Abstract
Kinetic studies indicate there are two phases to growth inhibition by ethylene for the hypocotyls of etiolated Arabidopsis seedlings. Phase I is transient, while phase II results in sustained growth inhibition. The EIN2 membrane protein is required for both the first and second phases of growth inhibition by ethylene, while the transcription factors EIN3 and EIL1 are required for the second phase but not the first phase. The first phase lasts no more than 2 h. It is less sensitive to the ethylene response inhibitor 1-methylcyclopropene and more sensitive to ethylene than the second phase. The first phase shows adaptation at low concentrations of ethylene (≤0.01 μL L−1) with a relative refractory period of 5 h after ethylene is added. A modified signal transduction model is proposed that accounts for the two phases of growth inhibition.
Among the myriad processes in plants that are influenced by the plant hormone ethylene, much attention has been focused on the inhibitory affects of ethylene on the growth of etiolated seedlings. This sensitive and easily quantified bioassay has been used to characterize the relationship between ethylene dose and physiological response, providing information of rate-limiting steps in the signal transduction pathway (Goeschl and Kays, 1975; Chen and Bleecker, 1995). Kinetic studies with pea (Pisum sativum) seedlings indicate that the growth response shows a lag of less than 10 min and that seedlings return to pretreatment growth within 20 min of ethylene withdrawal (Warner and Leopold, 1971; Burg, 1973; Goeschl and Kays, 1975; Rauser and Horton, 1975).
This behavior of the growth response must be accommodated by the emerging model for ethylene signal transduction based on mutational analysis (Guo and Ecker, 2004). In this model, the five ethylene receptors in complex with a kinase, CTR1, negatively regulate response pathways in the absence of ethylene. Ethylene binding inhibits the receptor/CTR1 complex leading to ethylene responses (Hua and Meyerowitz, 1998). There is genetic evidence that ethylene responses require the presence of the Nramp-related protein EIN2 (Alonso et al., 1999). Ethylene responses require the activities of at least two transcription factors, EIN3 and the EIN3-like protein EIL1 that appear to act downstream of EIN2 (Chao et al., 1997). EIN3/EIL1 in turn activate other transcription factors (Solano et al., 1998), suggesting that at least two rounds of transcriptional activation are required for responses to ethylene. The involvement of this transcriptional cascade in the seedling growth response must be reconciled with reports that growth inhibition in etiolated pea seedlings shows a lag of only 10 min or less following treatment (Warner and Leopold, 1971; Burg, 1973; Goeschl and Kays, 1975; Rauser and Horton, 1975).
In this study, we used kinetic analysis of growth to further examine details of growth inhibition by ethylene in wild-type seedlings and seedlings with mutations for one or more genes in the ethylene signal transduction pathway. We tested whether rapid growth inhibition was altered in mutant seedlings lacking the two transcription factors EIN3 and EIL1 or the Nramp-related protein EIN2.
RESULTS
First-Phase Response Is Independent of EIN3 and EIL1
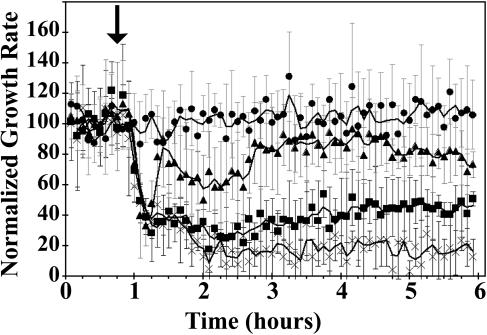
We confirmed our previous observation (see companion paper; Binder et al. [2004; pp. 2913–2920]) that the growth rate of etiolated Arabidopsis seedlings is rapidly inhibited by ethylene and appears to have two phases (Fig. 1). The first, rapid phase has a lag of approximately 15 min after ethylene is applied, followed by a deceleration in growth lasting an additional 15 min. After a brief plateau in growth rate, there is a second, slower phase in growth inhibition in which growth decelerates for 30 min until the growth rate reaches a new, low steady-state rate.
Figure 1.
Rapid kinetic analysis of growth in etiolated Arabidopsis seedlings. Measurements were made in air for 1 h prior to introducing 10 μL L−1 ethylene (↓). Seedling growth was measured for another 5 h. The rate of growth was determined throughout. Columbia (wild-type) seedlings (▴) were compared with ein3-1;eil1-1 (♦) and ein2-1 (○) seedlings.
To determine the role of transcription in ethylene signal transduction, we compared the response kinetics of hypocotyls of wild-type seedlings to a seed line carrying loss-of-function mutations in the EIN3 and EIL1 transcription factors (Fig. 1). The ein3-1;eil1-1 double mutant seedlings showed ethylene insensitivity to prolonged treatment with ethylene (data not shown) as reported previously (Alonso et al., 2003). However, we obtained surprising results when we examined the short-term growth responses to ethylene of the ein3-1;eil1-1 double mutant (Fig. 1). During the first hour of ethylene treatment, the ein3-1;eil1-1 mutants were indistinguishable from wild-type seedlings. The mutant seedling hypocotyls showed the same lag of approximately 15 min followed by rapid growth inhibition for 15 min and a plateau. After this first phase, mutant seedling hypocotyls behaved very differently from wild type and began to show an accelerated growth rate after 1 h in the continuing presence of saturating amounts of ethylene (Fig. 1). No such reversal in growth rate was seen in wild-type hypocotyls at this concentration of ethylene (Fig. 1). The mutant ein2-1 seedlings showed no transient responses to ethylene (Fig. 1). The growth rates in air prior to ethylene treatment were similar for wild-type (0.29 ± 0.08 mm h−1), ein2-1 (0.24 ± 0.11 mm h−1), and ein3-1;eil1-1 (0.31 ± 0.11 mm h−1) seedlings.
First-Phase Growth Inhibition Is Less Sensitive to 1-Methylcyclopropene and More Sensitive to Ethylene
To determine whether these two phases could be pharmacologically distinguished, Columbia wild-type seedlings were pretreated with various concentrations of 1-methylcyclopropene (1-MCP), a competitive inhibitor of ethylene (Sisler et al., 1996a, 1996b; Sisler and Serek, 1999) that is an effective antagonist of ethylene responses in etiolated Arabidopsis seedlings (Hall et al., 2000). The second phase of growth inhibition by ethylene showed higher sensitivity to 1-MCP than the phase I response (Fig. 2). Even the lowest concentration of 1-MCP used (10 nL L−1) reduced the amplitude of the second-phase inhibition by ethylene. No measurable effect on the amplitude of the first-phase response was seen until 100 nL L−1 1-MCP or more was used. At 1,000 nL L−1, all growth inhibition responses to ethylene were blocked.
Figure 2.
1-MCP prevents both phases of inhibition by ethylene. Seedlings were pretreated with 1-MCP for 17 h prior to growth measurements. At 45 min (↓), 10 μL L−1 ethylene was introduced. Concentrations of 1-MCP used were 0 (×), 10 (▪), 50 (▴), and 1,000 (•) nL L−1.
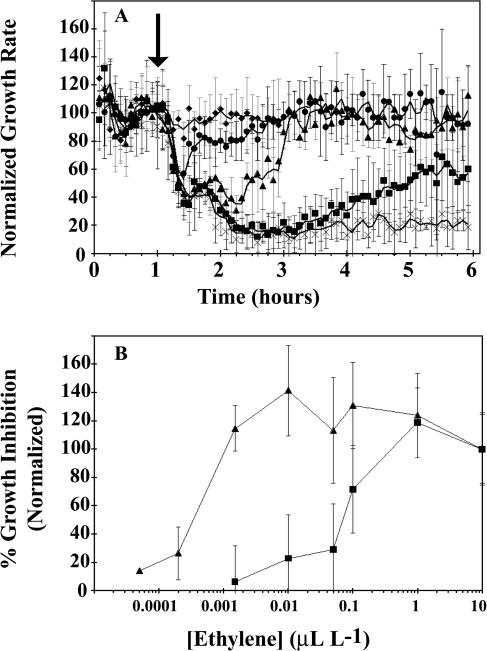
Since 1-MCP blocks ethylene binding to receptors, these results suggested that the first phase of growth inhibition was initiated at a lower concentration of ethylene than the second-phase response. To test this, we treated Columbia wild-type seedlings with ethylene concentrations ranging from 0.05 nL L−1 to 10 μL L−1 (Fig. 3). Figure 3A shows growth responses to representative ethylene concentrations. At high concentrations of ethylene (≥1 μL L−1), both phases of the ethylene response were observed and no reversal in growth rate was seen up to 7 h after ethylene treatment started (Figs. 1 and 3A). However, at lower concentrations of ethylene that still gave long-term growth inhibition (Fig. 3A), the hypocotyls initially showed maximal growth inhibition followed by a slow reversal in growth-rate inhibition to an intermediate growth rate. This intermediate growth rate was dependent upon the concentration of ethylene being used. Below 0.05 μL L−1 ethylene, no long-term growth inhibition was observed. However, the rapid, initial growth inhibition was still observed down to an ethylene concentration of 0.2 nL L−1. No response to ethylene was detected below 0.2 nL L−1. Similar results at these low ethylene concentrations were obtained with the ein3-1;eil1-1 mutants (data not shown).
Figure 3.
Growth inhibition by various concentrations of ethylene. A, Growth response kinetics at various concentrations of ethylene. Ethylene was introduced 1 h after measurements were initiated (↓). Concentrations used were: 1 μL L−1 (×), 100 nL L−1 (▪), 10 nL L−1 (▴), 1.5 nL L−1 (•), and 0.2 nL L−1 (♦). B, Dose-response relationship for phase I (▴) and phase II (▪) growth inhibition responses. Growth inhibition was normalized to inhibition obtained at 10 μL L−1 ethylene. For most doses of ethylene, the amount of growth inhibition was averaged from 20 to 35 min after ethylene was added to determine the percent inhibition for phase I. The exceptions to this were the two lowest doses of ethylene, which started to recover 30 min after ethylene was added. For these data points, the amount of inhibition between 20 and 25 min was averaged. For phase II responses, the amount of growth inhibition between 5 and 6 h was averaged and used to determine the percent inhibition.
Figure 3B shows the ethylene dose-response relationship for hypocotyls of wild-type seedling growth rates. The second phase of growth inhibition had a dose-response curve very similar to that reported for the long-term effects of ethylene upon hypocotyl length (Chen and Bleecker, 1995; Hall et al., 1999), while the first-phase growth inhibition continued to be observed at approximately 500-fold less ethylene. Interestingly, the amplitude of the first-phase response had no measurable change between 1 nL L−1 and 10 μL L−1 ethylene.
The First-Phase Response Shows Adaptation at Low Ethylene Concentrations
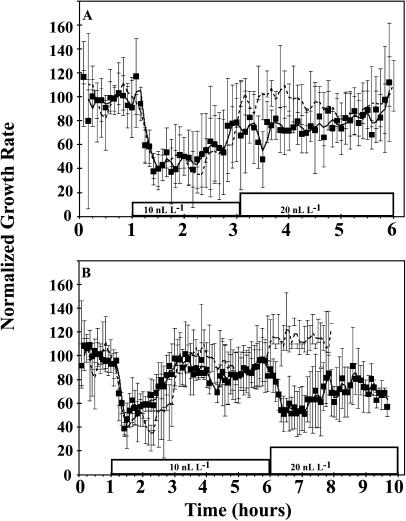
At ethylene concentrations ≤10 nL L−1, hypocotyl growth recovered to pretreatment rates in 2 h or less in the continuing presence of ethylene. The length of time for this recovery was dependent upon the concentration of ethylene present (Fig. 3A). This adaptation of the ethylene response has not been reported previously. Even after the growth rate recovered, the seedlings remained desensitized to ethylene. As shown in Figure 4, seedlings treated with 10 nL L−1 recovered normal growth within 2 h after ethylene was added. However, if the ethylene dose was increased to 20 nL L−1 immediately after the growth rate adapted to the first dose, the seedlings showed a diminished growth inhibition response. By contrast, if the increase in ethylene dose was given 5 h after the first dose was initiated, the seedlings showed a response similar to that seen for the initial 10 nL L−1 treatment in air (Fig. 4). Neither the length of the first phase response nor the resensitization were altered in ein3-1;eil1-1 mutant seedlings (data not shown).
Figure 4.
Etiolated seedlings adapt to low concentrations of ethylene. Seedlings were treated with 10 nL L−1 ethylene at 1 h. Growth rate returned to pretreatment rates approximately 2 h after ethylene was introduced. A second, equal dose of ethylene was introduced 2 h 10 min (A) or 5 h (B) after the first dose was initiated. The response profile of seedlings only given the initial dose of ethylene (dashed line) is shown in each section for comparison.
DISCUSSION
Using short-term growth analysis, we have examined kinetic details of ethylene signal transduction in etiolated Arabidopsis seedlings. This kinetic analysis has revealed that the seedling growth response to ethylene can be separated into two phases that display different characteristics. The first phase of growth inhibition (phase I) had a high sensitivity to ethylene, with a detectable response at 0.2 nL/L−1 ethylene and saturation at 1 nL L−1. The threshold ethylene concentration for this response is 5-fold lower than that for ethylene's effects on germination in manketti (Ricinodendron rautaneii) seeds (Keegan et al., 1989), cited as the most sensitive response to ethylene in the literature (Abeles et al., 1992). An unusual characteristic of the first phase was that the magnitude and time course of growth rate decrease was unaffected by ethylene dose, while the subsequent recovery to pretreatment growth rate showed ethylene dose dependence.
The second phase of growth-rate inhibition (phase II) was delayed in onset but sustained for the duration of the ethylene treatment time. This phase appeared to start at approximately 1 h after ethylene was added and was much less sensitive to ethylene. At high concentrations of ethylene (≥1 μL L−1), both phases of the ethylene response were observed, and no reversal in growth rate was seen up to 7 h after ethylene treatment started. However, at intermediate concentrations of ethylene that still gave long-term growth inhibition, the seedlings initially showed maximal growth inhibition followed by a slow reversal in growth inhibition rate to an intermediate growth rate. A similar reversal in long-term growth inhibition has been observed at lower ethylene concentrations in etiolated pea epicotyls (Goeschl and Kays, 1975). The dose-response characteristics of this second-phase response are very similar to the dose dependence of longer term inhibition of hypocotyl growth determined by end-point analysis after 3 to 4 d of continuous ethylene treatment (Chen and Bleecker, 1995; Hall et al., 1999).
It remains unresolved whether the phase I growth response involves control at the level of gene expression, given that the EIN3/EIL1 transcription factors are not required for this response. The 15-min lag between ethylene application and the initiation of the growth response may be sufficient for a gene induction mechanism, given that altered gene expression in response to ethylene within this time frame has been reported (Zegzouti et al., 1999). Additional members of the EIN3/EIL family of transcription factors are candidates, although no ethylene-related phenotypes are reported for knockouts in these genes (Guo and Ecker, 2004). Alternatively, other cellular processes such as changes in ion channels or enzymatic processes might lead to the initial, rapid growth inhibition. In this regard, the requirement for EIN2 activity for the phase I response is intriguing in that EIN2 contains a hydrophobic domain that is related to the Nramp family of ion transporters (Alonso et al., 1999), although no ion transport activity has been reported for EIN2 to date.
The ability of receptors to respond to very subtle changes in ethylene at concentrations well below the estimated Kd for ethylene binding indicates some form of signal amplification is occurring at these low ethylene concentrations for the phase I growth response. The threshold ethylene concentration for the phase I response is calculated to be 300-fold below the calculated Kd for the yeast-expressed ETR1 protein (Schaller and Bleecker, 1995). Based on the negative regulator model for ethylene binding, an equilibrium binding calculation using the reported Kd of 2.4 nm (Schaller and Bleecker, 1995) predicts that the receptor system can respond when only about 1 out of every 1,000 receptor molecules is turned off by ethylene binding. This ability to respond to subtle changes in signal concentration is reminiscent of behavior associated with the evolutionarily related two-component chemoreceptors from bacteria (Thomason et al., 2002). Current models for bacterial chemotaxis systems posit that amplification results from receptors forming higher-order clusters composed of receptor dimer subunits. Through direct contact, receptor dimers can influence the signaling states of neighboring dimers so that transmitters from many receptors may be altered by a single ligand-binding event. While there is no direct evidence for such a cooperative receptor-clustering mechanism associated with the ethylene receptor system, clustering models have been suggested as possible explanations for other features of ethylene signaling, such as the mechanisms for ethylene insensitivity of dominant mutant forms of the receptors (Gamble et al., 2002) and the ability of the system to sense small changes in receptor occupancy during recovery from the seedling growth response (see companion paper; Binder et al. [2004]). Recent biochemical evidence also indicates that receptors may form complex, nonstoichiometric interactions with the RAF-like CTR1 kinase at the endoplasmic reticulum membrane (Gao et al., 2003). Alternatively, evidence that CTR1 is an initiator MAPKKK for a MAP kinase cascade (Ouaked et al., 2003) provides an additional mechanism for signal amplification in the ethylene signal transduction pathway.
An additional property that the phase I growth response shares with bacterial chemotaxis is an adaptation phenomenon. The recovery to pretreatment rates of growth of seedlings at low ethylene concentrations can be considered a form of adaptation. The full phase I response can be reelicited by an additional incremental increase in ethylene concentration. The mechanisms for resetting ethylene sensitivity in this system appear to be complex. Even after seedlings have returned to pretreatment growth rate, an additional refractory period is needed before the system can respond to an additional incremental increase in ethylene dose. For bacterial chemotaxis, adaptation of this sort is mediated in part by a negative feedback loop involving transient methylation of a component of the receptor complex that modulates the signal output from a given dose of signal (Falke et al., 1997). However, despite the evolutionary relationship between the bacterial chemotaxis receptors and the ethylene receptors, no counterpart to the methyltransfer feedback system from bacteria has been identified as an ethylene signaling component.
The long-term growth inhibition by ethylene was eliminated or severely reduced in ein3-1;eil1-1 double mutant seedlings, indicating that phase II requires the presence of the EIN3/EIL1 transcription factors. Growth rates in the double mutant returned to pretreatment levels, indicating that the first-phase response lasts no more than approximately 2 h. Recent reports indicate that ethylene works by stabilizing EIN3 and EIL1 protein levels (Guo and Ecker, 2003; Potuschak et al., 2003; Yanagisawa et al., 2003; Gagne et al., 2004). One explanation for the delay in onset of the phase II response might have to do with the timing of accumulation of EIN3 and EIL protein. In addition, a downstream target of EIN3/EIL1, ERF1, is itself a transcription factor that mediates the seedling growth response (Solano et al., 1998), indicating that at least two rounds of transcriptional activation (ERF and its targets) must occur before proteins that mediate the phase II growth response can be produced.
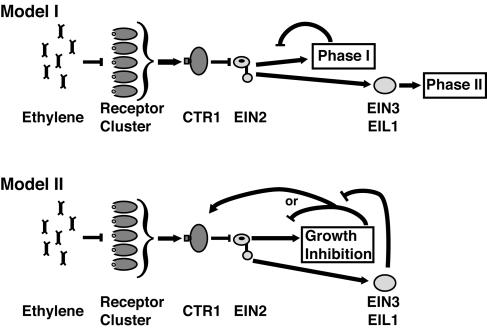
Loss-of-function mutations in EIN2 eliminated both phases of growth inhibition. We have also observed that the etr1-1 mutation eliminates phase I as well as phase II (data not shown), suggesting that the currently identified components in the ethylene signal transduction pathway are involved in the phase I as well as the phase II responses. Two models for EIN2 function can be considered in this case. One possibility is that EIN2 mediates the two phases of the seedling growth response independently: an EIN3/EIL1-independent first-phase response and an EIN3/EIL1-dependent second phase, each mediated by a different mechanism (Fig. 5, model I). Alternatively, both the first and second phases of growth may operate by a single EIN2-dependent mechanism, with EIN3/EIL1 operating in a feedback circuit on the growth response (Fig. 5, model II). In either case, the transient nature of the first-phase response implies a negative feedback loop that acts to reverse the growth inhibition mediated by EIN2. This feedback loop would act to negatively regulate the growth-specific pathway downstream of EIN2 in model I. For model II, the primary feedback loop could act directly to negatively regulate EIN2 or positively regulate the receptor/CTR complex. A candidate for the latter is the EER1 gene, a type-2 phosphatase that may regulate CTR1 activity (Larsen and Cancel, 2003). Model II requires a secondary feedback loop mediated by EIN3/EIL1 that negatively regulates the primary feedback loop, providing for the sustained growth response. The two models are not mutually exclusive; both mechanisms could contribute to the sustained growth response, with the EIN3/EIL1-independent pathway maintaining the physiological state of the cells and the EIN3/EIL1-dependent pathway adjusting the commensurate delivery of wall components via regulation of appropriate genes.
Figure 5.
Alternative models of ethylene signal transduction for the two phases of seedling growth inhibition. Feedback is invoked in both models to explain the observation that phase I shows adaptation. In model I, EIN2 mediates the two phases of seedling growth response through two independent mechanisms. Phase I is EIN3/EIL1 independent and subject to feedback inhibition, while phase II is EIN3/EIL1 dependent and not subject to feedback inhibition. In model II, both phases of growth response occur via a single EIN2-dependent mechanism. At very low ethylene concentrations, a feedback loop reverses the growth inhibition by either stimulating components upstream of EIN2 or inhibiting components at or downstream of EIN2, resulting in a transient growth inhibition. At higher ethylene concentrations, a secondary feedback loop, mediated through EIN3/EIL1, inhibits the primary feedback loop, resulting in sustained growth inhibition.
Previous studies with EIN2 may favor model II. EIN2 is composed of a membrane-associated domain and a cytoplasmic C-terminal domain (Alonso et al., 1999). Overexpression of the cytoplasmic domain CEND was sufficient to activate some ethylene-associated responses in an EIN3-dependent manner. However, CEND did not activate the etiolated hypocotyl growth response, implying that the membrane-associated portion of EIN2 may be essential for growth responses in the etiolated hypocotyl and presumably the primary root, given no constitutive root response was reported in CEND1 seedlings (Alonso et al., 1999). The prediction from model I would be that constitutive activation of EIN3/EIL1 by CEND would induce the sustained phase II growth inhibition in etiolated seedlings, while model II correctly predicts that activation of EIN3/EIL1 would have no consequence on seedling growth because it acts only in a secondary feedback loop in the system. It should be noted that CEND did inhibit growth in the adult plant in this previous study. However, this could be by a different mechanism than the growth response in etiolated hypocotyls and roots. For example, EIN3-dependent constitutive activation of defense genes could inhibit adult plant growth (Peterson et al., 2000).
MATERIALS AND METHODS
l-α-(2-Ammino ethoxyvinyl)-Gly and 1-MCP in the form of EthylBloc were kindly supplied by Rohm Haas (Philadelphia).
Seedling Growth-Rate Measurements
Arabidopsis seeds were surface sterilized and grown as previously described (see companion paper; Binder et al. [2004]). Growth-rate measurements were made on hypocotyls using images taken by a computer-driven digital camera as described previously (Parks et al., 1998; Parks and Spalding, 1999). After 1 h of air treatment, ethylene was introduced at a flow rate between 1 and 10 mL min−1. By using different concentrations of ethylene in the source cylinders, we could treat seedlings with ethylene concentrations between 0.05 nL L−1 and 10 μL L−1. Gas flow was regulated with either Side-Trak mass flow meters and controller (Sierra Instruments, Bolsuen, The Netherlands) or Hastings Instrument mass flow meters and controller (Teledyne Hastings Instruments, Hampton, VA). Overall gas flow during the experiments was maintained at 100 mL min−1. The concentration of ethylene in the chamber was confirmed by gas chromatography using a Carboxen 1000, 45/60-mesh size column (Supelco, Bellefonte, PA). For concentrations of ethylene 0.1 μL L−1 or above, the ethylene concentration was directly measured by taking a sample from the chamber. For experiments involving ethylene concentrations below 0.1 μL L−1, calibrations were made using an ethylene source cylinder 100- to 1,000-fold higher than that used during seedling treatments. The concentration of ethylene was then extrapolated from these measurements. Under these conditions, it took 4 min or less for the chamber to equilibrate to a steady-state ethylene concentration. All data represent the average of at least four seedlings total from a minimum of three separate experiments. Data were normalized to growth rate in air prior to treatment with ethylene.
Ethylene Response Inhibitor 1-MCP
In some experiments, seedlings were pretreated with the ethylene response inhibitor 1-MCP 17 h prior to growth-rate measurements. Agar plates with etiolated seedlings were placed in a sealed chamber containing Ethylbloc. 1-MCP was released by the addition of hot water through a septum in the sealed chamber. After 17 h of treatment, growth-rate analysis was carried out as described above, except seedlings were treated with air for 45 min prior to treatment with 10 μL L−1 ethylene.
Seedling Growth Measurements
For experiments examining the long-term effects of ethylene, the agar plates with seeds were wrapped in aluminum foil and placed in chambers with continuous air flow and grown for 3 d in the presence or absence of 40 μL L−1 ethylene. At the end of this time, seedlings were digitally scanned and analyzed using the public domain NIH Image program (developed at the United States National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image).
Acknowledgments
We thank Nicholas Dahl for technical assistance.
This work was supported by grants from the U.S. Department of Agriculture-HATCH (grant no. WIS04531 to A.B.B.), the American Floral Endowment, and the National Science Foundation (grant no. MCB–0131564).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.050393.
References
- Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in Plant Biology, Ed 2. Academic Press, San Diego
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR (2003) Five components of the ethylene-response pathway identified in a screen of weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA 100: 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, O'Malley RC, Wang W, Moore JM, Parks BM, Spalding EP, Bleecker AB (2004) Arabidopsis seedling growth response and recovery to ethylene: a kinetic analysis. Plant Physiol 136: 2913–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg SP (1973) Ethylene in plant growth. Proc Natl Acad Sci USA 70: 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chen QC, Bleecker AB (1995) Analysis of ethylene signal-transduction kinetics associated with seedling-growth response and chitinase induction in wild-type and mutant Arabidopsis. Plant Physiol 108: 597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke JJ, Bass RB, Butler SL, Chervitz SA, Danielson MA (1997) The two-component signaling pathway of bacterial chemotaxis: a molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu Rev Cell Dev Biol 13: 457–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo S-D, Yanagisawa S, Vierstra RD (2004) Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc Natl Acad Sci USA 101: 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Qu X, Schaller GE (2002) Mutational analysis of the ethylene receptor ETR1: role of the histidine kinase domain in dominant ethylene insensitivity. Plant Physiol 128: 1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Chen Y-F, Randlett MD, Zhao X-C, Findell JL, Kieber JJ, Schaller GE (2003) Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 278: 34725–34732 [DOI] [PubMed] [Google Scholar]
- Goeschl JD, Kays SJ (1975) Concentration dependencies of some effects of ethylene on etiolated pea, peanut, bean and cotton seedlings. Plant Physiol 55: 670–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ecker JR (2003) Plant responses to ethylene gas are mediated by SCFEBF1/EFB2-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- Guo H, Ecker JR (2004) The ethylene signaling pathway: new insights. Curr Opin Plant Biol 7: 40–49 [DOI] [PubMed] [Google Scholar]
- Hall AE, Chen QG, Findell L, Schaller GE, Bleecker AB (1999) The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor. Plant Physiol 121: 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Findell JL, Schaller GE, Sisler EC, Bleecker AB (2000) Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiol 123: 1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Keegan AB, Kelly KM, Van Staden J (1989) Ethylene involvement in dormancy release of Ricinodendron rautaneinii seeds. Ann Bot (Lond) 63: 229–234 [Google Scholar]
- Larsen PB, Cancel JD (2003) Enhanced ethylene responsiveness in the Arabidopsis eer1 mutant results from a loss-of-function mutation in the protein phosphatase 2A A regulatory subunit, RCN1. Plant J 34: 709–718 [DOI] [PubMed] [Google Scholar]
- Ouaked F, Rozhon W, Lecourieux D, Hirt H (2003) A MAPK pathway mediates ethylene signaling in plants. EMBO J 22: 1282–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Cho MH, Spalding EP (1998) Two genetically separable phases of growth inhibition induced by blue light in Arabidopsis seedlings. Plant Physiol 118: 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Spalding EP (1999) Sequential and coordinated action of phytochromes A and B during Arabidopsis stem growth revealed by kinetic analysis. Proc Natl Acad Sci USA 96: 14142–14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M, Brodersen P, Naested H, Adreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin JJ, Parker JE, et al (2000) Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 103: 1111–1120 [DOI] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P (2003) EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F Box proteins: EBF1 and EBF2. Cell 115: 679–689 [DOI] [PubMed] [Google Scholar]
- Rauser WE, Horton RF (1975) Rapid effects of indoleacetic acid and ethylene on growth in intact pea roots. Plant Physiol 55: 443–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB (1995) Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270: 1809–1811 [DOI] [PubMed] [Google Scholar]
- Sisler EC, Dupille E, Serek M (1996. a) Effect of 1-methycyclopropene and methylenecyclopropane on ethylene binding and ethylene action on cut carnations. Plant Growth Regul 18: 79–86 [Google Scholar]
- Sisler EC, Serek M (1999) Compounds controlling the ethylene receptor. Bot Bull Acad Sin 40: 1–7 [Google Scholar]
- Sisler EC, Serek M, Dupille E (1996. b) Comparison of cyclopropene, 1-methylcyclopropene, and 3,3-dimethylcyclopropene as ethylene antagonists in plants. Plant Growth Regul 18: 169–174 [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason PA, Wolanin PM, Stock JB (2002) Signal transduction: receptor clusters as information processing arrays. Curr Biol 12: 339–401 [DOI] [PubMed] [Google Scholar]
- Warner HL, Leopold AC (1971) Timing of growth regulation responses in peas. Biochem Biophys Res Commun 44: 989–994 [DOI] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo S-D, Sheen J (2003) Differential regulation of EIN3 stability by glucose and ethylene signaling in plants. Nature 425: 521–525 [DOI] [PubMed] [Google Scholar]
- Zegzouti H, Jones B, Frasse P, Marty C, Maitre B, Latche A, Pech J-C, Bouzayen M (1999) Ethylene-regulated gene expression in tomato fruit: characterization of novel ethylene-responsive and ripening-related genes isolated by differential display. Plant J 18: 589–600 [DOI] [PubMed] [Google Scholar]