Abstract
Background
Antiretroviral (ARV) drug treatment benefits the treated individual and can prevent HIV transmission. We assessed ARV drug use in a community-randomized trial that evaluated the impact of behavioral interventions on HIV incidence.
Methods
Samples were collected in a cross-sectional survey after a 3-year intervention period. ARV drug testing was performed using samples from HIV-infected adults at four study sites (Zimbabwe; Tanzania; KwaZulu-Natal and Soweto, South Africa; survey period 2009–2011), using an assay that detects 20 ARV drugs (6 nucleoside/nucleotide reverse transcriptase inhibitors [NRTIs]; 3 non-nucleoside reverse transcriptase inhibitors [NNRTIs]; 9 protease inhibitors; maraviroc; raltegravir).
Results
ARV drugs were detected in 2,011 (27.4%) of 7,347 samples; 88.1% had 1 NNRTI +/− 1–2 NRTIs. ARV drug detection was associated with sex (women>men), pregnancy, older age (>24 years), and study site (p<0.0001 for all four variables). ARV drugs were also more frequently detected in adults who were widowed (p=0.006) or unemployed (p=0.02). ARV drug use was more frequent in intervention versus control communities early in the survey (p=0.01), with a significant increase in control (p=0.004) but not in intervention communities during the survey period. In KwaZulu-Natal, a 1% increase in ARV drug use was associated with a 0.14% absolute decrease in HIV incidence (p=0.018).
Conclusions
This study used an objective, biomedical approach to assess ARV drug use on a population level. This analysis identified factors associated with ARV drug use and provided information on ARV drug use over time. ARV drug use was associated with lower HIV incidence at one study site.
Keywords: HIV, antiretroviral drug use, Africa
INTRODUCTION
Antiretroviral treatment (ART) has health benefits for HIV-infected individuals1–3 and prevents sexual transmission of HIV in serodiscordant couples.4,5 High coverage of ART in a population can increase life expectancy6 and may lower HIV incidence.7–9 ART coverage in population studies is usually assessed using data from HIV treatment and care settings.7,10,11 This approach is limited, since some individuals may be non-compliant with their treatment regimen, and some may acquire antiretroviral (ARV) drugs from other sources.12,13 Self-report of ARV drug use has also shown to be unreliable in some research and clinic settings.14–17
ARV drug testing provides an objective biomedical measure of ARV drug use. However, large surveys of ARV drug use in populations based on ARV drug testing have been limited because of the cost and effort of traditional ARV drug testing. Our research group developed low cost, high-throughput methods for multi-drug ARV testing that have been used to assess ARV drug use in clinical trials and cohort studies.14,15,18 In this report, we evaluated ARV drug use in a large, cross-sectional survey of African adults using samples collected in the National Institute of Mental Health (NIMH) Project Accept study (HIV Prevention Trials Network 043 trial [HPTN 043]).19,20
HPTN 043 was a large multi-national, phase 3, cluster-randomized, controlled trial in Africa and Thailand that evaluated the effect of behavioral interventions on HIV incidence at a community-level.19 Control communities received standard voluntary counseling and testing. Intervention communities received enhanced community-based voluntary counseling and testing over a 3-year period (2006–2009). The intervention included community-mobilization to increase testing and awareness of HIV status, accessible HIV testing in the community and increased post-test support services.19 At the end of the intervention period, HIV incidence was assessed in a cross-sectional household survey of >50,000 adults (2009–2011).20 At the African sites, the intervention package was associated with a modest overall reduction in HIV incidence (1.52% in the intervention communities vs. 1.81% in the control communities, p=0.082), with a significant reduction in HIV incidence among older women (p=0.0085).19 During the HPTN 043 trial, ART was scaled up in many resource-limited settings, including the countries where the study was conducted.10,21–24 In addition, more HIV-infected individuals became eligible for ART after 2009, when the World Health Organization (WHO) raised the recommended CD4 cell count threshold for ART initiation from 200 cells/mm3 to 350 cells/mm3.25
In this report, we used a high-throughput, qualitative, multi-drug ARV assay to evaluate ARV drug use among HIV-infected adults in HPTN 043 communities in South Africa, Zimbabwe, and Tanzania.
METHODS
Study cohort
HPTN 043 was conducted at four sites in Africa (Mutoko, Zimbabwe; Kisarawe, Tanzania; KwaZulu-Natal and Soweto, South Africa) and in Chiang Mai, Thailand (NCT00203749).19 Forty-eight communities (34 in Africa, 14 in Thailand) were randomized to receive either standard voluntary counseling and testing for HIV (control) or community-based voluntary counseling and testing for HIV (intervention). After the intervention period, samples were collected from eligible adults 18–32 years of age from randomly-sampled households in the communities. Samples were frozen within 24 hours of sample collection. Methods used to determine HIV status and estimate HIV incidence in HPTN 043 are described in previous reports.20,26 Samples were tested in-country with HIV rapid tests. Further testing was performed at the HPTN Laboratory Center (Baltimore, MD) to determine final HIV status. HIV incidence was assessed using a multi-assay algorithm that included the BED capture immunoassay, an antibody avidity assay, CD4 cell count, and HIV viral load.20 In this study, samples from HIV-infected adults from the African sites were tested for ARV drugs at the HPTN Laboratory Center. The site in Thailand had low HIV prevalence and was excluded from analyses.
Laboratory methods
Plasma samples from HIV-infected adults were analyzed retrospectively for the presence of 20 ARV drugs, including nine protease inhibitors (PIs; amprenavir, atazanavir, darunavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, and tipranavir), six nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs; abacavir, emtricitabine, lamivudine, stavudine, tenofovir, and zidovudine), three non-nucleoside reverse transcriptase inhibitors (NNRTIs; efavirenz, nevirapine, and rilpivirine), a CCR5 receptor antagonist (maraviroc), and an integrase inhibitor (raltegravir). Briefly, 100 μL of sample and 200 μL of internal standard solution (abacavir-d4 and lopinavir-d8) were prepared using simplified solid phase extraction on Strata-X plates. Drugs were detected using high performance liquid chromatography (HPLC) coupled with high resolution accurate mass (HRAM) mass spectrometry (MS; Q Exactive; Thermo Scientific, Pittsburgh, PA). The mobile phase system for the HPLC included 10 mM ammonium acetate (aqueous phase) and 0.05% ammonium hydroxide in methanol (organic phase). Samples were introduced onto a 5 μm Hypersil Gold perfluorinated phenyl column at 100% aqueous composition and elution occurred during a 2.5-minute step-and-hold isocratic step to 100% organic phase (methanol). The MS analysis was performed in targeted MS2 mode; fragments were detected at a resolution of 17,500 at m/z of 200. Multiplexing with a 4-channel chromatography system allowed for an effective analysis time of 1.5 minutes/sample. The lower limit of detection was 10 ng/mL for all 20 drugs.
Statistical analysis
Factors associated with ARV drug use were modeled by logistic regression at subject level. The factors were adjusted for each other as well as for site, study arm, and survey period (split into 6-month intervals). Fixed community effects were included in the form of zero-sum contrasts nested within the site-by-intervention interaction. Comparison of ARV drug use in control vs. intervention communities was done by a weighted paired t-test performed on community-level data. The weights were proportional to harmonic means of the numbers of HIV-positive adults in the paired communities. Degrees of freedom were adjusted to take into account the unequal weights.19 Association of HIV incidence with ARV drug use was modeled by linear regression at the community level with ARV prevalence, site, and intervention as predictors.
Ethical Approval
The work was carried out in accordance with the Declaration of Helsinki. HPTN 043 was conducted in partnership with established community advisory boards and local government departments. Consent was obtained at the community level for trial participation. Oral consent was obtained from each participant for collection and testing of blood samples. The study was approved by participating academic institutions and ethics committees for each site.19
RESULTS
Samples used for analysis
Blood samples were collected from adults at the four African sites (34 communities) during the post-intervention survey. Communities were matched into pairs based on shared attributes prior to randomization. Samples were collected around the same time period for each community pair (Supplemental Digital Content 1). A total of 46,693 samples were collected. The sample set analyzed in this report included samples from 7,354 (99.8%) of 7,366 HIV-infected individuals in the trial;19,20 12 samples were not included in the analysis (7 from participants with acute/early HIV infection; 5 from participants with missing CD4 cell count data).
Detection of ARV drugs
Results were obtained from 7,347 (99.9%) of 7,354 samples tested (Table 1). At least one ARV drug was detected in 2,011 (27.4%) samples; 88.1% of those samples had one NNRTI with or without one or two NRTIs. In 40.3% of the samples where one or more ARV drug was detected, we detected a single ARV drug (81.7% NNRTI, 16.7% NRTI, 1.6% PI or raltegravir [Table 1]). The most commonly detected NNRTI was efavirenz (detected in 62.3% of the samples with one or more ARV drug detected). Efavirenz was detected alone in 30.2% of the samples; 48.1% of those samples were from men aged 18–24 years. The most commonly detected NRTI was lamivudine (detected in 57.3% of the samples). PIs were detected in 0.8% of the samples and raltegravir (an integrase inhibitor) was detected in 0.1% of the samples; maraviroc (a CCR5 receptor antagonist) was not detected in any samples. For the analyses below, ARV drug use was defined as detection of at least one ARV drug.
Table 1.
Antiretroviral drugs detected in study samples*.
| Zimbabwe N=1,529 |
Tanzania N=531 |
KwaZulu-Natal N=3,640 |
Soweto N=1,647 |
All sites N=7,347 |
|
|---|---|---|---|---|---|
| Samples with ≥1 ARV drug detected, N (%) | 326 (21.3) | 114 (21.5) | 1,154 (31.7) | 417 (25.3) | 2,011 (27.4)a |
|
| |||||
| NNRTI, N (%) | 305 (19.9) | 85 (16.0) | 1,064 (29.2) | 371 (22.5) | 1,825 (24.8)b |
| NRTI, N (%) | 203 (13.3) | 85 (16.0) | 788 (21.6) | 246 (14.9) | 1,322 (18.0)c |
| PI, N (%) | 7 (0.5) | 1 (0.2) | 29 (0.8) | 20 (1.2) | 57 (0.8)d |
| Raltegravir, N (%) | 1 (0.1) | 0 (0.0) | 3 (0.1) | 0 (0.0) | 4 (0.1) |
| Maraviroc, N (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Stored plasma samples collected from HIV-infected participants during the post-intervention survey in HPTN 043 were tested for the presence of 20 antiretroviral (ARV) drugs. ARV drug use was defined as detection of at least one ARV drug. The table shows the number and percentage of HIV-infected study participants at each site who had at least one ARV drug detected in a given drug class.
Abbreviations: N: number; NNRTI: non-nucleoside reverse transcriptase inhibitor; NRTI: nucleoside/nucleotide reverse transcriptase inhibitors; PI: protease inhibitor.
Among the samples with one or more ARV drug detected, 40.3% had only one ARV drug detected; 81.7% had a NNRTI alone (91.7% had efavirenz, 8.3% had nevirapine), 16.7% had a NRTI alone (56.3% had zidovudine, 22.2% had tenofovir, 10.4% had lamivudine, 8.9% had stavudine, 1.5% had emtricitabine, 0.7% had abacavir), 1.4% had a PI (100% had lopinavir), and 0.2% had raltegravir.
NNRTIs were detected in 90.8% of the samples that had at least one ARV drug detected; 62.3% had efavirenz and 29.9% had nevirapine (1% of samples had both drugs detected).
NRTIs were detected in 65.7% of samples that had at least one ARV drug detected; 57.3% had lamivudine, 16.5% had stavudine, 10.7% had zidovudine, 8.3% had tenofovir, 1.0% had emtricitabine, and 0.2% had abacavir.
PIs were detected in 2.8% of samples with at least one ARV drug detected; 2.3% had lopinavir, 1.5% had ritonavir, 0.3% had darunavir, 0.1% had atazanavir, 0.1% had amprenavir, and 0.05% had saquinavir.
Factors associated with ARV drug use
Multivariate logistic regression analysis was used to evaluate factors associated with ARV drug use (Table 2). Several variables were independently associated with ARV drug use, as described below. Significant differences in the frequency of ARV drug use were observed at the different study sites (p<0.0001, Table 2). The highest prevalence of ARV drug use was observed in South Africa (31.7% in Kwazulu-Natal; 25.3% in Soweto), with lower prevalence in Tanzania and Zimbabwe (21.5% and 21.3%, respectively, Table 1). ARV drug use was significantly higher in non-pregnant women than men (p<0.0001, Table 2); ARV drugs were detected in samples from 29.4% of women (regardless of pregnancy status) and in 21.5% of men (Supplemental Digital Content 2). ARV drug use was also associated with pregnancy (p<0.0001, Table 2). Overall, 6.7% of the women were pregnant. The prevalence of ARV drug use among pregnant women was 39.5%, with the highest prevalence in Kwazulu-Natal (58.4%) and the lowest prevalence in Zimbabwe (19%, Supplemental Digital Content 2). ARV drug use was also associated with age (p<0.0001, Table 2). ARV drugs were detected more frequently in older adults (30.2%; ages 25–32) than younger adults (21.0%; ages 18–24) with the highest prevalence of ARV drug use among older women (33.0%, Supplemental Digital Content 2). Significant associations were also observed with marital and employment status (higher in widowed compared to married or single adults, p=0.006; unemployed compared to employed adults, p=0.02, Table 2). ARV drug use was not associated with CD4 cell count (27.7% for those with <350 CD4 cells/mm3 vs. 27.4% for those with >350 CD4 cells/mm3) or with socioeconomic status or education level (data not shown).
Table 2.
Factors associated with antiretroviral drug use*.
| Variable | N (%) | Adjusted OR (95% CI) | P-valuec | |
|---|---|---|---|---|
| Sex | Men Non-pregnant women |
1867 (25.8) 4996 (69.1) |
1.00 (ref.) 1.44 (1.26–1.64) |
<0.0001 |
| Pregnancy | Non-pregnant women Pregnant women |
4996 (69.1) 362 (5.0) |
1.00 (ref.) 2.03 (1.60–2.56) |
<0.0001 |
| Age | 18–24 years 25–32 years |
2213 (30.6) 5012 (69.4) |
1.00 (ref.) 1.93 (1.70–2.19) |
<0.0001 |
| Marital status | Single Married Separated Widowed |
5182 (71.7) 1547 (21.4) 329 (4.6) 167 (2.3) |
1.00 (ref.) 1.00 (0.80–1.24) 0.85 (0.60–1.20) 2.03 (1.33–3.08) |
0.002 |
| Employment status | Employed Unemployed |
3900 (54.0) 3325 (46.0) |
1.00 (ref.) 1.14 (1.02–1.28) |
0.02 |
| Site | Zimbabwe Tanzania KwaZulu-Natal Soweto |
1522 (21.0) 531 (7.3) 3556 (49.2) 1616 (22.4) |
1.00 (ref.) 0.99 (0.72–1.33) 2.29 (1.79–2.96) 1.54 (1.19–1.99) |
<0.0001 |
|
Study arma (Period <6 months) |
Control Intervention |
3662 (50.7) 3563 (49.3) |
1.00 (ref.) 1.58 (1.06–2.35) |
0.01 |
|
Survey periodb (Control arm) |
Period <6 months Period >6 months |
703 (19.2) 2959 (80.8) |
1.00 (ref.) 1.59 (1.16–2.17) |
0.004 |
|
Survey periodb (Intervention arm) |
Period <6 months Period >6 months |
546 (15.3) 3017 (84.7) |
1.00 (ref.) 0.91 (0.66–1.24) |
0.54 |
Antiretroviral (ARV) drug use was defined as detection of at least one ARV drug. The table shows results of multivariate analysis of factors associated with ARV drug use (see Methods). Additional data for the frequency of ARV drug detection in different demographic sub-groups is shown in Supplementary Table 1. The frequency of ARV drug detection in the control and intervention arms of the study is shown in Table 3. The frequency of ARV drug detection during different 6-month intervals of the post-intervention assessment is shown in Figure 1.
Abbreviations: OR: odds ratio; CI: confidence interval; N: number of HIV-infected participants in the subgroup.
Intervention: community-based voluntary HIV counseling and testing; Control: standard voluntary HIV counseling and testing. These results refer to the first 6 months of the survey. There was no significant intervention effect after the first 6 months.
Period: <6 months: first 6 months of the post-intervention survey period; >6 months: remainder of the post-intervention survey period.
The p-values summarize the overall association of the factor with ARV drug detection.
Temporal trends in ARV drug use
ARV drug use was assessed over the course of the 28-month post-intervention survey. ARV drug use increased 1.8-fold among all HIV-infected individuals during this period, from 19.7% in the first 6 months of the survey to 34.6% in the last 6 months of the survey (Figure 1). The increase in ARV drug use over time was highest in Zimbabwe (4.3-fold) and lowest in KwaZulu-Natal (1.3-fold), and was observed in both men and women and in both younger and older adults (data not shown).
Figure 1. Detection of antiretroviral drugs in samples collected during the HPTN 043 post-intervention survey.
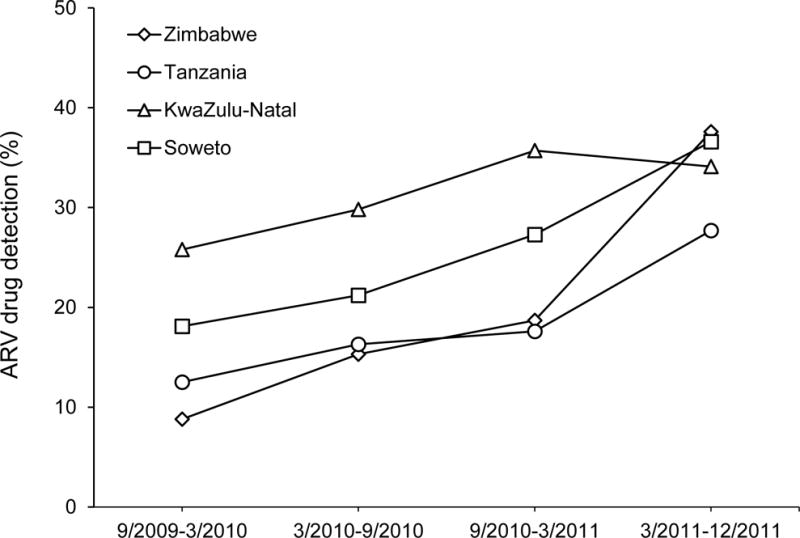
Blood samples were collected from HIV-infected participants in HPTN 043 in a post-intervention household survey. Stored plasma samples were retrospectively tested for the presence of 20 antiretroviral (ARV) drugs. The graph shows the percentage of individuals at each of the four African study sites who had at least one ARV drug detected in their study sample. Data are presented for 6-month intervals during the survey.
Comparison of ARV drug use in control vs. intervention communities
The study intervention did not include assistance accessing ART or provision of ART. Study participants accessed ART using locally-available services. We performed exploratory analyses to examine whether the study intervention was associated with increased ARV drug use. The prevalence of ARV drug use at the study sites ranged from 17.7% to 32.2% in the control communities and from 19.6% to 31.1% in the intervention communities (Table 3). There was no difference between ARV drug use in control vs. intervention communities (p=0.77, Table 3). Only Zimbabwe showed an intervention effect, with a 7.1% higher prevalence of ARV drug use in the intervention communities. The prevalence of ARV drug use was similar in the control and intervention communities for men and for women, and in both age groups (18–24 years and 25–32 years, data not shown).
Table 3.
Antiretroviral drug use in the control and intervention arms of HPTN 043 (intervention effect)*.
| Site | # with ARV test resultsa,b
|
# with ARV drugs detected (%)a,c
|
Effectd | Analytic weighte | ||
|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | |||
| Zimbabwe | 751 | 776 | 133 (17.7) | 192 (24.7) | 7.08 | 20.9 |
| Tanzania | 250 | 281 | 59 (23.6) | 55 (19.6) | −5.03 | 7.2 |
| KwaZulu-Natal | 1,777 | 1,834 | 572 (32.2) | 571 (31.1) | −0.90 | 49.8 |
| Soweto | 932 | 712 | 242 (26.0) | 175 (24.6) | −0.75 | 22.0 |
| All | 3,710 | 3,603 | 1,006 (27.1) | 993 (27.6) | 0.51 | 100.0 |
The table shows the number and percentage of individuals in the control and intervention arms of the HPTN 043 study who had at least one antiretroviral (ARV) drug detected.
Control: standard voluntary HIV counseling and testing; Intervention: community-based voluntary HIV counseling and testing.
Number of HIV-infected individuals with ARV test results.
Number of HIV-infected individuals with at least one ARV drug detected.
Weighted absolute difference in prevalence of ARV drug use between intervention and control communities.
Contribution of each site to the analysis (in %).
ARV drug use increased during the survey period in both control communities (from 18.0% to 32.4%) and intervention communities (from 22.3% to 37.7%). In control communities, ARV use increased significantly after the first 6 months of the survey (p=0.004); in contrast, the increase in ARV drug use over time in the intervention communities was not statistically significant. Prevalence of ARV drug use was higher in the intervention communities than the control communities in the first 6 months of the survey (p=0.01), but not in the later period (>6 months, Table 2).
Association of HIV incidence with ARV drug use
In HPTN 043, the impact of the study interventions on HIV incidence was estimated in the cross-sectional, post-intervention assessment.20 At three of the four study sites (Zimbabwe, Tanzania, and Soweto), there was no association between ARV drug use and HIV incidence (Figure 2). In contrast, in Kwazulu-Natal, ARV drug use was associated with lower HIV incidence (a 1% increase in ARV coverage was associated with a 0.14% absolute decrease in annual HIV incidence, p=0.018). However, this association may reflect a decrease in HIV incidence over time (direct effect of the time period related to factors other than ARV drug use), rather than an effect of ART on HIV incidence. In HPTN 043, the only subgroup that had a significant decrease in HIV incidence in the intervention arm was older women (25–32 years);19 in this subgroup, the prevalence of ARV use was similar in the control vs. intervention communities (32.5% vs. 33.5%, p=0.61).
Figure 2. Association of ARV drug use with HIV incidence.
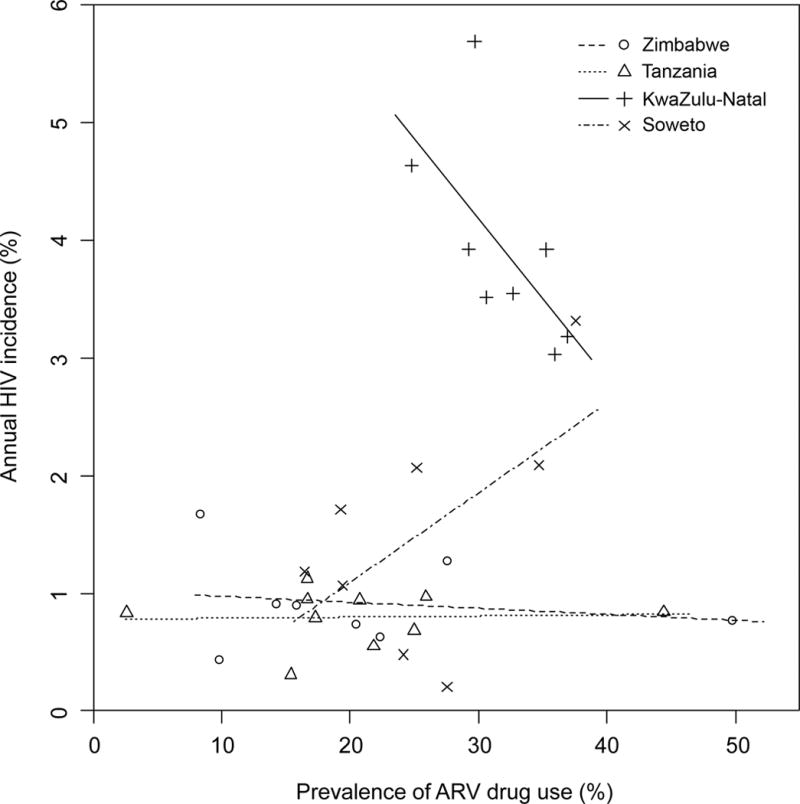
Stored plasma samples collected from HIV-infected participants during the post-intervention survey in HPTN 043 were tested for the presence of 20 antiretroviral (ARV) drugs. ARV drug use was defined as detection of at least one ARV drug. The graph shows the relationship between ARV drug use at each of the four African study sites and HIV incidence (determined in the HPTN 043 trial19,20). Site-specific regression lines are shown.
DISCUSSION
We used a novel, low-cost, high-throughput, multi-drug ARV assay to assess ARV drug use on a population-level in a large cross-sectional survey of African adults. By testing stored plasma samples for ARV drugs, we were able to obtain an objective, biomedical measure of ARV drug use. In HPTN 043, 27% of the HIV-infected adults from the four African sites had at least one ARV drug detected in their survey sample. The prevalence of ARV drug use in this population is lower than previous estimates of ARV coverage in these countries, which were based on surveillance data from patient and pharmacy monitoring systems.11 According to Joint United Nations Programme on HIV/AIDS (UNAIDS), <40% of eligible HIV-infected individuals in Zimbabwe, Tanzania, and South Africa received ART in 2009.27,28 By 2011, these estimates of ART coverage increased to 40–59% in Tanzania and 60–79% in Zimbabwe and South Africa.27,28 Our assessment is based on a random sample of the HIV-infected population, rather than surveillance data from known HIV-infected persons, which may account for the lower prevalence of ARV drug use in this study. Of note, the prevalence of ARV drug use in this study was slightly higher in KwaZulu-Natal (rural: 31.7%) than Soweto (urban, 25.3%).
The recommended first-line regimens at the time the study was conducted included one NNRTI (EFV or NVP) + two NRTIs (e.g., 3TC + ZDV or 3TC + d4T; newer guidelines introduced during the trial included the option of 3TC + FTC or 3TC + TDF). In this study, the majority (88%) of those with ARV drugs detected had an NNRTI with or without NRTIs, consistent with the recommended first-line ART regimens used in the study countries. Detection of drugs in other drug classes was rare; those drugs were not widely available and were not part of first-line ART regimens in the study countries at that time. Among the samples with any ARV drug detected, 40.3% had only one drug detected. Most of these individuals were likely taking multi-drug regimens for treatment, with only one drug detected by the multi-drug assay. Drugs that achieve higher levels in plasma or have longer half-lives are more likely to be detected in samples collected at random times after dosing, especially if an individual is not 100% adherent to a multi-drug treatment regimen. Even if fixed-dose drug combinations were used, NNRTIs would be detected longer than NRTIs because of their longer half-lives. Some reports from Africa have noted that EFV is used for recreational purposes, which could also explain detection of EFV in the absence of other drugs.29,30 Detection of an NRTI alone could reflect incomplete adherence to a multi-drug regimen or use of ARVs for another purpose (e.g., use of 3TC for hepatitis; use of a short course NRTI regimen for post-exposure prophylaxis [PEP]).
Several demographic factors were strongly associated with ARV drug use in this population. ARV drugs were more frequently detected in samples from South Africa than Tanzania and Zimbabwe. Of note, clinic-based voluntary HIV counseling and testing services were already available as standard-of-care at the sites in South Africa at the start of HPTN 043. These services were not available at the sites in Tanzania and Zimbabwe before the study started.31 Individuals who had access to HIV testing and counseling would have been more likely to be aware of their HIV status and linked to care. We also found a higher prevalence of ARV drug use in women compared to men, with a higher prevalence among pregnant women. This most likely reflects successful roll out of programs for prevention of mother-to-child transmission (PMTCT). Even so, ARV drug use among pregnant women varied from site to site, ranging from only 19% in Zimbabwe to 60% in KwaZulu-Natal. This may have reflected the type of regimens for PMTCT that were used in the study communities at the time the survey was conducted (i.e., women who were provided with short-course ARV regimens may not have been taking ARV drugs at the time of sample collection). Age was also strongly associated with ARV drug use, with more frequent detection of ARV drugs among older participants (ages 24–32 years). A study from rural Tanzania also cited higher ART coverage among older adults based on records from HIV care and treatment centers.32 In this study, ARV drug use was also associated with marital status (higher in widowed compared to married or single adults) and employment status (higher in unemployed compared to employed adults). Further studies are needed to evaluate the basis for these associations.
In addition to examining demographic trends in ARV drug use, a goal of this study was to explore the association of ARV drug use and HIV incidence, the primary outcome of the HPTN 043 study. While we did not observe an overall difference in ARV drug use in the control vs. intervention communities, we did find a significantly higher prevalence of ARV drug use in the intervention communities during the first 6 months of the survey period (9/2009–3/2010). This temporal pattern in intervention effect may be explained by the increases in ARV drug use that we observed in both the control and intervention communities during the survey period. These increases likely reflected both general scale-up of existing ART services through local government programs, PEPFAR, and other organizations10,21–24 and the 2009 changes in WHO guidelines for ART initiation, which increased the CD4 cell count threshold recommended for ART initiation. Of note, we did find a significant, association between higher prevalence ARV drug use and lower HIV incidence in KwaZulu-Natal which had both the highest prevalence of ARV drug use and the highest annual HIV incidence. At that site, a 1% increase in ARV drug use was associated with a 0.14% absolute decrease in HIV incidence; this corresponds to a 3.6% relative decline with the 3.9% annual HIV incidence observed in KwaZulu-Natal. A previous study of ART coverage in KwaZulu-Natal, based on analysis of the Hlabisa HIV Treatment and Care Programme’s database, reported that a 1% increase in ART coverage was associated with a 1.4% relative decline in HIV incidence.7 One limitation of our analysis is that the post-intervention survey periods in two of the 17 pairs of matched control and intervention communities did not completely overlap. Also, HIV incidence was determined using a multi-assay algorithm; participants who had one or more ARV drug detected in the survey sample were characterized as having non-recent (prevalent) HIV infection.20
In conclusion, using a low-cost, high-throughput, multi-drug ARV assay, we were able to assess the prevalence of ARV drug use in a large population survey, identify demographic factors associated with ARV drug use, and evaluate temporal trends in ARV drug use. We also observed an association of ARV prevalence with HIV incidence in KwaZulu-Natal, which suggest that ARV drug use could have been one of the factors contributing to reduced HIV incidence. This objective testing approach may aid other measurements of ARV drug use, such as analysis of public data sets for provision of ART or ART data provided by self-report, and may be useful in clinical trials, surveillance studies, and evaluation of public health programs using ART for HIV treatment and prevention.33
Supplementary Material
Supplemental Digital Content 1. Post-intervention survey periods for African sites in HPTN 043.
Supplemental Digital Content 2. Antiretroviral drug detection in demographic sub-groups.
Acknowledgments
The authors thank the NIMH Project Accept (HPTN 043) study team and study participants for providing samples for this study, and thank the laboratory staff at the study sites and at the HPTN Laboratory Center with assistance for sample management and testing. The authors thank Laura Robins-Morris for assistance with data management in the HPTN 043 study, and thank Dianne Rausch and Christopher Gordon (NIMH) for their support of the HPTN 043 study.
Source of Funding: This project was supported by the following awards: the HIV Prevention Trials Network, sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Mental Health (NMH), and the National Institute of Drug Abuse (NIDA), Office of AIDS Research, of the National Institutes of Health [NIH, grants UM1-AI068613 (Eshleman); UM1-AI068617 (Donnell); and UM1-AI068619 (El-Sadr). Additional support for NIMH Project Accept (HPTN 043) was provided by the NIMH (U01-MH066687, U01-MH066688, U01-MH066701, and U01-MH066702). Additional support provided by the Division of Intramural Research, NIAID.
Author roles
All authors meet the journal’s criteria for authorship. Individual contributions/author roles are listed below.
|
| |
| Jessica M. Fogel | Assisted with study design; analyzed data; wrote the manuscript |
|
| |
| William Clarke | Responsible for ARV testing; reviewed ARV test results |
|
| |
| Michal Kulich | Protocol statistician for HPTN 043; responsible for statistical analyses |
|
| |
| Estelle Piwowar-Manning | Responsible for overseeing laboratory activities in HPTN 043 |
|
| |
| Autumn Breaud | Performed ARV testing |
|
| |
| Matthew T. Olson | Assisted with analysis of ARV test data |
|
| |
| Mark A. Marzinke | Reviewed ARV test results |
|
| |
| Oliver Laeyendecker | Responsible for cross-sectional HIV incidence testing |
|
| |
| Agnès Fiamma | Study coordinator for HPTN 043 |
|
| |
| Deborah Donnell | Statistician for HPTN 043 |
|
| |
| Jessie K. K. Mbwambo | Principal Investigator for the HPTN 043 site in Tanzania |
|
| |
| Linda Richter | Principal Investigator for the HPTN 043 site KwaZulu-Natal |
|
| |
| Glenda Gray | Principal Investigator for the HPTN 043 site in Cape Town |
|
| |
| Michael Sweat | Study Investigator for the HPTN 043 |
|
| |
| Thomas J. Coates | Protocol Chair for HPTN 043 |
|
| |
| Susan H. Eshleman | Designed the study; analyzed data; wrote the manuscript |
|
| |
Footnotes
Note This work was presented in part at the Conference on Retroviruses and Opportunistic Infections (February, 2016), Boston, MA.
Conflict of Interests
None of the authors has a conflict of interest or potential conflict of interest, with the following exception: William Clarke is a consultant to Thermo Fisher Scientific.
Contributor Information
Jessica M. Fogel, Dept. of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
William Clarke, Dept. of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
Michal Kulich, Dept. of Probability and Statistics, Faculty of Mathematics and Physics, Charles Univ., Prague, Czech Republic.
Estelle Piwowar-Manning, Dept. of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
Autumn Breaud, Dept. of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
Matthew T. Olson, Dept. of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
Mark A. Marzinke, Dept. of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
Oliver Laeyendecker, Laboratory of Immunoregulation, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Baltimore, MD, USA; Dept. of Medicine, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
Agnès Fiamma, Program in Global Health, University of California at Los Angeles, Los Angeles, CA, USA.
Deborah Donnell, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; Dept. of Global Health, Univ. of Washington, Seattle, WA, USA.
Jessie K. K. Mbwambo, Muhimbili Univ. of Health and Allied Sciences, Muhimbili Univ. Teaching Hospital, Dar es Salaam, Tanzania.
Linda Richter, DST-NRF Centre of Excellence in Human Development, University of the Witwatersrand, Johannesburg, South Africa.
Glenda Gray, Perinatal HIV Research Unit, Chris Hani Baragwanath Hospital, Univ. of the Witwatersrand, Johannesburg, South Africa; South African Medical Research Council, Cape Town, South Africa.
Michael Sweat, Dept. of Psychiatry and Behavioral Sciences, the Medical Univ. of South Carolina, Charleston, SC, USA.
Thomas J. Coates, Center for World Health, David Geffen School of Medicine and UCLA Health, Los Angeles, CA, USA.
Susan H. Eshleman, Dept. of Pathology, Johns Hopkins Univ. School of Medicine, Baltimore, MD, USA.
References
- 1.Sterne JA, Hernan MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 2.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14:281–290. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Final results of the HPTN 052 randomized controlled trial: antiretroviral therapy prevents HIV transmission [MOAC0101LB]. Presented at: 8th IAS Conference on HIV Pathogenesis, Treatment & Prevention; 2015; Vancouver, Canada. [Google Scholar]
- 6.Bor J, Herbst AJ, Newell ML, et al. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339:961–965. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanser F, Barnighausen T, Grapsa E, et al. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 9.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klausner JD, Serenata C, O’Bra H, et al. Scale-up and continuation of antiretroviral therapy in South African treatment programs, 2005–2009. J Acquir Immune Defic Syndr. 2011;56:292–295. doi: 10.1097/QAI.0b013e3182067d99. [DOI] [PubMed] [Google Scholar]
- 11.Mahy M, Tassie JM, Ghys PD, et al. Estimation of antiretroviral therapy coverage: methodology and trends. Curr Opin HIV AIDS. 2010;5:97–102. doi: 10.1097/COH.0b013e328333b892. [DOI] [PubMed] [Google Scholar]
- 12.Rough K, Dietrich J, Essien T, et al. Whoonga and the abuse and diversion of antiretrovirals in Soweto, South Africa. AIDS Behav. 2014;18:1378–1380. doi: 10.1007/s10461-013-0683-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surratt HL, Kurtz SP, Cicero TJ, et al. Antiretroviral medication diversion among HIV-positive substance abusers in South Florida. Am J Public Health. 2013;103:1026–1028. doi: 10.2105/AJPH.2012.301092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fogel JM, Wang L, Parsons TL, et al. Undisclosed antiretroviral drug use in a multi-national clinical trial (HPTN 052) J Infect Dis. 2013;208:1624–1628. doi: 10.1093/infdis/jit390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzinke MA, Clarke W, Wang L, et al. Non-disclosure of HIV status in a clinical trial setting: antiretroviral drug screening can help distinguish between newly-diagnosed and previously-diagnosed HIV infection. Clin Infect Dis. 2014;58:117–120. doi: 10.1093/cid/cit672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan AK, Savage EJ, Lowndes CM, et al. Non-disclosure of HIV status in UK sexual health clinics–a pilot study to identify non-disclosure within a national unlinked anonymous seroprevalence survey. Sex Transm Infect. 2013;89:120–121. doi: 10.1136/sextrans-2012-050801. [DOI] [PubMed] [Google Scholar]
- 17.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen I, Clarke W, Ou SS, et al. Antiretroviral Drug Use in a Cohort of HIV-Uninfected Women in the United States: HIV Prevention Trials Network 064. PLoS One. 2015;10:e0140074. doi: 10.1371/journal.pone.0140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coates TJ, Kulich M, Celentano DD, et al. Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. Lancet Glob Health. 2014;2:e267–277. doi: 10.1016/S2214-109X(14)70032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laeyendecker O, Piwowar-Manning E, Fiamma A, et al. Estimation of HIV Incidence in a large, community-based, randomized clinical trial: NIMH Project Accept (HIV Prevention Trials Network 043) PLoS One. 2013;8:e68349. doi: 10.1371/journal.pone.0068349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larson E, O’Bra H, Brown JW, et al. Supporting the massive scale-up of antiretroviral therapy: the evolution of PEPFAR-supported treatment facilities in South Africa, 2005–2009. BMC Public Health. 2012;12:173. doi: 10.1186/1471-2458-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ojikutu B, Makadzange AT, Gaolathe T. Scaling up ART treatment capacity: lessons learned from South Africa, Zimbabwe, and Botswana. Curr Infect Dis Rep. 2008;10:69–73. doi: 10.1007/s11908-008-0012-0. [DOI] [PubMed] [Google Scholar]
- 23.Somi G, Matee M, Makene CL, et al. Three years of HIV/AIDS care and treatment services in Tanzania: achievements and challenges. Tanzan J Health Res. 2009;11:136–143. doi: 10.4314/thrb.v11i3.47700. [DOI] [PubMed] [Google Scholar]
- 24.WHO, UNAIDS, and UNICEF. Global HIV/AIDS response. Global HIV/AIDS response Epidemic update and health sector progress towards Universal Access. 2011 Available at: www.who.int/hiv/pub/progress_report2011/hiv_full_report_2011.pdf. Accessed: Nov 13, 2015.
- 25.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. Rapid advice. 2009 Available at: http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf. Accessed December 9, 2009. [PubMed]
- 26.Piwowar-Manning E, Fiamma A, Laeyendecker O, et al. HIV surveillance in a large, community-based study: results from the pilot study of Project Accept (HIV Prevention Trials Network 043) BMC Infect Dis. 2011;11:251. doi: 10.1186/1471-2334-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic 2012. Available at: http://www.unaids.org/en/resources/campaigns/20121120_globalreport2012/globalreport. Accessed: Nov 16, 2015.
- 28.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic. Available at: http://www.unaids.org/en/resources/documents/2010/20101123_globalreport. Accessed: Nov 16, 2015.
- 29.Gatch MB, Kozlenkov A, Huang RQ, et al. The HIV antiretroviral drug efavirenz has LSD-like properties. Neuropsychopharmacology. 2013;38:2373–2384. doi: 10.1038/npp.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grelotti DJ, Closson EF, Smit JA, et al. Whoonga: potential recreational use of HIV antiretroviral medication in South Africa. AIDS Behav. 2014;18:511–518. doi: 10.1007/s10461-013-0575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khumalo-Sakutukwa G, Morin SF, Fritz K, et al. Project Accept (HPTN 043): a community-based intervention to reduce HIV incidence in populations at risk for HIV in sub-Saharan Africa and Thailand. J Acquir Immune Defic Syndr. 2008;49:422–431. doi: 10.1097/QAI.0b013e31818a6cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levira F, Agnarson AM, Masanja H, et al. Antiretroviral treatment coverage in a rural district in Tanzania–a modeling study using empirical data. BMC Public Health. 2015;15:195. doi: 10.1186/s12889-015-1460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90. An ambitious treatment target to help end the AIDS epidemic. 2014 Available at: http://www.unaids.org/en/resources/documents/2014/90-90-90. Accessed: February 2, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Post-intervention survey periods for African sites in HPTN 043.
Supplemental Digital Content 2. Antiretroviral drug detection in demographic sub-groups.


