Abstract
Plants respond to proximate neighbors with a suite of responses that comprise the shade avoidance syndrome. These phytochrome-mediated responses include hyponasty (i.e. a more vertical orientation of leaves) and enhanced stem and petiole elongation. We showed recently that ethylene-insensitive tobacco (Nicotiana tabacum) plants (Tetr) have reduced responses to neighbors, showing an important role for this gaseous plant hormone in shade avoidance. Here, we investigate interactions between phytochrome signaling and ethylene action in shade avoidance responses. Furthermore, we investigate if ethylene acts in these responses through an interaction with the GA class of hormones. Low red to far-red light ratios (R:FR) enhanced ethylene production in wild-type tobacco, resulting in shade avoidance responses, whereas ethylene-insensitive plants showed reduced shade avoidance responses. Plants with inhibited GA production showed hardly any shade avoidance responses at all to either a low R:FR or increased ethylene concentrations. Furthermore, low R:FR enhanced the responsiveness of hyponasty and stem elongation in both wild-type and Tetr plants to applied GA3, with the stem elongation process being more responsive to GA3 in the wild type than in Tetr. We conclude that phytochrome-mediated shade avoidance responses involve ethylene action, at least partly by modulating GA action.
Plants exhibit increased shoot elongation rates as well as a number of other responses, including hyponasty (i.e. more upwardly orientated leaves) and early flowering, to avoid shading by neighbors (Ballaré, 1999; Smith, 2000; Pierik et al., 2003). The major light signal triggering these shade avoidance responses is the ratio of red (R) to far-red (FR) light, which is lowered by selective absorption of red light by chlorophyll (Ballaré, 1999; Aphalo et al., 1999). This R:FR is perceived by the phytochrome family of photoreceptors, which consists of five members (phyA–phyE) in Arabidopsis (Whitelam and Devlin, 1997; Smith, 2000). Of these, phytochromes B, D, and E perceive and transduce the reduced R:FR leading to shade avoidance responses in light-grown plants (Franklin et al., 2003).
Although the phytochrome system is very well described, surprisingly little is known about hormonal components involved in regulating the actual growth responses. Not only gibberellins are known to regulate phytochrome-mediated shoot elongation (Chory and Li, 1997), but also auxins (Morelli and Ruberti, 2000) are involved in shade avoidance responses. Several lines of evidence suggest a role for ethylene as well. Monochromatous red light inhibits ethylene production, whereas FR light can reverse this effect (Imaseki et al., 1971; Samimy, 1978; Vangronsveld et al., 1988). Ethylene production can also be increased by adding FR light to a white-light background in light-grown Sorghum bicolor (Finlayson et al., 1998, 1999). Furthermore, the Sorghum mutant null for PHYB (phyB-1) combines a constitutive shade avoidance phenotype with constitutive exaggerated ethylene production (Finlayson et al., 1998). More recent evidence shows that transgenic ethylene-insensitive tobacco (Nicotiana tabacum) plants have reduced shade avoidance responses to neighbors (Pierik et al., 2003). This is caused by the involvement of ethylene in shade avoidance responses to reduced blue light photon fluence rates and reduced R:FR (Pierik et al., 2004). In this study, we aim to identify the mechanism through which ethylene may modulate phytochrome-mediated shade avoidance responses. This includes an analysis of the effects of R:FR on ethylene production and responsiveness, an investigation of the responsiveness of ethylene-insensitive plants to low R:FR, and, finally, an analysis of the interactions between ethylene and GA.
The plant hormone family of GA has been subject of many studies that attempt to identify components in the regulation of growth responses to reduced R:FR. Elongation responses to low R:FR are mediated by increased endogenous levels of active GA (Beall et al., 1996; García-Martínez et al., 1987) or increased sensitivity to endogenous GA (Weller et al., 1994; López-Juez et al., 1995) in low R:FR. The resulting increased GA action then loosens the cell walls, thereby facilitating enhanced cell elongation (Weller et al., 1994; López-Juez et al., 1995). It is well established that ethylene can interact with GA to modify elongation growth (Rijnders et al., 1997; Kende et al., 1998; Achard et al., 2003), and we, therefore, hypothesized that ethylene stimulates shade avoidance responses to low R:FR by enhancing GA action.
We show here for tobacco that low R:FR stimulates ethylene production and ethylene-induced shade avoidance responses and that transgenic plants that cannot sense ethylene (Tetr) have reduced shade avoidance responses to low R:FR. Our data suggest that ethylene positively modulates phytochrome-mediated elongation responses, but not hyponastic bending of the leaves, at least partly by enhancing GA action.
RESULTS
Ethylene-Induced Growth Responses and Ethylene Production Are Stimulated by Low R:FR
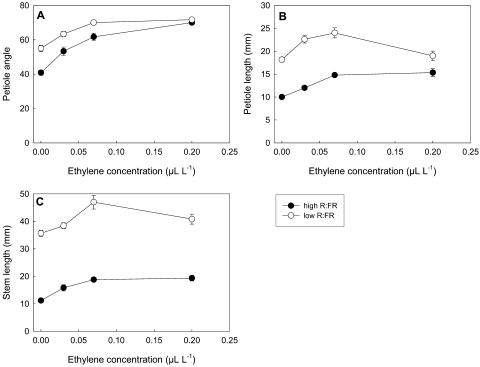
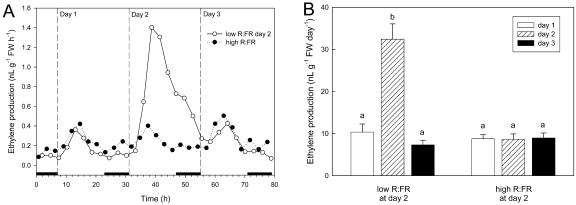
Exogenous ethylene induced an increase of petiole angle, petiole length, and stem length, and these responses were saturated already at an ethylene concentration of less than 0.10 μL L−1. The petiole angle, petiole length, and stem length of ethylene-treated plants were increased by a low compared to a high R:FR (Fig. 1), which may point to increased responsiveness to ethylene in a low R:FR (P < 0.05 for R:FR × ethylene interaction of petiole angle and length). Yet, this may as well have been the additive effect of the low R:FR and applied ethylene, and this low R:FR effect could even be mediated by increased ethylene levels. The latter is supported by Figure 2, which shows a stimulation of ethylene production by a low R:FR. Ethylene production showed a pronounced diurnal pattern with a higher production rate during the light period and a lower production in the dark, in both R:FR ratios (Fig. 2A). Therefore, ethylene production was calculated per diurnal cycle. Lowering the R:FR by switching on FR-emitting lamps (day 2) significantly increased ethylene production averaged over a 24-h period from 10 to more than 30 nL g−1 shoot fresh weight d−1 (Fig. 2B). This increased production disappeared when the FR lamps were switched off again (day 3).
Figure 1.
Effects of exogenous ethylene on petiole angle (A), petiole length (B), and stem length (C) of wild-type tobacco in a high or a low R:FR light environment. Data are means ± se (n = 6).
Figure 2.
Effects of R:FR of the incident light on ethylene production of wild-type tobacco. A, Patterns of ethylene production of individual representative plants in either the high or the low R:FR compartment. Black horizontal bars on the x axis indicate the dark periods. White symbol, Days 1 and 3, R:FR = 8.04; day 2, R:FR = 0.12. Black symbol, Days 1 to 3, R:FR = 8.04. B, Average ethylene production calculated over 24-h periods in a high (control) or a low (treatment) R:FR environment (data are means ± se [n = 5]). Note that the R:FR was lowered only at day 2 in the treatment group.
We conclude that ethylene action, determined by production of and responsiveness to the hormone, is controlled by sensing of the R:FR. Furthermore, hyponastic and stem and petiole elongation responses to low R:FR may be partly mediated by enhanced ethylene production rates.
Ethylene-Sensing Stimulates R:FR-Mediated Shade Avoidance Responses
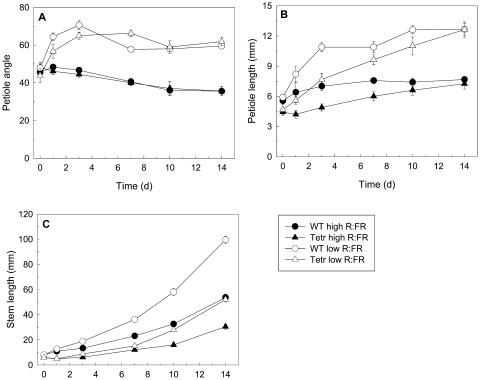
Although the results above indicate a clear interaction between the R:FR and ethylene action, it remained to be shown that ethylene played a role in R:FR-mediated shade avoidance responses. This was tested by investigating the response of transgenic plants that cannot sense ethylene (Tetr) to reduced R:FR. Within 1 d of treatment with a low R:FR, wild-type and Tetr petiole angles increased from approximately 40° to 65° and retained this stature during the entire experiment (Fig. 3A) with no significant difference between the two genotypes. Petiole elongation was significantly (P < 0.005) stimulated by a low R:FR (Fig. 3B), and this response was initially faster in wild type than in Tetr, but the two genotypes ultimately reached comparable petiole lengths. Stem elongation also increased upon low R:FR treatment and was always faster for the wild type than for Tetr. The stem length difference between wild type and Tetr was, however, more pronounced in a low compared to a high R:FR environment (Fig. 3C; P < 0.005 for Genotype × R:FR interaction). These data together show that low R:FR-induced hyponasty proceeds independently of ethylene, whereas ethylene sensing does determine the rate of low R:FR-induced stem and petiole elongation.
Figure 3.
Effects of R:FR of the incident light on petiole angles to the horizontal (A) and petiole (B) and stem (C) elongation of wild-type and ethylene-insensitive Tetr tobacco. Data are means ± se (n = 6).
GA Is Required for Ethylene-Induced Growth Responses
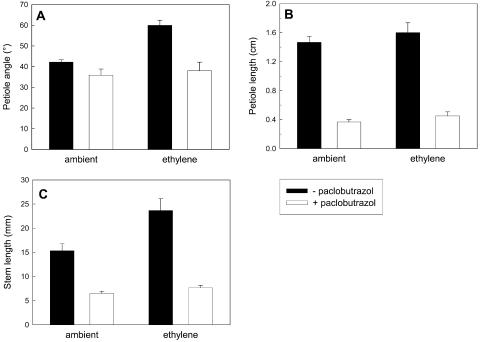
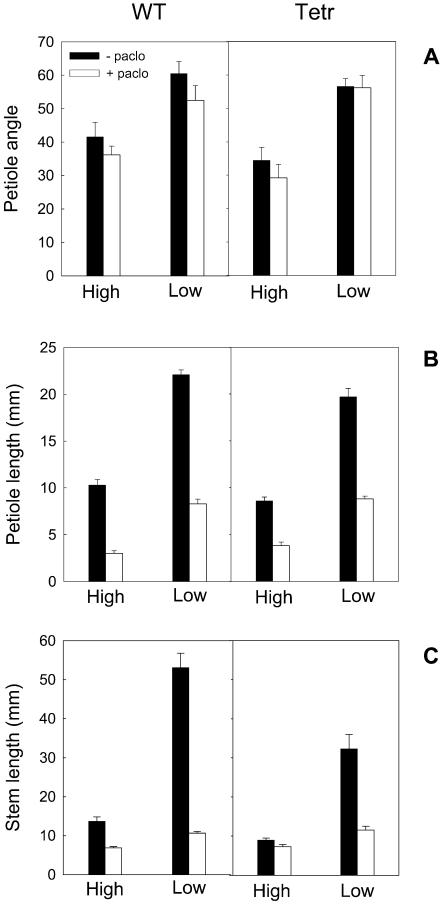
Since shade avoidance responses generally require GA action and GA can interact with ethylene, we investigated if the ethylene-induced shade avoidance-like responses described earlier (Fig. 1) also required GA. It appeared that the ethylene-induced elongation and hyponastic responses were absent when GA biosynthesis was inhibited (Fig. 4). Figure 4A shows that the petiole angles per se were not affected by inhibition of GA production with paclobutrazol, but this treatment completely prevented the hyponastic response to 0.6 μL L−1 ethylene. Interestingly, petiole length was not affected by the ethylene concentration used, but it was affected by GA action since application of paclobutrazol severely reduced petiole length (Fig. 4B). Finally, paclobutrazol severely suppressed stem elongation and also entirely prevented the ethylene-induced increase of stem elongation (Fig. 4C). It can, therefore, be concluded that ethylene-induced hyponasty and stem elongation require GA.
Figure 4.
Effects of exogenous ethylene and inhibition of GA biosynthesis on petiole angles to the horizontal (A) and petiole (B) and stem (C) elongation of wild-type tobacco. Plants were grown with or without added ethylene (ambient versus 0.6 μL L−1) and with (white bars) or without (black bars) the GA biosynthesis inhibitor paclobutrazol. Data are means (n = 6–9) ± se.
GA Involvement in Low R:FR-Induced Shade Avoidance Responses
So far, we have shown that ethylene production is increased by reduced R:FR, that increased ethylene levels induce responses that are similar to low R:FR-induced responses, and that the low R:FR-induced petiole and stem elongation responses require intact ethylene sensing. Since the ethylene-induced responses observed required GA, we investigated if the role of ethylene in low R:FR-induced shade avoidance also involved GA. First, the importance of GA in R:FR-induced shade avoidance responses was determined. Inhibition of GA biosynthesis with paclobutrazol severely reduced the low R:FR-induced stem and petiole elongation responses in both wild-type and Tetr plants (Fig. 5, B and C). Remarkably, low R:FR-induced hyponasty was not significantly affected by GA inhibition (Fig. 5A), which contrasts with the absolute requirement of GA for hyponasty induced by application of ethylene. Figure 6 shows that adding back GA3 to paclobutrazol-treated plants could rescue the original phenotype and the normal R:FR responses, showing that the effect of paclobutrazol on these responses is really through inhibited GA production, rather than a nonspecific effect.
Figure 5.
Effects of R:FR of the incident light and inhibition of GA biosynthesis on petiole angles to the horizontal (A) and petiole (B) and stem (C) elongation of wild-type and ethylene-insensitive Tetr tobacco. Plants were grown in light with a high or a low R:FR and with (white bars) or without (black bars) the GA biosynthesis inhibitor paclobutrazol (paclo). Data are means (n = 6–9) ± se.
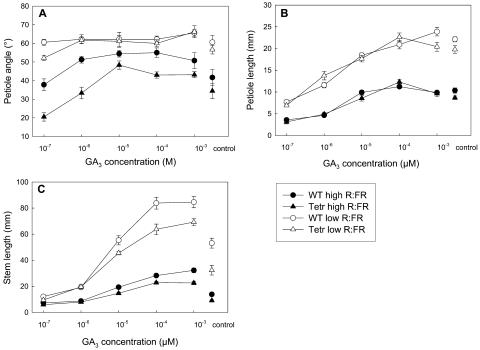
Figure 6.
Effects of exogenously applied GA3 concentrations on petiole angles to the horizontal (A) and petiole (B) and stem (C) elongation of wild-type and ethylene-insensitive Tetr tobacco in light with a high or a low R:FR. Data are means (n = 6–9) ± se. Data of control plants (i.e. plants that received no paclobutrazol and no GA3) from Figure 1 are replotted here for the clarity of comparison.
Since GA was crucial for stem and petiole elongation responses to low R:FR, the tissue responsiveness to GA was investigated in a low and a high R:FR. We also determined if Tetr plants had a different responsiveness to GA than wild type, in order to check if ethylene acts in R:FR-mediated shade avoidance by affecting plant sensitivity to GA. Plants grown at high R:FR showed a positive petiole angle response to GA that tended to increase to higher levels in wild type than in Tetr (Fig. 6A). However, petiole angles were stimulated by a low R:FR independently of GA, and in this low R:FR treatment, addition of GA did not further increase petiole angles (Fig. 6A). Elongation of the petioles increased with increasing GA concentrations in both genotypes, and this was significantly more pronounced in a low compared to a high R:FR (Fig. 6B; P < 0.001 for GA × R:FR interaction). The concentration-response curves of wild-type and Tetr petiole length are highly similar (Fig. 6B), suggesting a comparable GA sensitivity of petiole elongation in the wild type and Tetr. Stem elongation also increased significantly with increasing GA concentrations, comparable to the effects on petiole elongation. This elongation response was much stronger at low than at high R:FR (Fig. 6C; P < 0.001 for GA × R:FR interaction), showing that a low R:FR sensitizes the stem elongation process to GA. Furthermore, stem elongation of Tetr plants was less responsive to added GA than that of wild-type plants (Fig. 6C; P < 0.05 for GA × Genotype interaction), indicating reduced responsiveness of stem elongation to GA in Tetr. This is in contrast with petiole elongation, for which the wild type and Tetr have equal responsiveness to GA, even though petiole elongation does require GA.
We conclude that the involvement of ethylene in R:FR-mediated shade avoidance responses corresponds with a requirement for GA, and this may partly act through altered GA responsiveness.
DISCUSSION
We investigated the interactions between R:FR signaling by phytochromes, ethylene action, and GA action in regulating shade avoidance responses. These responses include hyponastic movement of the leaves and increased elongation rates of petioles and stems. Interestingly, the hormonal interactions involved in regulating these responses upon sensing of the R:FR were not always the same for the different responses studied. Especially, the regulation of hyponasty differed significantly from the stem and petiole elongation responses.
We found that ethylene production was enhanced by low R:FR (i.e. an inactivation of the phytochromes; Fig. 2), which is consistent with data by Finlayson and co-workers (1998, 1999), who showed similar effects in light-grown S. bicolor. The inhibitory effect of active phytochrome on ethylene production is also confirmed by studies on ethylene production in deetiolated plants (Vangronsveld et al., 1988), where red light, leading to phytochrome activation, did inhibit ethylene production. To our knowledge, our data are the first to show that rhythmic ethylene production is stimulated immediately upon lowering the R:FR and that this enhanced ethylene production also declines again upon restoring the light environment to a high R:FR. These fast responses make endogenous ethylene a potentially suitable regulator to control growth responses to dynamic conditions such as a changing light environment. The reduced R:FR that enhance ethylene production also induced hyponastic movement of the leaves and enhanced stem and petiole elongation (Fig. 3), as has been described before (Smith and Whitelam, 1997; Pierik et al., 2003). However, these stem and petiole elongation responses to low R:FR were delayed in plants that were insensitive to ethylene (Tetr), whereas the hyponastic response to low R:FR was not affected by ethylene insensitivity (Fig. 3). These data together suggest an interaction between ethylene action and R:FR sensing, which is strengthened by the observation that exogenously applied ethylene could induce hyponastic bending of the leaves and enhanced stem and petiole elongation (Fig. 1), comparable to the responses induced by a low R:FR. The responses of petiole angles and petiole and stem lengths in an ethylene atmosphere were even more pronounced in light with a low compared to a high R:FR (Fig. 1). In the low R:FR, the highest ethylene concentration used (0.20 μL L−1) was supraoptimal for petiole and stem length (Fig. 1, B and C). When an even higher ethylene concentration (0.60 μL L−1) in a high R:FR was used in a separate experiment, no stimulation of petiole length could be observed anymore (Fig. 4). Therefore, we suggest that a stimulation of elongation growth by ethylene may occur particularly at relatively low concentrations. This would explain why ethylene is classically described as a growth inhibitory hormone because often very high ethylene concentrations are used (more than 1 μL L−1), which is much higher than the concentrations used in the present experiment (0.03–0.2 μL L−1). Still, stimulatory effects of low ethylene concentrations on elongation growth have been described before (e.g. Fiorani et al., 2002, and references therein). Semiaquatic plants, such as Rumex palustris and rice (Oryza sativa), are especially well known for stimulation of shoot elongation by ethylene, even when applied at relatively high concentrations (Kende et al., 1998; Voesenek et al., 2003). From these studies on semiaquatic plants, it has also become clear that ethylene-induced stimulation of elongation growth is mediated through increased GA action (production and sensitivity; Hoffmann-Benning and Kende, 1992; Rijnders et al., 1997). In addition, the dwarf growth of the alpine ecotype of Stellaria longipes, as opposed to the fast ethylene-mediated elongation growth in the prairie ecotype (Emery et al., 1994), corresponds with reduced responsiveness of the dwarf plants to GA3 but not to other GA forms (Emery et al., 2001). We show here that in tobacco, growth-promoting effects of ethylene require GA action since these effects were absent in plants with limited GA production (Fig. 4). This was true for all responses investigated, albeit petiole elongation was not stimulated by the high ethylene concentration used (0.6 μL L−1), but it was still severely reduced by inhibition of GA biosynthesis both in the presence and absence of additional ethylene. Because ethylene-induced growth responses in tobacco thus appeared to involve GA, we investigated the relationship between ethylene and GA in low R:FR-induced shade avoidance responses. Interestingly, of the three responses to low R:FR investigated, only the two that involved sensing of ethylene (i.e. stem and petiole elongation; Fig. 3) were GA-dependent (Fig. 5). The involvement of GA in the R:FR-mediated petiole and stem elongation responses of tobacco consists at least partly of altered responsiveness to this hormone through light signaling by phytochrome (Fig. 6). This is especially clear for stem elongation, which is much more responsive to applied GA in a low compared to a high R:FR (Fig. 6C). This is in accordance with the literature (Reid et al., 1990) in which an increased sensitivity to GA by lowering of the R:FR has been described for pea (Pisum sativum) and cucumber (Cucumis sativus; Weller et al., 1994; López-Juez et al., 1995). The hyponastic response to low R:FR, which is independent of ethylene (Fig. 3), also appeared to be independent of GA (Fig. 5). The latter is remarkable since hyponasty induced by exogenous ethylene, instead of low R:FR, did require GA (Fig. 4). The absence of a role for ethylene in the hyponastic response to low R:FR does not mean that ethylene is not involved in leaf angle responses to neighbor plants in real canopies. We have recently shown that ethylene-insensitive plants do have reduced leaf angle responses to neighboring vegetation and even entirely absent leaf angle responses to reduced fluence rates of blue light (Pierik et al., 2004). It appears that hyponasty is regulated in a highly complex and diverse matter with, depending on the input signal that triggers the response, distinctive roles for ethylene and GA.
The involvement of ethylene in the low R:FR-induced stem and petiole elongation responses thus correlates obviously with a requirement of GA for those responses, and this can be partly explained by ethylene-enhanced GA responsiveness. Clearly, stem elongation was more responsive to GA in wild type than in Tetr (Fig. 6C), confirming the hypothesis that ethylene insensitivity reduces responsiveness to GA. Still, the difference between control (no paclobutrazol, no GA) wild-type and Tetr plants in low R:FR light was larger than at any of the GA concentrations (compare Figs. 5C and 6C), and this additional difference must, therefore, be attributed to a factor other than GA responsiveness. As ethylene can also control GA production (Rijnders et al., 1997), enhanced GA production may be a likely mechanism to explain the additional difference between wild type and Tetr. This also seems true for low R:FR-induced petiole elongation, which involves both ethylene and also GA action, but not through an ethylene-mediated increase of GA sensitivity since wild type and Tetr had similar GA sensitivity (Fig. 6). The model that arises now for stem and petiole elongation suggests that a reduced R:FR enhances production of and responsiveness to ethylene, which then modulates GA responsiveness and probably also production (Fig. 7A). Such a mechanism is supported by the fact that both the R:FR and ethylene have been shown in the literature to affect the sensitivity to and production of GA (García-Martínez et al., 1987; López-Juez et al., 1995; Rijnders et al., 1997). This mechanism is, however, certainly not applicable to the hyponastic response to low R:FR, as it proceeds independently of ethylene and GA, unless when induced by elevated ethylene (Fig. 7B).
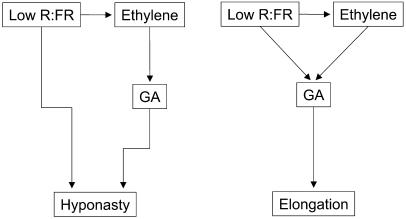
Figure 7.
Diagram describing interactions between R:FR signaling, ethylene, and GA in regulating hyponastic (A) and elongation (B) responses in tobacco. Reduced R:FR increases ethylene production. Hyponasty induced by low R:FR can proceed independently of ethylene and GA, but when induced by exogenous ethylene it does require GA. Elongation responses always require GA, regardless of the signal inducing the response (ethylene or low R:FR) and low R:FR-induced elongation is affected by sensitivity to ethylene and by GA.
Next to interactions between ethylene and GA, also interactions of ethylene with other growth factors may regulate shade avoidance responses. Auxins have, for example, been implicated in shade avoidance (Morelli and Ruberti, 2000), and interactions between ethylene and auxin are known to regulate hypocotyl elongation (Smalle et al., 1997) and apical hook opening (Lehman et al., 1996). The recent discovery of the DELLA proteins (Harberd, 2003) provides an excellent model to integrate auxin action with ethylene and GA action in modifying growth. All three hormones affect the abundance or stability of DELLA proteins, which are themselves downstream repressors of growth (Achard et al., 2003). As such, the DELLA proteins could be an important point of integration for different growth regulators, thereby determining the final growth responses.
In summary, wild type and Tetr respond differently to a low R:FR, showing the involvement of ethylene in phytochrome-mediated shade avoidance responses. This involvement of ethylene can at least partly be attributed to interactions between ethylene and GA action, and it is likely that GA acts downstream of ethylene in regulating shade avoidance responses. Part of this interaction seems to consist of ethylene-mediated changes in GA responsiveness.
MATERIALS AND METHODS
Plant Material
Wild-type and ethylene-insensitive (Tetr) tobacco (Nicotiana tabacum cv Samsun NN) seeds were germinated on moist sand (16 h light [90 μmol m−2 s−1; Philips TLD 36 W/840; Eindhoven, The Netherlands], 8 h dark, temperature 21°C). Tetr is an ethylene-insensitive, transgenic genotype that was obtained through introduction of the Arabidopsis etr1-1 mutant gene in tobacco (Knoester et al., 1998). After 9 d, seedlings were transferred to pots (height = 5 cm, diameter = 5.5 cm) filled with the same substratum and covered with transparent polyethylene sheets during the first week after transfer to avoid excessive water loss. After removal of the sheets, the plants were watered with full-strength Hoagland nutrient solution every other day and grown for an additional 3 weeks before they were used for experiments.
Light Sources
In several experiments plants received a high and low R:FR light ratio. This ratio was manipulated by adding FR light to the white light (high R:FR) background. In the shade avoidance comparison between wild type and Tetr, FR light was provided by halogen lamps (Osram Haloline Halogen R7s 500W; St. Helens, UK) with black acrylic filters (Black 901 Crylex; A.S.H. Plastics, Wolverhampton, UK), whereas in all other R:FR experiments FR was provided by FR-emitting incandescent lamps (Paulmann Schwarzlicht, 75 W; Paulmann Licht GmbH, Springe-Völksen, Germany), which did not affect photosynthetically active radiation levels. R:FR were calculated as the ratio between photon fluence rates in the 655 to 665 nm interval (R) and the 725 to 735 nm interval (FR), measured with a Licor1800 radiospectrometer (LI-COR, Lincoln, NE). White light sources were as described above in the “Plant Material” section, unless stated otherwise.
Ethylene Production and R:FR
Five-week-old wild-type plants were transferred to cylindrical glass cuvettes (one plant per cuvette; height 15 cm, diameter 6.5 cm) in the growth chamber (light intensity was 66 μmol m−2 s−1 and temperature was 20°C inside the cuvette). These closed cuvettes were flushed continuously (1 L h−1) with ethylene-free air, which was then led to a laser-driven photo-acoustic ethylene detection system (Voesenek et al., 1990) that monitored ethylene concentrations in the gas flow. The setup was divided into two light-tight parts with plants on one side receiving a high R:FR and plants in the other part receiving a reduced R:FR. After acclimatization of the plants in the cuvettes for 1 d in fluorescent-only light (R:FR = 8.04), ethylene production was measured in both compartments during the following day (day 1). On the subsequent day (day 2), ethylene production was measured with the FR lamps turned on during the light period in the low R:FR compartment (R:FR = 0.12). On the last day of measurements (day 3) ethylene production was monitored again with only white fluorescent light in both compartments. Ethylene production was calculated per gram shoot fresh weight per day.
Ethylene Responsiveness and R:FR
Five-week-old wild type plants were transferred to desiccators (six plants per desiccator) that were placed in a high (7.05) or a low (0.11) R:FR. There were 4 desiccators per light treatment, each receiving a continuous flow (0.5 L h−1) of a different ethylene concentration, obtained by mixing ethylene with atmospheric air (Mass flow controllers; Hi-Tech, Ruurlo, The Netherlands) to the desired concentrations (0.03, 0.07, 0.2 μL L−1) that were checked with a gas chromatograph (Chrompack, Middelburg, The Netherlands). After 1 d of treatment, petiole angles to the horizontal (fifth leaf) were measured with a protractor, and after 1 week of treatment stem and petiole length (fifth leaf) were determined.
R:FR-Induced Shade Avoidance Responses in Wild Type and Tetr
Wild type and Tetr were germinated on moist filter paper in petri dishes for 8 d (16 h light [175 μmol m−2 s−1; General Electric 65W/35; Fairfield, CT], 8 h dark, temperature 23°C). Thereafter, seedlings of both genotypes were transplanted to pots (height 5 cm, top diameter 5.5 cm) containing 1:1 sand:autoclaved potting soil. Three and a half weeks after sowing, plants were placed into growth cabinets (16 h light [180 μmol m−2 s−1; Osram Powerstar HQI-TS 150 W], 8 h dark; 21°C) with either a high (2.48) or low (0.32) R:FR. After transfer to the growth cabinets, plants acclimatized for 3 d before the actual R:FR treatments were started. Growth and morphology of these plants were then followed nondestructively for 14 d.
In a separate experiment, a different and independent ethylene-insensitive, transgenic line (Tetr20; Knoester et al., 1998) was tested for its low R:FR responsiveness, and it behaved similar to the present Tetr18 line, thus confirming that Tetr's phenotype is not an artifact caused by the genetic transformation of the plants but the effect of its insensitivity to ethylene.
Ethylene Effects in Relation to GA Action
We investigated if ethylene-induced shade avoidance responses require GA. Wild type plants received the GA biosynthesis inhibitor paclobutrazol (ICI Agrochemicals, Kent, UK) to inhibit GA production and were placed in an atmosphere with or without added ethylene (16 h light [210 μmol m−2 s−1; HPS 600 W; Philips, Eindhoven, The Netherlands], 8 h dark, temperature 20°C). Paclobutrazol was added to the soil substrate of each plant as 5 mL 10−5 m paclobutrazol in 0.033% ethanol 1 d before the start of the experiment and after 1 week during the experiment. Plants that received no GA inhibitor served as a control (n = 6), and these plants were treated with 5 mL 0.033% ethanol when the others received paclobutrazol. Experiments took place in glass containers that were flushed (0.5 L h−1) with either normal air (containing the atmospheric concentration of approximately 0.005 μL L−1 ethylene) or 0.6 μL L−1 ethylene (Air Liquide, Eindhoven, The Netherlands) in air (mixed with gas blenders; Brinkhorst, Veenendaal, The Netherlands). Concentrations were checked with a gas chromatograph (Chrompack, type 437A; Middelburg, Holland). Petiole angle to the horizontal (fifth leaf) were measured 1 d after start of the ethylene treatment; petiole length of the fifth leaf and stem length were measured 9 d after the start of the experiment.
GA Sensitivity at Low and High R:FR
The importance of GA for R:FR-induced shade avoidance responses was investigated, and the sensitivity to GA was determined for wild-type and Tetr plants in high and low R:FR. Plants containing a minimal endogenous GA concentration were obtained by treating wild-type and Tetr plants with paclobutrazol as described in the previous section. At the start of the experiment, shoots of paclobutrazol-treated wild-type and Tetr plants were sprayed with a range of GA3 concentrations (10−3 m, 10−4 m, 10−5 m, 10−6 m, 10−7 m, and 0 m GA3 in 1% ethanol; Sigma, St. Louis). The solution was sprayed on the leaves twice a week during the experiment. Plants were placed in a high (7.05) or low (0.11) R:FR compartment in the growth chamber. Stem length, petiole angle to the horizontal, and length of the fifth leaf were measured 11 d after the start of the experiment.
Statistical Analyses
Data of all experiments were analyzed with two- or three-way ANOVAs (repeated measures when applicable) with interactions. When needed, data were transformed (natural logarithm or arcsine square root) to obtain equal variances.
Acknowledgments
We thank Garry Whitelam (University of Leicester, UK) for giving R.P. the opportunity to carry out one of the experiments in his lab.
This work was supported by the Netherlands Organization for Scientific Research (grant no. 805.33.464 to R.P.) and by a PIONIER grant from the Netherlands Organization for Scientific Research (grant no. 800.84.470 to L.A.C.J.V.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.045120.
References
- Achard P, Vriezen WH, Van der Straeten D, Harberd N (2003) Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15: 2816–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aphalo PJ, Ballaré CL, Scopel AL (1999) Plant-plant signalling, the shade avoidance response and competition. J Exp Bot 50: 1629–1634 [Google Scholar]
- Ballaré CL (1999) Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci 4: 97–102 [DOI] [PubMed] [Google Scholar]
- Beall FD, Yeung EC, Pharis RP (1996) Far-red light stimulates internode elongation, cell division, cell elongation, and gibberellin levels in bean. Can J Bot 74: 743–752 [Google Scholar]
- Chory J, Li J (1997) Gibberellins, brassinosteroids and light-regulated development. Plant Cell Environ 20: 801–806 [Google Scholar]
- Emery RJN, Pearce DW, Pharis RP, Reid DM, Chinnappa CC (2001) Stem elongation and gibberellins in alpine and prairie ecotypes of Stellaria longipes. Plant Growth Regul 35: 17–29 [Google Scholar]
- Emery RJN, Reid DM, Chinnappa CC (1994) Phenotypic plasticity of stem elongation in two ecotypes of Stellaria longipes: the role of ethylene and response to wind. Plant Cell Environ 17: 691–700 [Google Scholar]
- Finlayson SA, Jung I-J, Mullet JE, Morgan PW (1999) The mechanism of rhythmic ethylene production in Sorghum: the role of phytochrome B and simulated shading. Plant Physiol 119: 1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson SA, Lee I-J, Morgan PW (1998) Phytochrome B and the regulation of circadian ethylene production in sorghum. Plant Physiol 116: 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorani F, Bogemann GM, Visser EJW, Lambers H, Voesenek LACJ (2002) Ethylene emission and responsiveness to applied ethylene vary among Poa species that inherently differ in leaf elongation rates. Plant Physiol 129: 1382–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Praekelt U, Stoddart WM, Billingham OE, Halliday KJ, Whitelam GC (2003) Phytochromes B, D and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol 131: 1340–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Martínez JL, Keith B, Bonner BA, Stafford AE, Rappaport L (1987) Phytochrome regulation of the response to exogenous gibberellins by epicotyls of Vigna sinensis. Plant Physiol 85: 212–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberd NP (2003) Relieving DELLA restraint. Science 299: 1853–1854 [DOI] [PubMed] [Google Scholar]
- Hoffmann-Benning S, Kende H (1992) On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol 99: 1156–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaseki H, Pjon C-H, Furya M (1971) Phytochrome action in Oryza sativa L. Plant Physiol 48: 241–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H, Van der Knaap E, Cho H-T (1998) Deepwater rice: a model plant to study stem elongation. Plant Physiol 118: 1105–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoester M, Van Loon LC, Van den Heuvel J, Hennig J, Bol JF, Linthorst HJM (1998) Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA 95: 1933–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman A, Black R, Ecker JR (1996) HOOKLESS1, an ethylene response gene is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85: 183–194 [DOI] [PubMed] [Google Scholar]
- López-Juez E, Kobayashi M, Sakurai A, Kamiya Y, Kendrick RE (1995) Phytochrome, gibberellins, and hypocotyl growth. Plant Physiol 107: 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli G, Ruberti I (2000) Shade avoidance responses: driving auxin along lateral routes. Plant Physiol 122: 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Visser EJW, de Kroon H, Voesenek LACJ (2003) Ethylene is required in tobacco to succesfully compete with proximate neighbours. Plant Cell Environ 26: 1229–1234 [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LACJ, de Kroon H, Visser EJW (2004) Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signalling. Plant J 38: 310–319 [DOI] [PubMed] [Google Scholar]
- Reid JB, Hasan O, Ross JJ (1990) Internode length in Pisum: gibberellins and the response to far-red-rich light. J Plant Physiol 137: 46–52 [Google Scholar]
- Rijnders JHGM, Yang YY, Kamiya Y, Takahashi N, Barendse GWM, Blom CWPM, Voesenek LACJ (1997) Ethylene enhances gibberellin levels and petiole sensitivity in flooding-tolerant Rumex palustris but not in flooding-intolerant R. acetosa. Planta 203: 20–25 [Google Scholar]
- Samimy C (1978) Effect of light on ethylene production and hypocotyl growth of soybean seedlings. Plant Physiol 61: 772–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Van der Straeten D (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94: 2756–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H (2000) Phytochromes and light signal perception by plants: an emerging synthesis. Nature 407: 585–591 [DOI] [PubMed] [Google Scholar]
- Smith H, Whitelam GC (1997) The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant Cell Environ 20: 840–844 [Google Scholar]
- Vangronsveld J, Clijsters H, Van Poucke M (1988) Phytochrome-controlled ethylene biosynthesis of intact etiolated bean seedlings. Planta 174: 19–24 [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Benschop JJ, Bou J, Cox MCH, Groeneveld HW, Millenaar FF, Vreeburg RAM, Peeters AJM (2003) Interactions between plant hormones regulate submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris. Ann Bot (Lond) 91: 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Harren FJM, Bogemann GM, Blom CWPM, Reuss J (1990) Ethylene production and petiole growth in Rumex plants induced by soil waterlogging: the application of a continuous flow system and a laser driven intracavity photoacoustic detection system. Plant Physiol 94: 1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Ross JJ, Reid JB (1994) Gibberellins and phytochrome regulation of stem elongation in pea. Planta 192: 489–496 [Google Scholar]
- Whitelam GC, Devlin PF (1997) Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ 20: 752–758 [Google Scholar]