Abstract
Responses to the plant hormone ethylene are mediated by a family of five receptors in Arabidopsis that act in the absence of ethylene as negative regulators of response pathways. In this study, we examined the rapid kinetics of growth inhibition by ethylene and growth recovery after ethylene withdrawal in hypocotyls of etiolated seedlings of wild-type and ethylene receptor-deficient Arabidopsis lines. This analysis revealed that there are two phases to growth inhibition by ethylene in wild type: a rapid phase followed by a prolonged, slower phase. Full recovery of growth occurs approximately 90 min after ethylene removal. None of the receptor null mutations tested had a measurable effect on the two phases of growth inhibition. However, loss-of-function mutations in ETR1, ETR2, and EIN4 significantly prolonged the time for recovery of growth rate after ethylene was removed. Plants with an etr1-6;etr2-3;ein4-4 triple loss-of-function mutation took longer to recover than any of the single mutants, while the ers1;ers2 double mutant had no effect on recovery rate, suggesting that receiver domains play a role in recovery. Transformation of the ers1-2;etr1-7 double mutant with wild-type genomic ETR1 rescued the slow recovery phenotype, while a His kinase-inactivated ETR1 construct did not. To account for the rapid recovery from growth inhibition, a model in which clustered receptors act cooperatively is proposed.
Ethylene regulates a number of developmental processes in higher plants, including growth in etiolated seedlings. Inhibition of growth in etiolated seedlings by ethylene is a convenient and useful bioassay that has been used to quantify the dose-response characteristics of ethylene (Chen and Bleecker, 1995) and, in mutant screens, to identify components in the ethylene signal transduction pathway (Bleecker et al., 1988; Guzman and Ecker, 1989). Mutational analysis of the ethylene signaling pathway has led to an increasingly refined model for signaling (Guo and Ecker, 2004). According to this model, responses to ethylene are mediated by a family of five receptors in Arabidopsis that are related to bacterial two-component receptors (Chang et al., 1993; Hua et al., 1998; Hua and Meyerowitz, 1998; Sakai et al., 1998). The ethylene receptors are thought to transduce signal via Ser/Thr kinase activity in CTR1 (Kieber et al., 1993; Huang et al., 2003). CTR1 may negatively regulate the ethylene response pathway by inhibiting activity of an Nramp-related protein, EIN2, which is required for responses to ethylene (Alonso et al., 1999). Ethylene binding to the receptors reduces the activity of the receptors, leading to reduced activity of CTR1 protein and an increase in activity of EIN2 protein along with subsequent signaling associated with it. At least some responses to ethylene, including the seedling growth response, are mediated by activation of a transcriptional cascade (Solano et al., 1998), suggesting that ethylene responses are mediated by differential gene activation and inactivation. In support of this, a number of genes have been shown to be ethylene responsive (Schenk et al., 2000; Van Zhong and Burns, 2003), including the genes for the ERS1, ERS2, and ETR2 ethylene receptors (Hua et al., 1998).
The ethylene receptors can be divided into two subfamilies. Subfamily I consists of ETR1 and ERS1, which contain all amino acid residues thought to be needed for His-kinase activity (Chang et al., 1993; Hua et al., 1995; Gamble et al., 1998), although kinase activity does not appear to be required for ethylene signaling (Wang et al., 2003). Subfamily II consists of ETR2, EIN4, and ERS2, which contain degenerate His-kinase domains (Hua et al., 1998; Sakai et al., 1998). Although all ethylene receptor isoforms appear to contribute to signaling, as evidenced by the observations that each receptor isoform binds ethylene with high affinity (F.I. Rodriguez, unpublished data), single loss-of-function receptor mutants lack a discernable phenotype, and various combinations of triple loss-of-function receptor mutants show a constitutive response phenotype (Hua and Meyerowitz, 1998; Hall and Bleecker, 2003). However, the five receptor isoforms are not entirely redundant in function (Hall and Bleecker, 2003; Wang et al., 2003), and the function of His kinase in ethylene signal transduction is still uncertain.
Despite our growing understanding of the ethylene signal transduction pathway, questions remain as to the role(s) of each receptor isoform in the control of etiolated seedling growth by ethylene. Previous kinetic studies indicate that ethylene rapidly inhibits the growth rate of etiolated pea (Pisum sativum) seedlings within 10 min and seedlings return to pretreatment growth within 20 min after ethylene is removed (Warner and Leopold, 1971; Burg, 1973; Goeschl and Kays, 1975; Rauser and Horton, 1975). This rapid response must be reconciled with the evidence that at least two rounds of transcriptional activation appear to be required for the sustained seedling growth inhibition by ethylene (Solano et al., 1998). The rapid recovery from inhibition must also be reconciled with the observation that ethylene dissociation from the ETR1 receptor has a half-life of 11 h (Schaller and Bleecker, 1995). To gain a better understanding of the roles that individual receptor isoforms play in ethylene signal transduction, we have initiated studies of short-term growth responses of etiolated Arabidopsis seedlings to ethylene. Using high-resolution, time-lapse imaging of individual seedlings growing in darkness, we monitored the kinetics of both the growth inhibition caused by the application of ethylene and the recovery to pretreatment growth once exogenous ethylene is removed.
RESULTS
Growth Inhibition and Recovery Kinetics
Short-term changes in growth rates of etiolated Arabidopsis seedlings were obtained using 2-d-old seedlings growing along the surface of a vertical agar plate. Under the conditions used in this study, we found that the growth rate of wild-type and mutant hypocotyls in air prior to ethylene treatment ranged from 0.17 to 0.42 mm h−1 (Table I). Control experiments carried out in air showed that hypocotyls had linear growth for at least 12 h under the conditions used in these experiments (data not shown).
Table I.
Average growth rate (mm h−1) of etiolated seedling hypocotyls in air
| Seedling Type | Hypocotyl Growth Rate |
|---|---|
| mm h−1 | |
| Columbia (wild type) | 0.30 ± 0.08 |
| WS (wild type) | 0.34 ± 0.07 |
| etr1-7 | 0.29 ± 0.10 |
| etr2-3 | 0.42 ± 0.13 |
| ers1-2 | 0.39 ± 0.10 |
| ers2-3 | 0.39 ± 0.12 |
| ein4-4 | 0.37 ± 0.11 |
| ers1-2; ers2-3 | 0.32 ± 0.12 |
| etr1-6;etr2-3;ein4-4 | 0.17 ± 0.11 |
| etr1-6;etr2-3;ein4-4 gETR-1 | 0.20 ± 0.07 |
| etr1-6 etr2-3;ein4-4 getr1-[D] | 0.23 ± 0.15 |
| ers1-2;etr1-7 | 0.26 ± 0.10 |
| ers1-2;etr1-7gETR1 | 0.33 ± 0.14 |
| ers1-2;etr1-7getr1-[HGG] | 0.38 ± 0.12 |
The average ± sd was calculated using data from the 1-h air pretreatment.
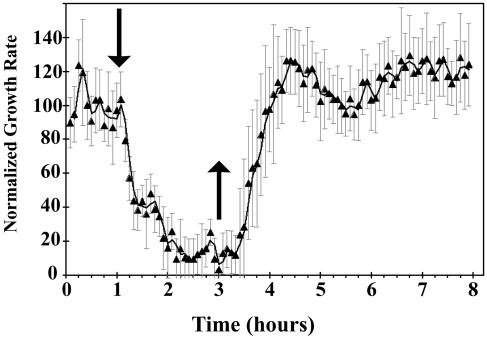
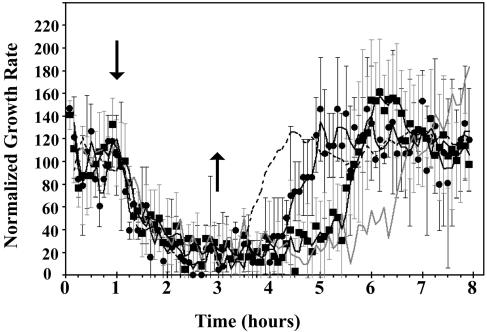
Figure 1 shows typical growth responses of etiolated wild-type Arabidopsis (Columbia) hypocotyls to a treatment with 10 μL L−1 ethylene. Addition of exogenous ethylene caused the growth rate to decrease within 15 min, reaching a new steady-state growth rate approximately 75 min after ethylene was added. Closer examination of the response kinetics showed that there appears to be at least two phases to growth inhibition by ethylene. The first, rapid deceleration phase had a lag of approximately 15 min after ethylene was applied, lasted approximately 15 min, and resulted in a steady-state growth rate of 0.13 mm h−1. Following this, growth rate was stable for approximately 20 min before a second, slower phase of growth inhibition ensued. The rate of change for this slower phase was approximately one-sixth the rate of change observed with the onset of the first phase. This lasted another 20 min until the growth rate reached the new steady-state rate of 0.03 mm h−1. Growth rate was suppressed to this low level until ethylene was removed.
Figure 1.
Rapid kinetic analysis of growth in etiolated Arabidopsis hypocotyls. Etiolated hypocotyls respond to ethylene within 15 min of applying 10 μL L−1 ethylene. There is a rapid decrease in growth rate, followed by a plateau in the change in growth rate. This is followed by a slow decrease in growth rate until a new steady-state growth rate is reached approximately 75 min after applying ethylene. When ethylene is removed, there is a lag of approximately 30 min before a measurable increase in growth rate is observed. Hypocotyls return to pretreatment growth rates approximately 90 min after ethylene is removed. In this and Figures 2, 4, and 5, measurements were made in air for 1 h prior to introducing 10 μL L−1 ethylene (↓). Ethylene was removed 2 h later (↑).
Following the withdrawal of ethylene from the treatment chamber, hypocotyls that had been growing in the presence of ethylene for 2 h began to recover, attaining pretreatment growth rates approximately 90 min later (Fig. 1). It is interesting to note that the time needed for growth recovery was much faster than the time required for dissociation of ethylene to occur from yeast-expressed ETR1 or ETR2 receptors (Schaller and Bleecker, 1995; F.I. Rodriguez, unpublished data).
Loss-of-Function Mutants in Some Ethylene Receptor Isoforms Affect Recovery from Growth Inhibition
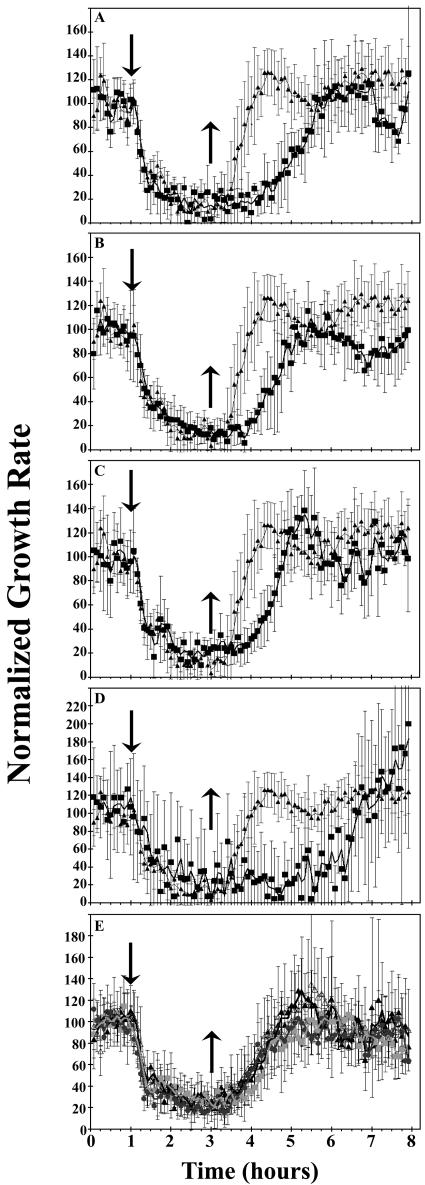
To study the role(s) of specific receptors in response to and recovery from ethylene, we examined growth-rate kinetics in various ethylene receptor null mutants. Of all the single null mutations tested, the etr1-7 null mutant showed the strongest phenotype. Figure 2 shows that this mutant took significantly longer to recover from ethylene-induced growth inhibition following a 2-h ethylene treatment. This longer recovery time was also noted for shorter (30-min) ethylene treatment (data not shown). Following ethylene removal, the etr1-7 mutant required more than 2.5 h to return to initial growth rates compared to 1.5 h for wild-type seedlings (Fig. 2A). Both the etr2-3 (Fig. 2B) and ein4-4 (Fig. 2C) mutants also displayed a slowed growth-rate recovery, each needing slightly over 2 h. Seedlings which contained only the ERS1 and ERS2 ethylene receptors (etr1-6;etr2-3;ein4-4 triple mutants) showed a very slow recovery of approximately 4 h (Fig. 2D). In general, the main effect of these receptor null mutations on the growth-rate response profile appeared to be a delay in the onset of measurable growth recovery. The exception to this was the etr2-3 mutant where the lag time and the rate of growth recovery contributed equally to the slower recovery to pretreatment growth rates. Growth-rate recovery following ethylene treatment was unaltered in ers1-2 and ers2-3 mutants, as well as the ers1-2;ers2-3 double null mutant (Fig. 2E). Thus, these two receptor isoforms that lack a receiver domain do not appear to contribute to the rapid recovery of seedlings from ethylene treatments.
Figure 2.
Comparisons of growth response kinetics in wild-type and loss-of-function receptors mutants. The responses of wild-type seedlings (▴) are shown in each section compared to etr1-7 mutants (A); etr2-3 mutants (B); ein 4-4 mutants (C); etr1-6;etr2-3;ein4-4 triple mutants (D); and ers1-2 (▪), ers2-3 (•), and ers1-2;ers2-3 (▵) mutants (E). Columbia (wild-type) seedlings were used for comparison with etr1-7, etr2-3, ein4-4, and etr1-6;etr2-3;ein4-4 (A–D), while WS (wild-type) seedlings were used for comparison with the ers1-2, ers2-3, and ers1-2;ers2-3 mutants (E). Ethylene was introduced at 1 h (↓) and removed 2 h later (↑).
Ethylene Receptor Transcript Levels
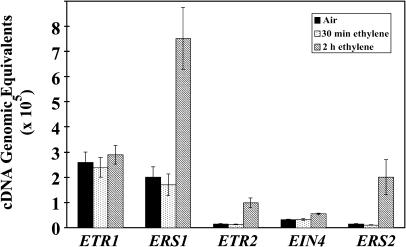
We have previously found that receptor transcript levels correlate with ethylene binding (R.C. O'Malley and J. Esch, unpublished data). We used quantitative reverse transcription (RT)-PCR to measure the mRNA levels of each receptor isoform in etiolated seedlings treated with ethylene for various amounts of time to examine whether the altered growth-rate response profiles observed for the various receptor null mutants correlated with any altered expression of receptor isoform genes. Results from this survey are shown in Figure 3.
Figure 3.
RT-PCR analysis from Columbia (wild-type) seedlings treated with ethylene. The mRNA from individual ethylene receptor isoforms was analyzed from etiolated seedlings before and during treatment with ethylene for 30 min or 2 h. All measurements were made in triplicate.
In air prior to ethylene treatment, the ETR1 transcript comprised approximately 50% and ERS1 approximately 37% of total receptor transcript levels. The other 3 receptor isoforms each comprised 6% or less of total receptor transcript levels. Upon treatment with 10 μL L−1 ethylene, transcript levels for each receptor isoform remained constant for the first 30 min, but after 2 h in the presence of ethylene the mRNA for ERS1, ERS2, and ETR2 showed large increases of approximately 3.8-, 6.6-, and 14.3-fold, respectively (Fig. 3). The mRNA levels of ETR1 and EIN4 remained constant in the presence of ethylene as reported previously (Chang et al., 1993; Hua et al., 1998). At this point, mRNA for ERS1 comprised approximately 54%, ETR1 approximately 20%, and ERS2 approximately 14% of total receptor transcript. The other 2 receptor isoforms each comprised 7% or less of total transcript levels (Fig. 3). The lack of contribution to recovery by ERS1, despite its high level of expression at the time of recovery in these experiments, further highlights a special role for receiver domain-containing receptor isoforms in the recovery process.
A Role for His-Kinase Activity and Receptors Containing a Receiver Domain in Recovery from the Hypocotyl Growth Response
In a previous study, it was shown that a kinase-deficient mutant of ETR1 could rescue the etr1;ers1 double mutant for a number of ethylene responses, indicating that canonical His-kinase activity was not essential for signaling by the receptor (Wang et al., 2003). Evidence for a specific role for receiver domain-containing receptor isoforms in the growth recovery from ethylene prompted us to explore the possibility that the His-kinase activity of ETR1 contributed to the recovery process.
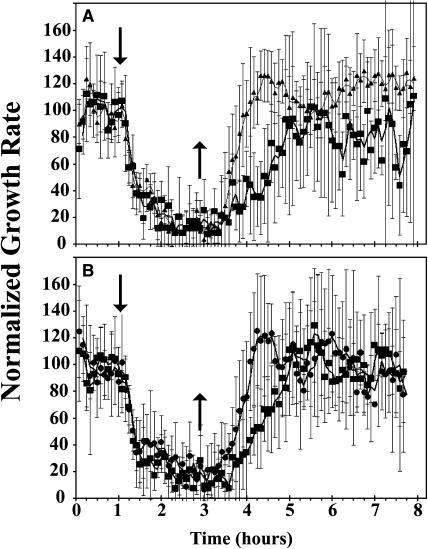
The double loss-of-function mutant, ers1-2;etr1-7, had slow growth recovery (Fig. 4). However, its recovery was faster than that observed in the etr1-7 mutant. This faster-than-expected recovery in the ers1-2;etr1-7 mutant plants is probably due to our observation that ETR2 transcript levels are elevated in the double mutant plants but not the etr1-7 mutants (data not shown). Transformation of the double mutant with wild-type genomic ETR1 (gETR1) rescued the slow growth recovery phenotype. However, when transformed with a kinase-inactivated ETR1 genomic clone (getr1-[HGG]), the slow growth recovery phenotype was not rescued, supporting a specific role for His-kinase activity in growth recovery.
Figure 4.
The slow growth recovery phenotype of plants deficient in His-kinase receptor isoforms was rescued by a wild-type ETR1 transgene but not a kinase-inactivated ETR1 transgene. A, The ers1-2;etr1-7 double loss-of-function receptor mutant (▪) had slower recovery than wild-type plants (▴). B, Transformation of this double mutant with genomic ETR1 (gETR1; •) rescued this slow recovery while a kinase-inactivated ETR1 transgene (getr1-[HGG]; ▪) did not. Ethylene was introduced at 1 h (↓) and removed 2 h later (↑).
To determine whether phosphotransfer to the receiver domain is required for normal growth recovery, we used plants deficient in receptor isoforms containing receiver domains. When etr1-6;etr2-3;ein4-4 triple loss-of-function mutant seedlings were transformed with genomic ETR1 (gETR1), the slow recovery phenotype was rescued (Fig. 5). This was seen in two independent plant lines. As would be predicted, the time for recovery in these transformants was similar to recovery seen in either the etr2-3 or the ein4-4 single loss-of-function mutants. A mutant ETR1 lacking the conserved Asp-659, getr1-[D], transformed into the triple null seedlings showed only a partial rescue of the recovery phenotype (Fig. 5). This result was seen in three independent transgenic plant lines. This reduced rescue by the getr1-[D] transgene could be due to lower expression levels of getr1-[D] compared to gETR1. However, using end-point analysis, growth in air of the etr1-6;etr2-3;ein4-4 triple loss-of-function mutant seedlings was rescued equally well by either the gETR1 or getr1-[D] transgene (data not shown), suggesting that both transgenes are functioning equally well in this context.
Figure 5.
The slow growth recovery phenotype of plants deficient in receiver domain receptor isoforms was rescued by a wild-type ETR1 transgene but rescued poorly by a phosphotransfer-inactivated ETR1 mutant. Transformation of the etr1-6;etr2-3;ein4-4 triple mutant with genomic ETR1 (gETR1; •) results in rescue of the slow recovery phenotype. However, transformation with an ETR1 transgene mutated at Asp-659 (getr1-[D]; ▪) rescued the slow recovery phenotype poorly. The responses of wild type (dashed line) and the triple mutant (gray line) are shown for comparison. Ethylene was introduced at 1 h (↓) and removed 2 h later (↑).
DISCUSSION
In this study, we examined the process of Arabidopsis hypocotyl growth inhibition and recovery in response to ethylene treatment and removal, respectively. This kinetic analysis uncovered details of these processes that would have been unavailable through the use of long-term ethylene treatments and end-point growth analysis. Kinetic analysis of the ethylene response has found that growth inhibition occurs in two distinct phases. An initial rapid phase occurs shortly after the onset of ethylene treatment and is then supplanted by a more prolonged phase of stronger growth inhibition. None of the receptor null mutations used in this study measurably altered the kinetics of this biphasic ethylene response. Previous studies showed that the ethylene receptors have redundant function in the continuing, long-term presence of air or ethylene (Hua et al., 1995, 1998; Hua and Meyerowitz, 1998; Hall and Bleecker, 2003) but may also have unique functions or properties (Hall and Bleecker, 2003; Wang et al., 2003). Results from this study showed that receptors have redundant function during the initial responses to ethylene since no single, receptor null mutation altered responses to ethylene.
Differences in the ethylene growth response that occurred as a consequence of deficiencies in specific receptor isoforms were clearly exposed during the recovery phase after ethylene was purged from the treatment chamber. Here, there appears to be a transition where ETR1, ETR2, and EIN4 are more important for rapid recovery of growth than ERS1 or ERS2. The most prominent structural difference between these two groups of receptors is that ETR1, ETR2, and EIN4 each contain a receiver domain. While loss-of-function mutations in these three receptors all caused a delay in growth recovery, the largest delay was noted for etr1-7. This correlated with the observation that of these three isoforms, ETR1 mRNA accounted for approximately 85% of the transcript in etiolated seedlings in air and approximately 65% of the transcript after 2 h in ethylene, when ethylene withdrawal was initiated. The delay in growth recovery in some receptor-deficient lines does not appear to simply reflect diminished growth rates in air. For instance, the largest delay in recovery for single loss-of-function mutants was observed in the etr1-7 mutants, yet this mutation had no measurable effect on growth in air. Also, while plants with the etr1-6;etr2-3;ein4-4 triple loss-of-function mutation had slower growth in air and took longer to recover than any of the single mutants, the etr1-6;etr2-3;ein4-4 gETR-1 plants also had slow growth in air, yet had recovery comparable to etr2-3 or ein4-4 mutant plants.
The relatively rapid rate of recovery from ethylene treatment exhibited by etiolated seedlings must be reconciled with binding studies of the ethylene receptors indicating that the ETR family of receptors show very slow release kinetics for ethylene (half-life 10–12 h; Schaller and Bleecker, 1995; F.I. Rodriguez, unpublished data). Based on the negative regulator model for ethylene signaling, it is predicted that ethylene treatment should result in receptors almost exclusively in the ethylene-bound, inactive state. Recovery from growth inhibition should then involve an increase in receptors in the active signaling state. The similarities of the dose-response curve for the hypocotyl response and the dose-binding curve for ethylene binding to yeast-expressed ETR1 (Schaller and Bleecker, 1995) indicate that receptor output is directly proportional to receptor occupancy, leading to the prediction that recovery of pretreatment growth should occur when the majority of receptors have reverted to the activated unbound state. Unbound and activated receptors could result from dissociation of ethylene, resulting in a reversion to the activated state. Alternatively, once exogenous ethylene has been removed, synthesis of new receptors would likely be in the active state and thus contribute to suppression of the growth-inhibiting signaling pathway.
While both of these processes presumably contribute to a shift in the proportions of total receptors in the signaling and nonsignaling states, it should require a very rapid ethylene dissociation rate and/or an extremely rapid receptor turnover rate to significantly shift the equilibrium of total receptors from the bound inactive state to the unbound active state within 90 min of exogenous ethylene removal. However, existing evidence suggests that ethylene release occurs on a much longer time scale for the ETR family of receptors (Schaller and Bleecker, 1995). Given that the ethylene release would be a function of both ethylene dissociation from intact receptors and release from receptors that are being turned over, the bulk turnover rate for receptors also appears to be on the slower time scale for the slow-release ethylene binding components in plants (Sanders et al., 1991). Furthermore, the efficacy and longevity of 1-methylcyclopropene, a competitive inhibitor of ethylene binding (Hall et al., 2000) and response (Blankenship and Dole, 2003), indicates that ethylene receptor turnover is too slow to account in a simple model for the rapid recovery from ethylene-induced growth inhibition.
Positing a model in which receptors act cooperatively offers one way to reconcile the relatively slow changes in receptor occupancy with the more rapid changes in response output found in this study. Receptor-clustering models are currently being utilized to describe the behavior of the two-component bacterial chemoreceptors that are evolutionarily related to the ethylene receptors (Bray et al., 1998; Duke and Bray, 1999; Shimizu et al., 2003). According to these models, receptor dimers cluster to form higher-order complexes. Through direct interactions, the occupancy state of one dimer can act to shift the signaling states of surrounding receptor dimers within a cluster. The consequence of this arrangement is that subtle changes in receptor occupancy are amplified, leading to a large change in total receptor output. While there is currently no direct evidence for ethylene receptor clustering, recent evidence indicates that signal amplification may also be occurring at very low ethylene concentrations in the phase I growth inhibition response of etiolated seedlings (see companion paper; Binder et al. [2004; pp. 2921–2927]). A model that incorporates the idea of receptor clustering in the rapid recovery from ethylene-induced growth inhibition is provided in Figure 6. While signal amplification could also occur at some point in the signal transduction pathway downstream of receptors, the demonstration that His-kinase activity and the receiver domains of hybrid receptors are required for maximum recovery rates suggests that receptors play some direct role in amplification of signal during recovery.
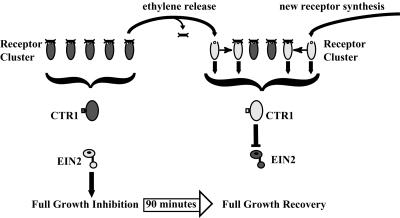
Figure 6.
Receptor-clustering model for recovery of growth after ethylene is removed. Based on the negative regulator model for ethylene signaling, ethylene treatment should result in receptors almost exclusively in the ethylene-bound, inactive state, leading to an inactive CTR1. This releases EIN2 from inhibition and leads to growth inhibition in etiolated seedlings. When ethylene is removed, recovery from growth inhibition should involve an increase in receptors in the active signaling state. Accumulation of active receptors can result directly from dissociation of ethylene from existing ethylene-bound receptors or synthesis of new unbound receptors. To account for the rapidity of growth recovery after ethylene withdrawal, a receptor interaction model is proposed in which unbound active receptors can alter the signaling status of neighboring ethylene-bound receptors as denoted by the arrows (→), resulting in an amplification of overall receptor signal output. Inactive proteins are dark gray, active proteins light gray, bound receptors shown by the presence of ethylene on the receptor, and unbound receptors shown by the absence of ethylene on the receptor.
The mechanisms by which His-kinase activity and phosphotransfer to the receiver domain might accelerate recovery are unknown. One possibility consistent with the proposed receptor cooperativity model is that transphosphorylation of receptors between receptor dimers in a cluster could favor the active (signaling) conformational state of the receptors. Imagine that receptors are normally in equilibrium between the active (unbound) and inactive (bound) conformational states with regard to downstream signaling. Unbound receptors could exist primarily in the active (signaling) state, while ethylene binding shifts the equilibrium to the inactive (nonsignaling) state. If receptor phosphorylation shifts the equilibrium of ethylene-bound receptors back to the active signaling state, the cooperative signaling proposed in the model would occur.
An alternative role for receiver domains in growth recovery could involve the rate at which newly synthesized receptors are incorporated into active signaling complexes. All five receptor isoforms form homodimers (Schaller et al., 1995; Hall et al., 1999; F.I. Rodriguez, unpublished data), consistent with data that two-component receptors generally function as dimers (Falke, 2002). Crystallographic evidence indicates that the receiver domain of ETR1 can dimerize in vitro (Müller-Dieckmann et al., 1999), providing a mechanism by which ETR1, ETR2, and EIN4 might dimerize faster than the receptors (ERS1 and ERS2) lacking this domain, thereby leading to more rapid formation of biologically active receptors. An interesting feature of receiver domains thought to be important in molecular interactions is the γ-loop, which is located next to the conserved Asp-659. The γ-loop of ETR1 has an atypical orientation compared to those in other structurally characterized receiver domains (Müller-Dieckmann et al., 1999), suggesting that it might have a function distinct from that shown for bacterial receptors. This loop could be important for molecular interactions involving receptor coupling in a cluster or synthesis of the final, biologically active receptor.
MATERIALS AND METHODS
l-α-(2-Ammino ethoxyvinyl)-Gly was kindly supplied by Dr. Tarlochan S. Dhadialla at Rohm Haas (Philadelphia). Mutants were in the Columbia background except for ers1-2 and ers2-3, which were in the Wassilewskija (WS) background.
Seedling Preparation
Arabidopsis seeds were surface sterilized by treatment with 70% alcohol for 30 s, placed on sterile filter paper to dry, and then placed on agar plates containing half-strength Murashige and Skoog basal salt mixture, pH 5.7 (Murashige and Skoog, 1962), 0.8% agar, and B5 vitamins consisting of inositol (100 mg mL−1), nicotinic acid (1 mg mL−1), pyridoxin HCl (1 mg mL−1), and thiamine HCl (10 mg mL−1) with no added sugar. In addition, 5 μm l-α-(2-ammino ethoxyvinyl)-Gly was added to inhibit endogenous ethylene production. Seeds were treated for 2 to 4 d at 4°C, light treated for 4 to 8 h under continuous fluorescent lights, then grown on vertically oriented plates in darkness at 22°C to be used for growth-rate measurements.
Growth-Rate Measurements in Hypocotyls
Seedlings were allowed to grow in darkness to a height of 3 to 4 mm (42–46 h) before the beginning of growth-rate measurements. The agar plates were fitted with a lid that allowed for continuous gas flow and placed vertically in a holder mounted on a micromanipulator. These manipulations were done in the dark or under dim, green light. Following 1 h of treatment with air to establish a basal growth rate, ethylene was introduced at a flow of 10 mL min−1. Overall gas flow was maintained at 100 mL min−1 throughout the experiment using Side-Trak mass flow meters and controller (Sierra Instruments, Bolsuen, The Netherlands). Gas chromatography using a Carboxen 1000, 45/60-mesh size column (Supelco, Bellefonte, PA) was used to measure the ethylene concentration in the effluent from the chamber. Under these conditions it took 4 min for the chamber to equilibrate to a steady-state concentration of 10.6 ± 0.6 μL L−1, and it took approximately 6 min for ethylene to dissipate to nondetectable levels after the ethylene flow was turned off.
Hypocotyl growth rates were measured in darkness using infrared radiation, an electronic camera, and custom software as previously described (Parks and Spalding, 1999; Folta and Spalding, 2001). Briefly, electronic images were captured every 5 min using a DEC-1000N CCD camera (Electrim, Princeton) equipped with a close-focus lens (K52-274; Edmund Scientific, Barrington, NJ) controlled by an IBM-compatible computer using the imaging software (version 1.24) supplied for Windows (Microsoft, Redmond, WA). Light for imaging produced by an infrared light-emitting diode was channeled through a fiber-optic cable to illuminate the seedlings from behind. Maximum zoom and magnification were used to provide an image resolution of 168 pixels per millimeter. The height in pixels of each seedling in each frame was analyzed using one of two methods. One method was to manually measure the height of each seedling in each frame using the public domain NIH Image program (version 1.62; developed at the United States National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image). Alternatively, seedling height was analyzed using custom software written in LabVIEW 5.0 (National Instruments, Austin, TX) and used in previous studies of blue light-inhibited growth (Folta and Spalding, 2001). Both methods yielded similar results. From these measurements we calculated growth rates for each 5-min increment using Microsoft Excel. All data presented in figures related to seedling growth represent the average of at least four seedlings total from a minimum of three separate experiments. Data were normalized to growth rate in air prior to treatment with ethylene.
Genomic DNA Purification
Genomic DNA was purified from 1 g of 5-d-old seedlings using the 10 columns of the DNeasy kit (Qiagen, Valencia, CA) and recombining all the samples. DNA concentration was determined by gel electrophoresis with ethidium bromide and confirmed by fluorometry using Hoechst reagent (Sigma-Aldrich, St. Louis). Size determination by gel electrophoresis indicated that the size of the genomic DNA was typically 15 to 10 kb.
RNA Extraction and cDNA Synthesis
Seedlings were grown in the absence or presence of ethylene under the conditions used for growth-rate measurements. To facilitate rapid harvest of seedlings, a piece of sterile, Whatman Number 2 filter paper (Clifton, NJ) was placed on the agar plate, and seeds were plated on top of this paper. At the end of air or ethylene treatment, seedlings were rapidly harvested by scraping the paper with a razor blade and placing the seedlings into liquid N2.
Total RNA was extracted from 50 to 100 mg of frozen Arabidopsis seedlings with the Qiagen RNeasy kit. The quantity of total RNA was determined by UV spectrometry. Subsequent denaturing PAGE analysis confirmed the concentration and provided a qualitative check of RNA integrity. A total of 1.0 μg of Arabidopsis RNA from seedlings was digested with 1 unit of μg−1 DNase (Invitrogen, Carlsbad, CA) and used to prepare cDNA by RT (Superscript II; Invitrogen) with 500 nm oligo(dT)18 + oligo(N)3 in a 20-μL reaction with a 50-min RT step. The cDNA samples from treated and untreated seedlings were diluted 5-fold to 100 μL final volume and split into three equal 33.3-μL aliquots. A genomic DNA 4-fold dilution series of 40 × 103, 10 × 103, and 2.5 × 103 molecules/μL was prepared, and 33.3 μL of each genomic DNA dilution was added to one of the cDNA aliquots.
Quantification of mRNA
For each receptor isoform, the gene-specific primers spanned the last intron and were designed to generate cDNA and genomic PCR products that differed in size between 12% and 16%. The sense primers were designed to include approximately 700 bp of the 3′end of the mRNA, which ensures that all the primers are measuring cDNA that are greater than 700 bp in length.
The cDNA and genomic DNA mixtures were amplified for 26 cycles using PCR (45 s at 94°C, 60 s at 55°C, 70 s at 72°C, and a final 7 min at 72°C for extension) with specific primers for each of the 5 ethylene receptors. The products were separated on a 1% agarose gel (100 V, 45 min) containing ethidium bromide. Products were visualized with UV illumination, photographed with a digital camera (Kodak DC120; Eastman-Kodak, Rochester, NY), and quantified with ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Analysis of the data was performed as described previously (Pfaffl et al., 1998).
Generation of Transgenic Lines
The genomic ETR1 was previously cloned into pBluescript II SK− (Wang et al., 2003). To generate the mutation of D659 to A (GAC to GCC), site-directed mutagenesis was performed on the cDNA of ETR1 in pBluescript II SK− using the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). The StyI-EcoRV fragment containing the mutation was removed from the cDNA to replace the corresponding region of the wild-type genomic ETR1 in pBluescript II SK−. The wild-type and mutant genomic ETR1 fragments were then removed from pBluescript II SK− and inserted into pPZP211. The mutations were confirmed by sequencing. The two constructs were transformed into Agrobacterium tumefaciens strain ABI and transferred into the triple null mutant etr1-6;etr2-3;ein4-4 using the floral dipping method. The transgenic plants were selected on agar plates containing 100 μg mL−1 gentamycin. Multiple transgenic lines were obtained for each construct. Two transgenic lines were selected etr1-6;etr2-3;ein4-4 gETR-1 and three for etr1-6 etr2-3;ein4-4 getr1-[D] based on their ability to rescue the slow growth phenotype as observed with end-point analysis.
Acknowledgments
We thank Matthew Touton for technical assistance.
This work was supported by the National Science Foundation (grant no. MCB–0131564 to A.B.B.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.050369.
References
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Binder BM, Mortimore LA, Stephanova AN, Ecker JR, Bleecker AB (2004) Short-term growth responses to ethylene in Arabidopsis seedlings are EIN3/EIL1 independent. Plant Physiol 136: 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241: 1086–1089 [DOI] [PubMed] [Google Scholar]
- Bray D, Levin MD, Morton-Firth CJ (1998) Receptor clustering as a cellular mechanism to control sensitivity. Nature 393: 85–88 [DOI] [PubMed] [Google Scholar]
- Blankenship SM, Dole JM (2003) 1-Methylcyclopropene: a review. Postharvest Biol Tec 28: 1–25 [Google Scholar]
- Burg SP (1973) Ethylene in plant growth. Proc Natl Acad Sci USA 70: 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM (1993) Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262: 539–543 [DOI] [PubMed] [Google Scholar]
- Chen QC, Bleecker AB (1995) Analysis of ethylene signal-transduction kinetics associated with seedling-growth response and chitinase induction in wild type and mutant Arabidopsis. Plant Physiol 108: 597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke TAJ, Bray D (1999) Heightened sensitivity of a lattice of membrane receptors. Proc Natl Acad Sci USA 96: 10104–10108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke JJ (2002) Cooperativity between bacterial chemotaxis receptors. Proc Natl Acad Sci USA 99: 6530–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Gamble RL, Coonfield ML, Schaller GE (1998) Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc Natl Acad Sci USA 95: 7825–7829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeschl JD, Kays SJ (1975) Concentration dependencies of some effects of ethylene on etiolated pea, peanut, bean and cotton seedlings. Plant Physiol 55: 670–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ecker JR (2004) The ethylene signaling pathway: new insights. Curr Opin Plant Biol 7: 40–49 [DOI] [PubMed] [Google Scholar]
- Guzman P, Ecker JR (1990) Exploting the triple response of Arabidopsis to identify ethylene-mediated mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Bleecker AB (2003) Analysis of combinatorial loss-of-function mutants in the Arabidopsis ethylene receptors reveals that the ers1;etr1 double mutant has severe developmental defects that are EIN2 dependent. Plant Cell 15: 2032–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Chen QG, Findell L, Schaller GE, Bleecker AB (1999) The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor. Plant Physiol 121: 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Findell JL, Schaller GE, Sisler EC, Bleecker AB (2000) Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiol 123: 1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM (1995) Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269: 1712–1714 [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM (1998) EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Li H, Hutchison CE, Laskey J, Kieber JJ (2003) Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J 33: 221–233 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Müller-Dieckmann N-J, Grantz AA, Kim S-H (1999) The structure of the signal receiver domain of the Arabidopsis thaliana ethylene receptor ETR1. Structure 7: 1547–1556 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Parks BM, Spalding EP (1999) Sequential and coordinated action of phytochromes A and B during Arabidopsis stem growth revealed by kinetic analysis. Proc Natl Acad Sci USA 96: 14142–14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M, Meyer HHD, Saurwein H (1998) Quantification of insulin-like growth factor-1 (IGF-1) mRNA: development and validation of an internally standardized competitive reverse transcription-polymerase chain reaction. Endocrinol Diabetes 106: 506–513 [DOI] [PubMed] [Google Scholar]
- Rauser WE, Horton RF (1975) Rapid effects of indoleacetic acid and ethylene on growth in intact pea roots. Plant Physiol 55: 443–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM (1998) ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA 95: 5812–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders IO, Harpham NVJ, Raskin I, Smith AR, Hall MA (1991) Ethylene binding in wild type and mutant Arabidopsis thaliana (L.) Heynh. Ann Bot (Lond) 68: 97–103 [Google Scholar]
- Schaller GE, Bleecker AB (1995) Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270: 1809–1811 [DOI] [PubMed] [Google Scholar]
- Schaller GE, Ladd A, Lanahan MB, Spanbauer JM, Bleecker AB (1995) The ethylene response mediator ETR1 from Arabidopsis forms disulfide-linked dimer. J Biol Chem 270: 12526–12530 [DOI] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Sommerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97: 11655–11660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu TS, Aksenov SV, Bray D (2003) A spatially extended stochastic model of the bacterial chemotaxis signalling pathway. J Mol Biol 329: 291–309 [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zhong G, Burns JK (2003) Profiling ethylene-regulated gene expression in Arabidopsis thaliana by microarray analysis. Plant Mol Biol 53: 117–131 [DOI] [PubMed] [Google Scholar]
- Wang W, Hall AE, O'Malley R, Bleecker AB (2003) Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc Natl Acad Sci USA 100: 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner HL, Leopold AC (1971) Timing of growth regulation responses in peas. Biochem Biophys Res Commun 44: 989–994 [DOI] [PubMed] [Google Scholar]