Abstract
Our objective was to determine the effects of SCH 57068 alone and with 17β-estradiol (E2) on bone, lipids and uteri in ovariectomized (OVX) rats. In OVX animals lumbar vertebral and femoral bone mineral density (BMD) were significantly higher after 12 weeks of treatment with SCH 57068 than in untreated OVX controls. Similarly BMD was superior in OVX + E2 + SCH 57068 treated animals than in OVX + E2 controls. SCH 57068 also significantly reduced the increase in bone turnover markers, serum pyridinoline and serum osteocalcin levels, induced by OVX, and increased mechanical bone strength. SCH 57068 also significantly reduced the rise in serum cholesterol and low-density lipoprotein cholesterol induced by OVX. SCH 57068 had no stimulatory effect on uterine epithelium when given alone in OVX rats. SCH 57068 (1 and 2.5 mg/kg) reduced uterine weight and blocked endometrial stimulation induced by E2. In summary, SCH 57068 adds to the positive effects of E2 on bone and lipid metabolism but blocks the stimulatory effects of E2 on the uterus. Potentially, E2 + SCH 57068 could be combined for the treatment and prevention of breast cancer or as a novel hormone replacement therapy.
Keywords: SERM, SCH 57068, Hormone replacement therapy, Breast cancer, Osteoporosis, Serum lipids
1. Introduction
SCH 57068 is a potent new generation selective estrogen receptor modulator (SERM). The lead compound in this class of agents is tamoxifen which has been extensively used as chronic therapy for both the treatment and prevention of breast cancer. While tamoxifen is effective against breast cancer, it also has other non-breast effects which are important. These include significant provocation of vasomotor symptoms and stimulation of the endometrium causing endome-trial bleeding and cancer [1]. Estrogen replacement therapy has been used in combination with tamoxifen but at standard doses is insufficient to overcome hot flashes and abrogates the anti-breast cancer effects of tamoxifen [1].
In healthy women hormone replacement therapy (HRT) consisting of estrogen plus progestin alleviates vasomotor symptoms and has beneficial effects on bone metabolism but increases the risk of breast cancer and cardiovascular events. The progestin is included to prevent estrogen stimulation and cancer of the endometrium.
SCH 57068 has a higher affinity for the estrogen receptor than estradiol, ICI 182780, 4-hydroxytamoxifen, raloxifene, droloxifene, and hydroxytoremifene [2]. SCH 57068 and its pro-drug, SCH 57050, significantly inhibit growth and prevent development of DMBA-induced mammary carcinomas in the Sprague–Dawley rat [3–5]. SCH 57050 also prevents bone loss and decreases serum cholesterol levels in the ovariectomized (OVX) rat [2,6].
SCH 57068 therefore has potential as a breast cancer drug given as monotherapy. Because of its greater potency than tamoxifen, however, it is of interest to determine whether estrogen could be combined with SCH 57068 to alleviate vasomotor symptoms without reducing its efficacy against breast cancer and having additive benefits on bone and lipid metabolism. In addition it is of interest to determine whether estrogen plus SCH 57068 could be a novel HRT.
The OVX rat is a widely used osteopenic animal model that mimics the development of estrogen deficiency-induced osteopenia in humans [7–10]. For example OVX caused significant decrease in femoral bone density, and 4 months of therapy with antiestrogens tamoxifen and keoxifene did not further decrease the bone density of OVX rats but rather helped to maintain bone density [7]. The model is also useful to study the lipid profile resulting from treatment with various endocrine therapies [11]. For these reasons we investigated the effects of SCH 57068 either alone or in combination with estrogen on organ functions other than the breast including bone, serum lipids and uterine histology in this model.
2. Materials and methods
2.1. Animals and experimental design
All experimental procedures were performed under the guidelines established by the Canadian Council on Animal Care. Ten-month-old female Sprague–Dawley rats were obtained from Harlan (Indianapolis, IN, USA). They were housed in pairs on a 12 h light/dark cycle, with room temperature set at 22 °C. The animals were allowed to acclimatize for 4 weeks, with ad lib access to both food (TD 89222 diet, 0.5% calcium and 0.4% phosphorus; Teklad, Madison, WI, USA) and tap water. Rats were matched according to body weight and assigned to 9 experimental groups of 9–12 animals each, as follows: group 1, intact controls; group 2, OVX controls; group 3–5, OVX + SCH 57068 0.01, 1 or 2.5 mg/kg; group 6, OVX + 17β-estradiol (E2, implant); group 7–9, OVX + E2 (implant) + SCH 57068 0.01, 1 or 2.5 mg/kg. Rats were anesthetized using ketamine hydrochloride (120mg/kg) and xylazine hydrochloride (24mg/kg) intramuscularly, and a bilateral ovariectomy was performed via a dorsal midline incision just caudal to the 13th rib [12]. The OVX + E2 animals were implanted with a slow-release pellet containing 0.085 mg E2 (Innovative Research of America, Sarasota, FL, USA). This dose of estradiol has previously been shown to result in physiologic proestrus serum concentrations of estradiol [13]. The intact control group was subjected to the same general surgical procedure as OVX animals except that the ovaries were not excised. SCH 57068 (Fig. 1) was provided by Schering Plough Research Institute, NJ, USA. SCH 57068 or vehicle (0.4% methylcellulose in water) was given once daily by oral gavage in a volume 0.1 ml/100 g of body weight for 12 weeks.
Fig. 1.
Molecular structure of SCH 57068.
After 12 weeks of treatment, the animals were euthanized by cardiac puncture under ketamine anesthesia. All animals were fasted overnight before blood collection for bone marker and serum lipid assays. The whole lumbar spine and femora from each animal were excised for subsequent measurements of bone mineral density, mechanical tests and histomorphometric analysis. The uteri were removed for histological examination.
2.2. Bone densitometry
The cleaned, excised lumbar spine and left femur of individual animals were scanned by dual energy X-ray absorptiometry (Hologic QDR; 4500 A) using the regional high resolution scan mode (0.311 mm ×0.311 mm pixels) with a line spacing of 0.0311 cm and a point resolution of 0.0311 cm. Whole left femur and lumbar vertebrae (first through sixth) were used in the analysis. The bone mineral content (BMC) and area were measured, and bone mineral density (BMD) was calculated automatically as BMC/area.
2.3. Biochemical markers of bone turnover
Bone turnover markers were measured using commercially available kits as specified by the manufacturers. All assays were performed in duplicate.
The bone resorption marker serum pyridinoline (PYD) was measured by competitive enzyme immunoassay (EIA) using a serum PYD kit (Metra Biosystems Inc., USA). Briefly, serum samples were filtrated by a 30k MWCO Spin filter, and incubated at 4 °C for 22 h in the dark. The bone formation marker serum osteocalcin (OC) was measured using a rat OC EIA Kit (Biomedical Technologies Inc., USA). Briefly, serum samples were diluted 1/10 with sample buffer, and incubated at 4 °C for 22 h.
2.4. Mechanical tests
The mechanical failure properties of the femora and vertebrae were conducted using an Instron 8501 material testing system (Instron Corp., Canton, Massachusetts, USA). In both experiments, force and deformation data were collected at a rate of 25 Hz using a 12-bit data acquisition card (National Instruments, USA), Labview 5.0 data acquisition software (National Instruments, USA) and a Pentium™ II computer (Compaq Canada).
The diaphysis of the right femur was tested to failure in a three-point bending test according to a procedure described previously [14]. Briefly, samples were subjected to a preload of 1 N at a rate of 1 mm/min until failure. The body of the fifth lumbar vertebra was tested to failure in unconfined compression using a procedure similar to that described previously [15]. Briefly, a preload of 2 N was applied to the vertebral body and then deformed at a rate of 2 mm/min until failure occurred. In these experiments, the point of failure was defined as a successive drop in load greater than 5%.
2.5. Specimen processing
Left femora from the rats were cleaned of soft tissue and fixed in 70% ethanol. The femora were bisected using an Isomet 1000 slow speed saw (Buehler Ltd., USA) and further fixed in 70% ethanol before plastic processing. Both halves of the femora were dehydrated in ascending grades of acetone and processed in ascending grades of Spurr (resin)/acetone before being embedded and polymerized in Spurr resin at 50 °C. Five-micron sections were cut serially using a Leica RM 2165 rotary microtome (Leica Canada) equipped with a tungsten carbide knife. The 5 μm sections were stained with Toluidine Blue and Goldner’s Trichrome for static histomorphometric assessment.
2.6. Histomorphometric analysis
Static histomorphometry was performed on a 5 μm undecalcified Goldner’s Trichrome stained section of each proximal femur. All quantitative assessment was performed by a single trained technician using a semiautomated image analysis system (Bioquant, R&M Biometrics, USA). A Metalux microscope (Leica Canada) equipped with a 10× objective (total magnification 125×) was used to assess the stained slides. Measurements of bone were taken from a 15 mm2 area in the central region beginning 0.2 mm distal to the growth plate. The area selected for measurement covered most of the trabecular bone available for measurement. For each sample the following parameters were measured: trabecular bone volume (BV), mineralized trabecular bone volume (Md.V), osteoid volume (OV), osteoid surface (OS), and eroded surface (ES). In addition, from the above measurements trabecular thickness (Tb.Th), osteoid thickness (O.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp) were calculated. All measurements and calculations were done according to the American Society for Bone and Mineral Research (ASBMR) nomenclature and guidelines [16], and the evaluation methods previously described [17–20].
2.7. Serum lipids
Blood samples were allowed to clot at 4 °C for 2 h, and then centrifuged at 2000 ×g for 10 min. The serum was transferred to new tubes for lipid assays. Total serum cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol and triglyceride levels were measured using the Bayer Advia Reagent Packs and assayed on an ADVIA® 1650 chemistry system analyzer (Bayer Diagnostics, Bayer Inc., USA).
2.8. Uterine weight and histology
2.8.1. Uterine wet weight
The uteri were excised, trimmed free of fat, pierced and blotted to remove excess fluid. The body of the uterus was cut just above its junction with the cervix and at the junction of the uterine horns with the ovaries. The uterus was then weighed (wet weight).
2.8.2. Uterine epithelial lining cells
10% phosphate buffered formalin-fixed uteri were processed for conventional paraffin embedding. Cross sections (4 μm in thickness) were prepared from both horns of each uterus and stained with hematoxylin and eosin. The epithelial lining cells were measured at a magnification of ×125, using a Quantimet 500 MC automated image analysis system (Leica Canada). The image analysis system is attached to an Orthoplan microscope equipped with a 25× objective and a JVC color camera.
2.9. Statistical analysis
Data are expressed as the mean ±standard error of the mean (S.E.M.). All the data were analyzed using a one-way ANOVA with statistical software (Analyse-it Software Ltd., UK). Pair-wise comparisons between groups were performed using Fisher’s PLSD post hoc test. Significance was considered at P < 0.05.
3. Results
3.1. Bone mineral density (BMD)
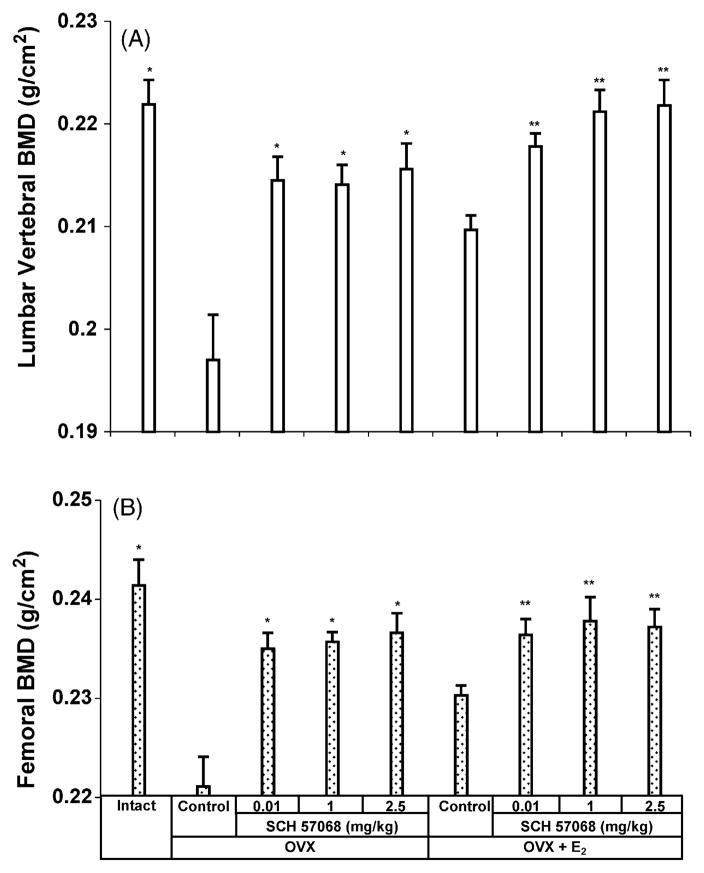
The effect of 12 weeks of treatment with SCH 57068 on lumbar spine BMD is illustrated in Fig. 2A. Lumbar spine BMD was 11.2% lower in OVX rats than in intact controls (P < 0.0001). After 12 weeks of treatment, the OVX animals given SCH 57068 at doses of 0.01–2.5 mg/kg had 96–97% of the BMD observed in intact rats. The lumbar spine BMD was 8.8–9.4% higher in OVX rats given 0.01–2.5 mg/kg of SCH 57068 than in OVX controls (all P <0.0001). The lumbar spine BMD was 3.8–5.7% higher in OVX plus E2-treated animals given 0.01–2.5 mg/kg of SCH 57068 than in OVX rats given E2 alone (all P < 0.0001). Similar effects were observed on femoral BMD (Fig. 2B). Twelve weeks after ovariectomy, femoral BMD had decreased by 8.4% (P <0.0001 versus intact controls). The animals given SCH 57068 at doses of 0.01–2.5 mg/kg had 97–98% of the BMD observed in intact rats. The femoral BMD was 6.3–7.0% higher in OVX rats given 0.01–2.5 mg/kg of SCH 57068 than in OVX controls (all P <0.0001). The femoral BMD was 2.6–3.0% higher in OVX plus E2-treated animals given 0.01–2.5 mg/kg of SCH 57068 than in OVX rats given E2 alone (all P < 0.0001).
Fig. 2.
Bone mineral density of the lumbar vertebrae (A) and whole femora (B) after 12 weeks of treatment with SCH 57068. Scale bars represent the mean ±S.E.M. (n = 9–12). *P < 0.0001 vs. OVX controls;**P < 0.0001 vs. OVX + E2 controls.
3.2. Biochemical markers of bone turnover
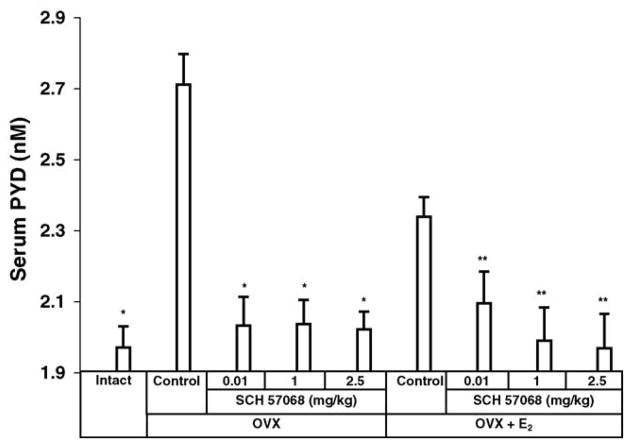
The preventive effects on bone loss of SCH 57068 were also correlated with serum PYD excretion, a biochemical marker of bone resorption. Twelve weeks after ovariectomy, the serum PYD excretion was 38% higher in OVX rats than in intact controls (P <0.0001), suggesting excessive bone resorption in OVX rats. In OVX animals given 0.01–2.5 mg/kg of SCH 57068 the OVX-induced increase of PYD was reduced by 91–93% (all P <0.0001), indicating prevention of bone resorption by treatment with SCH 57068. Also, the OVX plus E2-treated animals given 0.01–2.5 mg/kg of SCH 57068 had a reduction in the OVX-induced increase of serum PYD of 66–99% (all P <0.0001) in comparison with OVX rats given E2 alone (Fig. 3).
Fig. 3.
Serum pyridinoline (PYD) levels after 12 weeks of treatment with SCH 57068. Scale bars represent the mean ±S.E.M. (n = 9–12). *P < 0.0001 vs. OVX controls; **P < 0.0001 vs. OVX + E2 controls.
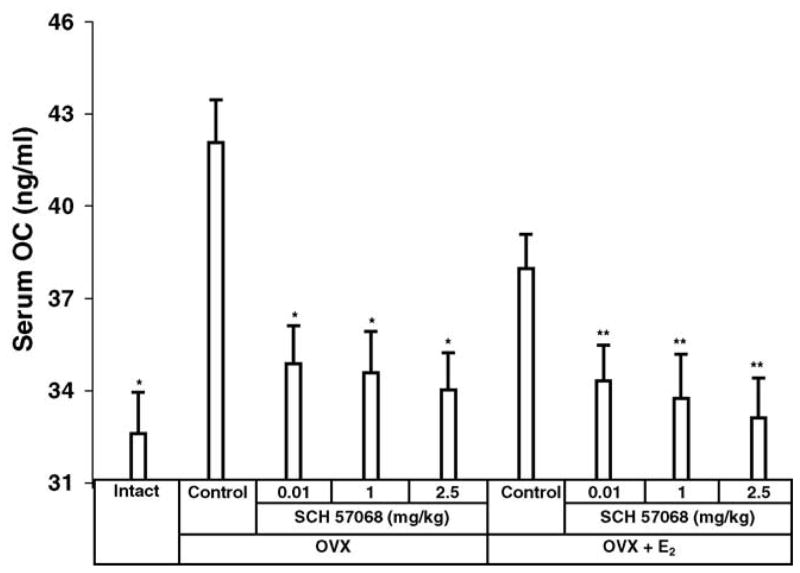
The results of serum OC, a biochemical marker of bone formation, are shown in Fig. 4. Twelve weeks after ovariectomy, serum OC was increased by 23% in OVX rats compared with intact controls (P <0.005). This indicates an increase in bone turnover. The OVX animals given 0.01–2.5 mg/kg of SCH 57068 had a 76–85% (all P <0.005) reduction in the OVX-induced increase of serum OC compared to OVX controls. Also, the OVX plus E2-treated animals given 0.01–2.5 mg/kg of SCH 57068 had a 68–90% (all P < 0.005) reduction in the OVX-induced increase of serum OC in comparison with OVX rats given E2 alone.
Fig. 4.
Serum osteocalcin (OC) levels after 12 weeks of treatment with SCH 57068. Scale bars represent the mean ±S.E.M. (n = 9–12). *P < 00.005 vs. OVX controls; **P < 0.005 vs. OVX + E2 controls.
3.3. Mechanical properties
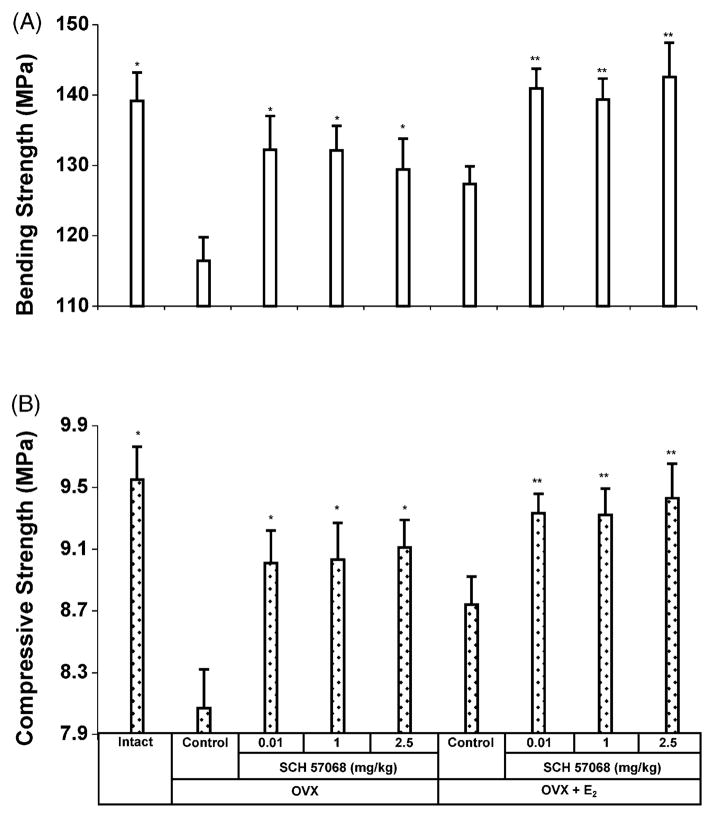
Ovariectomy or administration of SCH 57068 significantly affected the failure properties of the femur as indicated by three-point bending (Fig. 5A). Ovariectomy caused a 16.3% decrease in three-point bending strength (P < 0.0005 versus intact controls). The bending strengths were 11.1–13.5% higher in OVX rats given 0.01–2.5 mg/kg of SCH 57068 than in OVX controls (all P < 0.0005) and the bending strengths were 6.2–9.9% higher in OVX plus E2-treated animals given 0.01–2.5 mg/kg of SCH 57068 than in OVX rats given E2 alone (all P < 0.0005).
Fig. 5.
Mechanical properties of the femora and lumbar vertebrae after 12 weeks of treatment with SCH 57068. Three-point bending strength of the femora (A). Compressive strength of the fifth lumbar vertebrae (B). Scale bars represent the mean ±S.E.M. (n = 9–12). *P < 0.0005 vs. OVX controls; **P < 0.0005 vs. OVX + E2 controls.
The effect of SCH 57068 on compressive strength of the fifth lumbar vertebra is shown in Fig. 5B. Ovariectomy caused a 15.5% decrease in compressive strength (P < 0.0005 versus intact controls). The compressive strengths were 11.6–12.9% higher in OVX rats given 0.01–2.5 mg/kg of SCH 57068 than in OVX controls (all P < 0.0005) and the compressive strengths were 5.7–7.9% higher in OVX plus E2-treated animals given 0.01–2.5 mg/kg of SCH 57068 than in OVX rats given E2 alone (all P < 0.0005).
3.4. Bone histomorphometry
A summary of the structural parameters from the static histomorphometry is shown in Table 1. The OVX rats exhibited a significantly lower trabecular bone volume (BV), mineralized trabecular bone volume (Md.V) and trabecular number (Tb.N) (all P < 0.0001), as well as higher trabecular separation (Tb.Sp) (P < 0.0001), than the intact controls. The OVX rats and OVX plus E2-treated animals given 0.01–2.5 mg/kg of SCH 57068 had a significantly higher BV, Md.V and Tb.N (all P < 0.0001), as well as lower Tb.Sp (P < 0.0001), than the OVX and OVX plus E2-treated controls, respectively.
Table 1.
Histomorphometric values of the various groups
| Group | BV (%) | Md.V (%) | Tb.N (mm−1) | Tb.Sp (μm) | Tb.Th (μm) | OV (%) | OS (%) | O.Th (μm) |
|---|---|---|---|---|---|---|---|---|
| Intact | 23.33 ±1.18a | 23.26 ±1.19a | 3.80 ±0.22a | 204.9 ±13.2a | 62.1 ±4.2 | 0.27 ±0.05a | 2.72 ± 0.41a | 3.22 ±0.28 |
| OVX | 11.87 ±1.02 | 11.62 ±1.00 | 2.00 ±0.13 | 449.2 ±35.5 | 59.0 ±1.9 | 2.15 ±0.17 | 16.22 ± 0.90 | 3.99 ±0.51 |
| OVX + SCH57068 (0.01 mg/kg) | 19.03 ±0.11a | 18.91 ±1.10a | 2.87 ±0.17a | 286.8 ±18.9a | 66.5 ±2.2 | 0.60 ±0.09a | 5.83 ± 0.46a | 3.54 ±0.49 |
| OVX + SCH57068 (1 mg/kg) | 20.65 ±1.24a | 20.53 ±1.23a | 2.76 ±0.09a | 289.5 ±12.0a | 74.9 ±3.9 | 0.56 ±0.07a | 6.08 ± 0.46a | 3.45 ±0.15 |
| OVX + SCH57068 (2.5 mg/kg) | 20.68 ±0.87a | 20.56 ±0.86a | 2.70 ±0.14a | 297.3 ±19.0a | 77.0 ±3.5 | 0.56 ±0.04a | 6.41 ± 0.56a | 3.54 ±0.29 |
| OVX + E2 | 18.08 ±1.03a | 17.95 ±1.02a | 2.49 ±0.13a | 334.2 ±22.5a | 72.7 ±0.8 | 0.71 ±0.07a | 8.03 ± 0.79a | 3.40 ±0.23 |
| OVX + E2 + SCH 57068 (0.01 mg/kg) | 22.89 ±1.47b | 22.79 ±1.46b | 3.08 ±0.18b | 254.8 ±18.4b | 74.7 ±4.1 | 0.42 ±0.06b | 5.10 ± 0.49b | 3.18 ±0.50 |
| OVX + E2 + SCH57068 (1 mg/kg) | 21.58 ±0.80b | 21.48 ±0.79b | 3.16 ±0.09b | 249.6 ±9.3b | 68.5 ±2.1 | 0.45 ±0.04b | 4.28 ± 0.44b | 3.67 ±0.26 |
| OVX + E2 + SCH57068 (2.5 mg/kg) | 23.07 ±0.75b | 22.96 ±0.74b | 3.29 ±0.07b | 234.2 ±6.4b | 70.1 ±1.9 | 0.45 ±0.04b | 3.88 ± 0.54b | 4.63 ±0.46 |
Data are means ±S.E.M. of 5 animals.
P < 0.0001 vs. OVX rats.
P < 0.0001 vs. OVX + E2 rats.
The osteoid results, a bone formation parameter, are also summarized in Table 1. The osteoid volume (OV) and osteoid surface (OS) of the OVX rats were significantly higher than in intact controls and OVX rats given 0.01–2.5 mg/kg of SCH 57068 (all P < 0.0001). Also the OV and OS of the OVX plus E2-treated rats were significantly higher than in the OVX plus E2-treated animals given 0.01–2.5 mg/kg of SCH 57068 (all P < 0.0001).
3.5. Serum lipids
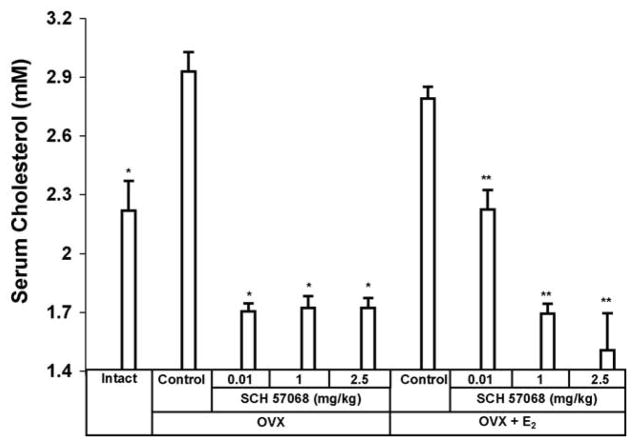
The effect of SCH 57068 on serum cholesterol is shown in Fig. 6. Twelve weeks after ovariectomy, a 32% increase in total serum cholesterol was observed in OVX rats compared with intact controls (P <0.0001). After 12 weeks of treatment, the administration of 0.01–2.5 mg/kg SCH 57068 to OVX rats caused 41–42% inhibition of serum cholesterol levels (all P <0.0001 versus OVX controls). OVX plus E2-treated animals given 0.01–2.5 mg/kg of SCH 57068 had a reduction in serum cholesterol of 20–46% compared to OVX rats given E2 alone (all P < 0.0001).
Fig. 6.
Serum cholesterol levels after 12 weeks of treatment with SCH 57068. Scale bars represent the mean ±S.E.M. (n = 9–12). *P < 0.0001 vs. OVX controls; **P < 0.0001 vs. OVX + E2 controls.
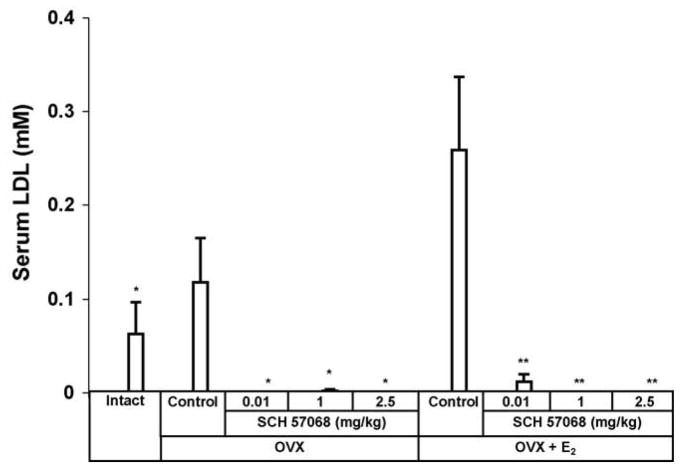
Results of the effect of SCH 57068 on serum LDL levels are shown in Fig. 7. OVX animals given 0.01–2.5 mg/kg of SCH 57068 had a reduction in serum LDL of > 95% (P < 0.05) compared with OVX controls. Also, OVX plus E2-treated animals given 0.01–2.5 mg/kg of SCH 57068 had a reduction in serum LDL of over 95% (P <0.05) compared to OVX plus E2 controls. No significant change was observed in serum triglyceride levels with any of the treatments used (Table 2).
Fig. 7.
Serum LDL levels after 12 weeks of treatment with SCH 57068. Scale bars represent the mean ±S.E.M. (n = 9–12). *P < 0.05 vs. OVX controls; **P < 0.05 vs. OVX + E2 controls.
Table 2.
Effect of 12-week treatment with SCH 57068 on body weight gain, uterine wet weight, serum HDL and triglyceride levels in the ovariectomized rat
| Group | Body weight gain (g) | Uterine weight (mg) | HDL (mM) | Triglycerides (mM) |
|---|---|---|---|---|
| Intact | 0.9 ± 3.6a | 652 ±23a | 1.98 ±0.10a | 0.60 ±0.08 |
| OVX | 33.3 ± 2.7 | 171 ±5 | 2.55 ±0.07 | 0.57 ±0.04 |
| OVX + SCH57068 (0.01 mg/kg) | 12.5 ± 2.6a | 184 ±7 | 1.67 ±0.04a | 0.63 ±0.03 |
| OVX + SCH57068 (1 mg/kg) | 10.8 ± 2.6a | 167 ±2 | 1.60 ±0.06a | 0.71 ±0.07 |
| OVX + SCH57068 (2.5 mg/kg) | 10.4 ± 2.5a | 160 ±3 | 1.68 ±0.04a | 0.66 ±0.07 |
| OVX + E2 | −12.0 ± 3.7 | 533 ±19 | 2.35 ±0.06 | 0.43 ±0.03 |
| OVX + E2 +SCH57068 (0.01 mg/kg) | −15.9 ± 1.6 | 530 ±21 | 2.12 ±0.11b | 0.51 ±0.03 |
| OVX + E2+ SCH57068 (1 mg/kg) | −16.3 ± 3.1 | 308 ±14b | 1.63 ±0.04b | 0.43 ±0.04 |
| OVX + E2+ SCH57068 (2.5 mg/kg) | −16.7 ± 4.8 | 241 ±6b | 1.70 ±0.04b | 0.39 ±0.03 |
Data are mean ±S.E.M. of 9–12 animals.
P < 0.0001 vs. OVX rats.
P < 0.0001 vs. OVX + E2 rats.
3.6. Uterine wet weight and epithelial lining cells
The effect of SCH 57068 on uterine wet weight in OVX rats and OVX plus E2-treated rats is presented in Table 2. Twelve weeks after ovariectomy, an approximate 74% decrease in uterine wet weight was observed (P < 0.0001 versus intact controls). No significant change was observed in uterine wet weight in OVX rats receiving 0.01–2.5 mg/kg SCH 57068. OVX plus E2-treated animals given 1.0 and 2.5 mg/kg of SCH 57068 had a reduction in uterine wet weight of 42% (P <0.0001) and 55% (P <0.0001), respectively, compared to the OVX plus E2 controls.
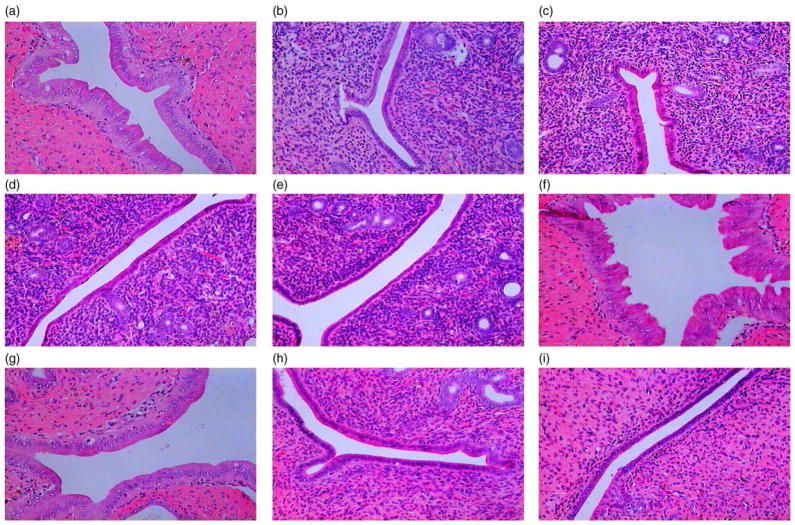
In Fig. 8, the uterine histology illustrates the absence of any stimulatory effect of 12 weeks of treatment with 0.01–2.5 mg/kg SCH 57068 on the uterine epithelial cells compared to OVX controls. The higher dose of SCH 57068 (1.0 mg and 2.5 mg/kg) significantly negated the E2 stimulatory effect on the uterine epithelial cells.
Fig. 8.
Hematoxylin and eosin-stained sections of rat uteri illustrating epithelial lining cells obtained from intact controls (a), OVX controls (b), OVX rats treated for 12 weeks with SCH 57068 0.01 mg/kg (c), 1 mg/kg (d) or 2.5 mg/kg (e), as well as OVX animals bearing an implant of 17 β-estradiol (E2) (f), OVX rats bearing an E2 implant treated for 12 weeks with SCH 57068 0.01 mg/kg (g), 1 mg/kg (h) or 2.5 mg/kg (i). Note the absence of stimulatory effect of SCH 57068 on the uterine epithelial cells in the OVX rat, as well as the inhibitory effect of SCH 57068 (1.0 mg and 2.5 mg/kg) on uterine epithelial cells in the OVX plus E2-treated rat (magnification of ×125).
3.7. Body weight gain
As shown in Table 2, 12 weeks after ovariectomy, gain in body weight in OVX rats was significantly greater than that seen in intact controls (33.3 g versus 0.9 g). However, 0.01–2.5 mg/kg of SCH 57068 given to OVX rats caused 62.5–68.8% decrease in weight gain compared with OVX controls (all P < 0.0001).
4. Discussion
SERMs are compounds with both estrogenic and antiestrogenic activities at different sites in the body. At present, two SERMs, tamoxifen for the prevention of breast cancer and raloxifene for the prevention of osteoporosis, are clinically available [21]. An ideal SERM would have an anti-proliferative antagonist action on the breast, lowering the risk of breast cancer and causing no endometrial proliferation, no menstrual bleeding and no endometrial cancer. An ideal SERM should also have estrogen-like actions in reducing osteoporosis, lowering the risk of cardiovascular disease by lowering total cholesterol and LDL, increasing HDL, and improving other markers of cardiovascular function [1]. SCH 57068 has many of these properties. SCH 57068 shows structural similarity with other benzopyrans and raloxifene analogues. The structure–function relationship of these molecules is described in detail [22].
The OVX rat model mimics changes in bone metabolism observed in human postmenopausal osteoporosis. Biochemical markers of bone resorption and formation reflecting bone turnover are elevated after OVX and return to low levels after estrogen repletion or after treatment with antiresorptive agents [23–26]. OVX also causes a reduction in BMD, bending strength of the femur and compressive strength of the vertebral bodies. Our results concerning biochemical markers of bone turnover, bone strength and histomorphometry in the OVX rats are therefore consistent with published studies of this model [23–30]. Administration of SCH 57068 to the OVX-treated animals in our experiment appeared to markedly reduce the increase in overall bone turnover induced by OVX. Primarily, bone resorption was reduced, reflected by a reduction in serum PYD. Consequently, the reflex rise in bone formation seen after OVX, as reflected by an increase in serum OC and osteoid volume and surface, was not seen after SCH 57068 administration. The overall reduction in bone turnover seen with SCH 57068 was also reflected by an expected increase in bending strength of the femora and compressive strength of the vertebrae, as well as trabecular bone number and volume, suggesting that SCH 57068 improved both cortical and trabecular bone metabolism. Of importance, SCH 57068 exerted a positive effect on bone metabolism not only as a single agent but also over and above the positive effect of estradiol. Changes in bone biomarkers have not always correlated with improvement in bone quality so it is important that bone strength was improved by SCH 57068.
The rat model is also useful for detecting the pharmacological effects of estrogens and antiestrogens on total serum cholesterol and LDL cholesterol. OVX causes a marked rise in total serum cholesterol and LDL cholesterol levels. In addition, as shown in Table 2, the administration of 0.01–2.5 mg/kg SCH 57068 to OVX rats caused a 34–37% decrease of HDL cholesterol compared to OVX controls (all P < 0.0001), and OVX plus E2-treated animals given 0.01–2.5 mg/kg of SCH 57068 had a reduction in HDL cholesterol of 10–31% compared to OVX rats given E2 alone (all P < 0.0001). To understand the changes in HDL cholesterol in rats, one has to contrast the changes seen in the animals compared to humans. In both humans and rodents estrogen lowers cholesterol by upregulating the hepatic LDL receptor, thus resulting in an increased removal of serum cholesterol from the circulation [31]. This effect results in a preferential reduction of LDL cholesterol in humans. However, in the rat, both HDL and LDL cholesterol are reduced, because rat HDL contains apoprotein E (not found in human HDL), which also binds to the hepatic LDL receptor [32]. Thus, in the rat, as opposed to humans, HDL cholesterol is a predominant form of circulating cholesterol, and estrogen therapy lowers both HDL cholesterol and LDL cholesterol [6]. Thus the reduction in both HDL and LDL cholesterol seen with SCH 57068 in the OVX rat is in the desired direction.
Estradiol has a stimulatory effect on the endometrium leading to increased vaginal bleeding and endometrial cancer [1]. Tamoxifen, through a partial estrogen agonist effect shares these characteristics [1,33,34]. Progestin is used to protect the endometrium when given as part of HRT but further increases the risk of breast cancer [35–37]. SCH 57068 appears to have a strong pure anti-estrogenic effect on the uterus and its endometrium and is sufficient to completely block the stimulatory effect of estradiol [2,38,39].
In summary, SCH 57068 has potent anti-breast cancer effects in pre-clinical models and our results have now confirmed its strong positive effect on bone and lipid metabolism and its negative effect on the endometrium. In addition when combined with estradiol we have now demonstrated that SCH 57068 adds advantage to estradiol on bone and lipid metabolism as well as blocking the stimulatory effects of estradiol on the endometrium. Importantly however, this did not occur at the lowest dose of SCH 57068.
Potentially SCH 57068 could be useful as monotherapy against breast cancer. In addition it may be possible to combine it with estradiol for alleviation of hot flashes and still retain its full anti-breast cancer effects. Furthermore in healthy postmenopausal women with intractable hot flashes the use of estrogen replacement therapy in combination with SCH 57068 might be possible, again alleviating vasomotor symptoms, while not increasing the risk of breast or endome-trial cancer and having additive benefits on bone and lipid metabolism. Of importance, however, despite these beneficial effects, the potential for thromboembolism seen with tamoxifen needs to be investigated for SCH 57068. Furthermore, these effects will also need to be delineated in combination with estrogen. The profile of SCH 57068 demonstrated in the rat model merits further testing in breast cancer patients. It would also be of interest to test it in combination with estrogen in symptomatic menopausal women.
Acknowledgments
The authors gratefully thank Dr. Fernand Labrie and Dr. Céline Martel, Molecular Endocrinology and Oncology Research Center, Laval University Medical Center (CHUL), Laval University, Canada, for consulting. The authors also gratefully thank Dr. Jian Wang of the Mechanical Testing Laboratory, Institute of Biomaterials and Biomedical Engineering, University of Toronto, for his assistance in bone mechanical testing, and Christina Djokoto of the Osteoporosis Program, University Health Network, for her assistance in measurement of bone mineral density, as well as Ionela Mitroi of the Department of Pathology and Laboratory Medicine, Mount Sinai Hospital, for her technical assistance in histological sample preparation. This study was supported in part by Schering Plough Research Institute, NJ, USA.
References
- 1.Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5:207–213. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 2.Labrie F, Labrie C, Bélanger A, Simard J, Gauthier S, Luu-The V, Mérand Y, Giguère V, Candas B, Luo S, Martel C, Singh SM, Fournier M, Coguet A, Richard V, Charbonneau R, Charpenet G, Tremblay A, Tremblay G, Cusan L, Veilleux R. EM-652 (SCH 57068), a third generation SERM acting as pure antiestrogen in the mammary gland and endometrium. J Steroid Biochem Mol Biol. 1999;69:51–84. doi: 10.1016/s0960-0760(99)00065-5. [DOI] [PubMed] [Google Scholar]
- 3.Luo S, Sourla A, Labrie C, Bélanger A, Labrie F. Combination effects of dehydroepiandrosterone and EM-800 on bone mass, serum lipids, and the development of dimethylbenz(a)anthracene-induced mammary carcinoma in the rat. Endocrinology. 1997;138:4435–4444. doi: 10.1210/endo.138.10.5429. [DOI] [PubMed] [Google Scholar]
- 4.Luo S, Stojanovic M, Labrie C, Labrie F. Inhibitory effect of the novel anti-estrogen EM-800 and medroxyprogesterone acetate on estrone-stimulated growth of dimethylbenz(a)anthracene-induced mammary carcinoma in the rat. Int J Cancer. 1997;73:580–586. doi: 10.1002/(sici)1097-0215(19971114)73:4<580::aid-ijc20>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Luo S, Labrie C, Bélanger A, Candas B, Labrie F. Prevention of development dimethylbenz(a)anthracene (DMBA)-induced mammary carcinoma in the rat by the new nonsteroidal antiestrogen EM-800 (SCH57050) Breast Cancer Res Treat. 1998;49:1–11. doi: 10.1023/a:1005928814521. [DOI] [PubMed] [Google Scholar]
- 6.Martel C, Picard S, Richard V, Bélanger A, Labrie C, Labrie F. Prevention of bone loss by EM-800 and raloxifene in the ovariectomized rat. J Steroid Biochem Mol Biol. 2000;74:45–56. doi: 10.1016/s0960-0760(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 7.Jordan VC, Phelps E, Lindgren JU. Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat. 1987;10:31–35. doi: 10.1007/BF01806132. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki I, Yamaguchi H. Characteristics of an ovariectomized osteopenic rat model. J Bone Miner Res. 1989;4:13–22. doi: 10.1002/jbmr.5650040104. [DOI] [PubMed] [Google Scholar]
- 9.Wronski TJ, Yen C-F. The ovariectomized rat as an animal model for postmenopausal bone loss. Cells Mater suppl. 1991;1:69–74. [Google Scholar]
- 10.Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–191. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- 11.Lundeen SG, Carver JM, Mckean ML, Winneker RC. Characterization of the ovariectomized rat model for the evaluation of estrogen effects on plasma cholesterol levels. Endocrinology. 1997;138:1552–1558. doi: 10.1210/endo.138.4.5083. [DOI] [PubMed] [Google Scholar]
- 12.Black LJ, Sato M, Rowley ER, Magee DE, Bekele A, Williams DC, Cullnan GJ, Bendele R, Kauffman RF, Bensch WR, Frolik CA, Termine JD, Bryant HU. Raloxifene (LY139481 HCl) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest. 1994;93:63–69. doi: 10.1172/JCI116985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanin CM, MacLusky NJ, Grynpas MD, Casper RF. The effect of three hormone replacement regimens on bone density in the aged ovariectomized rat. Fertil Steril. 1995;63:643–651. [PubMed] [Google Scholar]
- 14.Kasra M, Vanin CM, MacLusky NJ, Casper RF, Grynpas MD. Effect of different estrogen and progestin regimens on the mechanical properties of rat femur. J Orthop Res. 1997;15:118–123. doi: 10.1002/jor.1100150117. [DOI] [PubMed] [Google Scholar]
- 15.Chachra D, Vanin CM, MacLusky NJ, Casper RF, Grynpas MD. The effect of different hormone replacement therapy regimens on the mechanical properties of rat vertebrae. Calcif Tissue Int. 1995;56:130–134. doi: 10.1007/BF00296344. [DOI] [PubMed] [Google Scholar]
- 16.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization nomenclature, symbols and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 17.Quarles LD, Lobaugh B. Equivalency of various methods for estimating osteoid seam width. J Bone Miner Res. 1989;4:671–677. doi: 10.1002/jbmr.5650040505. [DOI] [PubMed] [Google Scholar]
- 18.Huffer WE, Lepoff RB. An indirect method of measuring widths suitable for automated bone histomorphometry. J Bone Miner Res. 1992;7:1417–1427. doi: 10.1002/jbmr.5650071209. [DOI] [PubMed] [Google Scholar]
- 19.Huffer WE, Ruegg P, Zhu JM, Lepoff RB. Semiautomated methods for cancellous bone histomorphometry using a general-purpose video image analysis system. J Microsc. 1994;173:53–66. doi: 10.1111/j.1365-2818.1994.tb03427.x. [DOI] [PubMed] [Google Scholar]
- 20.Steiniche T. Bone histomorphometry in the pathophysiological evaluation of primary and secondary osteoporosis and various treatment modalities. APMIS suppl. 1995;51:1–44. [PubMed] [Google Scholar]
- 21.Jordan VC, Gapstur S, Morrow M. Selective estrogen receptor modulation and reduction in risk of breast cancer, osteoporosis, and coronary heart disease. J Natl Cancer Inst. 2001;93:1449–1457. doi: 10.1093/jnci/93.19.1449. [DOI] [PubMed] [Google Scholar]
- 22.Jordan VC. Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 2. Clinical considerations and new agents. J Med Chem. 2003;46:1081–1111. doi: 10.1021/jm020450x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshitake K, Yokota K, Kasugai Y, Kagawa M, Sukamoto T, Nakamura T. Effects of 16 weeks of treatment with tibolone on bone mass and bone mechanical and histomorphometric indices in mature ovariectomized rats with established osteopenia on a low-calcium diet. Bone. 1999;25:311–319. doi: 10.1016/s8756-3282(99)00172-6. [DOI] [PubMed] [Google Scholar]
- 24.Lane NE, Kumer JL, Majumdar S, Khan M, Lotz J, Stevens RE, Klein R, Phelps KV. The effects of synthetic conjugated estrogens, a (cenestin) on trabecular bone structure and strength in the ovariectomized rat model. Osteoporos Int. 2002;13:816–823. doi: 10.1007/s001980200113. [DOI] [PubMed] [Google Scholar]
- 25.Li XX, Hara I, Matsumiya T. Effects of osthole on postmenopausal osteoporosis using ovariectomized rats; comparison to the effects of estradiol. Biol Pharm Bull. 2002;25:738–742. doi: 10.1248/bpb.25.738. [DOI] [PubMed] [Google Scholar]
- 26.Hornby SB, Evans GP, Hornby SL, Pataki A, Glatt M, Green JR. Long-term zoledronic acid treatment increases bone structure and mechanical strength of long bones of ovariectomized adult rats. Calcif Tissue Int. 2003;72:519–527. doi: 10.1007/s00223-002-2015-4. [DOI] [PubMed] [Google Scholar]
- 27.Dempster DW, Birchman R, Xu R, Lindsay R, Shen V. Temporal changes in cancellous bone structure of rats immediately after ovariectomy. Bone. 1995;16:157–161. [PubMed] [Google Scholar]
- 28.Ohnishi H, Nakamura T, Narusawa K, Murakami H, Abe M, Barbier A, Suzuki K. Bisphosphonate tiludronate increases bone strength by improving mass and structure in established osteopenia after ovariectomy in rats. Bone. 1997;21:335–343. doi: 10.1016/s8756-3282(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 29.Ammann P, Bourrin S, Bonjour JP, Brunner F, Meyer JM, Rizzoli R. The new selective estrogen receptor modulator MDL 103,323 increases bone mineral density and bone strength in adult ovariectomized rats. Osteoporos Int. 1999;10:369–376. doi: 10.1007/s001980050242. [DOI] [PubMed] [Google Scholar]
- 30.Lee LL, Lee JSC, Waldman SD, Casper RF, Grynpas MD. Polycyclic aromatic hydrocarbons present in cigarette smoke cause bone loss in an ovariectomized rat model. Bone. 2002;30:917–923. doi: 10.1016/s8756-3282(02)00726-3. [DOI] [PubMed] [Google Scholar]
- 31.Windler EE, Kovanen PT, Chao YS, Brown MS, Havel RJ, Goldstein JL. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J Biol Chem. 1980;255:10464–10471. [PubMed] [Google Scholar]
- 32.Chao YS, Windler EE, Chen GC, Havel RJ. Hepatic catabolism of rat and human lipoproteins in rats-treated with 17 β-ethynyl estra-diol. J Biol Chem. 1979;254:11360–11366. [PubMed] [Google Scholar]
- 33.Dowsett M. Origin and characteristics of adverse events in aromatase inhibition therapy for breast cancer. Semin Oncol. 2003;30(suppl 14):58–69. doi: 10.1016/s0093-7754(03)00300-2. [DOI] [PubMed] [Google Scholar]
- 34.Haskell SG. Selective estrogen receptor modulators. South Med J. 2003;96:469–476. doi: 10.1097/01.SMJ.0000051146.93190.4A. [DOI] [PubMed] [Google Scholar]
- 35.Sitruk-Ware R. Progestogens in hormonal replacement therapy: new molecules, risks, and benefits. Menopause. 2002;9:6–15. doi: 10.1097/00042192-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Hamada AL, Maruo T, Samoto T, Yoshida S, Nash H, Spitz IM, Johansson E. Estradiol/progesterone-releasing vaginal rings for hormone replacement therapy in postmenopausal women. Gynecol Endocrinol. 2003;17:247–254. [PubMed] [Google Scholar]
- 37.Luukkainen T. Issues to debate on the Women’s Health Initiative: failure of estrogen plus progestin therapy for prevention of breast cancer risk. Hum Reprod. 2003;18:1559–1561. doi: 10.1093/humrep/deg305. [DOI] [PubMed] [Google Scholar]
- 38.Labrie F, Labrie C, Bélanger A, Simard J, Giguère V, Tremblay A, Tremblay G. EM-652 (SCH57068), a pure SERM having complete antiestrogenic activity in the mammary gland and endometrium. J Steroid Biochem Mol Biol. 2001;79:213–225. doi: 10.1016/s0960-0760(01)00139-x. [DOI] [PubMed] [Google Scholar]
- 39.Gutman M, Couillard S, Roy J, Labrie F, Candas B, Labrie C. Comparison of the effects of EM-652 (SCH57068), tamoxifen, toremifene, droloxifene, idoxifene, GW-5638 and raloxifene on the growth of human ZR-75-1 breast tumors in nude mice. Int J Cancer. 2002;99:273–278. doi: 10.1002/ijc.10302. [DOI] [PubMed] [Google Scholar]