Abstract
Plant-emitted ethylene has received considerable attention as a stress hormone and is considered to play a major role at low concentrations in the tolerance of several species to biotic and abiotic stresses. However, airborne ethylene at high concentrations, such as those found in polluted areas (20–100 nL L−1) for several days, has received far less attention in studies of plant stress tolerance, though it has been shown to alter photosynthesis and reproductive stages (seed germination, flowering, and fruit ripening) in some species. To assess the potential effects of airborne ethylene on plant stress tolerance in polluted areas, the extent of oxidative stress, photo- and antioxidant protection, and visual leaf area damage were evaluated in ethylene-treated (approximately 100 nL L−1 in air) and control (without ethylene fumigation) holm oak (Quercus ilex) plants exposed to heat stress or to a combination of heat and drought stress. Control plants displayed tolerance to temperatures as high as 50°C, which might be attributed, at least in part, to enhanced xanthophyll de-epoxidation and 2-fold increases in α-tocopherol, and they suffered oxidative stress only when water deficit was superimposed on temperatures above 45°C. By contrast, ethylene-treated plants showed symptoms of oxidative stress at lower temperatures (35°C) than the controls in drought, as indicated by enhanced malondialdehyde levels, lower α-tocopherol and ascorbate concentrations, and a shift of the redox state of ascorbate to its oxidized form. In addition, ethylene-treated plants showed higher visual leaf area damage and greater reductions in the maximum efficiency of the PSII photochemistry than controls in response to heat stress or to a combination of heat and drought stress. These results demonstrate for the first time that airborne ethylene at concentrations similar to those found in polluted areas may reduce plant stress tolerance by altering, among other possible mechanisms, antioxidant defenses.
Ethylene, the simplest unsaturated hydrocarbon, regulates many diverse metabolic and developmental processes in plants, ranging from seed germination to organ senescence, and it is considered to play a major role as a signal molecule at low concentrations in the tolerance of several species to environmental stresses (for review, see Abeles et al., 1992; Morgan and Drew, 1997; Bleecker and Kende, 2000). Ethylene is a volatile hormone that diffuses freely in air and from cell to cell across membranes, and it therefore easily reaches target cells of the same organ, organs other than those responsible for its biosynthesis, or even target cells of neighbor plants acting in plant-plant signaling (Pierik et al., 2004). Ethylene concentrations in free air in rural areas may well be below 2 nL L−1. However, in a greenhouse replete with plants, airborne ethylene may increase to 6 nL L−1 and reach 25 to 80 nL L−1 within dense canopies of ethylene-emitting plants (Heilman et al., 1971; Pierik et al., 2004). Irrespective of plant ethylene emission, airborne ethylene may reach 20 nL L−1 on annual averages and 1 μL L−1 at hourly maximum concentrations as a result of industrial activity in polluted areas (as a reference, ethylene concentrations may be as high as 3 μL L−1 close to polyethylene factories or 1,000 μL L−1 in vehicle exhaust; for review, see Cape, 2003).
Although high ethylene concentrations of industrial origin in air may be dispersed fairly rapidly by the wind, their source at ground level means that there may be local fumigation of the natural vegetation in surrounding areas at concentrations ranging from 20 to 100 nL L−1 (or even higher) for several days, and effects of ethylene of industrial origin on vegetation should not be disregarded. In fact, pollutant-specific effects of ethylene have been reported in the field downwind from major industrial sources, showing significant effects on epinasty (Tonneijck et al., 1999, 2000) and reproductive stages, i.e. seed germination, flowering, and fruit ripening (for review, see Cape, 2003). Squier et al. (1985) also showed that ethylene at concentrations found in polluted areas may reduce CO2 assimilation in some species. However, very little information on the effects of airborne ethylene at concentrations found in polluted areas on the response of plants to environmental stresses has been provided so far.
Environmental stresses, such as heat and drought stress, may lead to an imbalance between antioxidant defenses and the amount of activated oxygen species (AOS) resulting in oxidative stress (Smirnoff, 1993; Gong et al., 1997; Larkindale and Knight, 2002; Pastori and Foyer, 2002; Xiong et al., 2002). Several AOS are thought to be involved at low concentrations in inter- and intracellular signaling in plant responses to stress (Doke, 1997; Foyer and Noctor, 1999; Op den Camp et al., 2003), but they can also cause damage at various levels of organization, including chloroplasts, when present at high concentrations (Asada, 1999; Halliwell and Gutteridge, 1999; Apel and Hirt, 2004). Apart from the xanthophyll cycle, photorespiration, and other changes in metabolic activity, which may protect the chloroplasts from oxidative damage (Demmig-Adams and Adams, 1992; Kozaki and Takeba, 1996; Eskling et al., 1997; Osmond et al., 1997), a number of enzymatic and non-enzymatic antioxidants that serve to control oxygen toxicity are present in chloroplasts. Of these, tocopherols (vitamin E), carotenoids, and ascorbate play an important role in maintaining the integrity of the photosynthetic membranes under oxidative stress (Havaux, 1998; Smirnoff and Wheeler, 2000; Munné-Bosch and Alegre, 2002).
Holm oak (Quercus ilex) is one of the most typical dominant Mediterranean forest species and shows tolerance to heat stress (Larcher, 2000). CO2 assimilation rates in holm oak decrease only at temperatures as high as 35°C, and it survives on sites where the maximum diurnal air temperatures may occasionally reach 45°C to 50°C (southern Spain). However, holm oak is relatively sensitive to drought stress, at least compared to other Mediterranean species, which explains its presence in subhumid areas of Mediterranean ecosystems (Tetriach, 1993; Peñuelas et al., 1998). Climatic change is presently affecting ecological distribution of the holm oak in Mediterranean ecosystems, and, except for the ozone effect (Bussotti and Ferretti, 1998; Eichhorn and Paar, 2000), it is still uncertain what the impact of increasing air pollutants will be on the tolerance of this widely distributed Mediterranean tree to heat and drought stress.
In an attempt to study the effects of airborne ethylene at concentrations found in polluted areas on stress tolerance in holm oak, photosynthesis, the extent of oxidative stress, photo- and antioxidant protection, and visual leaf area damage were evaluated in ethylene-treated (approximately 100 nL L−1 in air) and control (without ethylene fumigation) plants exposed to heat stress, or to a combination of heat and drought stress. It is shown here that airborne ethylene may reduce tolerance of holm oak plants to heat and drought stress by altering, among other processes, antioxidant defenses.
RESULTS
Airborne Ethylene Reduces Photosynthesis and Increases Stress-Induced Damage
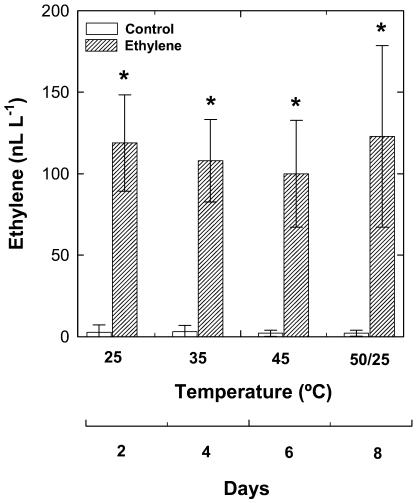
The effects of airborne ethylene on leaf gas exchange and several indicators of leaf injury were investigated in holm oak plants exposed to heat stress or to a combination of heat and drought stress. Plants were progressively exposed to increasing temperatures up to 45°C under irrigated conditions, or to a combination of high temperatures and drought stress (up to 45°C and 6 d of water deficit). After these treatments, each group of plants (irrigated and water stressed) were subject to strong temperature fluctuations (50/25°C), i.e. exposure at 50°C for 1 d followed by recovery at 25°C for another day, to evaluate the ability of holm oak to prevent oxidative damage under extreme climatic conditions. Ethylene fumigation allowed us to compare the response of plants to stress under contrasting ethylene concentrations in the air. The airborne ethylene concentrations in the fumigated chamber ranged from around 100 to 125 nL L−1, whereas those in the control chamber ranged between 2 and 3 nL L−1 throughout the experiment (Fig. 1). In addition, measurements of ethylene emitted by leaves revealed that ethylene production by holm oak leaves did not increase under heat or drought stress, and if any increase occurred it remained below detectable amounts (<0.1 nL [g dry wt h]−1) throughout the experiment in ethylene-treated and control plants.
Figure 1.
Airborne ethylene concentrations in non-fumigated (control, white bars) and fumigated (ethylene, filled bars) chambers throughout the experiment. Asterisk (*) indicates statistical difference (Student's t test, P < 0.05) between non-fumigated and fumigated chambers.
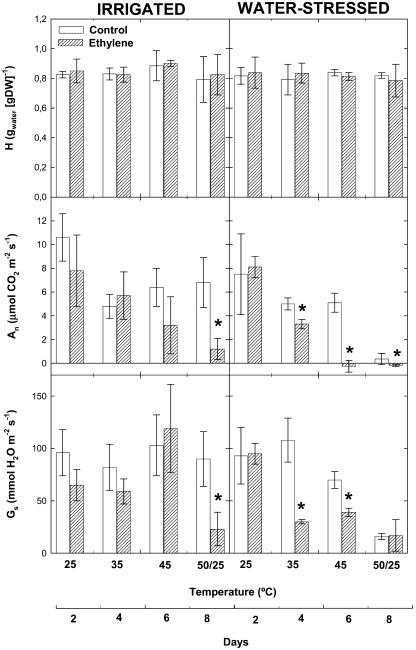
Leaf hydration was constant throughout the experiment (around 0.8 gwater [gdry wt]−1) in irrigated and water-stressed plants exposed to increasing temperatures (from 25°C–45°C) or temperature fluctuations (50/25°C), thereby indicating that neither airborne ethylene nor drought stress resulted in leaf water loss during the experiment (Fig. 2). The maintenance of leaf water content was associated with significant reductions in stomatal conductance in water-stressed plants, and ethylene-treated plants showed earlier stomatal closure under water deficit than controls as temperatures increased (Fig. 2). Net CO2 assimilation rates decreased as stomatal conductance was progressively reduced in drought. However, while stomatal conductance was constant, net CO2 assimilation rates decreased at 35°C in irrigated plants, thus indicating heat-induced non-stomatal limitations of photosynthesis. Airborne ethylene also caused non-stomatal limitations of photosynthesis, as indicated by the reductions in net CO2 assimilation accompanied by constant stomatal conductance rates between 35°C and 45°C in drought (Fig. 2).
Figure 2.
Effect of airborne ethylene (approximately 100 nL L−1) on leaf hydration (H), net CO2 assimilation (An), and stomatal conductance (Gs) rates in holm oak exposed to heat stress under irrigated and water-stressed conditions. Measurements were made on leaves of non-fumigated (control, white bars) and ethylene-treated plants (filled bars) progressively exposed to increasing temperatures (from 25°C–45°C), followed by a final temperature fluctuation treatment (50/25°C). Asterisk (*) indicates statistical difference (Student's t test, P < 0.05) between ethylene-treated and control plants.
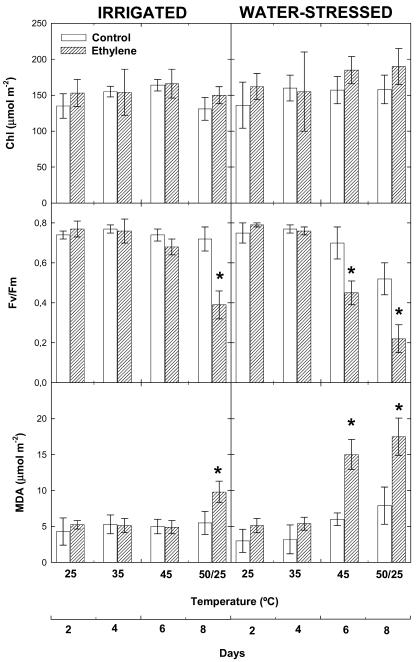
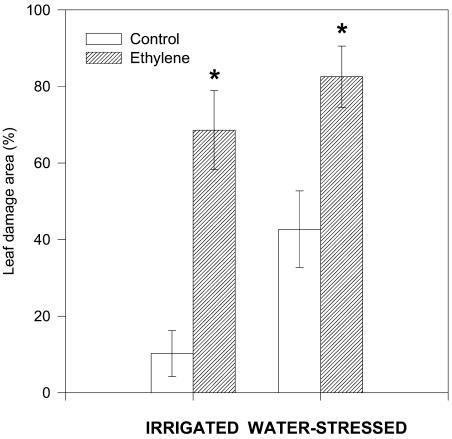
Indicators of heat- and drought-induced damage were measured in ethylene-treated and control plants (Fig. 3). While chlorophyll levels remained unaltered throughout the experiment in both plant groups, the maximum efficiency of PSII photochemistry (Fv/Fm ratio), which is an indicator of photoinhibitory damage to PSII, was significantly reduced under heat and drought stress, especially in ethylene-treated plants. The Fv/Fm ratio decreased concomitantly with lipid peroxidation, as indicated by malondialdehyde concentrations, which increased in both plant groups. In control plants, malondialdehyde levels increased significantly under strongly fluctuating temperatures (50/25°C) in drought stress only (Fig. 3), which was associated with strong reductions in photosynthesis (Fig. 2). Ethylene-treated plants showed higher malondialdehyde levels than controls at 45°C under drought stress and also under strong temperature fluctuations (50/25°C) both in irrigated and water-stressed plants (Fig. 3). The higher malondialdehyde levels were associated with higher visual leaf area damage at the end of the experiment in ethylene-treated plants as compared to controls. Control plants showed some discolored and brown areas (approximately 10%) as a result of heat stress under irrigated conditions, and leaf area damage increased 4-fold when drought was superimposed on heat stress. Levels of injury were much higher in ethylene-treated plants both under irrigated and water-stress conditions, and maximum levels of leaf damage area (approximately 83%) were attained under a combination of heat and drought stress in ethylene-treated plants (Fig. 4).
Figure 3.
Effect of airborne ethylene (approximately 100 nL L−1) on chlorophyll a + b (Chl) concentrations, Fv/Fm, and malondialdehyde (MDA) levels in holm oak exposed to heat stress under irrigated and water-stressed conditions. Measurements were made on leaves of non-fumigated (control, white bars) and ethylene-treated plants (filled bars) progressively exposed to increasing temperatures (from 25°C–45°C), followed by a final temperature fluctuation treatment (50/25°C). Asterisk (*) indicates statistical difference (Student's t test, P < 0.05) between ethylene-treated and control plants.
Figure 4.
Effect of airborne ethylene (approximately 100 nL L−1) on visual leaf damage area in holm oak exposed to heat stress under irrigated and water-stressed conditions. Measurements were made on leaves of non-fumigated (control, white bars) and ethylene-treated plants (filled bars) at the end of the experiment. Asterisk (*) indicates statistical difference (Student's t test, P < 0.05) between ethylene-treated and control plants.
Airborne Ethylene Alters Photo- and Antioxidant Protection under Stress
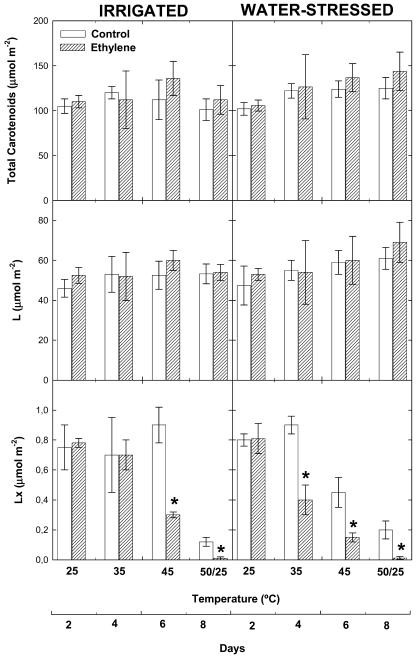
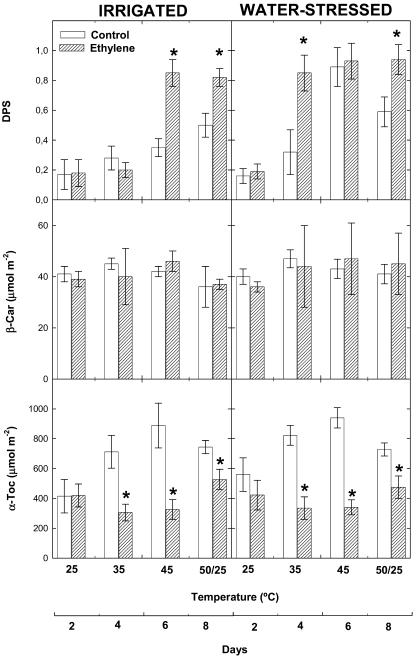
The effects of airborne ethylene on mechanisms of photo- and antioxidant protection were investigated in holm oak plants exposed to heat stress or to a combination of heat and drought stress. The concentrations of total carotenoids and those of the major carotenoids, lutein and β-carotene, did not change significantly in ethylene-treated plants as compared to controls, and remained unaltered throughout the experiment in response to heat and drought stress (Figs. 5 and 6). By contrast, lutein-epoxide levels and the de-epoxidation state of the xanthophyll cycle (level of violaxanthin de-epoxidation) were affected by heat and drought stress, especially in ethylene-treated plants. In agreement with previous stress indicators, ethylene-treated plants showed significantly lower lutein epoxide levels and higher de-epoxidation of violaxanthin than controls both under heat stress and under a combination of heat and drought stress. Ethylene-treated plants showed higher xanthophyll de-epoxidation than controls at 35°C under drought stress and at 45°C under irrigated conditions (Figs. 5 and 6).
Figure 5.
Effect of airborne ethylene (approximately 100 nL L−1) on total carotenoids, lutein (L), and lutein epoxide (Lx) concentrations in holm oak exposed to heat stress under irrigated and water-stressed conditions. Measurements were made on leaves of non-fumigated (control, white bars) and ethylene-treated plants (filled bars) progressively exposed to increasing temperatures (from 25°C–45°C), followed by a final temperature fluctuation treatment (50/25°C). Asterisk (*) indicates statistical difference (Student's t test, P < 0.05) between ethylene-treated and control plants.
Figure 6.
Effect of airborne ethylene (approximately 100 nL L−1) on the de-epoxidation state of the xanthophyll cycle (DPS), and β-carotene (β-Car) and α-tocopherol (α-Toc) concentrations in holm oak exposed to heat stress under irrigated and water-stressed conditions. Measurements were made on leaves of non-fumigated (control, white bars) and ethylene-treated plants (filled bars) progressively exposed to increasing temperatures (from 25°C–45°C), followed by a final temperature fluctuation treatment (50/25°C). DPS was estimated as (Z + 0.5A)/(Z + A + V), where Z is zeaxanthin, A is antheraxanthin, and V is violaxanthin. Asterisk (*) indicates statistical difference (Student's t test, P < 0.05) between ethylene-treated and control plants.
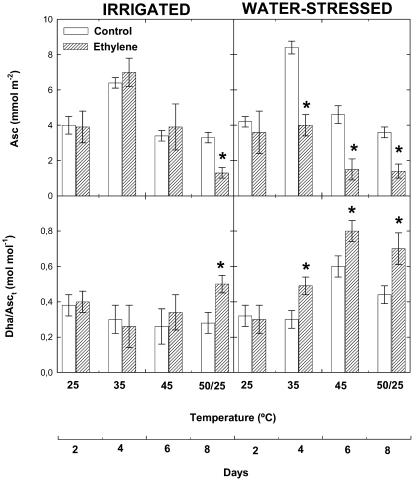
The extent of antioxidant protection differed in ethylene-treated and control plants (Figs. 6 and 7). In control plants, levels of α-tocopherol increased 2-fold in response to heat stress and then decreased under strong temperature fluctuations (50/25°C). By contrast, α-tocopherol levels did not increase under heat stress in ethylene-treated plants, and stayed at levels similar to (under strong temperature fluctuations) or even lower (at 35°C and 45°C) than those that appeared at 25°C (Fig. 6). Changes in α-tocopherol levels under a combination of heat and drought stress did not differ from those observed under heat stress in either ethylene-treated or control plants. Ascorbate levels increased as well in response to heat stress, but the response differed from that of α-tocopherol (Fig. 7). Maximum ascorbate levels were attained at 35°C and then decreased progressively as temperature increased. Ethylene-treated plants showed lower levels of ascorbate than controls under strong temperature fluctuations (50/25°C) and also above 35°C under drought stress. This was associated with higher oxidation of ascorbate in ethylene-treated plants as compared to controls, as indicated by the redox state of ascorbate. In irrigated plants, maximum oxidation of ascorbate was attained in ethylene-treated plants exposed to strong temperature fluctuations. In drought, the redox state of ascorbate shifted toward its oxidized form at 35°C in ethylene-treated plants, while this shift was not observed until 45°C in control plants.
Figure 7.
Effect of airborne ethylene (approximately 100 nL L−1) on ascorbate (Asc) concentrations and the redox state of ascorbate (estimated as Dha/Asct, where Asct = Dha + Asc) in holm oak exposed to heat stress under irrigated and water-stressed conditions. Measurements were made on leaves of non-fumigated (control, white bars) and ethylene-treated plants (filled bars) progressively exposed to increasing temperatures (from 25°C–45°C), followed by a final temperature fluctuation treatment (50/25°C). Asterisk (*) indicates statistical difference (Student's t test, P < 0.05) between ethylene-treated and control plants.
DISCUSSION
Plant-emitted ethylene has been shown to be involved in plant responses to environmental stresses, conferring protection against heat and drought stress in several, although not all, species (Abeles et al., 1992; Weyers and Paterson, 2001; Larkindale and Knight, 2002). Ethylene mediates the response to stress, acting as a signal molecule at low concentrations, and, in cooperation with other signaling compounds (abscisic acid, salicylic acid, and calcium), it may reduce heat-stress-induced oxidative damage in some species (Larkindale and Knight, 2002). However, the effect of airborne ethylene at high concentrations such as those found in polluted areas (20–100 nL L−1) for several days on plant stress tolerance has been poorly studied so far, giving contrasting results (for review, see Cape, 2003). While increasing airborne ethylene concentrations to 15 to 60 nL L−1 for 11 d resulted in reduced wilting under drought stress in Helianthus annuus (Reid and Watson, 1985), it has been shown that the reaction of ozone with ethylene in the substomatal cavity may increase ozone-induced damage in plants (Mehlhorn et al., 1991). Furthermore, the effects of ethylene at concentrations found in polluted areas on plant thermotolerance have not been investigated so far. Other studies on exogenous applications of ethylene or ethylene-releasing compounds and their effects on plant responses to environmental stresses have been done, but the ethylene concentrations applied are not representative of those found in nature (for review, see Cape, 2003).
For many physiological effects, the dose-response curves for airborne ethylene have thresholds around 10 nL L−1 and half-maximal responses in the range of 100 to 500 nL L−1 (Gunderson and Taylor, 1988; Abeles et al., 1992). Thus, airborne ethylene at concentrations found in polluted areas (20–100 nL L−1 or even higher) for several days may potentially influence plant physiology and alter plant responses to environmental stresses. We show here, for the first time to our knowledge, that airborne ethylene at concentrations found in polluted areas (100 nL L−1) for several days may negatively affect plant responses to environmental stresses, namely heat and drought stress, by altering, among other possible mechanisms, plant antioxidant defenses. Interestingly, the negative effects of airborne ethylene were only observed under stress and were more evident as the stress was more severe. Plants treated with the same airborne ethylene concentrations for 8 d and kept at 25°C under irrigated conditions did not show any symptoms of oxidative damage or alterations in photo- and antioxidant protection (data not shown), which indicates that ethylene alters the physiology of holm oak plants only under stress. Although more evidence is needed to fully understand this stress-specific response to airborne ethylene in holm oak, there is evidence in other species of stress effects on perception of ethylene, and it is possible that some steps of the ethylene signal transduction pathway may be influenced by stress (for review, see Morgan and Drew, 1997).
Holm oak plants without ethylene treatment displayed tolerance to temperatures as high as 50°C, which might be attributed, at least in part, to enhanced xanthophyll de-epoxidation (of both lutein epoxide and violaxanthin) and transient increases in α-tocopherol and ascorbate, which are known to protect chloroplasts from excess excitation energy resulting from reduced CO2 assimilation (Demmig-Adams and Adams, 1992; Asada, 1999; Munné-Bosch and Alegre, 2002). The photo- and antioxidant protection exerted by carotenoids, tocopherols, and ascorbate has been previously reported in sun leaves of holm oak exposed to low temperatures in the field (García-Plazaola et al., 1999). Here, we show that the concerted action of these antioxidants may also play a significant biological role in the tolerance of this species to heat stress.
Holm oak plants without ethylene treatment suffered oxidative stress, as indicated by a shift of the redox state of ascorbate toward its oxidized form and enhanced malondialdehyde levels, only when water deficit was superimposed on temperatures above 45°C. In addition, drought stress for 8 d increased visual leaf area damage 4-fold under heat stress as compared to irrigated plants. This is indicative of this species' high degree of tolerance for heat stress and of its sensitivity to drought when combined with heat stress, which is in agreement with previous studies (Tetriach, 1993; Peñuelas et al., 1998; Larcher, 2000).
To our knowledge, ethylene emission by holm oak leaves has not been studied so far. In this study, no increase in ethylene emission in holm oak under heat stress or under a combination of heat and drought stress was detected, which indicates that hormones other than ethylene may be directly involved in the activation of mechanisms of photo- and antioxidant protection in this species under stress. The absence of stress-induced ethylene synthesis in this study is in agreement with previous studies on other Mediterranean plants, including other species of the genus Quercus, which do not show enhanced ethylene synthesis under various stress treatments (Finch-Savage et al., 1996; Pezeshki et al., 1996; Munné-Bosch et al., 2002).
By contrast, holm oak plants exposed to airborne ethylene concentrations found in polluted areas (100 nL L−1) showed reduced stress tolerance and alterations in mechanisms of photo- and antioxidant protection, thus indicating a sensitivity of this species to airborne ethylene under stress. Ethylene-treated plants showed symptoms of oxidative stress at lower temperatures (35°C) than controls in drought, as indicated by enhanced malondialdehyde levels, lower α-tocopherol and ascorbate concentrations, and a shift of the redox state of ascorbate to its oxidized form. In addition, ethylene-treated plants showed higher visual leaf area damage and stronger reductions in the Fv/Fm ratio than controls in response to heat stress or to a combination of heat and drought stress.
Since it is known that oxidative damage occurs during recovery from heat stress in some species (Gong et al., 1997; Larkindale and Knight, 2002), plants were subjected to strong temperature fluctuations (50/25°C), i.e. exposure at 50°C for 1 d followed by recovery at 25°C for another day, to evaluate the ability of holm oak to prevent oxidative damage under extreme climatic conditions. While control plants were able to withstand this treatment under irrigated conditions, ethylene-treated plants were especially sensitive to it and suffered oxidative damage and leaf injury, as indicated by the significant increases in malondialdehyde concentrations and visual leaf area damage. The higher ethylene-induced sensitivity to environmental stress and oxidative damage in holm oak indicates that this species responds negatively to airborne ethylene at concentrations found in polluted areas. This confirms previous studies that support the contention that ethylene, as occurs with other plant hormones, may be detrimental to plant function when present at high concentrations in leaves (for review, see Morgan and Drew, 1997; Weyers and Paterson, 2001). In that sense, it has been shown that Arabidopsis mutants eto1 and eto3, which produce high levels of ethylene, develop necrotic lesions in response to an acute ozone exposure (Rao et al., 2002).
The mechanisms by which airborne ethylene may reduce tolerance of holm oak plants to heat and drought stress may provoke uncontrolled production of AOS, as indicated by increased lipid peroxidation and reduced antioxidant defenses, under stress. This is partly in agreement with previous studies, which have shown that ethylene at high concentrations (>1 μL L−1) may reduce antioxidant defenses, increase lipid peroxidation and induce cell death in some species (Lee et al., 1998; Hodges and Forney, 2000). However, the biological significance of these studies is difficult to ascertain since native vegetation is not exposed to ethylene concentrations above 1 μL L−1 for several days in nature, even in polluted areas. Thus, any similarity between these studies and ours may be accidental, especially considering that responses to airborne ethylene may largely depend on ethylene concentrations, and further research is needed to better understand the relationship between airborne ethylene, AOS production, and cell death in plants found in polluted areas.
CONCLUSION
In conclusion, we have shown that (1) holm oak plants activate several mechanisms of photo- and antioxidant protection to withstand heat and drought stress; (2) these mechanisms are able to protect the leaves from oxidative damage, when stress is not extremely severe; (3) ethylene reduces photosynthesis and alters antioxidant protection under heat and drought stress; and (4) ethylene at concentrations found in polluted areas may decrease the stress tolerance of native Mediterranean vegetation. The mechanisms by which airborne ethylene at concentrations found in polluted areas may reduce plant stress tolerance are still not fully understood, but our results support the contention that airborne ethylene may trigger the uncontrolled production of AOS, overwhelm antioxidant defenses, and induce cell death under stress. Further studies are needed to better understand the signal transduction pathway involved in the response of stressed holm oak plants to airborne ethylene, especially considering the effect of ethylene in conjunction with other air pollutants.
MATERIALS AND METHODS
Experimental System, Ethylene Treatment, and Variables Measured
Two-year-old holm oak plants (Quercus ilex), which were obtained from a nursery (Forestal Catalana S.A., Breda, Spain), were grown in a greenhouse in 2-L pots with peat and sand (2:1, v/v) containing slow-release fertilizer (2 g L−1, 15:8:11 N,P,K + 2Mg) under irrigated conditions. For each experimental set, 20 plants were transferred into a 1.59-m3 (1.75 m high × 1.25 m wide × 0.725 m deep) chamber (Vötsch-industrietechnik Bio Line model VB 1014; Balingen-Frommern, Germany), in which environmental conditions (photosynthetically active photon flux density, relative humidity, and air temperature) were programmed independently by an automated control mechanism. Light was supplied by 8 400-W halogen lamps (model HQI-T; Vötsch-industrietechnik), each supplying a photosynthetically active photon flux density of 500 to 600 μmol m−2 s−1 at a height of 0.9 m on a plane over the plant during a 12-h photoperiod; relative humidity in the chamber was kept at around 50% throughout the experiment. Plants were exposed to two watering regimes: one-half of the plants (10 individuals) were watered daily, once with tap water and once with Hoagland solution (irrigated plants), while the other half did not receive water at all for 8 d (water-stressed plants). During this period, each group of plants was exposed to temperature increases from 25°C to 45°C, followed by a final temperature fluctuation (50/25°C) treatment. For high temperature treatments, plants were progressively exposed to from 25°C to 35°C and then to 45°C and were kept at such temperatures for 2 d before measurements. After exposure to 45°C, plants were subjected to 50°C for 1 d, followed by a sharp decrease to 25°C, at which temperature plants were kept for one more day before measurements.
Two different atmospheres were assayed as a result of ethylene fumigation and non-fumigation (control) in four experimental sets, two sets for each treatment, in plants of the same height (approximately 0.9 m) transferred to the environmental growth chamber between May 19, 2003, and July 22, 2003. Ethylene treatment was applied by regulating the entrance of 1% (v/v) ethylene (Abelló Linde S.A., Barcelona) with a manometer-coupled valve (model VXG41.15R; Landis & Gyr., Geneva) controlled by a Sho-rate rotameter (model 1350/D1A9D1B00000; Brooks Instruments B.V., Veenendaal, The Netherlands). The airflow was measured with a mass flow meter (model 5811 N; Brooks Instruments B.V.) attached to the chamber. The airborne ethylene concentrations in the fumigated chamber were kept at around 100 to 125 nL L−1, whereas those in the control chamber ranged between 2 and 3 nL L−1 throughout the experiment, as estimated by gas chromatography coupled to mass spectrometry (GC-MS; see below). Airflow in the chamber was 0.125 m3/min, and air in the chamber was completely renewed every 12.7 min.
Ethylene emission, leaf water status, gas exchange, chlorophyll fluorescence, chlorophylls, lipid peroxidation, and the extent of photo- and antioxidative protection (levels of carotenoids, tocopherols, and ascorbate) were determined in fully developed young leaves of randomly chosen plants grown under irrigated and water-stress conditions in non-fumigated (control) and ethylene-treated atmospheres. For measurements of lipid peroxidation, photosynthetic pigments, and antioxidants, leaves were collected around 1.5 h after the start of the light period, frozen in liquid nitrogen, and stored at −20°C until analyses. Measurements of ethylene emission, leaf water status, gas exchange, and chlorophyll fluorescence were performed around 2 h after the start of the light period. Estimation of leaf area damage in plants was performed at the end of the experiment. Leaves showing necrotic lesions were discarded for all other measurements. Measurements at each temperature were made on four to six individuals belonging to duplicated experiments for each assayed atmosphere.
Ethylene Measurements
Ethylene emission by leaves and ethylene concentrations in fumigated and non-fumigated chambers were determined by GC-MS. A Ciras-2 gas exchange system (PP Systems, Hertfordshire, England) was used for sampling foliar emissions. Intact leaves were clamped in an automatic Parkinson leaf cuvette (Std Broad 2.5). Air coming out of the cuvette flowed through a T system to a glass tube (11.5 cm long and 0.4 cm i.d.) filled with adsorbents Carbotrap C (300 mg), Carbotrap B (200 mg), and Carbosieve S-III (125 mg) from Supelco (Bellefonte, PA) separated by plugs of quartz wool. The tubes' hydrophobic properties minimized sample displacement by water and produced no chemical transformation as checked with standards. Prior to use, they were conditioned for 3 min at 350°C with a stream of purified helium. The sampling time was 5 min and the flow was 500 mL min−1 with a calibrated sampler pump (Escort Elf Pump; Mine Safety Appliances, Pittsburgh). Blanks of 15 min free air sampling without clamping leaves were carried out immediately before and after each measurement. The glass tubes (with trapped volatiles) were stored at −28°C until analysis (no more than 24–48 h later). For sampling airborne ethylene in fumigated and non-fumigated chambers, part of the air exiting the chamber flowed through a T system to a glass tube filled with adsorbents Carbotrap C, Carbotrap B, and Carbosieve S-III (Supelco) as described above.
Ethylene analyses were performed by using a GC-MS system (Hewlett Packard HP59822B; Palo Alto, CA). Samples of trapped ethylene were desorbed (Thermal Desorption Unit, model 890/891; Supelco) at 250°C for 2 min and injected into a 30 m × 0.25 mm × 0.25 mm film thickness capillary column (Supelco HP-5, Crosslinked 5% Me Silicone; Supelco). After sample injection, the initial temperature (46°C) was increased at 30°C min−1 up to 70°C and thereafter at 10°C min−1 up to 150°C, a temperature that was maintained for another 5 min. Helium flow was 1 mL min−1. The identification of ethylene was conducted by comparison with the mass spectra of an authentic standard from Abelló Linde S.A.
The absence of ethylene emission by holm oak leaves under heat and drought stress was confirmed by using a high-sensitivity Proton-Transfer-Reaction Mass Spectrometer (PTR-MS-FTD hs) system with a fast drift tube (Ionicon Analytik GesmbH, Innsbruck, Austria). Sampling was performed as described before, except that part of the air exiting the leaf cuvette flowed through a T system to the PTR inlet. The PTR-MS system was operated as described elsewhere (Lindinger et al., 1998).
Water Status and Leaf Mass per Area Ratio
Leaves were weighed (fresh wt) and leaf area was immediately measured using a LI-COR LI-3100 area meter (LI-COR, Lincoln, NE). Then leaves were oven dried at 60°C to constant weight (dry wt). The hydration of leaves (H) and leaf mass per area ratio (LMA) were determined as (fresh wt − dry wt)/dry wt and dry wt/leaf area, respectively.
Leaf Gas Exchange and Chlorophyll Fluorescence Measurements
Calibrated Ciras-2 porometers (PP Systems) were used for determination of CO2 and water exchange. Intact leaves were clamped into a Parkinson leaf cuvette (Std Broad 2.5), and net CO2 assimilation and stomatal conductance rates were calculated from variations in gas exchange concentrations according to Von Caemmerer and Farquhar (1981).
Measurements of Fv/Fm were conducted in situ on attached leaves with a portable pulse-modulated fluorimeter PAM-2000 (Walz, Effeltrich, Germany). Leaves were dark adapted with leaf holder clips for 20 min prior to measurements. The Fv/Fm ratio was calculated as (Fm − Fo)/Fm, where Fm and Fo are the maximum and basal fluorescence yields, respectively, of dark-adapted leaves (Genty et al., 1989).
Estimation of Lipid Peroxidation
The extent of lipid peroxidation in leaves was estimated by measuring the amount of malondialdehyde using the method described by Hodges et al. (1999), which takes into account the possible influence of interfering compounds in the assay for thiobarbituric acid (TBA)-reactive substances. In short, samples were repeatedly extracted (4 times) with 80:20 (v/v) ethanol/water containing 1 μg mL−1 butylated hydroxytoluene (BHT) using ultrasonication (Vibra-Cell Ultrasonic processor; Sonics & Materials, Canbury, CT). After centrifugation, supernatants were pooled and an aliquot of appropriately diluted sample was added to a test tube with an equal volume of either (1) −TBA solution containing 20% (w/v) trichloroacetic acid and 0.01% (w/v) BHT, or (2) +TBA solution containing the above plus 0.65% (w/v) TBA. Samples were heated at 95°C for 25 min and, after cooling, absorbance was read at 440 nm, 532 nm, and 600 nm. Malondialdehyde equivalents (nmol mL−1) were calculated as 106 × ((A − B)/157,000), where A = ((Abs 532+TBA) − (Abs 600+TBA) − (Abs 532−TBA − Abs 600−TBA)), and B = ((Abs 440+TBA − Abs 600+TBA) × 0.0571).
Photosynthetic Pigments and Antioxidants
The extraction and HPLC analyses of photosynthetic pigments and antioxidants (chlorophylls, carotenoids, tocopherols, and reduced and oxidized ascorbate) were carried out essentially as described by Munné-Bosch and Alegre (2003). For pigment and tocopherol analyses, leaves were repeatedly extracted (4 times) with ice-cold 85 (v/v) and 100% acetone using ultrasonication (Vibra-Cell Ultrasonic processor). For ascorbate analyses, the same extraction procedure was followed by using ice-cold extraction buffer (40% [v/v] methanol, 0.75% [w/v] m-phosphoric acid, 16.7 mm oxalic acid, 0.127 mm diethylenetriaminepentaacetic acid) instead of acetone. Following extraction, 0.1 mL of the pooled supernatants were transferred to 0.9 mL of the mobile phase (24.25 mm Na-acetate/acetic acid, pH 4.8; 0.1 mm diethylenetriaminepentaacetic acid; 0.015% [w/v] m-phosphoric acid; 0.04% [w/v] octylamine; 15% [v/v] methanol) for determination of reduced ascorbate. For determination of total ascorbate (reduced plus oxidized), 0.1 mL of the pooled supernatants were incubated for 10 min at room temperature in darkness with 0.25 mL of 2% (w/v) dithiothreitol and 0.5 mL of 200 mm NaHCO3. The reaction was stopped by adding 0.25 mL of 2% (v/v) sulfuric acid and 0.8 mL of the mobile phase. After extraction of photosynthetic pigments, tocopherols, and reduced and oxidized ascorbate, HPLC analyses were performed immediately as described.
Chlorophylls and carotenoids (neoxanthin, lutein, lutein epoxide, violaxanthin, antheraxanthin, zeaxanthin, and α- and β-carotene) were separated on a DuPont non-endcapped Zorbax ODS-5 μm column (250 × 4.6 mm, 20%C; Scharlau, Barcelona) at 30°C at a flow rate of 1 mL min−1. The solvents consisted of (A) acetonitrile/methanol (85:15, v/v) and (B) methanol/ethyl acetate (68:32, v/v). The gradient used was: 0 to 14 min 100% A, 14 to 16 min decreasing to 0% A, 16 to 28 min 0% A, 28 to 30 min increasing to 100% A, and 30 to 38 min 100% A. Detection was carried out at 445 nm (Diode array detector 1000S; Applied Biosystems, Foster City, CA). Compounds were identified by their characteristic spectra and by coelution with authentic standards, which were obtained from Fluka (Buchs, Switzerland).
α-Tocopherol was separated on a Partisil 10 ODS-3 column (250 × 4.6 mm; Scharlau) at a flow rate of 1 mL min−1. The solvents consisted of (A) methanol/water (95:5, v/v) and (B) methanol. The gradient used was: 0 to 10 min 100% A, 10 to 15 min decreasing to 0% A, 15 to 20 min 0% A, 20 to 23 min increasing to 100% A, and 23 to 28 min 100% A. α-Tocopherol was quantified through its A283 (Diode array detector 1000S) and was identified by its characteristic spectrum and by coelution with an authentic standard, which was obtained from Fluka.
Ascorbate was isocratically separated on a Spherisorb ODS C8 column (Teknokroma, St. Cugat, Spain) using the mobile phase (24.25 mm Na-acetate/acetic acid, pH 4.8; 0.1 mm diethylenetriaminepentaacetic acid; 0.015% [w/v] m-phosphoric acid; 0.04% [w/v] octylamine; 15% [v/v] methanol) at a flow rate of 0.8 mL min−1. Detection was carried out at 255 nm (Diode array detector 1000S). Ascorbate was identified by its characteristic spectrum and by coelution with an authentic standard from Sigma (Steinheim, Germany).
Visual Leaf Area Damage
The percentage of damaged (discolored or brown) leaves was measured at the end of the experiment by measuring the damaged area of all the plant leaves. Leaf area (separating damaged and healthy leaves) was measured using a LI-COR LI-3100 area meter.
Statistical Analyses
Statistical differences between controls and ethylene-treated plants were analyzed following the Student's t test using SPSS (Chicago). Differences were considered significant at a probability level of P < 0.05. Data shown in the figures correspond to the overall treatment mean ± the range observed between the two chambers for either controls (non-fumigated) or ethylene-treated plants progressively exposed to increasing temperatures under irrigated and water-stress conditions.
Acknowledgments
We are very grateful to the Serveis Científico-Tècnics (Universitat de Barcelona) for technical assistance.
This work was supported by the Spanish Government (grant nos. REN2001–0003 and REN2003–04871).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.050005.
References
- Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in Plant Biology. Academic Press, San Diego
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Bussotti F, Ferretti M (1998) Air pollution, forest condition and forest decline in Southern Europe: an overview. Environ Pollut 101: 49–65 [DOI] [PubMed] [Google Scholar]
- Cape JN (2003) Effects of airborne volatile organic compounds on plants. Environ Pollut 122: 145–157 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol 43: 599–626 [Google Scholar]
- Doke N (1997) The oxidative burst: role in signal transduction and plant stress. In SG Scandalios, ed, Oxidative Stress and the Molecular Biology of Antioxidant Defences. Spring Harbor Laboratory Press, New York, pp 785–813
- Eichhorn J, Paar U (2000) Oak decline in Europe. Methods and results of assessments in the ICP forests. In T Oszako, C Delatour, eds, Recent Advances on Oak Health in Europe. Forest Research Institute, Warsaw, pp 41–51
- Eskling M, Arvidsson PO, Akerlund HE (1997) The xanthophyll cycle, its regulation and components. Physiol Plant 100: 806–816 [Google Scholar]
- Finch-Savage W, Blake P, Clay H (1996) Desiccation stress in recalcitrant Quercus robur L. seeds results in lipid peroxidation and increased synthesis of jasmonates and abscisic acid. J Exp Bot 47: 661–667 [Google Scholar]
- Foyer CH, Noctor G (1999) Leaves in the dark see the light. Science 284: 599–601 [DOI] [PubMed] [Google Scholar]
- García-Plazaola JI, Artexte U, Becerril JM (1999) Diurnal changes in antioxidant and carotenoid composition in the Mediterranean schlerophyll tree Quercus ilex (L) during winter. Plant Sci 143: 125–133 [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Gong M, Chen SN, Song YQ, Li ZG (1997) Effect of calcium and calmodulin on intrinsic heat tolerance in relation to antioxidant systems in maize seedlings. Aust J Plant Physiol 24: 371–379 [Google Scholar]
- Gunderson CA, Taylor GE (1988) Kinetics of inhibition of foliar gas exchange by exogenous ethylene: an ultrasensitive response. New Phytol 110: 517–524 [Google Scholar]
- Halliwell B, Gutteridge JMC (1999) Free Radicals in Biology and Medicine. Clarendon, Oxford
- Havaux M (1998) Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci 3: 147–151 [Google Scholar]
- Heilman MD, Meredith FI, González CL (1971) Ethylene production in the cotton plant (Gossypium hirsutum L.) canopy and its effect on fruit abscission. Crop Sci 11: 25–27 [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reacting substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604–611 [DOI] [PubMed] [Google Scholar]
- Hodges DM, Forney CF (2000) The effects of ethylene, depressed oxygen and elevated carbon dioxide on antioxidant profiles of senescing spinach leaves. J Exp Bot 51: 645–655 [DOI] [PubMed] [Google Scholar]
- Kozaki A, Takeba G (1996) Photorespiration protects C3 plants from photooxidation. Nature 384: 557–560 [Google Scholar]
- Larcher W (2000) Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosyst 134: 279–295 [Google Scholar]
- Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128: 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Chae HS, Lee TK, Kim SH, Shin SH, Cho BH, Cho SH, Kang BG, Lee WS (1998) Ethylene-mediated phospholipid catabolic pathway in glucose-starved carrot suspension cells. Plant Physiol 116: 223–229 [PMC free article] [Google Scholar]
- Lindinger W, Hansel A, Jordan A (1998) On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS): medical applications, food control, and environmental research. Int J Mass Spectrom Ion Process 173: 191–241 [Google Scholar]
- Mehlhorn H, Oshea JM, Mellburn AR (1991) Atmospheric ozone interacts with stress ethylene formation by plants to cause visible plant injury. J Exp Bot 42: 17–24 [Google Scholar]
- Morgan PG, Drew MC (1997) Ethylene and plant responses to stress. Physiol Plant 100: 620–630 [Google Scholar]
- Munné-Bosch S, Alegre L (2002) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21: 31–57 [Google Scholar]
- Munné-Bosch S, Alegre L (2003) Drought-induced changes in the redox state of α-tocopherol, ascorbate, and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiol 131: 1816–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, López-Carbonell M, Alegre L, van Onckelen HA (2002) Effect of drought and high solar radiation on 1-aminocyclopropane-1-carboxylic acid and abscisic acid concentrations in Rosmarinus officinalis plants. Physiol Plant 114: 380–386 [DOI] [PubMed] [Google Scholar]
- Op den Camp RGL, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Göbel C, Feussner I, et al (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15: 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond B, Badger M, Maxwell K, Björkman O, Leegod R (1997) Too many photons: photorespiration, photoinhibition and photooxidation. Trends Plant Sci 2: 119–120 [Google Scholar]
- Pastori G, Foyer CH (2002) Common components, networks, and pathways of cross-tolerance to stress: the central role of “redox” and abscisic acid-mediated controls. Plant Physiol 129: 460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñuelas J, Filella I, Llusià J, Siscart D, Piñol J (1998) Comparative field study of spring and summer leaf gas exchange and photobiology of the Mediterranean trees Quercus ilex and Phillyrea latifolia. J Exp Bot 49: 229–238 [Google Scholar]
- Pezeshki SR, Pardue JH, DeLaune RD (1996) Leaf gas exchange and growth of flood-tolerant and flood-sensitive tree species under low soil redox conditions. Tree Physiol 16: 453–458 [DOI] [PubMed] [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LACJ, de Kroon H, Visser EJW (2004) Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signalling. Plant J 38: 310–319 [DOI] [PubMed] [Google Scholar]
- Rao MV, Lee H, Davis KR (2002) Ozone-induced ethylene production is dependent on salicylic acid, and both salicylic acid and ethylene act in concert to regulate ozone-induced cell death. Plant J 32: 447–456 [DOI] [PubMed] [Google Scholar]
- Reid DM, Watson K (1985) Ethylene as an air pollutant. In JA Roberts, G Tucker, eds, Ethylene and Plant Development. Butterworths, London, pp 277–286
- Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125: 27–58 [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Plant Sci 19: 267–290 [DOI] [PubMed] [Google Scholar]
- Squier SA, Taylor GE, William TJ, Selvidge WJ, Gunderson CA (1985) Effect of ethylene and related hydrocarbons on carbon assimilation and transpiration in herbaceous and woody species. Environ Sci Technol 19: 432–437 [DOI] [PubMed] [Google Scholar]
- Tetriach M (1993) Photosynthesis and transpiration of evergreen Mediterranean and deciduous trees in an ecotone during a growing season. Acta Oecol 14: 341–360 [Google Scholar]
- Tonneijck AEG, Jansen BP, Bakker C (2000) Assessing the effects of atmospheric ethylene on epinasty and tuber yield of potato (Solanum tuberosum L.) near polyethylene manufacturing plants. Environ Monit Assess 60: 57–69 [Google Scholar]
- Tonneijck AEG, ten Berge WF, Jansen BP, Bakker C (1999) Epinastic response of potato to atmospheric ethylene near polyethylene manufacturing plants. Chemosphere 39: 1617–1628 [Google Scholar]
- Von Caemmerer S, Farquhar GD (1981) Some relations between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- Weyers JDB, Paterson NW (2001) Plant hormones and the control of physiological processes. New Phytol 152: 375–407 [DOI] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu J (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14 (Suppl): S165–S183 [DOI] [PMC free article] [PubMed] [Google Scholar]