were 69,774 men who did not receive any ADT. An additional 28,833 men were excluded because their age at diagnosis or year of diagnosis was outside the ranges of ADT users for hard-matching purposes. This left 40,941 men, of whom an additional 143 were excluded due to prior bone metastases, leaving 40,798 eligible ADT nonusers.
Of the 24,518 ADT users, we were able to match 19,079 with appropriate nonusers. Baseline characteristics were well matched between ADT users and nonusers (Table 1). The median duration of follow-up for both groups was 6.47 years.
Table 1.
Characteristics of ADT Users and Nonusers in Matched Cohort at Index Date
| Characteristic | ADT Users (%; n = 19,079) | ADT Nonusers (%; n = 19,079) |
|---|---|---|
| Age, years | ||
| Mean | 75.0 | |
| SD | 6.3 | |
|
| ||
| Collapsed ambulatory diagnostic group | ||
| Acute minor | 79.0 | 80.0 |
| Acute major | 84.4 | 84.1 |
| Likely to recur (includes myocardial infarction) | 78.9 | 78.6 |
| Asthma | 6.2 | 6.9 |
| Chronic medical unstable | 92.9 | 92.3 |
| Chronic medical stable (includes hypertension and diabetes) | 84.2 | 84.3 |
| Chronic specialty unstable | 17.3 | 17.5 |
| Chronic specialty stable | 8.7 | 9.3 |
| Eye/dental | 28.2 | 29.0 |
| Psychosocial | 33.9 | 34.1 |
| Prevention, administration | 32.3 | 32.3 |
|
| ||
| Use of bone-thinning medication | 8.9 | 9.6 |
|
| ||
| Prior bone mineral density test | 3.1 | 3.1 |
|
| ||
| Prior diagnosis of osteoporosis | 4.5 | 4.6 |
|
| ||
| Prior fragility fracture | 3.1 | 3.4 |
|
| ||
| Prior prostate cancer treatment | ||
| Radical prostatectomy | 6.5 | 6.5 |
|
| ||
| Rural/small town | 17.7 | 17.2 |
|
| ||
| Income quintile | ||
| 1 (highest) | 17.5 | 18.3 |
| 2 | 16.0 | 15.9 |
| 3 | 16.7 | 16.9 |
| 4 | 17.0 | 17.0 |
| 5 (lowest) | 14.0 | 13.7 |
|
| ||
| At least 51% of primary care visits to same physician | 60.3 | 61.8 |
Abbreviations: ADT, androgen deprivation therapy; SD, standard deviation.
Primary Outcomes in Matched Cohort
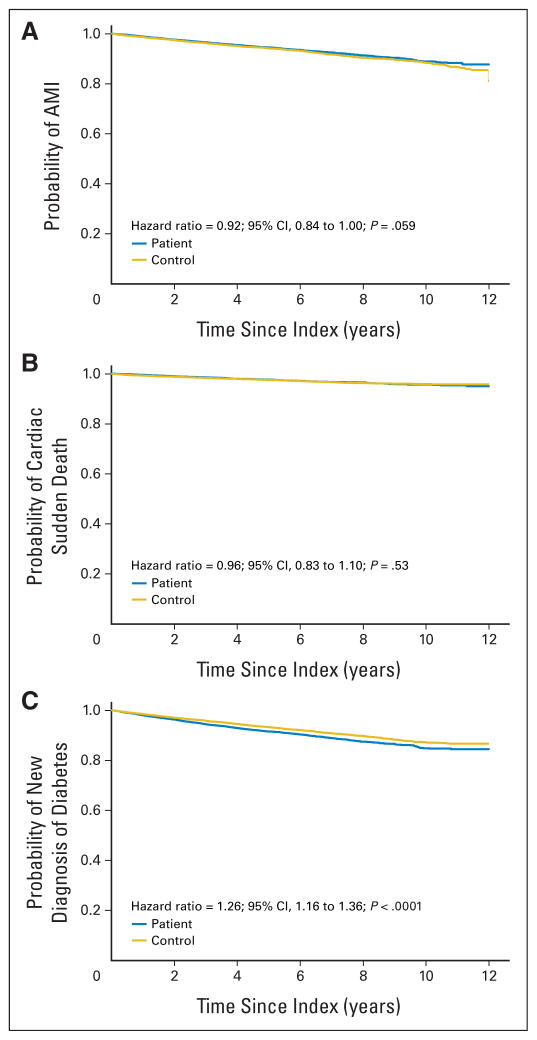
A total of 949 ADT users (4.8%) suffered a new MI compared with 1,085 nonusers (5.5%), with an HR of 0.92 (95% CI, 0.84 to 1.00; P = .059). Significant predictors of time to MI included prior diabetes, prior hypertension, prior dyslipidemia, prior MI, prior coronary artery disease, prior aspirin use, and prior chronic kidney disease (Table 2). For sudden cardiac death, 399 ADT users (2.0%) and 436 nonusers (2.2%) experienced an event, with an adjusted HR of 0.96 (95% CI, 0.83 to 1.10; P = .53). Other significant predictors of time to sudden cardiac death included prior diabetes, prior hypertension, prior dyslipidemia, prior MI, prior coronary artery disease, prior aspirin use, prior chronic kidney disease, and regular access to a primary care physician (Table 2). On the basis of Kaplan-Meier plots, there was no evidence to suggest earlier time to MI or sudden cardiac death among ADT users (Fig 2).
Table 2.
Multivariable Regression Model Results for Primary Outcomes
| Variable | Myocardial Infarction
|
Sudden Cardiac Death
|
Diabetes
|
|||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| ADT use | 0.92 | 0.84 to 1.00 | 0.96 | 0.83 to 1.10 | 1.24 | 1.15 to 1.35 |
|
| ||||||
| Prior diabetes | 1.47 | 1.33 to 1.63 | 1.30 | 1.10 to 1.54 | NA | |
|
| ||||||
| Prior hypertension | 1.27 | 1.16 to 1.39 | 1.31 | 1.13 to 1.51 | 1.31 | 1.21 to 1.42 |
|
| ||||||
| Prior dyslipidemia | 0.89 | 0.80 to 0.99 | 0.74 | 0.62 to 0.87 | 1.08 | 0.99 to 1.18 |
|
| ||||||
| Prior myocardial infarction | 1.72 | 1.45 to 2.03 | 1.54 | 1.20 to 1.98 | 0.87 | 0.69 to 1.08 |
|
| ||||||
| Prior coronary artery disease | 1.76 | 1.35 to 2.28 | 2.40 | 1.64 to 3.51 | 1.13 | 0.85 to 1.50 |
|
| ||||||
| Prior congestive heart failure | 1.07 | 0.78 to 1.46 | 1.35 | 0.88 to 2.07 | 1.24 | 0.87 to 1.77 |
|
| ||||||
| Prior aspirin use | 1.70 | 1.54 to 1.87 | 1.57 | 1.35 to 1.84 | 1.04 | 0.94 to 1.14 |
|
| ||||||
| Prior peripheral vascular disease | 1.18 | 0.89 to 1.57 | 1.39 | 0.97 to 2.01 | 1.04 | 0.76 to 1.43 |
|
| ||||||
| Prior cerebrovascular disease | 1.12 | 0.91 to 1.39 | 1.26 | 0.94 to 1.69 | 0.89 | 0.69 to 1.13 |
|
| ||||||
| Prior chronic kidney disease | 1.59 | 1.18 to 2.15 | 1.76 | 1.21 to 2.57 | 0.92 | 0.63 to 1.36 |
|
| ||||||
| Regular access to primary care | 0.92 | 0.83 to 1.01 | 0.65 | 0.56 to 0.76 | 0.96 | 0.88 to 1.05 |
NOTE. Each of the outcomes was estimated using multivariable Cox proportional hazards models with pair-specific random effects. All models were also adjusted for income quintile and rurality. Values in bold type are statistically significant at P < .05.
Abbreviations: HR, hazard ratio; ADT, androgen deprivation therapy; NA, not applicable (covariates not included in model for this outcome measure).
Fig 2.
Kaplan-Meier time-to-event curves for the primary outcomes comparing users of androgen deprivation therapy to a matched cohort of nonusers. Outcomes are as follows: (A) acute myocardial infarction (AMI), (B) sudden cardiac death, (C) diabetes.
With respect to diabetes, 1,392 ADT users (7.1%) versus 1,181 nonusers (6.0%) developed incident diabetes, with an adjusted HR of 1.26 (95% CI, 1.16 to 1.36; P < .0001). The only other significant predictor of time to incident diabetes was prior hypertension (Table 2). Kaplan-Meier time-to-event plots for each of the primary outcomes are shown in Figure 2.
Secondary Outcomes
A total of 1,778 ADT users (9.0%) compared with 1,157 nonusers (5.9%) had a fragility fracture, with an adjusted HR of 1.65 (95% CI, 1.53 to 1.78; P < .0001). With respect to any fracture, 3,387 ADT users (17.2%) suffered a fracture compared with 2,495 nonusers (12.7%), with an adjusted HR of 1.46 (95% CI, 1.39 to 1.54; P < .0001).
A new diagnosis of congestive heart failure was noted in 2,496 ADT users (12.7%) and 2,715 controls (13.8%), with an adjusted HR of 0.95 (95% CI, 0.90 to 1.00; P = .057). Stroke was noted in 1,057 ADT users (5.4%) and 1,251 nonusers (6.4%), with an adjusted HR of 0.88 (95% CI, 0.81 to 0.96; P = .001).
Diagnostic cardiac catheterization was undertaken in 1,173 ADT users (6.0%) and 1,411 nonusers (7.2%), with an adjusted HR of 0.88 (95% CI, 0.81 to 0.95; P = .001). Revascularization was performed in 702 ADT users (3.6%) and 866 nonusers (4.4%), with an adjusted HR of 0.87 (95% CI, 0.78 to 0.96; P = .005).
Duration of ADT Use and Outcomes
Duration of ADT use was associated with a decreased risk of sudden cardiac death and an increased risk of fragility fracture but was not associated with MI risk in analyses where the duration of ADT use was considered as either a continuous or a categoric variable (Table 3). Increasing duration of ADT use was associated with a trend toward an increased risk of diabetes (P = .079 for > 24 months of exposure compared with < 3 months of exposure; Table 3).
Table 3.
Duration of ADT Use and Selected Outcomes
| Variable | Myocardial Infarction
|
Sudden Cardiac Death
|
Diabetes
|
Fragility Fracture
|
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Duration of ADT use, months | ||||||||
| < 3 | Referent | Referent | Referent | Referent | ||||
| 3–6 | 0.99 | 0.88 to 1.12 | 0.95 | 0.79 to 1.14 | 1.00 | 0.90 to 1.10 | 0.96 | 0.87 to 1.05 |
| 6–24 | 0.92 | 0.83 to 1.03 | 1.00 | 0.85 to 1.17 | 0.99 | 0.90 to 1.08 | 1.11 | 1.03 to 1.21 |
| > 24 | 0.93 | 0.84 to 1.04 | 0.81 | 0.69 to 0.96 | 1.09 | 0.99 to 1.19* | 1.21 | 1.11 to 1.31 |
|
| ||||||||
| Duration of ADT use per month of ADT | 0.999 | 0.998 to 1.001 | 0.998 | 0.997 to 1.000 | 1.001 | 1.000 to 1.001 | 1.002 | 1.001 to 1.003 |
|
| ||||||||
| P value | .30 | .0408 | .26 | < .0001 | ||||
NOTE. Primary outcomes were estimated using multivariable Cox proportional hazards models. Androgen deprivation therapy (ADT) was modeled as both a categorical variable and a continuous variable. All models were also adjusted for age, comorbidity, medication use, income quintile, primary care access, and rurality. Values in bold type are statistically significant at P < .05. P values refer to models containing duration of ADT use as a continuous variable.
Abbreviations: ADT, androgen deprivation therapy; HR, hazard ratio.
P = .079.
Sensitivity Analyses of Primary Cardiovascular Outcomes
The proportions of ADT users and nonusers taking aspirin, angiotensin antagonists, beta-blockers, and cholesterol-lowering agents were similar at baseline (data not shown). Including these agents as dichotomous exposure variables before the index date or as time-dependent covariates did not alter either of the HRs for MI or sudden cardiac death (data not shown).
DISCUSSION
There has been intense interest in the vascular and endocrine adverse effects of ADT, given the large number of men with prostate cancer who are treated with ADT.1 Using a series of linked administrative databases in Ontario, Canada, to examine outcomes in a population-based cohort of men with prostate cancer given ADT, we found ADT use was associated with an increased risk of incident diabetes, but not with an excess risk of MI or sudden cardiac death after risk adjustment. Secondary outcomes showed an increased risk of fragility fractures but no excess risk of congestive heart failure among ADT users, and a lower likelihood of stroke and of undergoing invasive diagnostic or therapeutic cardiac procedures among ADT users.
These findings are best interpreted in the context of prior studies. Our finding of an increased incidence of diabetes in ADT users was similar to that of Keating et al.14 The finding is also in keeping with those of smaller studies that examined physiologic effects of ADT on measures of glucose homeostasis and insulin resistance.22,23,35 The number needed to harm for an incident case of diabetes was moderately large at 91.
We also found a decreased risk of stroke with ADT use. This was a surprising finding, and given the lack of a plausible biologic explanation and that this was a secondary outcome and a novel finding, it is best viewed as hypothesis generating and requires confirmation.
Our findings are more contentious with respect to MI and sudden cardiac death. Whereas several studies have variously suggested an increased risk of MI, time to fatal MI, and sudden cardiac death among ADT users, we found no such excess risk in either our matched cohort analysis or in duration of ADT use analysis. Three of the four prior studies were limited by a small number of end points (in the two studies that reported risk of MI, the number of events were 5115 and 6117). Our study featured a large number of events, more extensive adjustment for comorbidities (including cardioprotective medication use), and inclusion of all hospitals in the province, which may explain the discrepant findings. We also used a definition for MI that had a lower false-positive rate than simple ICD-9 diagnostic codes.31
It may seem paradoxical that ADT is associated with an increased risk of diabetes (an established cardiovascular risk factor) yet no increased risk of cardiovascular outcomes. Two lines of reasoning may help clarify this discrepancy. First, the increased risk of cardiovascular outcomes from diabetes takes many years to develop, whereas we examined incident diabetes. With even longer follow-up, the impact of incident diabetes on cardiovascular outcomes may be seen. Second, numerous comorbidities and competing causes of mortality may obscure any increased risk of cardiovascular outcomes from diabetes among older ADT users. In particular, in an observational study such as ours, ADT users may have more adverse disease characteristics and greater comorbidity than other men with prostate cancer, which increases the baseline risk of noncancer mortality.
Our study features many strengths. It includes a large sample size and a population-based cohort of men given ADT. We were able to systematically capture a variety of vascular, endocrine, and bone events using a rich set of linked administrative databases, which provided for extensive adjustment for potential confounding variables. Most of our primary outcomes were captured with a high level of accuracy using validated algorithms and near 100% data capture. Our fracture results are similar to those of published studies,9–13 supporting the validity of our findings. Our outcomes also included several secondary vascular outcomes that were not previously assessed among men given ADT. However, several limitations must also be kept in mind. First, we were limited to studying men age 66 years or older in order to obtain prescription medication information. Although this age group represents the vast majority of men who start ADT, we cannot comment on whether similar toxicities would be seen in younger men. Second, despite using comprehensive and well-validated databases, there may be some degree of undercoding of comorbidities. Any undercoding of comorbidity would likely be seen equally among ADT users and nonusers. In support of our findings, several other risk factors for various outcomes were in the anticipated direction, such as an increased risk of MI among men with diabetes or hypertension.
In conclusion, ADT use is associated with an excess risk of incident diabetes and fractures but not MI or sudden cardiac death. These results should help clinicians in discussing the risks of ADT with their patients.
Supplementary Material
Acknowledgments
Supported in part by the Toronto General & Toronto Western Research Foundation and a research scientist award from the Canadian Cancer Society (S.M.H.A.) and by a midcareer scientist award from the Canadian Institutes of Health Research (A.M.C.).
Footnotes
The Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Shabbir M.H. Alibhai, Minh Duong-Hua, Rinku Sutradhar, Neil E. Fleshner, Padraig Warde, Angela M. Cheung, Lawrence F. Paszat
Financial support: Shabbir M.H. Alibhai
Administrative support: Shabbir M.H. Alibhai, Lawrence F. Paszat
Provision of study materials or patients: Minh Duong-Hua, Lawrence F. Paszat
Collection and assembly of data: Minh Duong-Hua, Lawrence F. Paszat
Data analysis and interpretation: Shabbir M.H. Alibhai, Minh Duong-Hua, Rinku Sutradhar, Neil E. Fleshner, Padraig Warde, Angela M. Cheung, Lawrence F. Paszat
Manuscript writing: Shabbir M.H. Alibhai, Minh Duong-Hua, Rinku Sutradhar
Final approval of manuscript: Shabbir M.H. Alibhai, Minh Duong-Hua, Rinku Sutradhar, Neil E. Fleshner, Padraig Warde, Angela M. Cheung, Lawrence F. Paszat
References
- 1.Meng MV, Grossfeld GD, Sadetsky N, et al. Contemporary patterns of androgen deprivation therapy use for newly diagnosed prostate cancer. Urology. 2002;60:7–11. doi: 10.1016/s0090-4295(02)01560-1. [DOI] [PubMed] [Google Scholar]
- 2.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Grossfeld GD, Lubeck DP, et al. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 5.Alibhai SM, Gogov S, Allibhai Z. Long-term side effects of androgen deprivation therapy in men with non-metastatic prostate cancer: A systematic literature review. Crit Rev Oncol Hematol. 2006;60:201–215. doi: 10.1016/j.critrevonc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Greenspan SL, Coates P, Sereika SM, et al. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90:6410–6417. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 7.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med. 2006;166:465–471. doi: 10.1001/.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 9.Daniell HW. Osteoporosis after orchiectomy for prostate cancer. J Urol. 1997;157:439–444. [PubMed] [Google Scholar]
- 10.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 11.Smith MR, Boyce SP, Moyneur E, et al. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136–139. doi: 10.1016/S0022-5347(05)00033-9. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: A claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ, III, Alothman KI, Khosla S, et al. Fracture risk following bilateral orchiectomy. J Urol. 2003;169:1747–1750. doi: 10.1097/01.ju.0000059281.67667.97. [DOI] [PubMed] [Google Scholar]
- 14.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 15.D’Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 16.Saigal CS, Gore JL, Krupski TL, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 17.Tsai HK, D’Amico AV, Sadetsky N, et al. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 18.Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: Analysis of RTOG 92-02. Eur Urol. 2008;54:816–823. doi: 10.1016/j.eururo.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Roach M, III, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: Long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 20.Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol. 2009;27:92–99. doi: 10.1200/JCO.2007.12.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–4267. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 22.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 23.Basaria S, Muller DC, Carducci MA, et al. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–588. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 24.Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–2367. [PubMed] [Google Scholar]
- 25.Institute for Clinical Evaluative Sciences (ICES) ICES data holdings. http://www.ices.on.ca/webpage.cfm?site_id=1&org_id=26&morg_id=0&gsec_id=5314&item_id=5322.
- 26.Holowaty EJ, Fehringer G, Marrett LD. Cancer Incidence in Ontario: Trends and Regional Variations. Toronto, Ontario, Canada: Ontario Cancer Treatment and Research Foundation; 1995. [Google Scholar]
- 27.Canadian classification of diagnostic, therapeutic, and surgical procedures. Ottawa, Canada: Statistics Canada; 1986. [Google Scholar]
- 28.Petersen LA, Wright S, Normand SL, et al. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med. 1999;14:555–558. doi: 10.1046/j.1525-1497.1999.10198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starfield B, Weiner J, Mumford L, et al. Ambulatory care groups: A categorization of diagnoses for research and management. Health Serv Res. 1991;26:53–74. [PMC free article] [PubMed] [Google Scholar]
- 30.Reid RJ, MacWilliam L, Verhulst L, et al. Performance of the ACG case-mix system in two Canadian provinces. Med Care. 2001;39:86–99. doi: 10.1097/00005650-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Tu JV, Austin P, Naylor CD, et al. Acute myocardial infarction outcomes in Ontario: Methods appendix. In: Naylor CD, Slaughter PM, editors. Cardiovascular Health and Services in Ontario: An ICES Atlas. Toronto, Ontario, Canada: Institute for Clinical Evaluative Sciences; 1999. pp. 25–30. [Google Scholar]
- 32.Tu JV, Austin PC, Walld R, et al. Development and validation of the Ontario acute myocardial infarction mortality prediction rules. J Am Coll Cardiol. 2001;37:992–997. doi: 10.1016/s0735-1097(01)01109-3. [DOI] [PubMed] [Google Scholar]
- 33.Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: Determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25:512–516. doi: 10.2337/diacare.25.3.512. [DOI] [PubMed] [Google Scholar]
- 34.Oakes D. Bivariate survival models induced by frailties. J Am Stat Assoc. 1989;84:487–493. [Google Scholar]
- 35.Smith MR, Lee H, Fallon MA, et al. Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology. 2008;71:318–322. doi: 10.1016/j.urology.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.