The plant hormone ethylene has wide-ranging and dramatic effects on the growth, development, and stress responses of the plant throughout its life (Abeles et al., 1992). The proliferation of papers and review articles on ethylene in recent years has been largely due to the tremendous role that mutational analysis has played in dissecting the molecular intricacies of the ethylene synthesis and response pathways (Bleecker and Kende, 2000; Guo and Ecker, 2004). The nine papers in this Focus Issue on Ethylene contribute important discoveries and novel directions regarding ethylene receptor function, as well as ethylene production, response, signaling, and ecophysiology. These are only a small sampling of the many areas of ethylene biology, as numerous topics in biotic and abiotic stresses and other aspects of plant growth and development are not represented here. Thus, several exciting fronts, such as ethylene cross-talk with other hormone signaling pathways, are not contained in this issue and hopefully can be featured in a future issue.
BIOSYNTHESIS AND PERCEPTION
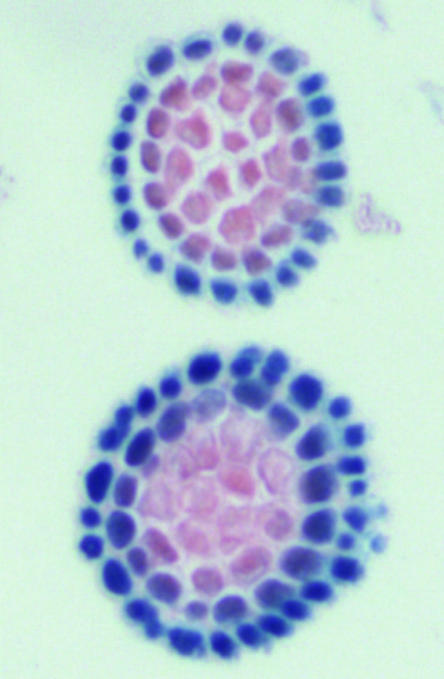
Ethylene biosynthesis is regulated by developmental processes as well as by numerous external stresses. The rate-limiting step of ethylene biosynthesis is the production of 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC synthase, which is followed by the conversion of ACC to ethylene by ACC oxidase (Bleecker and Kende, 2000). In this issue, a comparative analysis by Tsuchisaka and Theologis (2004; pp. 2982–3000) reveals a complex spatial and temporal regulation of the entire family of ACC synthase genes in Arabidopsis. During development and under various stresses, unique as well as overlapping expression patterns are observed (e.g. Fig. 1), and these potentially reflect a combinatorial code for functional ACC synthase heterodimers. This analysis contains beautiful images that illustrate ethylene's pervasiveness in plant development and physiological processes and provides a foundation for future studies on the mechanics of ACC synthase action and the roles of ethylene.
Figure 1.
Effect of auxin on the expression of ACS-GUS in the lateral root cap cell layers of Arabidopsis from the work by Tsuchisaka and Theologis (this issue). Top, −IAA; bottom, +IAA.
In the past decade, outstanding progress has been made toward elucidating the ethylene signaling pathway, which is currently modeled as an essentially linear pathway consisting of membrane-associated ethylene receptors leading to transcription factors regulating gene expression in the nucleus (for review, see Guo and Ecker, 2004). Five of the nine papers in this issue address questions concerning ethylene signaling, and three of these papers are focused on ethylene receptor function. To provide the appropriate context for these papers, an overview of the ethylene receptors is given here. The ethylene receptors are derived from the family of two-component His protein kinase receptors, which transmit their signal through His autophosphorylation on the His kinase domain, followed by transfer of the phosphate to a conserved aspartate residue in the cognate receiver domain (which is the carboxyl-terminal domain for some of the ethylene receptors; Chang and Stadler, 2003). In Arabidopsis, there are five ethylene receptors in two subfamilies, all of which have been shown to bind ethylene (Schaller and Bleecker, 1995; Hall et al., 2000; F. Rodriguez and A. Bleecker, unpublished data). Subfamily 1 receptors (ETR1 and ERS1) contain all five of the functionally defined His kinase sequence motifs, whereas subfamily 2 members (ETR2, EIN4, and ERS2) are substantially diverged, carrying only one or two of the motifs. One member of each subfamily (ERS1 and ERS2) lacks the receiver domain. Despite the differences between the five receptors, they all exhibit redundancy with one another in that the single null mutant of each receptor appears as wild type (Hua and Meyerowitz, 1998; Zhao et al., 2002; Hall and Bleecker, 2003; Wang et al., 2003). Combinations of receptor null mutants result in constitutive ethylene responses, indicating that the receptors are negative regulators of ethylene response (Hua and Meyerowitz, 1998). Interestingly, the double null mutant of subfamily 1 generally exhibits much more severe phenotypes than any other double or triple receptor null mutant combination (Zhao et al., 2002; Hall and Bleecker, 2003; Wang et al., 2003). These genetic studies are consistent with a model in which the receptors actively repress responses in the absence of ethylene (such that ethylene binding turns off signaling) and dominant ethylene insensitivity results from gain-of-function mutations in the receptor genes, allowing the receptor system to continue repressing responses even in the presence of ethylene. The ETR1 receptor has been shown to have His kinase activity in vitro (Gamble et al., 1998), but transfer of the phosphate to the receiver domain has not been demonstrated. In fact, His kinase activity of the ethylene receptors has been the subject of much scrutiny, as described below.
WHAT ARE THE ETHYLENE RECEPTORS DOING, AND HOW ARE THEY DOING IT?
Of the many fascinating questions in ethylene signaling and response, one of the most intriguing is how do the receptors transmit the signal? This question was initially difficult to address due to the redundancy of the ethylene receptor genes. However, the discovery of a severe and distinct phenotype for light-grown seedlings of the subfamily 1 receptor loss-of-function mutant (ers1 etr1) immediately opened the door to analyzing the in vivo effects of key mutations that are known to abolish His kinase activity in vitro. The surprising result was that a transgene encoding a His kinase-inactive form of the ETR1 receptor was capable of rescuing the subfamily 1 mutant phenotype to wild type, indicating that canonical His kinase activity in ETR1 does not serve a primary function in ethylene signal transduction (Wang et al., 2003).
Now, two papers in this issue uncover potential functions for His kinase activity as well as the receiver domain, based on studies of dark-grown Arabidopsis seedlings. Qu and Schaller tested mutated forms of the ETR1 receptor for the ability to rescue the constitutive ethylene response of a triple receptor null mutant (Qu and Schaller, 2004; pp. 2961–2970). Both wild-type ETR1 and ETR1 lacking the receiver domain rescued the seedling constitutive ethylene response in air, but ETR1 lacking the receiver gave ethylene hypersensitivity at low doses of ethylene. Moreover, an ETR1 point mutation that blocks His kinase activity in vitro conferred only partial rescue of the constitutive phenotype in air and at low doses, suggesting that His kinase activity is required for efficient repression of ethylene responses. These results indicate that the ETR1 His kinase and receiver domains play a quantitative role in the repression of ethylene responses.
Another role is revealed in the first of two companion papers from the Bleecker lab. In this paper, Binder et al. (2004a; pp. 2913–2920) introduce an innovative dissection of the rapid kinetics of ethylene response in etiolated Arabidopsis seedlings, employing a highly sensitive imaging technique, which was developed for examining the response of hypocotyls to light (Parks and Spalding, 1999; Folta and Spalding, 2001). By analyzing short-term growth inhibition in etiolated hypocotyls in response to ethylene treatment on the order of minutes, Binder et al. (2004a) uncovered two phases of ethylene inhibition: a transient rapid phase followed by a sustained slower phase. In addition to the rate of growth inhibition, the rate of growth recovery was measured upon ethylene removal. Using this novel and sensitive assay, loss-of-function receptor mutants were then examined to assess the role of each of the ethylene receptor isoforms in the three kinetic phases. None of the loss-of-function receptor mutants were altered in the kinetics of either growth inhibition phase. Interestingly, however, mutants of the receptor isoforms that possess a receiver domain had a slower rate of recovery, suggesting that the receiver domain plays a role in recovery from growth inhibition in etiolated seedlings. Furthermore, the recovery defect in the subfamily 1 double mutant could be rescued by a wild-type ETR1 transgene, but not by a His kinase-inactive form of ETR1. These findings, together with those of Qu and Schaller, indicate a quantitative role for the His kinase and receiver domains in recovery from ethylene treatment and in ethylene signaling, respectively.
Adding another twist to the story, in vitro evidence suggests that most of the ethylene receptors possess Ser/Thr kinase activity instead of, or perhaps in addition to, His kinase activity. This was first reported by Xie et al. (2003), who demonstrated Ser/Thr kinase activity for a subfamily 2 ethylene receptor, NTHK1, from tobacco (Nicotiana tabacum). Now Zhang et al. (2004; pp. 2971–2981) find that another tobacco subfamily 2 ethylene receptor, NTHK2, displays either Ser/Thr or His kinase activity depending upon whether Mn2+ or Ca2+ is present in the reaction respectively. In conjunction with this finding, a paper by Moussatche and Klee (2004) was just published showing that all of the Arabidopsis ethylene receptors, except for ETR1, possess Ser/Thr kinase activity, while ERS1 possesses both activities depending upon which ions are present in the reaction.
It thus remains an open question whether phosphotransfer by the receptors is the primary mode of signal transmission to downstream components in the pathway. The current view is that phosphotransfer might be required, although there is no definitive proof either way. The work of Wang et al. (2003), Gamble et al. (2002), and Qu and Schaller (this issue) argue against a canonical His phosphorelay being the primary signaling mechanism, but they do not rule out the possibility that His kinase activity plays a role in certain kinetic or physiological situations. The papers of Xie et al. (2003), Zhang et al. (this issue), and Moussatche and Klee (2004) raise the distinct possibility that novel forms of phosphotransfer could be at play. The testing of point mutants (that disrupt kinase activity) for the ability to rescue the appropriate receptor-deficient lines will ultimately establish the biological relevance of these novel activities. In any case, the new findings and approaches featured in this issue bring us closer to the point where specific roles of the ethylene receptors can be identified in connection with their biochemical activities.
At the same time, several lines of evidence seem to be converging on a model of synergistic action by the ethylene receptors. For one, several dominant mutant isoforms of the receptors confer strong ethylene insensitivity (Hua et al., 1998) even though they are expressed at a very low level (Binder et al., 2004a). Secondly, the paper by Qu and Schaller (this issue) demonstrates that the His-kinase domain of ETR1 is needed for signaling in a receptor deficient background, yet it was previously shown that the His kinase domain is not needed for the dominant mutant form of ETR1 to transmit suppression of ethylene responses when other receptor isoforms are present (Gamble et al., 2002). Thirdly, the receptors act over a wide dynamic range suggestive of cooperative action. The kinetic analyses by the Bleecker lab indicate that the response system can work for ethylene concentrations ranging over seven orders of magnitude. The initial rapid response phase for growth inhibition was highly sensitive to ethylene and could be obtained by ethylene concentrations as low as 0.2 nL L−1. Response occurs at an ethylene concentration that is 300-fold below the estimated Kd for ethylene binding, and it was calculated that an ethylene response can be obtained when only 1 out of 1,000 receptors is occupied with ethylene (Binder et al., 2004b; pp. 2921–2927). The same system appears capable of responding to changes in ethylene concentrations between 100 and 1,000 μL L−1 (Chen and Bleecker, 1995). These observations are consistent with a model in which receptors interact synergistically to transmit the signal, reminiscent of the current models of bacterial chemotaxis where the receptors form higher order clusters of receptor dimers.
Binder et al. (2004b) also detected an interesting adaptation phenomenon at low doses of ethylene. When low ethylene concentrations were applied continuously to dark-grown seedlings, there was at first a transient response, but then growth rates recovered to pretreatment rates within 2 h, and the seedlings remained desensitized to ethylene. If a second, higher dose of ethylene was applied immediately after the adaptation, then a reduced response was obtained. If the second dose was given 5 h after the first dose, then the response was normal. This again has parallels with adaptation in bacterial chemotaxis, for which there are elaborate models involving synergistic interactions between receptor dimers in large clustered arrays, as discussed in Binder et al. (2004b).
ACTION DOWNSTREAM OF THE RECEPTORS
Ethylene signaling requires the transcription factors EIN3 and EIL1, which regulate the expression of additional transcription factors such as ERF1 (Chao et al., 1997; Solano et al., 1998). EIN3 and EIL1 are important players in ethylene signaling, since the ein3 eil1 double loss-of-function mutant shows complete insensitivity to ethylene (Alonso et al., 2003). A recent exciting discovery was that stabilization of EIN3 is a key regulatory step in ethylene signaling (Guo and Ecker, 2004; Potuschak et al., 2003; Yanagisawa et al., 2003; Gagne et al., 2004). Here in this issue, EIN3 and EIL1 are examined in the rapid growth response assay in Binder et al.'s second paper (2004b). Surprisingly, the ein3 eil1 double mutant was indistinguishable from the wild type in the rapid response phase and displayed insensitivity only in the slower response phase. A dominant ethylene-insensitive receptor mutant was blocked in both of the response phases. The finding that EIN3 and EIL1 are not required for the first response phase suggests that transcription induction may not be involved in the initial rapid response to ethylene. This is consistent with the fact that the first rapid response phase begins just 15 min after ethylene is applied. If correct, this raises the new and interesting question as to what mechanisms give rise to the rapid response.
Another key component in ethylene signaling is EIN2, a novel integral membrane protein that acts upstream of EIN3 (Alonso et al., 1999). EIN2 is thought to play a central role in ethylene responses, based on the fact that loss-of-function mutations in Arabidopsis EIN2 block ethylene responses completely. In the rapid growth response assay in Arabidopsis seedlings, EIN2 was found to be required for both phases of growth inhibition (Binder et al., 2004b). One question worth addressing is whether EIN2 has the same role in other plants, particularly in ethylene-mediated processes not typically examined in Arabidopsis, e.g. fruit ripening and adventitious root formation (Klee, 2004). In this issue, Shibuya et al. (2004; pp. 2900–2912) establish the universal role of EIN2 in ethylene signaling by generating and analyzing a collection of ein2 knock-down mutants in petunia (Petunia hybrida). Their findings confirm that EIN2 functions in a wide range of ethylene responses. One difference, however, is that expression of petunia EIN2 is regulated by ethylene (which is not seen for Arabidopsis EIN2), suggesting a level of control over ethylene responses that has not been detected, or does not exist, in Arabidopsis.
ECOPHYSIOLOGY OF ETHYLENE RESPONSE
Three of the papers in this issue examine ethylene response in different ecophysiological systems. Ethylene is required for the shade avoidance response, and previous work has shown that ethylene insensitive tobacco plants have reduced leaf movement and elongation response to plant neighbors resulting from reduced responsiveness to red (R):far-red (FR) light ratio and blue light intensity (Pierik et al., 2003, 2004b). In this issue, Pierik et al. (2004a; pp. 2928–2936) present several novel findings that build upon the previous work. They present evidence that ethylene production and responsiveness are under control of the phytochrome photoreceptors, indicating that ethylene is a critical element in the signaling pathway of phytochrome-mediated shade-avoidance responses. They also suggest that ethylene interacts with GA in determining the growth responses to reduced R:FR ratios. This work shows that the two elements of the shade avoidance response (hyponasty and elongation of petiole and stem) are differentially regulated and demonstrates that low R:FR can cause rhythmic ethylene production, which might represent an effective and ecologically relevant rapid response to a dynamic light environment.
Ethylene gas is not only produced by plants, but is a by-product of human industrial activities. The paper by Munné-Bosch et al. (2004; pp. 2937–2947) addresses the effects of ethylene (at concentrations found in polluted areas) on plant responses to environmental stresses such as heat and drought stress. Ethylene is typically thought to induce resistance to stresses, yet Munné-Bosch et al. discover that high levels of ethylene comparable to those generated in polluted areas actually reduce resistance to heat and drought stress in the tree holm oak, and they suggest that this might occur through the alteration of antioxidant defenses.
Another ecologically relevant system is the submergence-induced hyponastic response of petioles in the flood-tolerant plant Rumex palustris. The paper by Cox et al. (2004; pp. 2948–2960) presents new insights on the mechanisms of this response, which brings the leaf blade above the surface of the water. The detailed studies in this paper provide evidence that ethylene, together with auxin, abscisic acid, and GA, all play roles in various aspects of the hyponastic response.
The papers in this Focus Issue present new and exciting advances in ethylene research. Although they provide a selective view of a broad field, they hopefully will inspire thinking and discussion on the “simplest” of hormones. Sincere thanks go to all of the authors, reviewers, and editorial staff for producing this Focus Issue.
References
- Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in Plant Biology, Ed 2. Academic Press, New York
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR (2003) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA 100: 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, O'Malley RC, Wang W, Moore JM, Parks BM, Spalding EP, Bleecker AB (2004. a) Arabidopsis seedling growth response and recovery to ethylene: a kinetic analysis. Plant Physiol 136: 2913–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, Mortimore LA, Stepanova AN, Ecker JR, Bleecker AB (2004. b) Short term growth responses to ethylene in Arabidopsis seedlings are EIN3/EIL1 independent. Plant Physiol 136: 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Chang C, Stadler R (2003) Ethylene hormone receptor action in Arabidopsis. Bioessays 23: 619–627 [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chen QC, Bleecker AB (1995) Analysis of ethylene signal-transduction kinetics associated with seedling-growth response and chitinase induction in wild-type and mutant Arabidopsis. Plant Physiol 108: 597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MCH, Benschop JJ, Vreeburg RAM, Wagemaker CAM, Moritz T, Peeters AJM, Voesenek LACJ (2004) The roles of ethylene, auxin, abscisic acid and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiol 136: 2948–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo S-D, Yanagisawa S, Vierstra RD (2004) Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc Natl Acad Sci USA 101: 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Coonfield ML, Schaller GE (1998) Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc Natl Acad Sci USA 95: 7825–7829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Qu X, Schaller GE (2002) Mutational analysis of the ethylene receptor ETR1. Role of the histidine kinase domain in dominant ethylene insensitivity. Plant Physiol 128: 1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ecker JR (2004) The ethylene signaling pathway: new insights. Curr Opin Plant Biol 7: 40–49 [DOI] [PubMed] [Google Scholar]
- Hall AE, Bleecker AB (2003) Analysis of combinatorial loss-of-function mutants in the Arabidopsis ethylene receptors reveals that the ers1 etr1 double mutant has severe developmental defects that are EIN2 dependent. Plant Cell 15: 2032–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Finell JL, Schaller GE, Sisler EC, Bleecker AB (2000) Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiol 123: 1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 72: 261–271 [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM (1998) EIN4 and ERS2 are members of the putative ethylene-receptor gene family in Arabidopsis. Plant Cell 10: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ (2004) Ethylene signal transduction. Moving beyond Arabidopsis. Plant Physiol 135: 660–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussatche P, Klee HJ (2004) Autophosphorylation activity of the Arabidopsis ethylene receptor multigene family. J Biol Chem (in press) [DOI] [PubMed]
- Munné-Bosch S, Peñuelas J, Asensio D, Llusià J (2004) Airborne ethylene may alter antioxidant protection and reduce tolerance of holm oak to heat and drought stress. Plant Physiol 136: 2937–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Spalding EP (1999) Sequential and coordinated action of phytochromes A and B during Arabidopsis stem growth revealed by kinetic analysis. Proc Natl Acad Sci USA 96: 14142–14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Cuppens MLC, Voesenek LACJ, Visser EJW (2004. a) Interactions between ethylene and gibberellins in phytochrome-mediated shade avoidance responses in tobacco. Plant Physiol 136: 2928–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Visser EJW, de Kroon H, Voesenek LACJ (2003) Ethylene is required in tobacco to successfully compete with proximate neighbors. Plant Cell Environ 26: 1229–1234 [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LACJ, de Kroon H, Visser EJW (2004. b) Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signalling. Plant J 38: 310–319 [DOI] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P (2003) EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F-box proteins. Cell 115: 679–689 [DOI] [PubMed] [Google Scholar]
- Qu X, Schaller GE (2004) Requirement of the histidine kinase domain for signal transduction by the ethylene Receptor ETR1. Plant Physiol 136: 2961–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB (1995) Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270: 1809–1811 [DOI] [PubMed] [Google Scholar]
- Shibuya K, Barry KG, Ciardi JA, Loucas HM, Underwood BA, Nourizadeh S, Ecker JR, Klee HJ, Clark DG (2004) The central role of PhEIN2 in ethylene responses throughout plant development in petunia. Plant Physiol 136: 2900–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchisaka A, Theologis A (2004) Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol 136: 2982–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Hall AE, O'Malley R, Bleecker AB (2003) Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc Natl Acad Sci USA 100: 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Zhang JS, Zhou HL, Li J, Zhang ZG, Wang DW, Chen SY (2003) Serine/threonine kinase activity in the putative histidine kinase-like ethylene receptor NTHK1 from tobacco. Plant J 33: 385–393 [DOI] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo S-D, Sheen J (2003) Differential regulation of EIN3 stability by glucose and ethylene signaling in plants. Nature 425: 521–525 [DOI] [PubMed] [Google Scholar]
- Zhang Z-G, Zhou H-L, Chen T, Gong Y, Cao W-H, Wang Y-J, Zhang J-S, Chen S-Y (2004) Evidence for serine/threonine and histidine kinase activity in the tobacco ethylene receptor protein NTHK2. Plant Physiol 136: 2971–2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X-C, Xiang Q, Mathews DE, Schaller GE (2002) Effect of ethylene pathway mutations upon expression of the ethylene receptor ETR1 from Arabidopsis. Plant Physiol 130: 1983–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]