Abstract
Subclinical kidney disease is associated with developing hypertension in the general population, but data are lacking among HIV-infected persons. We examined associations of kidney function and injury with incident hypertension in 823 HIV-infected and 267 HIV-uninfected women in the Women’s Interagency HIV Study, a multicenter, prospective cohort of HIV-infected and uninfected women in the United States. Baseline kidney biomarkers included estimated glomerular filtration rate using cystatin C (eGFR), urine albumin-to-creatinine ratio (ACR), and seven urine biomarkers of tubular injury: alpha-1-microglobulin, interleukin-18 (IL-18), kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, liver fatty acid binding protein, N-acetyl-beta-D-glucosaminidase (NAG), and alpha1-acid-glycoprotein (AAG). We used multivariable Poisson regression to evaluate associations of kidney biomarkers with incident hypertension, defined as two consecutive visits of anti-hypertensive medication use. Over a median follow-up of 9.6 years, 288 HIV-infected women (35%) developed hypertension. Among the HIV-infected women, higher ACR was independently associated with incident hypertension (RR=1.13 per ACR doubling, 95%CI: 1.07, 1.20), as was lower eGFR (RR=1.10 per 10 ml/min/1.73m2 lower eGFR, CI: 1.04, 1.17). No tubular injury and dysfunction biomarkers were independently associated with incident hypertension in HIV-infected women. In contrast, among the HIV-uninfected women, ACR was not associated with incident hypertension, whereas higher IL-18, AAG and NAG were significantly associated with incident hypertension. These findings suggest that early glomerular injury and kidney dysfunction may be involved in the pathogenesis of hypertension in HIV-infected persons. The associations of the tubular markers with hypertension in HIV-uninfected women should be validated in other studies.
Keywords: HIV, hypertension, kidney disease, albuminuria, glomerular filtration rate, urine biomarker
Introduction
With the success of combination antiretroviral therapy, people with human immunodeficiency virus (HIV) infection have dramatically increased life expectancies and face an increasing burden of age-related comorbidities, including cardiovascular disease.1,2 Hypertension in HIV-infected individuals is common, with prevalence estimates ranging from 13–45%,3–7 and associated with a twofold increased risk of acute myocardial infarction.8 Despite these observations, our understanding of risk factors for hypertension in the HIV-infected population is limited.9–12
The kidneys play a central role in blood pressure regulation.13 Reduced kidney function is common among HIV-infected individuals, with prevalence estimates of chronic kidney disease ranging from 7–32%.14–17 Compared to uninfected individuals, HIV-infected individuals have a threefold risk of developing end-stage renal disease and have significantly higher cystatin C levels, an endogenous marker of filtration.18–20 While several cross-sectional studies show that chronic kidney disease is highly prevalent among HIV-infected individuals with hypertension,17,21 to our knowledge there are no studies in the HIV population that have evaluated whether reduced kidney function is associated with the development of hypertension.
Substantial kidney injury occurs subclinically – before a measurable reduction in kidney function – and is independently associated with hypertension among HIV-uninfected persons.22,23 The most common measure of kidney injury is the urinary albumin concentration, which reflects endothelial and hemodynamic changes within the glomerulus. HIV-infected individuals have a fivefold higher prevalence of microalbuminuria compared to uninfected individuals, and higher urinary albumin levels among HIV-infected individuals are associated with subsequent kidney function decline.24,25 Glomerular injury leading to the development of hypertension may explain the strong association between albuminuria and cardiovascular disease in HIV-infected individuals.26 Higher urinary albumin levels may also reflect widespread inflammation, which could be a particularly important mechanism of hypertension in HIV-infected individuals because of HIV-associated chronic immune system activation.27,28
Tubulointerstitial injury may also play an important role in the pathophysiology of hypertension. Several experimental studies have shown tubulointerstitial injury may lead to hypertension through abnormal tubuloglomerular feedback and impaired sodium handling.22,29 The proximal tubules are known to be a site of the latent HIV reservoir, and we have recently shown that several tubular injury markers are not only elevated in HIV-infected individuals compared to HIV-uninfected individuals, but are also associated with longitudinal decline in kidney function.25,30,31 To our knowledge, no epidemiologic studies in any population have evaluated the association of biomarkers for tubular dysfunction and injury with the development of hypertension.
In this study, we investigated whether markers of kidney function, glomerular injury and tubular dysfunction and injury are associated with incident hypertension in a large cohort of HIV-infected and HIV-uninfected women. We evaluated kidney function with estimated glomerular filtration rate (eGFR) by cystatin C, glomerular injury with the urinary albumin-to-creatinine ratio (ACR), and tubular dysfunction and injury with seven urine biomarkers: alpha-1-microglobulin (α1m), interleukin-18 (IL-18), kidney injury marker-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), liver fatty acid binding protein (L-FABP), N-acetyl-beta-D-glucosaminidase (NAG), and alpha1-acid-glycoprotein (AAG).
Methods
Study design
The Women’s Interagency HIV Study (WIHS) study design and methods have been described previously.32 In brief, 3,766 women (2,791 HIV-infected and 975 uninfected women) of similar backgrounds were enrolled in 1994–1995 (n=2,623) and 2001–2002 (n=1,143) from six sites (Bronx/Manhattan, Brooklyn, Chicago, Los Angeles, San Francisco and Washington, DC). The WIHS HIV Kidney Aging study was designed as a nested cohort study to investigate the onset of kidney disease in the setting of HIV. Between October 1999 and March 2000, 1,197 women (908 HIV-infected and 289 HIV-uninfected) had baseline urine and serum specimens collected. This study included the HIV-infected and HIV-uninfected women with available urine and serum specimens collected during this time interval. We excluded from our analysis women with prevalent hypertension at the time of specimen collection, defined as the use of anti-hypertensive medication at ≥2 consecutive visits prior to and including the nested study baseline visit. We also excluded participants without any follow-up blood pressure measurement after the baseline visit. Follow-up started at the nested study baseline visit and was truncated at 10 years.
WIHS was approved by the institutional review boards of all participating institutions, and informed consent was obtained from all study participants. This study of kidney injury was also approved by the Committee on Human Research of the University of California, San Francisco.
Predictors
We evaluated eGFR, ACR, and seven urine markers of tubular injury: IL-18, KIM-1, NGAL, NAG, AAG, α1m, and L-FABP. We calculated eGFR using the 2012 CKD-EPI cystatin C equation.33 Cystatin C was measured by a particle-enhanced immunoturbidimetry assay (Gentian). All urinary biomarkers were measured at the Cincinnati Children’s Hospital Medical Center Biomarker Laboratory (see supplementary material for details on urine biomarker measurements).
We analyzed urine biomarkers as continuous variables (log-transformed due to their right-skewed distribution) and categorical variables divided into tertiles. We additionally analyzed ACR as a dichotomous variable using the clinically meaningful cut-point (ACR≤30 versus >30 mg/g). We analyzed eGFR as a continuous variable, in tertiles, and dichotomized (eGFR ≤60 versus >60 ml/min/1.73m2).
Outcomes
Our primary outcome was incident hypertension, which we defined as use of an anti-hypertensive medication at two consecutive visits. Our secondary outcome was incident hypertension defined as presence of at least two of the following three conditions at two consecutive visits: (1) SBP>140 mmHg or DBP>90 mmHg, (2) use of an anti-hypertensive medication, or (3) self-reported history of hypertension. WIHS-trained personnel assessed blood pressure and medication use during each semiannual WIHS examination. After the participant sat for at least five minutes, three blood pressure measurements were obtained from the right arm using a DINAMAP 1846 SX automated oscillometric device (Critikon, Inc., Tampa, FL). Blood pressure measurements at each visit were averaged across the three readings.
Covariates
Demographics, traditional hypertension risk factors, and HIV-related characteristics were collected at each examination and analyzed as time-varying covariates. The following covariates were included in all models for the HIV-infected group: hepatitis C virus (HCV) infection (confirmed by detectable HCV RNA following a positive HCV antibody result), current CD4 lymphocyte count, current HIV viral load, and current highly active antiretroviral therapy (HAART) use. HCV infection was included in all models for the HIV-uninfected group. Tubular biomarker models also included urine creatinine to account for variations in urine concentration. We tested the following demographic characteristics and traditional hypertension risk factors as candidate covariates in all multivariable models: age; race/ethnicity; diabetes (defined using confirmatory criteria for fasting glucose ≥126mg/dL, self-reported diabetes, self-reported diabetes medication use, or HbA1c ≥6.5%), insulin resistance estimated using the homeostasis model assessment score (HOMA-IR) defined as (insulin × glucose)/405, cigarette smoking status (current, former, never), menopause status, LDL and HDL cholesterol, triglycerides, serum albumin, body mass index (BMI), waist circumference, current stimulant use, and alcohol use (none, light, moderate, heavy). We tested the following HIV-related characteristics as candidate covariates in the HIV-infected group: nadir CD4 lymphocyte count, history of AIDS diagnosis, current HAART use, current nucleoside reverse transcriptase inhibitor (NRTI) use, current non-nucleoside reverse transcriptase inhibitor (NNRTI) use, and current protease inhibitor (PI) use.
Statistical analysis
We performed all analyses separately in the HIV-infected and HIV-uninfected groups. We compared demographic and baseline clinical characteristics by ACR level (ACR≤30 vs. >30 mg/g). We used Poisson regression with a robust variance estimator to model the associations of each kidney biomarker with our primary and secondary definitions of hypertension. For covariate selection, we used Bayesian model averaging and retained variables in our final models with posterior probabilities >35%.34
We evaluated kidney biomarker associations both individually (without adjusting for other biomarkers) and after adjustment for ACR and eGFR. We performed interaction testing to evaluate whether associations of ACR and eGFR with incident hypertension varied by age, race, and diabetes. We also combined the HIV-infected and HIV-uninfected participants into one study population and performed interaction testing to evaluate whether associations of each kidney biomarker with incident hypertension varied by HIV status. We conducted a sensitivity analysis excluding patients with diabetes to evaluate whether kidney biomarker associations with incident hypertension were modified by diabetes.
Bayesian model averaging was performed using the BMA package for the R statistical computing language (R Development Core Team, Vienna, Austria). All other analyses were performed using the SAS system, version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Of the 1,197 women in WIHS with available urine samples collected in the nested study, we excluded 85/908 (9.4%) HIV-infected and 22/289 (7.6%) HIV-uninfected women with prevalent hypertension. The remaining 823 HIV-infected and 267 HIV-uninfected women represented our study population. All subjects had at least two follow up blood pressure measurements (median 19, interquartile range [IQR]: 14, 19).
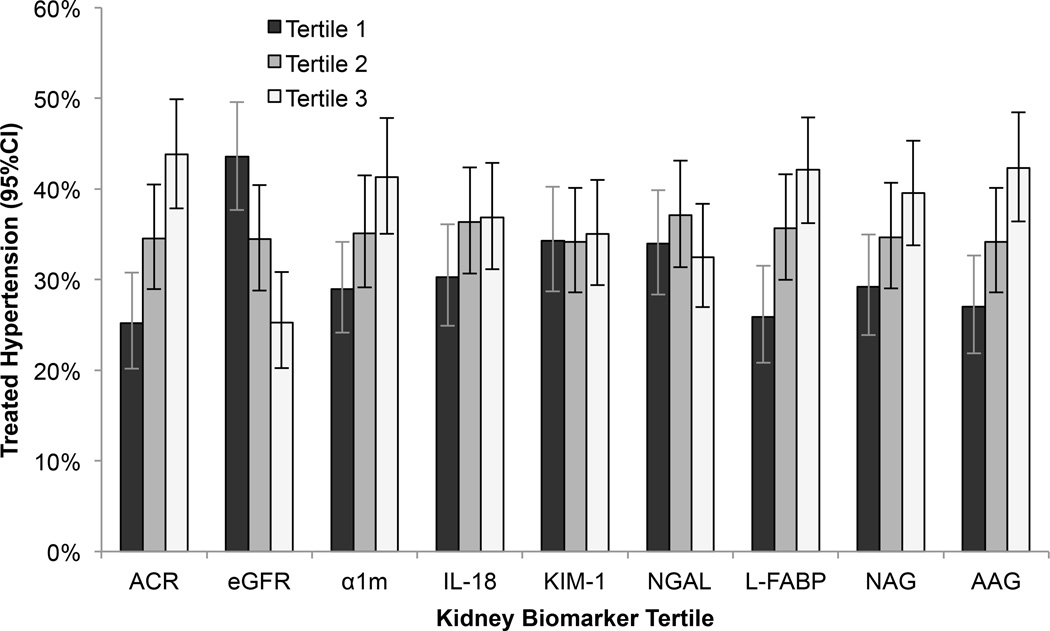
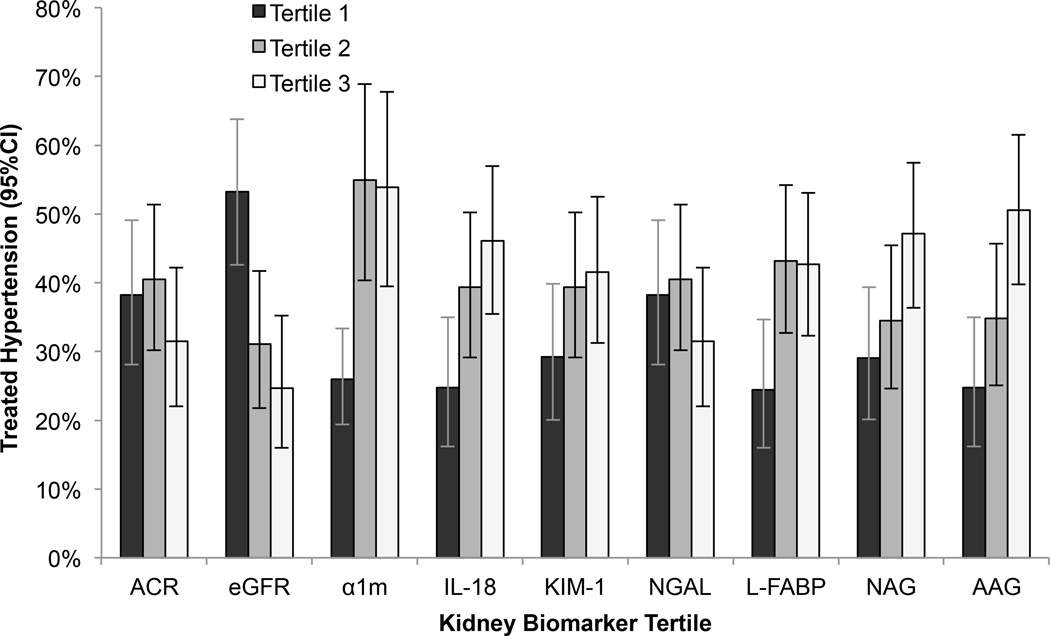
Among the HIV-infected women, the mean age at baseline was 40 years; 59% had a baseline SBP<120 mmHg and DBP<80 mmHg. HIV-infected participants with higher ACR at baseline were more often of African-American race and menopausal, had lower HDL, higher triglycerides, higher HIV viral load, and lower eGFR (Table 1). During a median follow up of 9.6 years (IQR: 9.3, 9.9), 288/823 (35%) HIV-infected and 98/267 (37%) HIV-uninfected women developed hypertension. In unadjusted analyses, lower eGFR and higher α1m, IL-18, L-FABP, NAG, and AAG concentrations were associated with increased risk of incident hypertension in both HIV-infected and HIV-uninfected women, whereas higher ACR concentrations were associated with increased risk of incident hypertension only in HIV-infected women (Figures 1 and 2).
Table 1.
Study population baseline characteristics by ACR
| Demographics | HIV infected women | HIV uninfected women | ||||
|---|---|---|---|---|---|---|
| ACR≤30 mg/g (N=670) |
ACR>30 mg/g (N=153) |
P- value |
ACR≤30 mg/g (N=247) |
ACR>30 mg/g (N=20) |
P- value |
|
| Age (years) | 40 (36–45) | 41 (36–46) | 0.10 | 39 (33–44) | 42 (37–46) | 0.19 |
| Race | ||||||
| African American | 358 (53%) | 101 (66%) | 0.01 | 148 (60%) | 12 (60%) | 0.57 |
| Caucasian | 144 (21%) | 21 (14%) | 30 (12%) | 1 (5%) | ||
| Other | 168 (25%) | 31 (20%) | 69 (28%) | 7 (35%) | ||
| Menopause | 122 (18%) | 41 (27%) | 0.02 | 26 (11%) | 2 (11%) | 0.99 |
| Current smoking | 336 (50%) | 79 (52%) | 0.52 | 139 (57%) | 14 (74%) | 0.33 |
| Diabetes mellitus | 139 (21%) | 39 (25%) | 0.20 | 59 (24%) | 10 (53%) | <0.01 |
| HOMA-IR | 2.8 (2.0–4.1) | 3.4 (2.4–5.6) | <0.01 | 2.7 (1.9–4.2) | 3.7 (2.2–6.5) | 0.10 |
| Systolic blood pressure (mmHg) | 115 (107–122) | 121 (111–130) | <0.01 | 117 (110–127) | 124 (114–133) | 0.15 |
| Diastolic blood pressure (mmHg) | 72 (67–77) | 77 (71–82) | <0.01 | 73 (67–80) | 76 (67–81) | 0.46 |
| LDL cholesterol (mg/dL) | 101 (87–113) | 103 (89–118) | 0.06 | 106 (93–120) | 108 (100–115) | 0.90 |
| HDL cholesterol (mg/dL) | 49 (43–54) | 46 (41–53) | 0.02 | 53 (47–59) | 51 (46–59) | 0.63 |
| TG (mg/dL) | 137 (114–168) | 156 (125–189) | <0.01 | 129 (101–155) | 124 (97–160) | 0.86 |
| BMI (kg/m2) | 26 (23–30) | 27 (23–31) | 0.92 | 29 (24–34) | 33 (25–37) | 0.27 |
| Waist Circumference (cm) | 87 (79–97) | 88 (79–102) | 0.44 | 92 (80–100) | 96 (79–109) | 0.27 |
| Active hepatitis C | 204 (30%) | 48 (31%) | 0.83 | 52 (21%) | 3 (16%) | 0.57 |
| Current stimulant use | 76 (11%) | 12 (8%) | 0.21 | 41 (17%) | 5 (26%) | 0.29 |
| eGFR (ml/min/1.73m2) | 92 (78–106) | 81 (66–96) | <0.01 | 103 (91–117) | 102 (88–108) | 0.46 |
| HAART use | 398 (59%) | 86 (56%) | 0.47 | |||
| NRTI use | 453 (68%) | 93 (61%) | 0.11 | |||
| NNRTI use | 181 (27%) | 47 (31%) | 0.36 | |||
| PI use | 287 (43%) | 58 (38%) | 0.27 | |||
| Current CD4 | 391 (246–555) | 384 (168–624) | 0.58 | |||
| Nadir CD4 | 222 (122–337) | 212 (97–339) | 0.46 | |||
| History of AIDS | 353 (53%) | 82 (54%) | 0.84 | |||
| HIV viral load | 203 (30%) | 41 (27%) | <0.01 | |||
| <=80 | ||||||
| 81–1,999 | 162 (24%) | 22 (15%) | ||||
| 2,000–9,999 | 115 (17%) | 24 (16%) | ||||
| >10,000 | 187 (28%) | 63 (42%) | ||||
Note: Data are presented as median (interquartile range) or percentage.
Abbreviations: ACR, albumin-creatinine ratio; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride; BMI, body mass index; NRTI, nucleoside reverse transcriptase inhibitor; HAART, highly active antiretroviral therapy; HOMA-IR, insulin resistance; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; eGFR, estimated glomerular filtration
Figure 1.
Incident hypertension by tertile of kidney biomarkers in HIV-infected women. In unadjusted analysis, the lowest tertile of estimated glomerular filtration rate using cystatin C (eGFR) was associated with increased risk of incident hypertension compared to the highest tertile, and the highest tertiles of urine albumin-to-creatinine ratio (ACR), alpha-1-microglobulin (α1m), interleukin-18 (IL-18), liver fatty acid binding protein (L-FABP), N-acetyl-beta-D-glucosaminidase (NAG), and alpha1-acid-glycoprotein (AAG) concentrations were associated with increased risk of incident hypertension compared to the lowest tertiles.
Figure 2.
Incident hypertension by tertile of kidney biomarkers in HIV-uninfected women. In unadjusted analysis, the lowest tertile of estimated glomerular filtration rate using cystatin C (eGFR) was associated with increased risk of incident hypertension compared to the highest tertile, and the highest tertiles of alpha-1-microglobulin (α1m), interleukin-18 (IL-18), liver fatty acid binding protein (L-FABP), N-acetyl-beta-D-glucosaminidase (NAG), and alpha1-acid-glycoprotein (AAG) concentrations were associated with increased risk of incident hypertension compared to the lowest tertiles.
After adjusting for traditional and HIV-related hypertension risk factors in the HIV-infected group, the highest tertile of ACR remained associated with an 80% higher risk of incident hypertension, relative to the lowest tertile (Table 2). ACR>30 mg/g was associated with a 73% increased risk of incident hypertension compared to an ACR≤30 mg/g. The association of higher ACR with incident hypertension risk showed little attenuation after adjusting for eGFR.
Table 2.
Association of kidney biomarkers with risk of incident hypertension in HIV-infected women
| Unadjusted | Multivariable Adjusted | |||
|---|---|---|---|---|
| Biomarker | Participants (N) |
RR (95%CI) | Individual Markers * RR (95%CI) |
Individual Markers + ACR + eGFR† RR (95%CI) |
| ACR | ||||
| Continuous | 823 | 1.18 (1.12, 1.25) | 1.15 (1.08, 1.22) | 1.13 (1.07, 1.20) |
| T1: < 6.9 mg/g | 274 | Reference | Reference | Reference |
| T2: 6.9–15 mg/g | 275 | 1.43 (1.05, 1.96) | 1.48 (1.08, 2.02) | 1.49 (1.09, 2.04) |
| T3: >15 mg/g | 274 | 2.11 (1.57, 2.84) | 1.80 (1.33, 2.43) | 1.72 (1.27, 2.33) |
| ≤ 30 mg/g | 670 | Reference | Reference | Reference |
| > 30 mg/g | 153 | 2.00 (1.54, 2.61) | 1.73 (1.32, 2.26) | 1.68 (1.28, 2.21) |
| eGFR | ||||
| Continuous‡ | 823 | 1.16 (1.10, 1.22) | 1.12 (1.06, 1.19) | 1.10 (1.04, 1.17) |
| T1: < 81 ml/min/1.73m2 | 280 | 2.13 (1.59, 2.86) | 1.73 (1.26, 2.38) | 1.65 (1.20, 2.28) |
| T2: 81–99 ml/min/1.73m2 | 270 | 1.43 (1.05, 1.96) | 1.32 (0.96, 1.81) | 1.33 (0.97, 1.83) |
| T3: >99 ml/min/1.73m2 | 273 | Reference | Reference | Reference |
| < 60 ml/min/1.73m2 | 69 | 1.85 (1.28, 2.67) | 1.48 (1.00, 2.18) | 1.32 (0.89, 1.96) |
| ≥ 60 ml/min/1.73m2 | 754 | Reference | Reference | Reference |
| α1m | ||||
| Continuous | 823 | 1.19 (1.08, 1.30) | 1.14 (1.03, 1.26) | 0.98 (0.87, 1.10) |
| T1§ | 339 | Reference | Reference | Reference |
| T2 | 242 | 1.20 (0.89, 1.62) | 1.08 (0.80, 1.46) | 0.98 (0.72, 1.32) |
| T3 | 242 | 1.55 (1.15, 2.10) | 1.22 (0.90, 1.66) | 0.95 (0.69, 1.32) |
| IL-18 | ||||
| Continuous | 823 | 1.09 (0.98, 1.21) | 1.11 (0.99, 1.24) | 1.05 (0.94, 1.18) |
| T1 | 274 | Reference | Reference | Reference |
| T2 | 275 | 1.17 (0.85, 1.59) | 1.19 (0.87, 1.63) | 1.13 (0.82, 1.55) |
| T3 | 274 | 1.15 (0.82, 1.63) | 1.27 (0.89, 1.80) | 1.15 (0.80, 1.64) |
| KIM-1 | ||||
| Continuous | 823 | 1.03 (0.93, 1.14) | 1.00 (0.89, 1.12) | 0.90 (0.80, 1.01) |
| T1 | 274 | Reference | Reference | Reference |
| T2 | 275 | 0.83 (0.61, 1.14) | 0.70 (0.50, 0.97) | 0.68 (0.49, 0.94) |
| T3 | 274 | 0.86 (0.61, 1.19) | 0.78 (0.55, 1.12) | 0.69 (0.48, 0.98) |
| NGAL | ||||
| Continuous | 823 | 1.01 (0.94, 1.10) | 1.01 (0.94, 1.10) | 0.96 (0.89, 1.04) |
| T1 | 274 | Reference | Reference | Reference |
| T2 | 275 | 1.03 (0.77, 1.37) | 1.03 (0.77, 1.38) | 1.03 (0.77, 1.38) |
| T3 | 274 | 0.87 (0.64, 1.19) | 0.90 (0.66, 1.22) | 0.81 (0.59, 1.11) |
| L-FABP | ||||
| Continuous | 823 | 1.11 (1.05, 1.17) | 1.06 (1.00, 1.13) | 1.02 (0.97, 1.08) |
| T1 | 274 | Reference | Reference | Reference |
| T2 | 275 | 1.32 (0.96, 1.83) | 1.14 (0.82, 1.57) | 1.12 (0.81, 1.55) |
| T3 | 273 | 1.77 (1.27, 2.47) | 1.38 (0.98, 1.94) | 1.23 (0.87, 1.75) |
| NAG | ||||
| Continuous | 823 | 1.22 (1.09, 1.35) | 1.14 (1.02, 1.27) | 0.97 (0.86, 1.10) |
| T1 | 274 | Reference | Reference | Reference |
| T2 | 271 | 1.13 (0.81, 1.57) | 0.99 (0.71, 1.39) | 0.92 (0.65, 1.29) |
| T3 | 278 | 1.45 (1.01, 2.06) | 1.19 (0.83, 1.72) | 0.92 (0.63, 1.36) |
| AAG | ||||
| Continuous | 823 | 1.17 (1.10, 1.24) | 1.13 (1.06, 1.20) | 1.03 (0.96, 1.11) |
| T1 | 274 | Reference | Reference | Reference |
| T2 | 275 | 1.31 (0.96, 1.79) | 1.17 (0.85, 1.61) | 1.10 (0.80, 1.52) |
| T3 | 274 | 1.78 (1.31, 2.41) | 1.46 (1.07, 2.00) | 1.10 (0.78, 1.54) |
AAG, α1-acid-glycoprotein; ACR, albumin-to-creatinine ratio; α1m, alpha-1 microglobulin; CI, confidence interval; eGFR, estimated glomerular filtration rate by cystatin C; IL-18, interleukin 18; KIM-1, kidney injury molecule 1; L-FABP, liver fatty acid-binding protein; NAG, N-acetyl-β-D-glucosaminidase; NGAL, neutrophil-gelatinase associated lipocalin; RR, relative risk; T, tertile.
Adjusted for age, race/ethnicity, DM, HOMA-IR, stimulant use, CD4, HIVRNA, NRTI, HAART, HCV, and urine creatinine. Biomarkers included individually, not simultaneously
Adjusted for age, race/ethnicity, DM, HOMA-IR, stimulant use, CD4, HIVRNA, NRTI, HAART, HCV, urine creatinine, ACR, and eGFR.
Continuous eGFR is modeled per 10 ml/min/1.73m2 decrease. All other continuous predictors are modeled per doubling.
All below detectable
Lower eGFR was also associated with incident hypertension in HIV-infected participants after controlling for traditional and HIV-related hypertension factors. Each 10 ml/min/1.73m2 decrement in baseline eGFR was associated with a 12% increased risk of incident hypertension, while the lowest eGFR tertile was associated with a 73% higher risk of developing hypertension compared to the highest eGFR tertile.
In HIV-uninfected women, ACR had minimal association with hypertension after multivariate adjustment including eGFR (Table 3). The test for HIV by ACR interaction was marginally significant (p=0.08). By contrast, lower eGFR was associated with increased risk of incident hypertension in HIV-uninfected women after multivariate adjustment including ACR.
Table 3.
Association of kidney biomarkers with risk of incident hypertension in HIV-uninfected women
| Unadjusted | Multivariable Adjusted | |||
|---|---|---|---|---|
| Biomarker | Participants (N) |
RR (95%CI) | Individual Markers * RR (95%CI) |
Individual Markers + ACR + eGFR† RR (95%CI) |
| ACR | ||||
| Continuous | 267 | 1.04 (0.88, 1.23) | 0.98 (0.84, 1.13) | 0.97 (0.84, 1.11) |
| T1: < 6.1 mg/g | 89 | Reference | Reference | Reference |
| T2: 6.1–10 mg/g | 89 | 1.09 (0.69, 1.75) | 1.11 (0.69, 1.78) | 1.10 (0.68, 1.76) |
| T3: >10 mg/g | 89 | 0.92 (.56, 1.52) | 0.96 (0.58, 1.61) | 1.00 (0.60, 1.68) |
| ≤ 30 mg/g | 247 | Reference | Reference | Reference |
| > 30 mg/g | 20 | 1.63 (0.82, 3.23) | 1.01 (0.50, 2.04) | 0.98 (0.48, 1.98) |
| eGFR | ||||
| Continuous‡ | 267 | 1.32 (1.19, 1.47) | 1.18 (1.06, 1.32) | 1.18 (1.06, 1.32) |
| T1: <96 ml/min/1.73m2 | 92 | 2.65 (1.59, 4.42) | 1.89 (1.09, 3.27) | 1.87 (1.08, 3.25) |
| T2: 96–112 ml/min/1.73m2 | 90 | 1.44 (0.82, 2.54) | 1.27 (0.71, 2.26) | 1.25 (0.70, 2.24) |
| T3: >112 ml/min/1.73m2 | 85 | Reference | Reference | Reference |
| < 90 ml/min/1.73m2 | 63 | 2.49 (1.66, 3.74) | 1.81 (1.16, 2.83) | 1.81 (1.16, 2.83) |
| ≥ 90 ml/min/1.73m2 | 204 | Reference | Reference | Reference |
| α1m | ||||
| Continuous | 267 | 1.37 (1.08, 1.74) | 1.07 (0.83, 1.37) | 1.03 (0.77, 1.36) |
| T1§ | 164 | Reference | Reference | Reference |
| T2 | 51 | 2.38 (1.47, 3.87) | 1.99 (1.21, 3.26) | 1.94 (1.16, 3.23) |
| T3 | 52 | 2.45 (1.47, 4.08) | 1.65 (0.96, 2.83) | 1.51 (0.87, 2.64) |
| IL-18 | ||||
| Continuous | 267 | 1.25 (1.06, 1.47) | 1.18 (1.00, 1.41) | 1.16 (0.97, 1.38) |
| T1 | 89 | Reference | Reference | Reference |
| T2 | 89 | 2.07 (1.18, 3.62) | 1.78 (1.00, 3.16) | 1.80 (1.00, 3.24) |
| T3 | 89 | 2.43 (1.33, 4.45) | 2.11 (1.10, 4.03) | 2.28 (1.17, 4.46) |
| KIM-1 | ||||
| Continuous | 267 | 1.21 (0.99, 1.49) | 1.19 (0.96, 1.48) | 1.17 (0.94, 1.45) |
| T1 | 89 | Reference | Reference | Reference |
| T2 | 89 | 1.41 (0.79, 2.52) | 1.25 (0.68, 2.29) | 1.33 (0.72, 2.44) |
| T3 | 89 | 1.77 (0.96, 3.26) | 1.42 (0.73, 2.80) | 1.50 (0.75, 3.02) |
| NGAL | ||||
| Continuous | 267 | 0.93 (0.80, 1.07) | 1.04 (0.90, 1.21) | 1.04 (0.89, 1.21) |
| T1 | 89 | Reference | Reference | Reference |
| T2 | 89 | 1.12 (0.69, 1.83) | 1.20 (0.72, 1.99) | 1.17 (0.71, 1.95) |
| T3 | 89 | 0.84 (0.48, 1.46) | 1.19 (0.68, 2.08) | 1.20 (0.68, 2.11) |
| L-FABP | ||||
| Continuous | 267 | 1.15 (1.02, 1.29) | 1.05 (0.94, 1.18) | 1.04 (0.92, 1.16) |
| T1 | 90 | Reference | Reference | Reference |
| T2 | 88 | 2.04 (1.16, 3.58) | 1.91 (1.09, 3.36) | 1.80 (1.02, 3.18) |
| T3 | 89 | 1.96 (1.05, 3.67) | 1.52 (0.80, 2.89) | 1.45 (0.76, 2.78) |
| NAG | ||||
| Continuous | 267 | 1.48 (1.20, 1.82) | 1.31 (1.05, 1.63) | 1.31 (1.05, 1.64) |
| T1 | 93 | Reference | Reference | Reference |
| T2 | 87 | 1.31 (0.74, 2.31) | 1.44 (0.81, 2.57) | 1.57 (0.87, 2.82) |
| T3 | 87 | 2.25 (1.22, 4.15) | 2.16 (1.16, 4.03) | 2.35 (1.23, 4.47) |
| AAG | ||||
| Continuous | 267 | 1.22 (1.08, 1.37) | 1.08 (0.96, 1.21) | 1.09 (0.96, 1.24) |
| T1 | 89 | Reference | Reference | Reference |
| T2 | 89 | 1.37 (0.79, 2.39) | 1.22 (0.69, 2.17) | 1.24 (0.70, 2.21) |
| T3 | 89 | 2.45 (1.44, 4.18) | 1.87 (1.08, 3.23) | 1.82 (1.04, 3.17) |
AAG, α1-acid-glycoprotein; ACR, albumin-to-creatinine ratio; α1m, alpha-1 microglobulin; CI, confidence interval; eGFR, estimated glomerular filtration rate by cystatin C; IL-18, interleukin 18; KIM-1, kidney injury molecule 1; L-FABP, liver fatty acid-binding protein; NAG, N-acetyl-β-D-glucosaminidase; NGAL, neutrophil-gelatinase associated lipocalin; RR, relative risk; T, tertile.
Adjusted for age, race/ethnicity, DM, HOMA-IR, stimulant use, HCV, and urine creatinine. Biomarkers included individually, not simultaneously
Adjusted for age, race/ethnicity, DM, HOMA-IR, stimulant use, HCV, urine creatinine, ACR, and eGFR.
Continuous eGFR is modeled per 10 ml/min/1.73m2 decrease. All other continuous predictors are modeled per doubling.
All below detectable
Higher α1m, IL-18, L-FABP, NAG, and AAG levels in HIV-infected women showed modest associations with incident hypertension after multivariate adjustment, but after adjustment for ACR and eGFR these associations were no longer statistically significant (Table 2). KIM-1 appeared to be associated with a decreased risk of hypertension after multivariate adjustment for ACR and eGFR. Among HIV-uninfected women, higher α1m, IL-18, NAG, and AAG levels were associated with incident hypertension after multivariate adjustment (Table 3). After adjustment for ACR and eGFR, associations of IL-18, NAG, and AAG remained statistically significant. Tests for interaction by HIV status for each tubular injury biomarker were not statistically significant (all interaction p-values > 0.20).
Results were similar in analyses using the secondary outcome definition for hypertension in HIV-infected women (Tables S1) and HIV-uninfected women (Table S2). Associations of ACR and eGFR with incident hypertension did not appear to vary by age or race (all interaction p-values >0.20). As a sensitivity analysis, we examined associations of kidney biomarkers with incident hypertension in non-diabetic HIV-infected participants (n=645). While the association of ACR with risk of hypertension did not vary by diabetes status (test for DM by ACR interaction: p=0.53), the association of low eGFR with incident hypertension appeared to be stronger in non-diabetics (test for DM by eGFR interaction: p=0.0086). Specifically, each 10 ml/min/1.73m2 decrement in eGFR was associated with a 17% increased risk of hypertension (95% CI: 1.08, 1.26) in the fully adjusted analysis. In addition, the lowest eGFR tertile was associated with a 99% increased risk of incident hypertension compared to the highest eGFR tertile (95% CI: 1.32, 2.99), and eGFR<60 ml/min/1.73m2 was associated with a 51% increased risk of hypertension (95% CI: 0.93, 2.46). In the HIV-infected population, tubular markers had no significant associations with hypertension among non-diabetics in fully adjusted models.
Discussion
Early identification of HIV-infected individuals at risk for hypertension is important because HIV infection is associated with a higher risk of cardiovascular disease and because of the growing burden of chronic diseases in the aging HIV population.1,2 In this cohort study, we found that higher ACR and lower eGFR were independently associated with a greater risk of incident hypertension in HIV-infected women, even after adjustment for traditional and HIV-related hypertension risk factors. Counter to our hypothesis, biomarkers for tubular dysfunction and injury had at most weak associations with incident hypertension in HIV-infected women, which were attenuated after adjustment for ACR and eGFR. In a similar group of HIV-uninfected women, lower eGFR and higher levels of several tubular injury biomarkers were associated with greater risk of incident hypertension, but we observed no association with higher ACR. To our knowledge, this is the first study to evaluate associations of kidney function and multiple markers of kidney injury with the development of hypertension in an HIV-infected cohort.
There are multiple studies in non-HIV populations that have reported associations between higher urinary albumin excretion and an increased risk of incident hypertension.35–39 Meanwhile, a cohort study of middle-aged, ethnically diverse men and women without clinically apparent cardiovascular disease found lower eGFR, but not albuminuria, was associated with incident hypertension.23 Urinary albumin excretion represents glomerular injury, and there is growing evidence suggesting that subclinical renal microvascular injury may lead to the development of hypertension.22 The association between higher ACR and incident hypertension in the HIV-infected group may reflect HIV-associated systemic endothelial dysfunction and arterial inflammation that produce glomerular injury and also lead to hypertension.27,28 Because few HIV-uninfected participants had an ACR>30 mg/g, our study may have been underpowered to detect an association between higher ACR and incident hypertension in the HIV-uninfected group.
Our finding of an association between kidney dysfunction and incident hypertension in both the HIV-infected and HIV-uninfected groups is similar to reports from the limited number of studies in non-HIV populations.23,39,40 Reduced kidney function may represent several mechanisms involved in the pathogenesis of hypertension, including congenitally reduced nephron numbers, impaired sodium handling, salt sensitivity, and vasoconstriction from activated sympathetic and renin-angiotensin systems.41 Higher levels of inflammation among HIV-infected individuals may also underlay the association of subclinical kidney disease and hypertension because elevated inflammatory markers are associated with kidney dysfunction in the HIV-infected population and with incident hypertension in the general population.42,43
To our knowledge, this is the first study in any population to evaluate associations of biomarkers for tubular injury and dysfunction with risk of incident hypertension. In the HIV-infected group, the weak associations across all tubular injury markers and the attenuation after including ACR and eGFR suggest that tubulointerstitial injury plays a smaller role in the development of hypertension compared to glomerular injury and kidney dysfunction. Although higher levels of IL-18, NAG, and AAG were associated with hypertension in the HIV-uninfected group, interaction testing by HIV status was not statistically significant. We hypothesized higher concentrations of α1m, IL-18, L-FABP, KIM-1 and NAG would be associated with hypertension because they represent injury specific to the proximal tubule, which is responsible for the majority of water and sodium reabsorption.44 The weak associations in the HIV-infected group may be because HIV-related kidney injury and inflammation are more pronounced in the glomerulus compared to the tubulointerstitium. Alternatively, our study may not have adequately captured tubulointerstitial injury occurring before or after the baseline measurements. Unexpectedly, KIM-1 was associated with decreased risk of developing hypertension in the HIV-infected group, although in models without adjustment for urine creatinine there was no association between KIM-1 or any of the tubular markers and incident hypertension. This finding requires confirmation in other cohorts.
Strengths of our study include the use of a multicenter, racially and ethnically diverse cohort representative of U.S. HIV-infected women with a mean follow-up period of 10 years and a frequently measured outcome. We had the ability to adjust for multiple traditional and HIV-related hypertension risk factors and to simultaneously assess multiple kidney injury biomarkers. We were also able to perform our analysis in a similar group of HIV-uninfected women. Our study also has several limitations. First, urinary albumin was quantified from a single spot urine sample instead of multiple samples or a 24-hour urine collection. However, despite the variations in ACR influenced by diet, muscle mass, and time of day, ACR has been shown to be an accurate estimator of albuminuria, and it’s use is supported by national guidelines.45 ACR may have non-differentially overestimated albuminuria because of lower muscle mass in HIV-infected women leading to lower urinary creatinine levels. Second, blood pressure was measured at biannual WIHS clinic visits instead of with ambulatory blood pressure monitoring, which may lead to non-differential outcome misclassification related to masked hypertension or white coat hypertension, and may not capture hypertension diagnosed and treated between visits.46 Third, anti-hypertensive medications may have been prescribed to diabetic participants with albuminuria but without hypertension. However, ACR associations did not substantially differ in our sensitivity analysis excluding diabetic participants. Fourth, because of the bidirectional association between kidney dysfunction and hypertension, our results could reflect hypertension not captured at baseline. However, we used data from up to five years prior to the baseline visit to exclude prevalent hypertension. Fifth, our observational study may not have accounted for all potential confounders given the numerous risk factors for hypertension and kidney injury. For example, we lacked data on exposure to nephrotoxic medications such as NSAIDs. Finally, our results may not be generalizable to HIV-infected men.
Supplementary Material
Perspectives.
We report independent associations of ACR and eGFR with incident hypertension in a large, diverse cohort of HIV-infected women, and independent associations of eGFR and several tubular injury biomarkers with incident hypertension in a similar group of HIV-uninfected women. Our findings suggest early glomerular injury and kidney dysfunction may be involved in the pathogenesis of hypertension in HIV, and may help explain their associations with cardiovascular disease in HIV-infected individuals.26 Additional studies are needed to evaluate whether HIV-associated inflammation plays a role in kidney injury and hypertension, and to corroborate whether ACR and eGFR could be used for targeting HIV-infected individuals at risk of hypertension. The associations of several tubular injury biomarkers with incident hypertension in HIV-uninfected women should be validated in other studies.
What Is New?
Novelty and Significance
This is the first study to evaluate associations of kidney function and multiple markers of kidney injury with the development of hypertension in an HIV-infected cohort.
Among HIV-infected women, biomarkers of kidney dysfunction and glomerular injury were independently associated with an increased risk of developing hypertension, whereas biomarkers of kidney tubular injury and dysfunction showed no associations.
In contrast, in a similar group of HIV-uninfected women, biomarkers of kidney dysfunction and kidney tubular injury and dysfunction were associated with an increased risk of developing hypertension, while glomerular injury showed no association.
What Is Relevant?
Our findings should help inform the pathogenesis of hypertension and the relationship between kidney disease and cardiovascular disease in both HIV-infected and HIV-uninfected persons.
Our findings could be used for targeting HIV-infected persons at risk of hypertension.
Summary.
Biomarkers of glomerular injury and kidney dysfunction are associated with incident hypertension in a large, diverse cohort of HIV-infected women. Our study suggests subclinical kidney disease may be involved in the pathogenesis of hypertension in HIV.
Acknowledgments
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Mary Young and Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV).
Sources of funding
The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA). Shlipak and Scherzer are funded by R01AG034853-08.
Footnotes
Disclosures
None.
References
- 1.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palella FJ, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD. HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 1999. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.Gazzaruso C, Bruno R, Garzaniti A, Giordanetti S, Fratino P, Sacchi P, Filice G. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. 2003;21:1377–1382. doi: 10.1097/01.hjh.0000059071.43904.dc. [DOI] [PubMed] [Google Scholar]
- 4.Bergersen BM, Sandvik L, Dunlop O, Birkeland K, Bruun JN. Prevalence of hypertension in HIV-positive patients on highly active retroviral therapy (HAART) compared with HAART-naïve and HIV-negative controls: results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2003;22:731–736. doi: 10.1007/s10096-003-1034-z. [DOI] [PubMed] [Google Scholar]
- 5.Mateen FJ, Kanters S, Kalyesubula R, Mukasa B, Kawuma E, Kengne AP, Mills EJ. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. J Hypertens. 2013;31:1372–1378. doi: 10.1097/HJH.0b013e328360de1c. discussion 1378. [DOI] [PubMed] [Google Scholar]
- 6.Jericó C, Knobel H, Montero M, Sorli ML, Guelar A, Gimeno JL, Saballs P, López-Colomés JL, Pedro-Botet J. Hypertension in HIV-infected patients: prevalence and related factors. Am J Hypertens. 2005;18:1396–1401. doi: 10.1016/j.amjhyper.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, Prins M, Reiss P. AGEhIV Cohort Study Group. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;59:1787–1797. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- 8.Armah KA, Chang C-CH, Baker JV, et al. Prehypertension, Hypertension, and the Risk of Acute Myocardial Infarction in HIV-Infected and -Uninfected Veterans. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;58:121–129. doi: 10.1093/cid/cit652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krauskopf K, Van Natta ML, Danis RP, Gangaputra S, Ackatz L, Addessi A, Federman AD, Branch AD, Meinert CL, Jabs DA. Correlates of hypertension in patients with AIDS in the era of highly-active antiretroviral therapy. J Int Assoc Provid AIDS Care. 2013;12:325–333. doi: 10.1177/2325957413491432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiébaut R, El-Sadr WM, Friis-Møller N, et al. Predictors of hypertension and changes of blood pressure in HIV-infected patients. Antivir Ther. 2005;10:811–823. doi: 10.1177/135965350501000706. [DOI] [PubMed] [Google Scholar]
- 11.De Arruda Junior ER, Lacerda HR, Vilela Moura LCR, de Albuquerque M, de FPM, de Barros Miranda Filho D, Nunes Diniz GT, de Albuquerque VMG, Zirpoli Amaral JC, de Arraes Ximenes RA, Monteiro VS. Risk factors related to hypertension among patients in a cohort living with HIV/AIDS. Braz J Infect Dis. 2010;14:281–287. doi: 10.1590/s1413-86702010000300014. [DOI] [PubMed] [Google Scholar]
- 12.Crane HM, Van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. AIDS Lond Engl. 2006;20:1019–1026. doi: 10.1097/01.aids.0000222074.45372.00. [DOI] [PubMed] [Google Scholar]
- 13.Guyton AC, Coleman TG, Cowley AV, Scheel KW, Manning RD, Norman RA. Arterial pressure regulation Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 14.Gupta SK, Mamlin BW, Johnson CS, Dollins MD, Topf JM, Dubé MP. Prevalence of proteinuria and the development of chronic kidney disease in HIV-infected patients. Clin Nephrol. 2004;61:1–6. doi: 10.5414/cnp61001. [DOI] [PubMed] [Google Scholar]
- 15.Gardner LI, Holmberg SD, Williamson JM, Szczech LA, Carpenter CCJ, Rompalo AM, Schuman P, Klein RS. Epidemiology Research Study Group. Development of proteinuria or elevated serum creatinine and mortality in HIV-infected women. J Acquir Immune Defic Syndr 1999. 2003;32:203–209. doi: 10.1097/00126334-200302010-00013. [DOI] [PubMed] [Google Scholar]
- 16.Fernando SK, Finkelstein FO, Moore BA, Weissman S. Prevalence of chronic kidney disease in an urban HIV infected population. Am J Med Sci. 2008;335:89–94. doi: 10.1097/MAJ.0b013e31812e6b34. [DOI] [PubMed] [Google Scholar]
- 17.Wyatt CM, Winston JA, Malvestutto CD, Fishbein DA, Barash I, Cohen AJ, Klotman ME, Klotman PE. Chronic kidney disease in HIV infection: an urban epidemic. AIDS Lond Engl. 2007;21:2101–2103. doi: 10.1097/QAD.0b013e3282ef1bb4. [DOI] [PubMed] [Google Scholar]
- 18.Eggers PW, Kimmel PL. Is there an epidemic of HIV Infection in the US ESRD program? J Am Soc Nephrol JASN. 2004;15:2477–2485. doi: 10.1097/01.ASN.0000138546.53152.A7. [DOI] [PubMed] [Google Scholar]
- 19.Odden MC, Scherzer R, Bacchetti P, Szczech LA, Sidney S, Grunfeld C, Shlipak MG. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Arch Intern Med. 2007;167:2213–2219. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estrella MM, Parekh RS, Astor BC, Bolan R, Evans RW, Palella FJ, Jacobson LP. Chronic Kidney Disease and Estimates of Kidney Function in HIV Infection: A Cross-Sectional Study in the Multicenter AIDS Cohort Study. JAIDS J Acquir Immune Defic Syndr. 2011;57:380–386. doi: 10.1097/QAI.0b013e318222f461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peck RN, Shedafa R, Kalluvya S, Downs JA, Todd J, Suthanthiran M, Fitzgerald DW, Kataraihya JB. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC Med. 2014;12:125. doi: 10.1186/s12916-014-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 23.Kestenbaum B, Rudser KD, de Boer IH, Peralta CA, Fried LF, Shlipak MG, Palmas W, Stehman-Breen C, Siscovick DS. Differences in Kidney Function and Incident Hypertension: The Multi-Ethnic Study of Atherosclerosis. Ann Intern Med. 2008;148:501–508. doi: 10.7326/0003-4819-148-7-200804010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szczech LA, Grunfeld C, Scherzer R, Canchola JA, van der Horst C, Sidney S, Wohl D, Shlipak MG. Microalbuminuria in HIV infection. AIDS Lond Engl. 2007;21:1003–1009. doi: 10.1097/QAD.0b013e3280d3587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shlipak MG, Scherzer R, Abraham A, et al. Urinary Markers of Kidney Injury and Kidney Function Decline in HIV-Infected Women. JAIDS J Acquir Immune Defic Syndr. 2012;61:565–573. doi: 10.1097/QAI.0b013e3182737706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121:651–658. doi: 10.1161/CIRCULATIONAHA.109.898585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 28.Hsue PY, Deeks SG, Hunt PW. Immunologic Basis of Cardiovascular Disease in HIV-Infected Adults. J Infect Dis. 2012;205:S375–S382. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson RJ, Schreiner GF. Hypothesis: the role of acquired tubulointerstitial disease in the pathogenesis of salt-dependent hypertension. Kidney Int. 1997;52:1169–1179. doi: 10.1038/ki.1997.442. [DOI] [PubMed] [Google Scholar]
- 30.Jotwani V, Scherzer R, Abraham A, Estrella MM, Bennett M, Devarajan P, Anastos K, Cohen MH, Nowicki M, Sharma A, Young M, Tien PC, Grunfeld C, Parikh CR, Shlipak MG. Does HIV infection promote early kidney injury in women? Antivir Ther. 2013;19:79–87. doi: 10.3851/IMP2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jotwani V, Scherzer R, Abraham A, Estrella MM, Bennett M, Cohen MH, Nowicki M, Sharma A, Young M, Tien PC, Ix JH, Sarnak MJ, Parikh CR, Shlipak MG. Association of urine α1-microglobulin with kidney function decline and mortality in HIV-infected women. Clin J Am Soc Nephrol CJASN. 2015;10:63–73. doi: 10.2215/CJN.03220314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, Waheed S, Coresh J. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis Off J Natl Kidney Found. 2011;58:682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoeting JA, Madigan D, Raftery AE, Volinsky CT. Bayesian model averaging: a tutorial (with comments by M Clyde, David Draper and E. I George, and a rejoinder by the authors. Stat Sci. 1999;14:382–417. [Google Scholar]
- 35.Wang TJ, Evans JC, Meigs JB, Rifai N, Fox CS, D’Agostino RB, Levy D, Vasan RS. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation. 2005;111:1370–1376. doi: 10.1161/01.CIR.0000158434.69180.2D. [DOI] [PubMed] [Google Scholar]
- 36.Brantsma AH, Bakker SJL, de Zeeuw D, de Jong PE, Gansevoort RT. Urinary albumin excretion as a predictor of the development of hypertension in the general population. J Am Soc Nephrol JASN. 2006;17:331–335. doi: 10.1681/ASN.2005111153. [DOI] [PubMed] [Google Scholar]
- 37.Forman JP, Fisher NDL, Schopick EL, Curhan GC. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol JASN. 2008;19:1983–1988. doi: 10.1681/ASN.2008010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jessani S, Levey AS, Chaturvedi N, Jafar TH. High normal levels of albuminuria and risk of hypertension in Indo-Asian population. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2012;27:ii58–ii64. doi: 10.1093/ndt/gfr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang M, Matsushita K, Sang Y, Ballew SH, Astor BC, Coresh J. Association of Kidney Function and Albuminuria With Prevalent and Incident Hypertension: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2015;65:58–66. doi: 10.1053/j.ajkd.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takase H, Dohi Y, Toriyama T, Okado T, Tanaka S, Sonoda H, Kimura G. Evaluation of risk for incident hypertension using glomerular filtration rate in the normotensive general population. J Hypertens. 2012;30:505–512. doi: 10.1097/HJH.0b013e32834f6a1d. [DOI] [PubMed] [Google Scholar]
- 41.Cowley AW, Roman RJ. The role of the kidney in hypertension. JAMA. 1996;275:1581–1589. [PubMed] [Google Scholar]
- 42.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano J, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–2951. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 43.Gupta S, Kitch D, Tierney C, Melbourne K, Ha B, McComsey G. AIDS Clinical Trials Group Study A5224s Team. Markers of renal disease and function are associated with systemic inflammation in HIV infection: Renal and inflammatory markers in HIV infection. HIV Med. 2015;16:591–598. doi: 10.1111/hiv.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Armando I, Upadhyay K, Pascua A, Jose PA. The regulation of proximal tubular salt transport in hypertension: an update. Curr Opin Nephrol Hypertens. 2009;18:412–420. doi: 10.1097/MNH.0b013e32832f5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313:837–846. doi: 10.1001/jama.2015.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Subcommittee of Professional Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.