Abstract
IL-35 is a recently identified cytokine exhibiting potent immunosuppressive properties. The therapeutic potential and effects of IL-35 on pathogenic T effector (Teff) cells and Foxp3+ Treg cells, however, are ill-defined. We tested the capacity of IL-35 to suppress ongoing autoimmunity in NOD mice. For this purpose, an adeno-associated virus vector in which IL-35 transgene expression is selectively targeted to β cells via an insulin promoter (AAV8mIP-IL35) was used. AAV8mIP-IL35 vaccination of NOD mice at a late preclinical stage of type 1 diabetes (T1D) suppressed β-cell autoimmunity and prevented diabetes onset. Numbers of islet-resident conventional CD4+ and CD8+ T cells, and DCs were reduced within 4 weeks of AAV8mIP-IL35 treatment. The diminished islet T-cell pool correlated with suppressed proliferation, and a decreased frequency of IFN-γ-expressing Teff cells. Ectopic IL-35 also reduced islet Foxp3+ Treg-cell numbers and proliferation, and protection was independent of induction/expansion of adaptive islet immunoregulatory T cells. These findings demonstrate that IL-35-mediated suppression is sufficiently robust to block established β-cell autoimmunity, and support the use of IL-35 to treat T1D and other T-cell-mediated autoimmune diseases.
Keywords: NOD mice, Type 1 diabetes, cytokine immunotherapy, IL-35, β cell
INTRODUCTION
Type 1 diabetes (T1D) is an autoimmune disease characterized by the destruction of the insulin producing β cells found in the pancreatic islets of Langerhans [1–4]. In the NOD mouse, a spontaneous model of T1D, the onset of diabetes is preceded by progressive infiltration of the islets that begins at 3–4 weeks of age [1, 2]. This insulitis consists of various immune effectors including dendritic cells (DCs), macrophages, B cells and T cells. Overt diabetes, typically first detected in NOD female mice at 12 weeks of age, develops once >80% of β cell mass has been rendered nonfunctional or destroyed by ongoing inflammation [1]. The primary mediators of β cell destruction are CD4+ and CD8+ T cells, which mostly exhibit a type 1 effector phenotype marked by IFN-γ production [1, 5]. Breakdown of β cell-specific self-tolerance within the T cell compartment is complex, and is partly due to aberrant immunoregulation within the islets. Diminished local levels of IL-2 in NOD mice for instance, leads to a progressive loss in the number and function of islet-resident Foxp3-expressing immunoregulatory CD4+ T cells (Foxp3+Treg) [6–9]. Temporal dysregulation of islet Foxp3+Treg in turn facilitates the expansion of pathogenic T effectors (Teff) leading to efficient β cell destruction [6–8]. The pathogenicity of islet Teff in NOD mice is further enhanced by a reduced sensitivity to Foxp3+Treg-mediated suppression [10, 11].
IL-35 is a recently discovered member of the IL-12 cytokine family consisting of Epstein-Barr-virus-induced gene 3 (Ebi3) and IL-12α subunits [12]. Unlike other members of this cytokine family, IL-35 exhibits only immunosuppressive properties and has been shown to mediate protection in several diseases in both humans and mouse models [13–17]. IL-35 is secreted by and contributes to the suppressor function of Foxp3+Treg [18–20]. Additionally, IL-35 promotes differentiation of conventional CD4+ T cells into induced regulatory Th35 suppressor (iTr35) cells, which secrete IL-35 but not IL-10 or TGFβ, and lack FoxP3 expression [21]. The inhibitory effects of IL-35 are largely mediated via cell cycle arrest in T cells, and by blocking Th1 and Th17 cell differentiation [20]. The use of Ebi3 deficient mice or a fusion protein has demonstrated a role for IL-35 in regulating autoimmunity and autoinflammation [12, 22]. Indeed, transgenic expression of IL-35 by β cells prior to the initiation of autoimmunity prevents diabetes in NOD mice [23]; how IL-35 impacts islet infiltrating Teff and Foxp3+Treg once β cell autoimmunity is established, however, remains ill-defined.
We and others have employed adeno-associated virus (AAV) vector gene delivery as a means to modify β cells and locally suppress islet autoimmunity [24, 25]. In general, AAV vectors are regarded as the safest and most efficient strategy to administer genes in vivo, and have been used to treat various genetic disorders in the clinic [26–30]. AAV vectors, in conjugation with a number of capsid protein serotypes with varying cell tropism and tissue specific promoters, offer an approach to selectively transduce distinct tissues and obtain long-term cell-specific transgene expression in vivo [27, 31, 32]. We have for instance, used an AAV vector containing a mouse preproinsulin II promoter (mIP) to transduce and selectively express IL-2 by β cells in NOD mice [24]. In this way, β cell autoimmunity is suppressed by increasing the islet Foxp3+Treg pool, while localizing IL-2 expression to the islets and therefore avoiding the complications associated with systemic IL-2 delivery [24, 33]. Notably, AAV vectors also provide tools to dissect the local effects of various cytokines and factors on islet/tissue resident immune effectors during ongoing inflammation [24]. In the current study, an IL-35 expressing AAVmIP vector was used to test if the inhibitory properties of IL-35 are sufficiently robust to suppress ongoing β cell autoimmunity. We show that targeting IL-35 expression to β cells in NOD mice at a late preclinical T1D stage prevents the development of diabetes. Protection is marked by significantly reduced numbers of islet T cells and DC, and a phenotypically distinct islet Foxp3+Treg pool, which in turn is needed to suppress CD4+ Teff differentiation.
RESULTS
β-cell-specific IL-35 expression prevents overt diabetes at a late preclinical stage in NOD mice
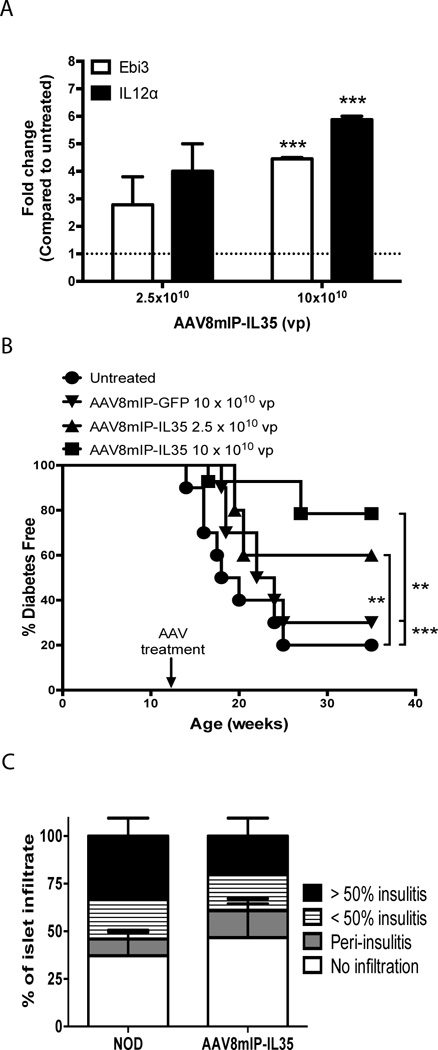
To assess the therapeutic potential of IL-35, AAVmIP-IL35 was engineered in which Ebi3 and Il12a are driven by mIP to restrict expression to β cells. In our hands long-term (e.g. up to 1 year) AAV8mIP transgene expression is detected in β cells but not other tissues including the liver, thymus and spleen [24]. The AAV capsid serotype 8 protein was used to package AAVmIP-IL35 to increase β cell transduction efficiency in vivo [25, 34, 35]. Injection of NOD mice with AAV8mIP-IL35 resulted in a dose-dependent increase in Ebi3 and IL-12α mRNA levels in pancreatic islets (Fig. 1A). To determine if ectopic IL-35 suppressed ongoing β cell autoimmunity, 12 week-old NOD female mice, reflecting a late preclinical stage at which islets are heavily infiltrated, were vaccinated i.p. with AAV8mIP-IL35, control AAV8mIP-GFP or were left untreated, and blood glucose levels monitored. The majority (≥70%) of untreated and AAV8mIP-GFP-treated control NOD mice developed diabetes with a similar time of onset by 35 weeks of age (Fig. 1B). In contrast, a dose-dependent increase in diabetes-free NOD female mice was observed following AAV8mIP-IL35 vaccination (Fig. 1B). After receiving 2.5×1010 and 10×1010 vector particles (vp) of AAV8mIP-IL35, 60% and 79% of NOD female mice remained nondiabetic, respectively (Fig. 1B). Furthermore, analyses of pancreatic sections from nondiabetic 35 week-old NOD female mice treated with 10×1010 vp AAV8mIP-IL35 showed a frequency of insulitis equivalent to that seen in unmanipulated, nondiabetic 12 week-old NOD female mice (Fig. 1C). These results demonstrate that β cell-specific expression of IL-35 suppresses the progression of insulitis and the development of overt diabetes.
Figure 1. β-cell-specific expression of IL-35 at a late preclinical stage prevents the onset of diabetes.
12 week-old NOD female mice were treated with AAV8mIP-IL35, AAV8mIP-GFP, or left untreated. (A) Ebi3 and Il12α mRNA levels in pancreases/islets were compared between AAV8mIP-IL35 and untreated cohorts; the ratio of expression for the respective genes between AAV8mIP-IL35 and untreated controls is depicted. Data represent average ± SEM of 2 independent experiments in which pancreas/islets were pooled from 2–3 mice per cohort in each experiment. ***p<0.001 (Student’s t test). (B) Cohorts treated with AAV8mIP-IL35 (10×1010 vp, n=14; 2.5×1010 vp, n=5), AAV8mIP-GFP (10×1010 vp, n=10), or left untreated (n=10) were monitored for overt diabetes by blood glucose levels. **p<0.01, ***p<0.001 (Kaplan-Meier log-rank test). (C) Insulitis was determined in hematoxylin and eosin stained pancreatic sections of 35-week old non-diabetic NOD female mice treated with AAV8mIP-IL35 (n=6) and compared to 12-week old NOD female mice (n=8). Insulitis was scored as the percentage of islets with no insulitis, peri-insulitis, <50% intra-insulitis, and >50% intra-insulitis. Data shown as average + SEM of the indicated number of mice pooled from 2 experiments.
Ectopic IL-35 expression reduces islet infiltration
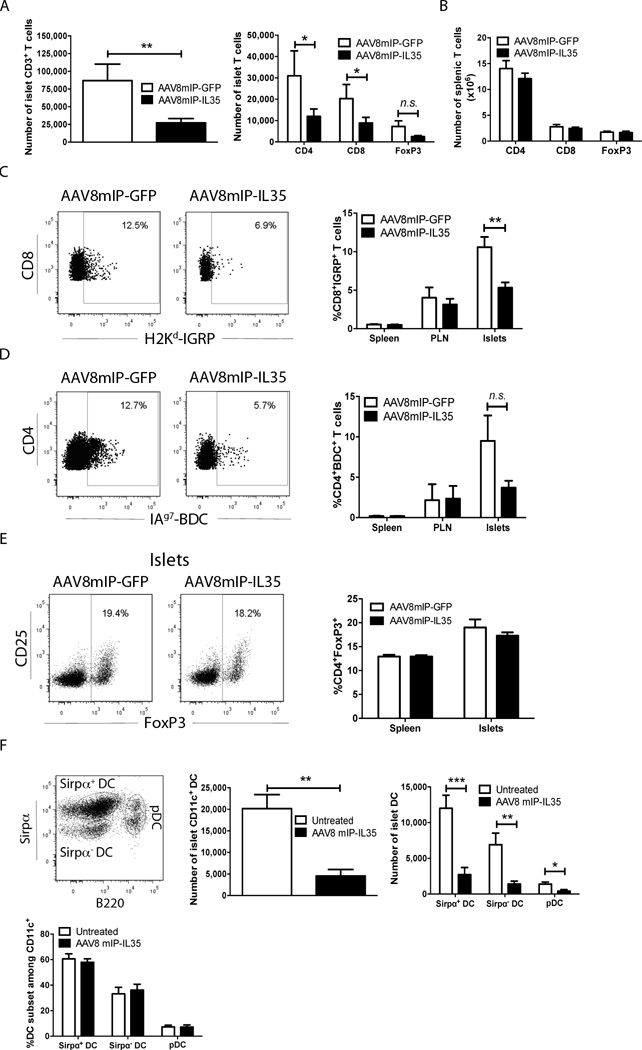
To gain insight into the mechanism(s) of protection, the effect of β cell-specific IL-35 on islet infiltrating cells was examined. NOD female mice 12 weeks of age were vaccinated with 10×1010 vp of AAV8mIP-IL35 or AAV8mIP-GFP, and islets isolated 4 weeks later. In agreement with the insulitis data for animals followed long-term (Fig. 1C), islet but not splenic T cell numbers were reduced in AAV8mIP-IL35-treated animals (Fig. 2A,B). Specifically, numbers of conventional CD4+ and CD8+ T cells infiltrating the islets were reduced 2.6- and 2.3-fold, respectively, in AAV8mIP-IL35 versus AAV8mIP-GFP NOD mice (Fig. 2A). The latter was reflected by a decreased frequency of H2Kd-IGRP and IAg7-BDC tetramer staining CD8+ T cells and CD4+ T cells, respectively, in the islets but not the draining pancreatic lymph nodes (PLN) or spleen (Fig. 2C,D). A trend in reduced islet Foxp3+Treg numbers was also detected in AAV8mIP-IL35 NOD mice, which correlated with no marked change in the frequency of Foxp3+Treg among the islet CD4+ T cell pool (Fig. 2A,E). These results indicated that the impact of ectopic IL-35 was local, consistent with findings obtained in NOD mice vaccinated with an IL-2 expressing AAV8mIP vector [24].
Figure 2. Ectopic IL-35 expression reduces islet infiltrating T cells and DCs.
(A–E) Groups of 3–6 12 week-old NOD female mice were treated with 10×1010 vp of AAV8mIP-IL35, AAV8mIP-GFP, or left untreated, and 4 weeks later islets, spleen and/or PLN examined. (A, B) The number of live CD3+-gated CD4+, CD8+, and FoxP3+ cells were determined in the (A) islets, and (B) spleen. Data shown as average ± SEM 5 separate experiments, analyzing a total of 26 AAV8mIP-GFP- and 33 AAV8mIP-IL35-treated mice. *p<0.05, **p<0.01 (Student’s t test). (C, D) Live CD3+ cells were gated on and the frequency of islet, PLN, and splenic (C) H2Kd-IGRP+ CD8+ and (D) IAg7-BDC+ CD4+FoxP3− T cells determined. Representative flow plots for tetramer staining of islet CD8+ and CD4+ T cells are provided (left). Staining of control H2Kd-HA and IAg7-control peptide was typically <6% of CD8+ and CD4+ T cells, respectively. Data shown as average ± SEM of 2 separate experiments with a total of 10–11 mice per group (right). (E) Frequency of islet and splenic CD3+-gated FoxP3+ cells. Data shown as average ± SEM of 5 separate experiments with a total 26–33 mice per cohort. (F) CD11c+ cells were gated on and the number of bulk islet CD11c+ DCs, and number and frequency of islet CD11c+Sirpα+ DCs, CD11c+Sirpα− DCs and CD11c+B220+ pDCs measured via flow cytometry. Data shown as average ± SEM of 2 separate experiments with a total of 6 mice per group. ***p<0.001, **p<0.01, *p<0.05 (Student’s t test).
Numbers of islet resident non-T cell types were also reduced by β cell-specific IL-35 expression. Islet DC were decreased ∼4-fold in the AAV8mIP-IL35 versus control groups (Fig. 2F). This decrease was seen for CD11c+ Sirpα+ and Sirpα− DC subsets, as well as plasmacytoid DC (pDC) (Fig. 2F). No marked changes in DC frequency (Fig. 2F) or maturation, based on expression levels of IAg7, H2Kd and CD80 were detected for the respective DC subsets (data not shown). Together, these results demonstrate that β cell-specific IL-35 affects islet cellularity, marked by reduced numbers of conventional CD4+ and CD8+ T cells, Foxp3+Treg, and other islet resident immune effectors such as DC.
Ectopic IL-35 expression suppresses islet T-cell expansion
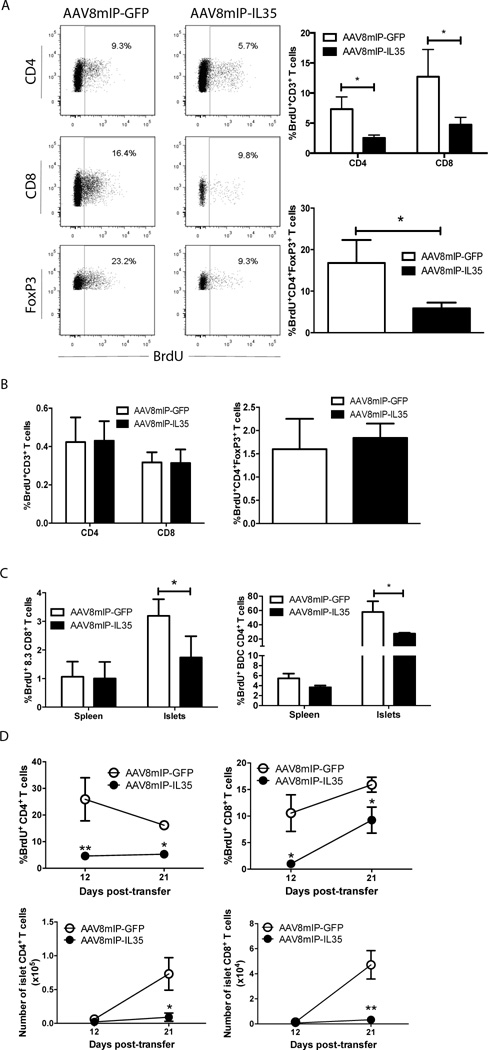
A reduced islet T cell pool may be due to suppressed proliferation, and/or increased cell death. To examine the proliferative status of islet T cells, 12 week-old NOD female mice were vaccinated with AAV8mIP-IL35 and AAV8mIP-GFP and 4 weeks later, BrdU uptake measured. An ∼2–4-fold reduction in BrdU incorporation by islet CD4+ and CD8+ T cells and Foxp3+Treg was detected in AAV8mIP-IL35-treated NOD mice (Fig. 3A), whereas proliferation of splenic T cells was unaffected (Fig. 3B). A similar decrease in BrdU incorporation was seen for islet CD4+ and CD8+ T cells in AAV8mIP-IL35-vaccinated TCR transgenic NOD.BDC2.5 and NOD.8.3 mice, respectively (Fig. 3C). Based on VAD-FMK staining, however, ectopic IL-35 had no effect on the frequency of apoptotic CD4+ and CD8+ T cells or Foxp3+Treg in the pancreas of NOD mice 10 d post-vaccination (Supporting Information Fig. 1). Similarly, the frequency of islet CD4+ and CD8+ T cells and Foxp3+Treg expressing the pro-apoptotic molecule Bim or the anti-apoptotic molecule Bcl-2 was unaffected by AAV8mIP-IL35-treatment relative to controls (Supporting Information Fig. 1).
Figure 3. β-cell-specific IL-35 reduces proliferation of islet resident conventional T cells and Foxp3+Treg cells.
Groups of 3–5 (A,B) 12 week-old NOD female mice, (C) 6 week-old NOD.BDC2.5 and NOD.8.3 female or (D) 10 week-old NOD.scid female mice were treated with 10×1010 vp of AAV8mIP-IL35, AAV8mIP-GFP, or left untreated. (A–D) 4 week post-AAV vector treatment, live CD3+ cells were gated on and BrdU incorporation by CD4+, CD8+, and CD4+FoxP3+ cells determined via flow cytometry in the (A) islets and (B) spleen of NOD mice, and in (C) NOD.BDC2.5 and NOD.8.3 mice. (A, B) Data shown as average ± SEM of 3 separate experiments with a total of 10 mice per cohort. (C) Data shown as average ± SEM of 2 independent experiments analyzing a total of 5 mice per cohort. *p<0.05 (Student’s t test). (D) NOD.FoxP3GFP-derived Thy1.2+ T cells were adoptively transferred and BrdU incorporation and number of live islet CD3+CD4+Foxp3− and CD3+CD8+ T cells determined via flow cytometry 12 days and 21 days post-transfer. Data represent an average ± SEM of 2 independent experiments, with a total of 10 mice per cohort. **p<0.01, *p<0.05 (Student’s t test).
In a second model, NOD.scid mice were treated with 10×1010 vp of AAV8mIP-IL35 or AAV8mIP-GFP, and 4 weeks later injected with FACS-sorted splenic T cells (Thy1.2+) from NOD.Foxp3GFP donors. At 12 and 21 d post-transfer, a similar number of CD4+ and CD8+ T cells and an equivalent frequency of BrdU+ T cells were detected in the spleen of the 2 experimental groups (Supporting Information Fig. 2). In contrast, a decrease in both the number of CD4+ and CD8+ T cells and percentage of proliferating cells was detected in the islets of AAV8mIP-IL35-versus AAV8mIP-GFP-treated recipients at the respective times post-transfer (Fig. 3D). At 6 d post-transfer, however, a similar number and frequency of BrdU+ T cells were detected in AAV8mIP-IL35 and AAV8mIP-GFP vaccinated NOD.scid recipients indicating that T cell trafficking into the islets was not markedly affected by IL-35 (data not shown). These results demonstrate that ectopic IL-35 effectively suppresses islet T cell proliferation and expansion independent of induction of apoptosis.
β− cell-specific IL-35 expression reduces the proinflammatory milieu of the islets
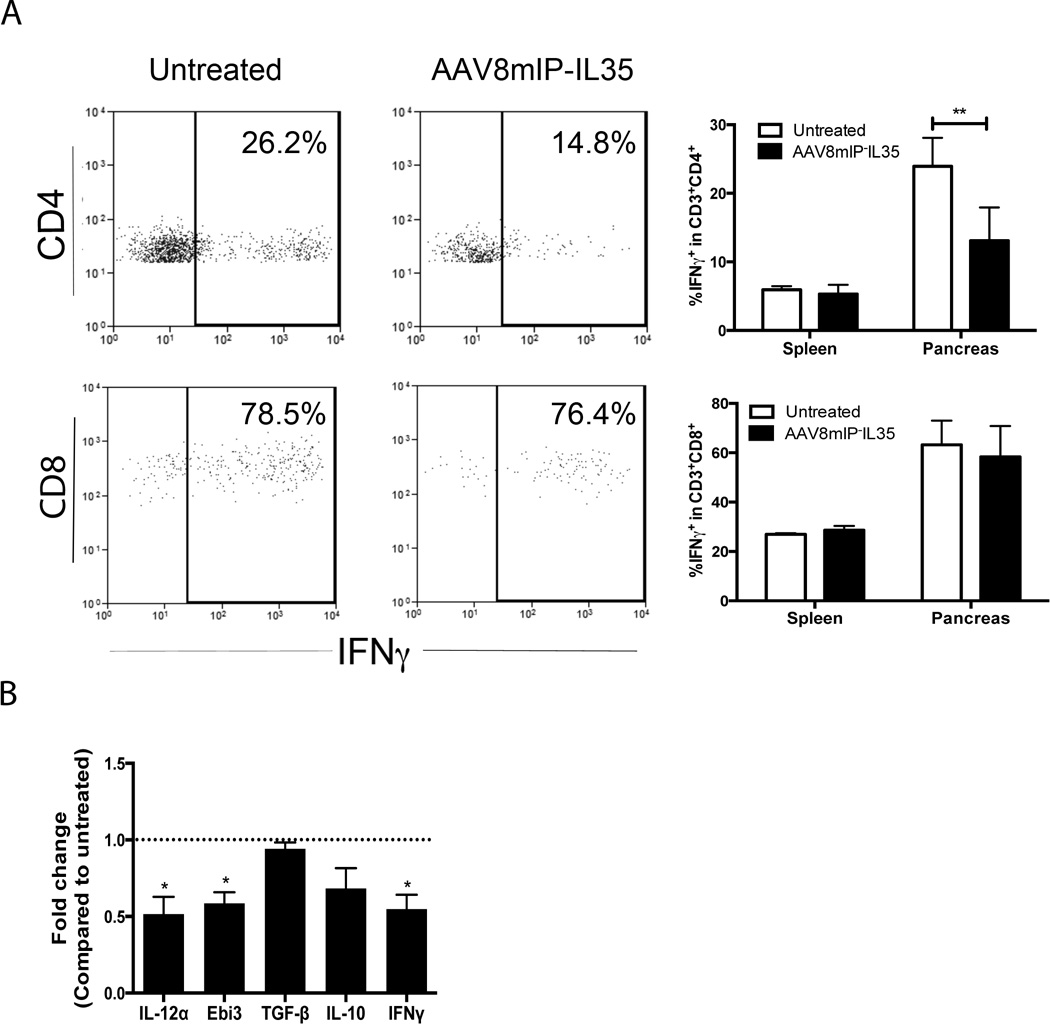
Whether ectopic IL-35 also induced qualitative changes within the islet T cell pool was assessed. Flow cytometric analyses showed that the frequency of pancreatic IFN-γ+CD4+ Teff was decreased ∼2 fold by ectopic IL-35 (Fig. 4A). Somewhat surprisingly, the frequency of IFN-γ+CD8+ Teff was unaffected by AAV8mIP-IL35 (Fig. 4A). Expression of IFN-γ mRNA by pancreatic conventional (GFP−) T cells FACS-sorted from NOD.Foxp3GFP female mice was reduced by ectopic IL-35 relative to the control group (Supporting Information Fig. 3).
Figure 4. Ectopic IL-35 induces a qualitatively distinct pool of islet conventional T cells.
(A, B) Groups of 3–4 NOD or NOD.BDC.Foxp3GFP female mice 12 weeks of age were treated with AAV8mIP-IL35 or left untreated, and spleen and pancreas examined 4 weeks later. (A) Live CD3+ cells were gated on and pancreatic CD4+FoxP3− and CD8+ T cells assessed for intracellular IFN-γ. Data shown as average ± SEM of 2 separate experiments with a total of 6 mice per cohort. **p<0.01 (two-way ANOVA with Bonferroni post-test). (B) Four 10 week-old NOD.BDC.Foxp3GFP female mice were treated with 10×1010 vp of AAV8mIP-IL35 or left untreated and pancreases pooled 4 weeks later. Thy1.2+CD3+GFP− cells were sorted, and mRNA levels of Ebi3, IL-12α, IFN-γ, TGFβ, and IL-10 compared. Data are the average ± SEM of 2 separate experiments. *p<0.05 (Student’s t test).
A diminished pool of IFN-γ+ CD4+ Teff may have in part been due to induction/expansion of adaptive CD4+ Treg, such as iTr35 cells. Accordingly, 10 week-old female NOD.BDC.Foxp3GFP mice were treated with AAV8mIP-IL35, conventional (GFP−) T cells FACS-sorted 4 weeks later, and cytokine mRNA expression measured by qRT-PCR. Notably, naïve BDC CD4+ T cells are found at a relatively high frequency in the islets (∼30%), which in turn may serve as precursors for iTr35 cells or other adaptive Treg subsets. Consistent with the data for AAV8mIP-IL35 treated NOD.Foxp3GFP mice (Supporting Information Fig. 3), the expression of IFN-γ mRNA by pancreatic BDC CD4+ T cells was reduced 2-fold by ectopic IL-35 (Fig. 4B). Furthermore, Ebi3 and IL-12α transcripts were decreased in BDC CD4+ T cells relative to the control group (Fig. 4B), indicating limited (if any) IL-35-mediated iTr35 cell differentiation or expansion in AAV8mIP-IL35 vaccinated mice. Ectopic IL-35 also failed to increase BDC CD4+ T cell expression of IL-10 and TGFβ1 mRNA (Fig. 4B). Similar results were obtained in female NOD.Foxp3GFP mice vaccinated with AAV8mIP-IL35 (Supporting Information Fig. 3).
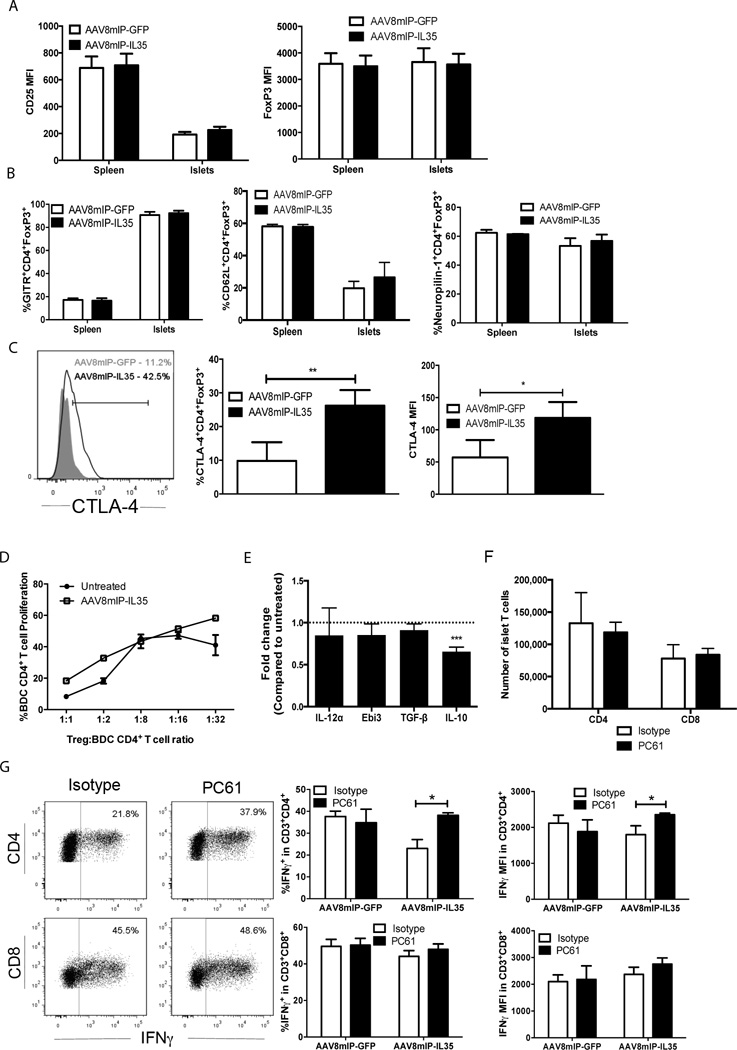
Next, the effects of ectopic IL-35 on the phenotype and function of islet Foxp3+Treg were examined in 12 week-old NOD female mice. CD25 and FoxP3 expression based on MFI (Fig. 5A), as well as the frequency of GITR+ and CD62Lhi islet Foxp3+Treg were unaffected by IL-35 (Fig. 5B); CD62Lhi Foxp3+Treg typically exhibit enhanced suppressor activity [9, 36–38]. In addition, ectopic IL-35 had no effect on the frequency of Foxp3+Treg expressing neuropilin-1 (Nrp-1), a marker for thymic-derived Foxp3+Treg (Fig. 5B) [39]. However, a 3-fold increase in the frequency of CTLA-4+ islet Foxp3+Treg coupled with a 2-fold increase in CTLA-4 MFI was observed in AAV8mIP-IL35-vaccinated NOD mice (Fig. 5C). To assess islet Foxp3+Treg suppressor activity, NOD.BDC.Foxp3GFP mice were treated with AAV8mIP-IL35, pancreatic CD4+GFP+ T cells FACS-sorted, and co-cultured with conventional BDC CD4+ T cells and sBDC peptide-pulsed APC. Using the same approach, ectopic IL-2 expression was found to increase the in vitro suppressor activity of islet BDC Foxp3+Treg FACS-sorted from AAV8mIP-IL2 vaccinated NOD.BDC.Foxp3GFP mice [24]. No difference in suppressor activity was detected between Foxp3+Treg isolated from AAV8mIP-IL35 versus control animals (Fig. 5D). Furthermore, with the exception of IL-10 mRNA which was reduced, similar levels of Ebi3, IL-12α, and TGFβ1 mRNA were detected in FACS-sorted pancreatic Foxp3+Treg (GFP+) from AAV8mIP-IL35 and control NOD.BDC.Foxp3GFP mice (Fig. 5E).
Figure 5. Islet Foxp3+Treg cells selectively suppress CD4+ Teff cells in AAV8mIP-IL35 treated mice.
(A–E) Groups of 3–4 12 week-old (A–C) NOD, (D,F,G) NOD.Foxp3GFP, or (E) 10 week-old NOD.BDC.Foxp3GFP female mice were treated with 10×1010 vp of AAV8mIP-IL35 or AAV8mIP-GFP or left untreated, and 4 weeks later, islet and splenic CD3+ T cells analyzed. (A) Level of CD25 and FoxP3 expression among islet CD3+CD4+ T cells. Data shown as average ± SEM of 5 separate experiments with a total of 20–23 mice per cohort. (B) Frequency of GITR, CD62L, and Nrp-1 expressing CD3+CD4+FoxP3+ cells. (C) The frequency (middle) and MFI (right) for CTLA-4 expression by islet CD3+CD4+FoxP3+ cells. (B–C) Data shown as average ± SEM of 2–3 separate experiments with a total of 6–8 mice per cohort. *p<0.05, **p<0.01 (Student’s t test). (D) In vitro suppressor activity of pancreatic FoxP3+/GFP+ BDC CD4+ T cells sorted from AAV8mIP-IL35 or untreated animals assessed via flow cytometry. (E) Cytokine mRNA levels were measured in Thy1.2+CD3+GFP+ cells sorted from NOD.BDC.Foxp3GFP pancreases, and the fold-difference between AAV8mIP-IL35 and control mice determined. Data represented are average average ± SEM of 2 independent experiments with pancreatic samples pooled from 4 mice per group in each experiment. ***p<0.001 (Student’s t test). (F–G) 4 weeks post-AAV8mIP-IL35 or AAV8mIP-GFP treatment, mice received PC61 or isotype control Ab, 12 days later live CD3+GFP−(FoxP3)− cells in the islets were gated on, and the (F) number and (G) frequency of IFN-γ+ CD4+ and CD8+ T cells by CD4+ and CD8+ T cells determined. Data presented as average ± SEM of 2 separate experiments with a total of 6 mice per group. *p<0.05 (Student’s t test).
To investigate the relative contribution of Foxp3+Treg in vivo following ectopic IL-35 expression, AAV8mIP-IL35 vaccinated NOD.Foxp3GFP female mice were treated with PC61, an anti-CD25 Ab known to deplete and/or block activity of Foxp3+Treg [40–42]. Islet and splenic Foxp3+Treg frequency and number were reduced at least 2-fold 12 d by PC61 compared to the isotype Ab control group (Supporting Information Fig. 4). No difference in the number of islet CD4+ and CD8+ T cells was detected between AAV8mIP-IL35 vaccinated animals treated with PC61 and isotype control Ab (Fig. 5F). On the other hand, the frequency and MFI of intracellular IFN-γ for islet IFN-γ+CD4+ but not IFN-γ+CD8+ T cells were increased in PC61 versus control group (Fig. 5G). In contrast, no further increase in IFN-γ+CD4+ Teff or IFN-γ MFI was detected in the islets of AAV8mIP-GFP vaccinated animals treated with PC61 (Fig. 5G). The latter suggests that Foxp3+Treg play only a limited role at this late preclinical stage of T1D. These results demonstrate that the frequency of islet IFN-γ+CD4+ Teff but not IFN-γ+CD8+ Teff is decreased in AAV8mIP-IL35 vaccinated NOD mice, which in turn is mediated, at least in part, by islet Foxp3+Treg. Furthermore, protection induced by ectopic IL-35 is independent of adaptive Treg (e.g. iTr35 cells).
DISCUSSION
Earlier work by the Vignali group demonstrated that transgenic NOD mice expressing IL-35 by β cells throughout ontogeny remain diabetes free [23]. Our findings show that the anti-inflammatory properties of ectopic IL-35 are sufficiently robust to suppress ongoing β cell autoimmunity at late preclinical T1D, and prevent the onset of diabetes. Notably, β cell-specific IL-35 had quantitative and qualitative effects locally on CD4+ and CD8+ Teff, Foxp3+Treg and other immune effectors residing in the islets.
A key aspect of the protection induced by ectopic IL-35 was the reduction in islet infiltrating β cell-specific CD4+ and CD8+ Teff (Figs. 1C, 2A,B). Insulitis in 35 week-old nondiabetic NOD mice vaccinated with AAV8mIP-IL35 was the equivalent of a 12 week-old animal, indicating that progression of islet infiltration was suppressed shortly after treatment. Indeed, conventional T cells were decreased ∼4-fold within 4 weeks of AAV8mIP-IL35 treatment (Fig. 2A). Analogous results were seen in the islets of AAV8mIP-IL35-treated NOD.scid mice after transfer of NOD T cells (Fig. 3D). The reduction in islet CD4+ and CD8+ T cells induced by ectopic IL-35 correlated with suppressed T cell proliferation but no effect on the frequency of apoptotic islet T cells (Supporting Information Fig. 1). Bettini et al. similarly reported diminished proliferation of pancreatic Teff in IL-35 transgenic NOD mice [23]. In addition to T cells, the number of islet DC (Fig. 2F) as well as islet B cells (data not shown) was reduced in AAV8mIP-IL35-vaccinated mice. A decrease in the islet APC pool is expected to aid in limiting local T cell stimulation, expansion and/or Teff differentiation. Whether ectopic IL-35 directly affected islet APC is unclear, although the absence of any change in activation and maturation suggests that islet DC were likely affected by IL-35 in an indirect manner. In this scenario, IL-35 suppresses CD4+ T cell-derived pro-inflammatory cytokines (e.g. IFN-γ, TNFα) which indirectly downregulates local chemokines and integrins needed for efficient retention of islet APC, as well as conventional T cells and Foxp3+Treg. This and the possibility that IL-35 indeed has direct effects on DC (and B cells) are currently being assessed.
Under the appropriate conditions IL-35 promotes differentiation of iTr35 cells. Our findings, however, indicate that ectopic IL-35 failed to induce detectable iTr35 cell differentiation. Levels of Ebi3 and IL-12α mRNA were in fact reduced in islet CD4+ T cells from AAV8mIP-IL35 mice (Fig. 4B; Supporting Information Fig. 3). Vignali and colleagues similarly reported a lack of iTr35 cell differentiation in the pancreas of IL-35 transgenic NOD mice [23]. These results argue against a key role for islet iTr35 cells in AAV8mIP-IL35-induced protection. Lack of an increase in iTr35 cells may be attributed to insufficient local levels of IL-10, which is needed in convert with IL-35 to drive efficient subset differentiation [21, 43]. No change in the expression of TGFβ1 and IL-10 by islet CD4+ T cells in AAV8mIP-IL35-treated animals (Fig. 4B) also indicates a minimal (if any) role for other subsets of adaptive (FoxP3−) CD4+ Treg in regulating β cell autoimmunity locally.
The in vivo effects of IL-35 on Foxp3+Treg in general, and specifically within the islets are ill-defined. Notably, ectopic IL-35 was seen to influence the islet Foxp3+Treg pool in a variety of ways. There was a trend towards a reduction in islet Foxp3+Treg numbers, which corresponded with decreased proliferation (Figs. 2,3), also reported in IL-35 transgenic NOD mice [23]. Similar to conventional CD4+ T cells, apoptosis was not increased and the frequency of Bcl-2+ and Bim+ cells unchanged in islet Foxp3+Treg (Supporting Information Fig. 1). Furthermore, levels of CD25 and FoxP3 expression, and the frequency GITR+, CD62Lhi and Nrp-1+ Foxp3+Treg were similar between AAV8mIP-IL35 and control groups (Fig. 5). On the other hand, both the MFI (∼2-fold) and frequency (∼3-fold) of CTLA-4-expressing islet Foxp3+Treg were significantly increased in AAV8mIP-IL35 vaccinated animals (Fig. 5C). Since iTr35 cells are characterized in part by a CTLA-4hi phenotype [21], it is possible that IL-35R signaling regulates CTLA-4 expression in the appropriate T cell subset. Other reported effects of IL-35 on Foxp3+Treg include down-regulation of expression of proinflammatory cytokines, and up-regulation of the transcriptional factor Eos, which is known to regulate Foxp3+Treg suppressor activity [44]. Despite the increase in CTLA-4, a potent effector molecule, the in vitro suppressor activity of pancreatic Foxp3+Treg from AAV8mIP-IL35 mice was not elevated (Fig. 5D). This result is consistent with a significant decrease in IL-10 expression but no marked increase in the expression of TGFβ1 or IL-35 (Ebi3, IL-12α) by pancreatic Foxp3+Treg from AAV8mIP-IL35 vaccinated mice (Fig. 5E). Results with PC61 Ab treatment, however, suggest that islet Foxp3+Treg play a selective role in suppressing differentiation of pathogenic CD4+ Teff in vivo. The frequency of islet IFN-γ+CD4+ but not IFN-γ+CD8+ Teff was increased ∼2-fold in AAV8mIP-IL35 vaccinated mice treated with PC61 Ab (Fig. 5G). The latter is consistent with >2-fold decrease in islet IFN-γ+CD4+ T cells not seen for IFN-γ+CD8+ following AAV8mIP-IL35 treatment (Fig. 4A). It is unclear why only expansion/differentiation of IFN-γ+ CD4+ Teff is affected by Foxp3+Treg. One possibility is that IL-35 enhances the sensitivity of CD4+ T cells to Foxp3+Treg-mediated suppression. The qualitative changes induced by IL-35 (Fig. 5) may also result in a Foxp3+Treg pool that is more effective at suppressing CD4+ Teff expansion/differentiation in vivo. Overall, however, our results support a model in which the dominant effect of ectopic IL-35 is to directly suppress expansion of islet CD4+ and CD8+ T cells with islet Foxp3+Treg playing a selective suppressor role. As discussed above a diminished pool of islet resident DC and APC would also be expected to aid in limiting T cell expansion.
Finally, our studies highlight the therapeutic potential of IL-35 either as a monotherapy or in combination with other immunotherapies to suppress ongoing β cell autoimmunity and T1D. The suppressor activity of IL-35 readily blocks the expansion of pathogenic CD4+ and CD8+ Teff. A recent study also showed that systemic administration of recombinant IL-35 prevents diabetes in a multiple low dose streptozotocin model of T1D, and induces remission in a portion of new onset diabetic NOD mice; recurrent diabetes develops, however, once IL-35 therapy is terminated [44]. On the other hand, i.v. injection of 8 week-old NOD female mice with an adenovirus recombinant encoding IL-35 had no marked protective effect [45]; whether sufficient systemic levels of IL-35 were achieved, however, is unclear. Nevertheless, systemic application of IL-35, particularly over an extended period, may lead to suppression of normal immune function. Indeed, unwanted systemic effects are a common concern for most cytokine-based immunotherapies. Our study provides further evidence that the use of AAV vectors is an effective strategy to manipulate the expression and tolerogenic properties of a cytokine in a highly tissue-specific manner. Engineering capsid proteins with enhanced tropism for certain cell types (e.g. β cells) and which lack reactivity to neutralizing Ab, coupled with sensitive inducible transgene systems will greatly enhance the clinical application of AAV vectors and cytokine therapy.
MATERIALS AND METHODS
Mice
NOD/LtJ, NOD.CB17-Prkdcscid/J (NOD.scid), NOD.Cg-Tg(TcraBDC2.5)1Doi Tg(TcrbBDC2.5)2Doi/DoiJ (NOD.BDC2.5), NOD.Cg-Tg(TcraTcrbNY8.3)1Pesa/DvsJ (NOD.8.3), NOD.129×1(Cg)-Foxp3tm2Tch/DVsJ (NOD.Foxp3GFP), NOD.BDC.Foxp3GFP, and NOD.129P2(C)-Tcratm1Mjo/DoiJ (NOD.Cαnull) mice were bred and maintained under specific pathogen-free conditions in an American Association for Laboratory accredited animal facility. NOD mice were diagnosed as diabetic after 3 consecutive blood glucose readings >250 mg/dL. All procedures were approved by the University of North Carolina Institutional Animal Use and Care Committee.
AAV vector engineering, packaging and vaccination
Full-length cDNAs encoding murine Ebi3 and Il12α separated by an elastin gene linker (pUNO1-mIL35elasti; Invivogen) or EGFP were subcloned into a single stranded AAV plasmid [46] containing mIP, and packaged with serotype 8 capsid protein as previously described [34]. Briefly, HEK 293T cells were triple-transfected with adeno helper plasmid (pXX6–80), AAV8 capsid plasmid, and plasmids encoding IL-35 or GFP via polyethylamine. 72 h post-transfection, nuclear fractions were harvested, packaged vector purified by cesium chloride (RPI) gradient, and titer determined by Southern dot blot.
Islet isolation and insulitis scoring
Pancreases were perfused with 2 mg/mL of collagenase P (Roche) prepared in HBSS (Sigma) and digested for 25 min at 37°C. Islets were purified over a Lympholyte 1.1 (Cedarlane) gradient, handpicked, and counted. Islet preparations from each individual animal consisted of 80–100 islets. Lymphocytes were collected from isolated islets following dissociation with an enzyme-free cell dissociation buffer (GIBCO) and filtered through a 70 µm cell strainer.
Formalin-fixed, paraffin embedded pancreases from non-diabetic 12 week-old or 35 week-old AAV8mIP-IL35-treated NOD female mice were serially sectioned 100 µm apart, and stained with hematoxylin and eosin. Insulitis was then scored for a minimum of 50 islets for each individual pancreas.
RNA, cDNA and quantitative real time PCR
RNA from sorted islet/pancreas-resident Thy1.2+GFP− or GFP+ T cells was isolated using Trizol (Invitrogen) or RNeasy Mini Kit (Qiagen), and cDNA reverse transcribed with the Super Script III first strand kit (Invitrogen) following the manufacturer’s recommendations. Primers for Ebi3 and Il12a were as previously described [12]: Ebi3 forward primer, AGCAGCAGCCTCCTAGCCT; Ebi3 reverse primer, ACGCCTTCCGGAGGGTC; Il12a forward primer, TGGCTACTAGAGAGACTTCTTCCACAA; Il12a reverse primer, GCACAGGGTCATCATCAAAGAC. Primers for IFN-γ, TGFβ1, TNFα, IL-10, and IL-2 are as follows: IFN-γ forward primer, TCAAGTGGCATAGATGTGGAAGAA; IFN-γ reverse primer, TGGCTCTGCAGGATTTTCATG; IL-2 forward primer, CCTGAGCAGGATGGAGAATTACA; IL-2 reverse primer, TCCAGAACATGCCGCAGAG; TNFα forward primer, CATCTTCTCAAAATTCGAGTGACAA; TNFα reverse primer, TGGGAGTAGACAAGGTACAACCC; IL-10 forward GCTCTTACTGACTGGCATGAG; IL-10 reverse primer, CGCAGCTCTAGGAGCATGTG; TGFβ1 forward primer, TGACGTCACTGGAGTTGTACGG; TGFβ1 reverse primer, GGTTCATGTCATGGATGGTGC. cDNA samples were amplified using a Maxima SYBR green master mix (Thermo Fisher) and an ABI Prism 7500 (Applied Biosystems). Relative mRNA expression was quantified by the comparative threshold (CT) method, where target mRNA expression is normalized to endogenous β-actin expression as determined by the formula 2−ΔΔCT.
Flow cytometry
Single cell suspensions from various tissues were treated with rat anti-mouse CD16/32 (2.4G2) (BD Biosciences) to block Fc receptors and subsequently stained with titrated fluorochrome-conjugated Ab specific for: CD3 (145-2C11), CD4 (GK1.5), CD8 (53.6.7), CD25 (PC61.5), CD62L (MEL-14), GITR (DTA-1), VAD-FMK (Promega), and Nrp-1 (3E12). Fluorochrome-conjugated H2Kd tetramers complexed to IGRP206–214 (VYLKTNVFL) or control influenza hemagglutinin HA (IYSTVGSSL) peptide, and IAg7 tetramers complexed to BDC mimetic (AHHPIWARMDA) or control (AMKRHGLDNYRGYSL) peptides were obtained from the National Institutes of Health Tetramer Core Facility. Intracellular staining of FoxP3 (FJK-16s; eBioscience), CTLA-4 (UC10-4F10–11), Bim (Cell Signaling Technology) and Bcl-2 (3F11; BD Biosciences) was done using the Fix/Perm and Perm/Wash reagents (eBioscience) according to the manufacturer’s recommendations.
For intracellular cytokine staining, lymphocytes were stimulated with 500 ng/ml PMA (Sigma) and 1000 ng/ml ionomycin (Sigma) for 5 h at 37°C, with 10 µg/ml Brefeldin A (Sigma) added for the last 4 h of incubation. After surface staining, cells were fixed and permeabilized with the Fix/Perm kit (eBioscience) and stained for intracellular IFN-γ (XMG1.2).
BrdU incorporation was analyzed via flow cytometry as described previously [47]. Animals were given an i.p. injection of 2 mg BrdU (Sigma), organs harvested 24 h later, and analyzed using mouse anti-BrdU PE Ab (BD Biosciences).
In addition, NOD.Foxp3GFP female mice were given i.p. 500 µg of PC61 5.3. (BioXcell) or rat IgG1 isotype control Ab. Organs were harvested 12 d later and biotinylated anti-CD25 (7D4) used for flow cytometric analysis.
Data were acquired on a Becton-Dickinson LSRII and Cyan flow cytometer (DakoCytomation) and analyzed using FlowJo Software and Summit software (DakoCytomation).
Cell adoptive transfer
Thy1.2+ cells (5×106) from spleens of non-diabetic NOD.Foxp3GFP female mice were sorted via FACS and injected i.p. into AAV8mIP-GFP or AAV8mIP-IL35 treated NOD.scid female mice. Purity of sorted Thy1.2+ cells was >95%.
In vitro suppressor assay
Pancreatic single cell suspensions prepared from NOD.BDC.FoxP3GFP female mice were stained with anti-CD4, and CD4+GFP+ T cells sorted on FACSAria II (BD Biosciences) at a cell purity of >95% post-sort. Splenocytes from NOD.Cα−/− mice were used as APC and pulsed with 1 µg/mL BDC peptide. CD4+CD25− T cells enriched from spleens of NOD.BDC2.5 mice using CD4+CD25+ Regulatory T cell Isolation Kit (Milltenyi Biotec), were labeled with 5 µM Cell Trace Violet (CTV; Invitrogen) and used as responders. BDC CD4+CD25− T cells (3×104) were co-cultured with varying ratios of CD4+GFP+ T cells and APC (3×104) for 72 h at 37°C. Proliferation was assessed by dilution of CTV measured via flow cytometry.
Statistical analysis
Graphpad Prism software was used to perform all statistical analyses, including Kaplan-Meier log-rank test, Student’s t test, and Two-way ANOVA, where appropriate.
Supplementary Material
Acknowledgments
This study was supported by funding from the National Institutes of Health (1R01DK103546) to R.T., F.M. and M.C.J. were supported by a National Institutes of Health Training Grant (T32 AI007273). The UNC Flow Cytometry Core Facility is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center, and North Carolina Biotech Center Institutional Support Grant 2005-IDG-1016.
Abbreviations
- AAV
adeno-associated virus
- CTV
Cell Trace Violet
- DC
dendritic cells
- Ebi3
Epstein-Barr-virus-induced gene 3
- iTr35
induced regulatory Th35 suppressor cells
- pDC
plasmacytoid DC
- T1D
type 1 diabetes
- Teff
effector T cells
- vp
vector particles
- IL-35
Interleukin-35
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors declare no financial or commercial conflict of interest.
REFERENCES
- 1.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 6.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 8.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–1414. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 9.Goudy KS, Johnson MC, Garland A, Li C, Samulski RJ, Wang B, Tisch R. Reduced IL-2 expression in NOD mice leads to a temporal increase in CD62Llo FoxP3+ CD4+ T cells with limited suppressor activity. Eur J Immunol. 2011;41:1480–1490. doi: 10.1002/eji.201040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171:4040–4047. doi: 10.4049/jimmunol.171.8.4040. [DOI] [PubMed] [Google Scholar]
- 11.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008;181:7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 13.Zhang XH, Zhou Y, Zhang JM, Zhou SY, Wang M, Feng R, Feng FE, Wang QM, Zhu XL, Zhao XS, Lv M, Kong Y, Chang YJ, Huang XJ. IL-35 inhibits acute graft-versus-host disease in a mouse model. Int Immunopharmacol. 2015;29:383–392. doi: 10.1016/j.intimp.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Ning X, Jian Z, Wang W. Low Serum Levels of Interleukin 35 in Patients with Rheumatoid Arthritis. Tohoku J Exp Med. 2015;237:77–82. doi: 10.1620/tjem.237.77. [DOI] [PubMed] [Google Scholar]
- 15.Filkova M, Vernerova Z, Hulejova H, Prajzlerova K, Veigl D, Pavelka K, Vencovsky J, Senolt L. Pro-inflammatory effects of interleukin-35 in rheumatoid arthritis. Cytokine. 2015;73:36–43. doi: 10.1016/j.cyto.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20:633–641. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai Z, Wong CK, Dong J, Chu M, Jiao D, Kam NW, Lam CW, Tam LS. Remission of systemic lupus erythematosus disease activity with regulatory cytokine interleukin (IL)-35 in Murphy Roths Large (MRL)/lpr mice. Clin Exp Immunol. 2015;181:253–266. doi: 10.1111/cei.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi J, Leung PS, Bowlus C, Gershwin ME. IL-35 and Autoimmunity: a Comprehensive Perspective. Clin Rev Allergy Immunol. 2015 doi: 10.1007/s12016-015-8468-9. [DOI] [PubMed] [Google Scholar]
- 19.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieuwenhuis EE, Neurath MF, Corazza N, Iijima H, Trgovcich J, Wirtz S, Glickman J, Bailey D, Yoshida M, Galle PR, Kronenberg M, Birkenbach M, Blumberg RS. Disruption of T helper 2-immune responses in Epstein-Barr virus-induced gene 3-deficient mice. Proc Natl Acad Sci U S A. 2002;99:16951–16956. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bettini M, Castellaw AH, Lennon GP, Burton AR, Vignali DA. Prevention of autoimmune diabetes by ectopic pancreatic beta-cell expression of interleukin-35. Diabetes. 2012;61:1519–1526. doi: 10.2337/db11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson MC, Garland AL, Nicolson SC, Li C, Samulski RJ, Wang B, Tisch R. beta-cell-specific IL-2 therapy increases islet Foxp3+Treg and suppresses type 1 diabetes in NOD mice. Diabetes. 2013;62:3775–3784. doi: 10.2337/db13-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehman KK, Trucco M, Wang Z, Xiao X, Robbins PD. AAV8-mediated gene transfer of interleukin-4 to endogenous beta-cells prevents the onset of diabetes in NOD mice. Mol Ther. 2008;16:1409–1416. doi: 10.1038/mt.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, Glader B, Chew AJ, Tai SJ, Herzog RW, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake AW, High KA. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 27.Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, Rouhani F, Conlon TJ, Calcedo R, Betts MR, Spencer C, Byrne BJ, Wilson JM, Flotte TR. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flotte TR, Trapnell BC, Humphries M, Carey B, Calcedo R, Rouhani F, Campbell-Thompson M, Yachnis AT, Sandhaus RA, McElvaney NG, Mueller C, Messina LM, Wilson JM, Brantly M, Knop DR, Ye GJ, Chulay JD. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 30.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grieger JC, Samulski RJ. Adeno-associated virus vectorology, manufacturing, and clinical applications. Methods Enzymol. 2012;507:229–254. doi: 10.1016/B978-0-12-386509-0.00012-0. [DOI] [PubMed] [Google Scholar]
- 32.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 33.Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, Ahmann A, Rabinovitch A, Aggarwal S, Phippard D, Turka LA, Ehlers MR, Bianchine PJ, Boyle KD, Adah SA, Bluestone JA, Buckner JH, Greenbaum CJ, Diabetes T the Immune Tolerance, N. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs beta-cell function. Diabetes. 2012;61:2340–2348. doi: 10.2337/db12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Zhu T, Rehman KK, Bertera S, Zhang J, Chen C, Papworth G, Watkins S, Trucco M, Robbins PD, Li J, Xiao X. Widespread and stable pancreatic gene transfer by adeno-associated virus vectors via different routes. Diabetes. 2006;55:875–884. doi: 10.2337/diabetes.55.04.06.db05-0927. [DOI] [PubMed] [Google Scholar]
- 36.Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201:1333–1346. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ermann J, Hoffmann P, Edinger M, Dutt S, Blankenberg FG, Higgins JP, Negrin RS, Fathman CG, Strober S. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 38.Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J Immunol. 2002;169:2461–2465. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- 39.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. S1711–S1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Setiady YY, Coccia JA, Park PU. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur J Immunol. 2010;40:780–786. doi: 10.1002/eji.200939613. [DOI] [PubMed] [Google Scholar]
- 41.Montero E, Nussbaum G, Kaye JF, Perez R, Lage A, Ben-Nun A, Cohen IR. Regulation of experimental autoimmune encephalomyelitis by CD4+, CD25+ and CD8+ T cells: analysis using depleting antibodies. J Autoimmun. 2004;23:1–7. doi: 10.1016/j.jaut.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 42.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 43.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh K, Kadesjo E, Lindroos J, Hjort M, Lundberg M, Espes D, Carlsson PO, Sandler S, Thorvaldson L. Interleukin-35 administration counteracts established murine type 1 diabetes - possible involvement of regulatory T cells. Sci Rep. 2015;5:12633. doi: 10.1038/srep12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flores RR, Kim E, Zhou L, Yang C, Zhao J, Gambotto A, Robbins PD. IL-Y, a Synthetic Member of the IL-12 Cytokine Family, Suppresses the Development of Type 1 Diabetes in NOD Mice. Eur J Immunol. 2015 doi: 10.1002/eji.201445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 47.Tough DF, Sprent J, Stephens GL. Measurement of T and B cell turnover with bromodeoxyuridine. Curr Protoc Immunol. 2007 doi: 10.1002/0471142735.im0407s77. Chapter 4: Unit 4 7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.