Abstract
Uric acid is a risk factor for coronary artery disease (CAD) in postmenopausal women but the association with inflammation and coronary microvascular endothelial dysfunction (CED) is not well-defined. The aim of this study was to determine the relationship of serum uric acid (SUA), inflammatory markers and CED. In this prospective cohort study, serum uric acid, hsCRP levels, and neutrophil count were measured in 229 postmenopausal women who underwent diagnostic catheterization, were found to have no obstructive CAD and underwent coronary microvascular function testing, to measure coronary blood flow (CBF) response to intracoronary acetylcholine.
The average age was 58 years (IQR 52, 66) years. Hypertension was present in 48%, type 2 diabetes mellitus in 5.6%, and hyperlipidemia in 61.8%. CED was diagnosed in 59% of postmenopausal women. Mean uric acid level was 4.7 ± 1.3 mg/dL. Postmenopausal women with CED had significantly higher SUA compared to patients without CED (4.9 ± 1.3 vs. 4.4 ± 1.3 mg/dL; p=0.02). There was a significant correlation between SUA and % change in CBF to acetylcholine (p=0.009), and this correlation persisted in multivariable analysis. SUA levels were significantly associated with increased neutrophil count (p=0.02) and hsCRP levels (p=0.006) among patients with CED, but not those without CED. Serum uric acid is associated with coronary microvascular endothelial dysfunction in postmenopausal women and may be related to inflammation. These findings link serum uric acid levels to early coronary atherosclerosis in postmenopausal women.
Keywords: uric acid, microvascular dysfunction, inflammation, postmenopausal, endothelial dysfunction, nonobstructive coronary artery disease
Background
Coronary artery disease (CAD) is a well-recognized leading cause of death in developed countries in both men and women. Premenopausal women have a lower cardiovascular risk when compared to men of the same age, and this is thought to be secondary to protective effects of estrogen. As estrogen levels decrease, cardiovascular risk may increase in postmenopausal women.(1, 2),
Elevated uric acid levels and inflammatory markers have been associated with a number of cardiovascular risk factors including hypertension, hyperlipidemia, obesity, and metabolic syndrome across the population but also in postmenopausal women.(3, 4) Serum uric acid levels are increased in women undergoing both natural and surgical menopause even after adjustment for potential confounders.(5) Uric acid has thus been studied in postmenopausal women and data shows that the absence of estrogen may be associated with hyperuricemia.(6) Additionally, higher levels of uric acid have been associated with increased coronary artery calcium deposits and progression of CAD, independent of other cardiovascular risk factors in postmenopausal women. (7) Uric acid has also been identified as an independent predictor of cardiovascular events.(7),(8) This may be secondary to inflammation as evidenced by increased inflammatory markers and oxidative stress, decreased release of endothelial nitric oxide, and evolution of endothelial dysfunction.(7),(9),(10) Interestingly, uric acid levels even at the upper limit of normal have been shown to be associated with increased cardiovascular risk. (11–16)
The mechanism explaining this association of uric acid and cardiovascular disease in patients with coronary endothelial dysfunction (CED) is poorly understood. Endothelial dysfunction, characterized by reduced nitric oxide activity, is an early phase of atherosclerosis and a predictor of cardiovascular events.(17–23),(24, 25) Preliminary data suggest peripheral endothelial dysfunction may be associated with elevated uric acid levels, however these findings are inconsistent. (24, 25),(26, 27) How uric acid may contribute to CED is unknown.
The association of elevated uric acid levels and peripheral endothelial dysfunction, CAD and all-cause mortality appears to be more pronounced in postmenopausal women.(8, 28),(29) Because increased inflammatory markers and uric acid levels are associated with CED and possibly increased cardiovascular risk, the aim of this study was to examine the relationship of serum uric acid, inflammation and CED in postmenopausal women and to assess predictors of coronary events in this population. We hypothesized that uric acid and inflammation were associated with endothelial dysfunction and adverse outcomes.
Methods
Patient population
Consecutive patients referred to Mayo Clinic between January 1992 and August 2012 for cardiac catheterization with no evidence of significant coronary artery disease on initial angiography were enrolled. All patients had blood work which included measurement of C-reactive protein, complete blood count with differential, basic chemistry panel, and uric acid level. We excluded any patient that was not a postmenopausal female or had not completed baseline laboratory testing and index angiography with complete acetylcholine study. On enrollment, all patients completed a standardized validated questionnaire to assess baseline characteristics.(21, 30, 31) Menopausal status was assessed on enrollment via questionnaire, and based on primary physician diagnosis. A total of 229 subjects who underwent comprehensive coronary endothelial function assessment and basic laboratory assessment were analyzed. All patients had provided informed consent as part of study enrollment, but there was no specific consent obtained to analyze uric acid specifically. Only postmenopausal women with complete laboratory testing as well as index angiography with complete acetylcholine study were included. Patients with history of percutaneous coronary intervention, coronary artery bypass graft surgery, unstable angina pectoris, valvular heart disease, peripheral vascular disease, or known congestive heart failure were excluded from the study. The study was approved by the Mayo Clinic Institutional Review Board and all patients provided informed consent.
Coronary endothelial function assessment
Endothelium-dependent coronary vasoreactivity was assessed according to standardized protocol as previously described. (21, 31, 32) The Doppler guidewire was advanced within the coronary-infusion catheter and placed in the mid-left anterior descending coronary artery. Acetylcholine was administered in the left anterior descending artery at incremental doses for 3 minutes each with increasing concentrations. During each infusion, Doppler measurements, hemodynamic tracings and coronary angiogram were recorded to be subsequently analyzed by a blinded, independent investigator. Coronary artery diameter was measured by having an investigator measure the segment 6 mm distal to the Doppler wire tip using a computer based image system. The percent change in coronary blood flow (CBF) in response to acetylcholine was indicative of endothelium-dependent coronary flow reserve. Coronary endothelial dysfunction was defined as less than 50% increase in CBF with acetylcholine.(21, 33, 34)
Subsequent evaluation
All patients received a standardized questionnaire for assessment of their overall health several years after their index coronary angiogram and endothelial function study. The standardized questionnaire was used to assess occurrence of major adverse cardiovascular events including stroke, rehospitalization, myocardial infarction or death after a median follow-up of 6.3 years (IQR 3.5, 10.7). Responses were verified by medical record review conducted by an investigator blinded to the results of the standardized questionnaire.
Statistical analysis
All data are displayed as mean ± standard deviation. Variables that have skewed distribution are reported as median with first and third quartiles listed in parenthesis. Demographic and baseline clinical data were compared using Fisher’s Exact Test, ANOVA and Wilcoxon tests for continuous data and Pearson chi squared test for categorical data after adjustment for potential confounders including traditional cardiovascular risk factors.
Results
Baseline characteristics
A total of 229 postmenopausal women without epicardial stenosis on coronary angiography underwent microvascular function testing with graded infusion of acetylcholine. Average age was 58 years (IQR 52, 66) years. Hypertension was present in 48%, type 2 diabetes mellitus in 5.6%, and hyperlipidemia in 61.8%. Mean uric acid level was 4.7 ± 1.3 mg/dL.
CED was diagnosed in 59% of postmenopausal women on invasive acetylcholine testing. There was no significant difference in follow-up duration between patients with and without CED (p=0.96).
Coronary blood flow and Uric acid
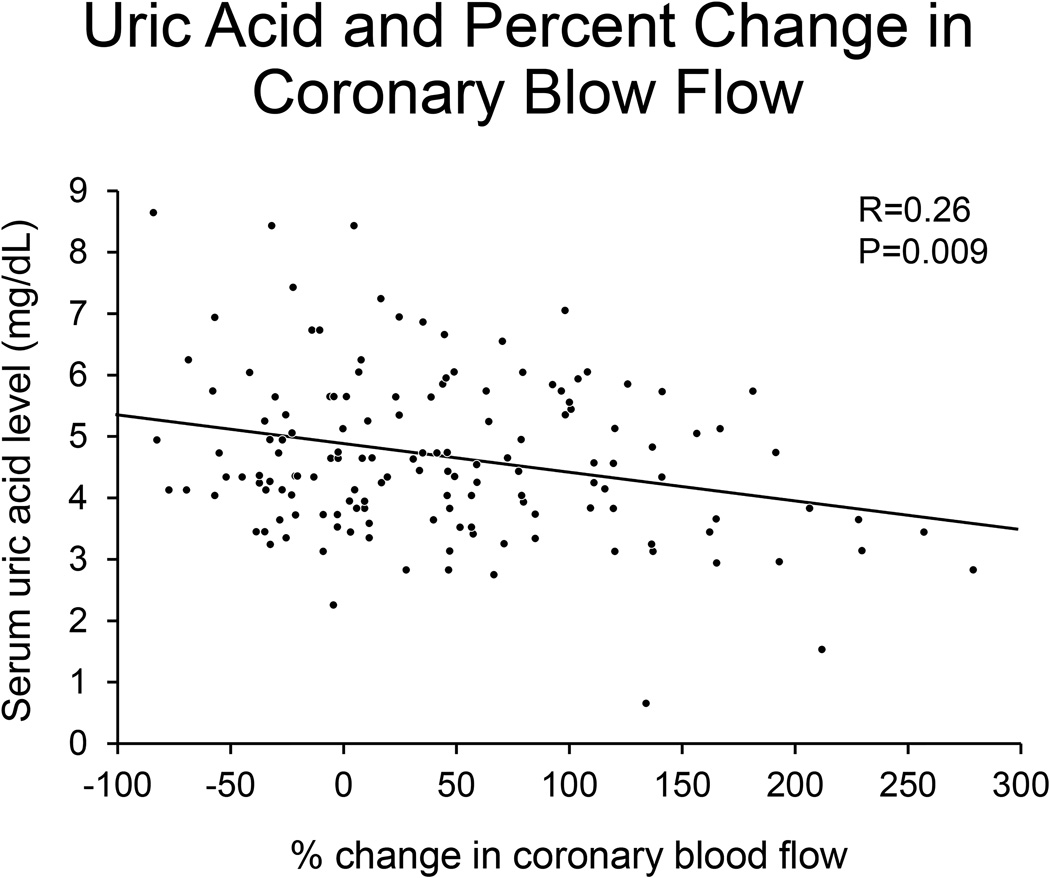
Patients with CED had significantly higher levels of uric acid when compared to patients without CED (4.9 ± 1.3 mg/dL vs. 4.4 ± 1.3 mg/dL; p=0.03). There was a significant correlation between serum uric acid level (SUA) and percent change in CBF with acetylcholine (p=0.009) (Figure 1).
Figure 1. Uric acid and percent change in coronary blood flow.
Figure 1 shows that increased serum uric acid levels are associated with decreased percent change in coronary blood flow and worse endothelial function.
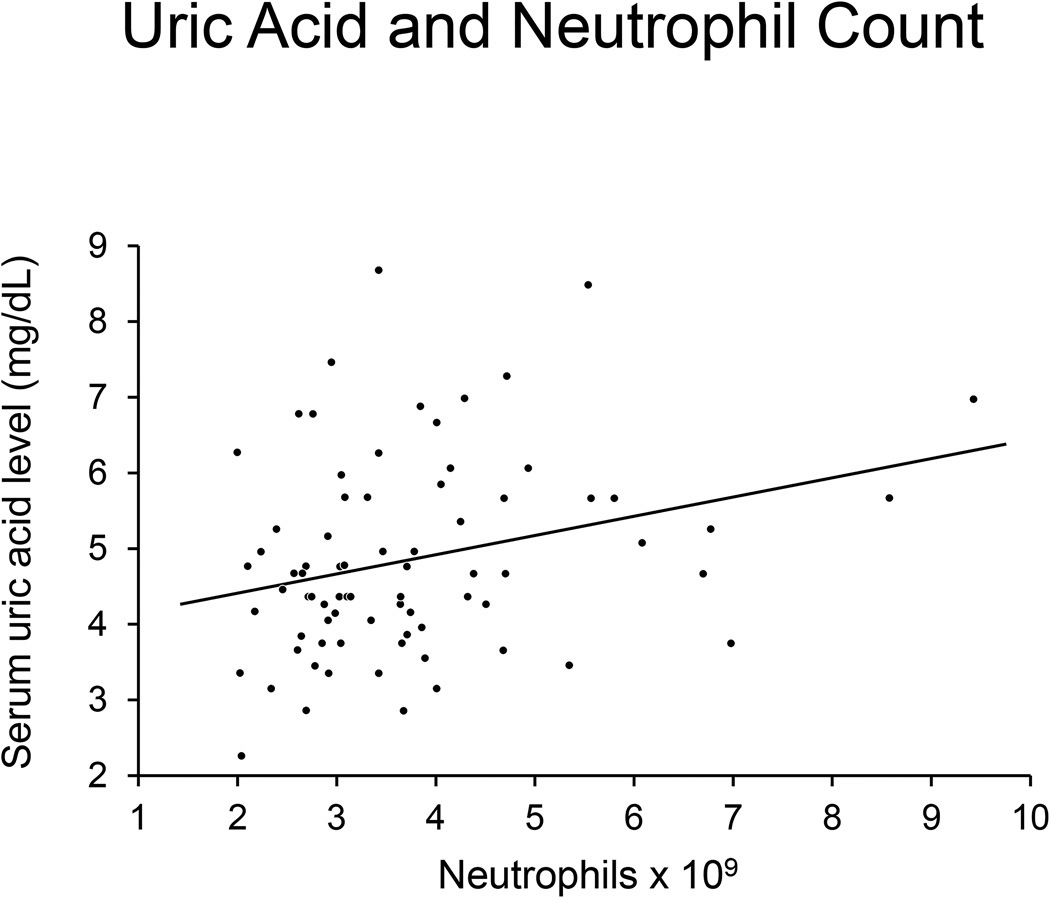
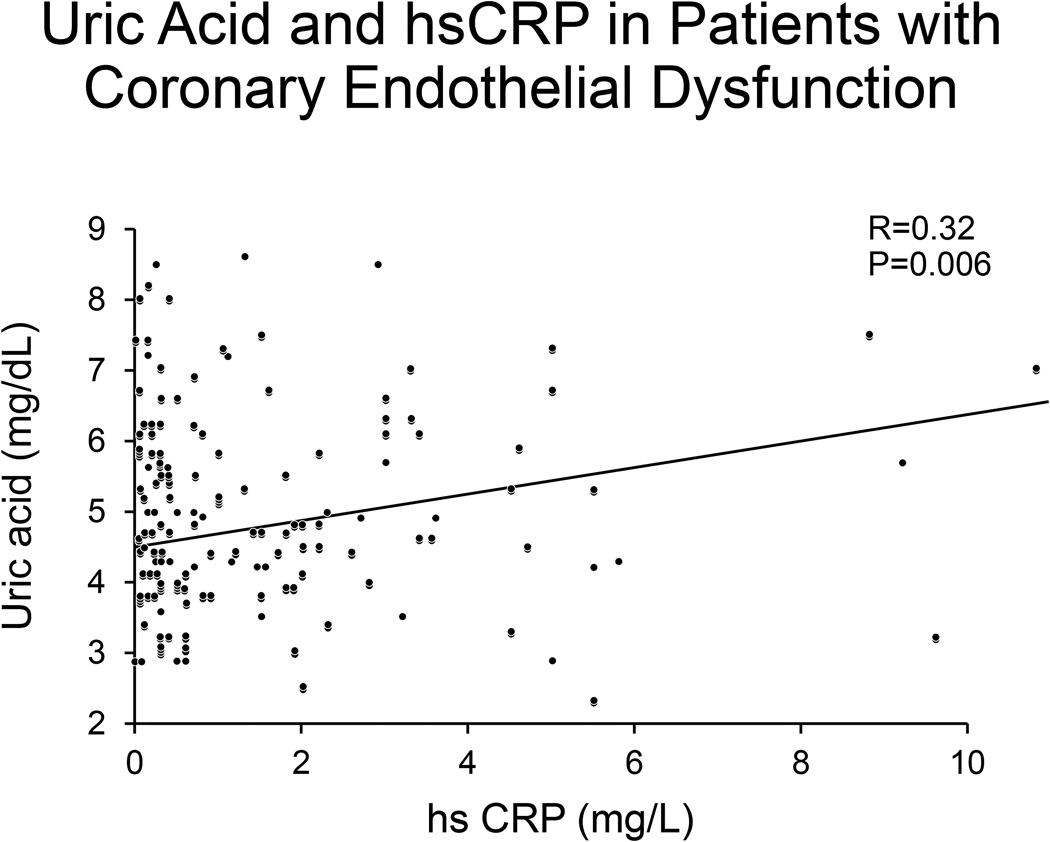
In a linear regression model, higher serum uric acid levels were significantly associated with higher neutrophil counts (p=0.01) (Figure 2), and higher hsCRP levels (p=0.006) in patients with CED (Figure 3). After adjustment for history of current estrogen use, glomerular filtration rate (GFR), hypertension, and body mass index (BMI), SUA remained significantly associated with CED (p=0.02). Neutrophil count and hsCRP were not significantly associated with CED.
Figure 2.
Figure 2 shows that neutrophil counts increase with increasing serum uric acid levels.
Figure 3. Uric acid and hsCRP in patients with Coronary Endothelial Dysfunction.
Figure 3 shows that increasing hsCRP levels are associated with increased uric acid levels.
Coronary blood flow and inflammatory markers
In a linear regression model, increasing uric acid levels were significantly associated with increased neutrophil count (p=0.01) (Figure 2), and increased hsCRP levels (p=0.006) (Figure 3) in patients with CED. Uric acid was not associated with increased levels of inflammatory markers in patients without CED (p>0.05). Higher SUA levels were associated with higher hsCRP in a linear regression model (R= 0.22; p=0.01).
Major Adverse Cardiovascular Events
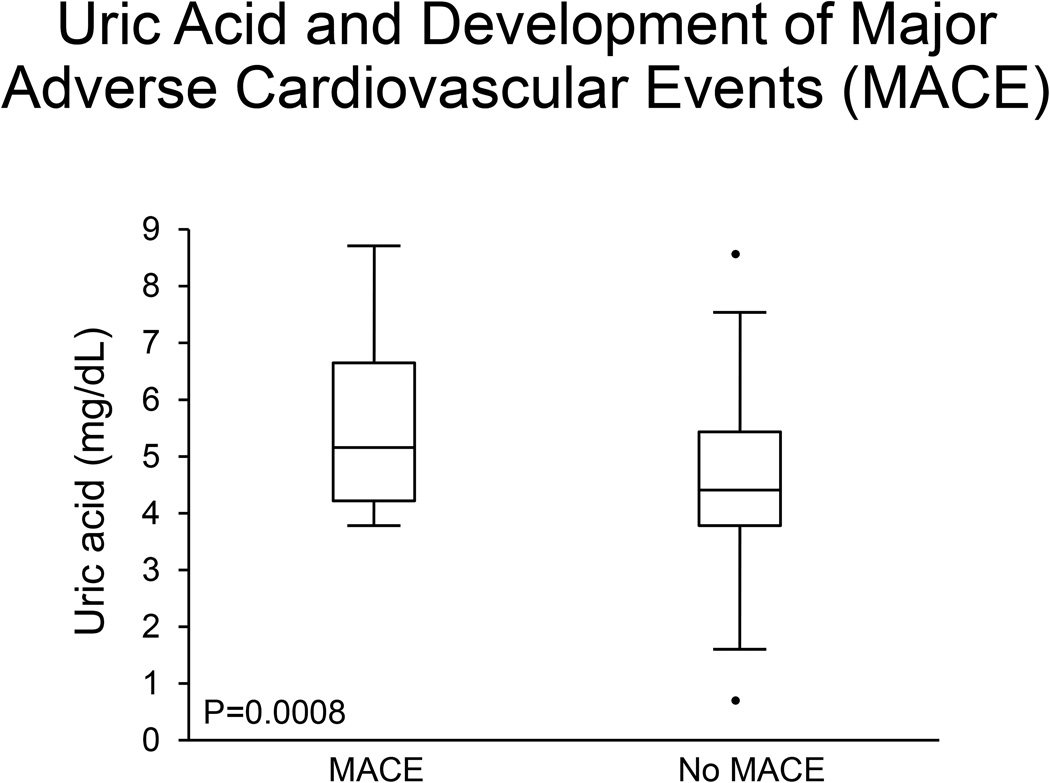
Patients were followed for a median of 7 years (IQR 4, 11) for the development of major adverse cardiovascular events (MACE), which included stroke, cardiovascular death, myocardial infarction, and cardiovascular hospitalization. There were a total of 25 MACE, 16 occurring in patients with CED on baseline acetylcholine study and 9 in patients without CED. Baseline uric acid levels were significantly higher in patients who developed MACE than in patients who did not (5.2 (IQR 4.2, 6.7) vs. 4.4 (3.8, 5.4); p=0.008) (Figure 4). When stratifying by patients with and without CED, uric acid levels were only significantly associated with MACE in patients with CED. Uric acid was an independent predictor of MACE after adjustment for potential confounders including age, hypertension, diabetes, hyperlipidemia, body mass index, and estrogen (LR 8.9 p=0.003). Given potential association of lipid levels and fasting blood glucose, a separate multivariable regression adjusting for age, hypertension, glucose, uric acid, body mass index, HDL, LDL, triglycerides, estrogen use, and antihypertensive use, showed that uric acid remained an independent predictor of major adverse cardiovascular events (LR 4.3, p=0.04).
Figure 4. Uric acid and Development of Major Adverse Cardiovascular Events (MACE).
Patients who developed MACE on follow-up had significantly higher uric acid levels than patients who did not develop MACE.
Discussion
The current study demonstrates an association between serum uric acid, coronary microvascular endothelial dysfunction, inflammatory markers, and cardiovascular outcomes. We have three main findings. First, CED in postmenopausal women is independently associated with increased serum uric acid levels after adjustment for traditional risk factors. Second, higher uric acid levels were associated with higher levels of inflammatory markers in patients with CED, and higher inflammatory markers were in turn associated with a reduced response of CBF to acetylcholine. Third, baseline uric acid levels were higher in patients who developed MACE on follow-up, and uric acid was an independent predictor of MACE after adjusting for potential confounders. Higher uric acid levels at baseline may play a role in development of adverse cardiovascular outcomes, although the mechanisms for this are unclear. These findings suggest that serum uric acid levels are associated with CED and potentially progression of atherosclerosis mediated by uric acid and inflammation.
CED is increasingly recognized as an initial predisposing step for development of atherosclerosis. Recognition of risk factors for early coronary atherosclerosis and CED can be important in identifying and treating vulnerable patients who might develop CAD. (17, 18, 20, 35) While uric acid is a recognized risk factor in the development of cardiovascular disease, the mechanism is not well-understood.
Uric acid and cardiovascular risk factors
Several studies have suggested that uric acid is associated with traditional cardiovascular risk factors and major adverse cardiovascular events, and several mechanisms have been postulated to explain these associations, including inflammatory and vascular changes in the microcirculation, endothelial dysfunction and dysregulation of glucose uptake.(9, 10, 36),(37) Adverse outcomes of uric acid are independent of crystal formation, and so even normal levels of uric acid may be associated with adverse cardiovascular events. Several studies have shown that uric acid levels at the upper limit of normal can be associated with cardiovascular risk factors and mortality, and suggest that a lower threshold may be considered to prevent cardiovascular events. (11, 14, 15)
Serum uric acid has also been associated with endothelial dysfunction in several studies. Acikgoz and colleagues found that SUA levels were higher in patients with microvascular angina and that SUA levels were predictive of carotid atherosclerosis.(38) Increased hsCRP levels and reduced flow-mediated vasodilation have also been reported in patients with higher uric acid levels.(39) This association between elevated uric acid levels and reduction in flow-mediated vasodilation have been corroborated by other studies.(40),(41) The current study thus extends these previous observations and demonstrates for the first time the relationship between SUA and coronary endothelial function.
Uric acid levels also may be associated with hypertension, which in turn has been associated with endothelial dysfunction. Zoccali and colleagues studied a cohort of patients with untreated essential hypertension reporting that uric acid levels were associated with endothelial dysfunction even after adjustment for potential confounders.(42) The role of hypertension is unclear, but given that hypertension may be associated with both microvascular dysfunction and uric acid levels, we adjusted for both hypertension as well as anti-hypertensive use, and found that uric acid remained an independent predictor of major adverse cardiovascular outcomes in this population. In a separate study of patients with untreated essential hypertension, inflammation was implicated as a potential key factor mediating the endothelial-renal function link.(43)
Treatment of hyperuricemia with allopurinol has been associated with improved cardiovascular outcomes, further supporting our findings.(44–46),(47) In a recent large prospective case-matched cohort study of patients with gout, urate-lowering therapy was found to improve overall cardiovascular outcomes.(47) Colchicine has also been shown to result in significant reduction in cardiovascular mortality in patients with gout. (48, 49)
Uric acid and CVD in women
Several studies have alluded to a sex-specific association between uric acid levels, inflammatory markers, early atherosclerosis and cardiovascular disease. Data suggest that the association between uric acid and cardiovascular risk factors and cardiovascular disease may be particularly prominent in postmenopausal women, further supporting our findings that CED is associated with increased uric acid levels and inflammation in postmenopausal women.(5, 50–53)
Hyperuricemia has been independently associated with non-invasive peripheral endothelial dysfunction in postmenopausal women.(40) Elevated uric acid levels have also been independently associated with increased coronary artery calcification in postmenopausal women without known cardiovascular risk factors and increased incident risk of MACE. (7),(8)
Potential mechanisms
There is a growing body of evidence suggesting an association between increased uric acid levels and postmenopausal status, and a causal link to increased cardiovascular risk in postmenopausal women. Endogenous estradiol plays a role in preserving endothelial function and in lowering serum uric acid level independent of cardiovascular risk factors, and with menopause, decreased estrogen levels and increased serum uric acid levels may promote endothelial dysfunction and development of cardiovascular disease.(5, 54–56) While the mechanism linking uric acid and cardiovascular disease has yet to be fully understood, it has been postulated that decreased estrogen levels may lead to increased uric acids levels, which in turn may cause endothelial dysfunction, predisposing patients to development of cardiovascular disease.(57)
Inflammation also plays a key role in the link between uric acid and endothelial dysfunction.(58–60) In the current study, we found that elevated inflammatory markers, including hsCRP and neutrophil count were associated with reduced percent change CBF with acetylcholine. Additionally, higher SUA levels were significantly associated with increased hsCRP and MACE in several studies of patients with CED, suggesting an association between inflammation and endothelial dysfunction.(61–63) These findings are supported by previous data linking elevated inflammatory markers to elevated uric acid levels.(64) Data suggest that uric acid may induce both endothelial dysfunction and vessel inflammation which concomitantly may lead to accelerated atherosclerosis, likely explaining the increased MACE seen in patients with higher uric acid levels and CED.(39, 64, 65) Uric acid appears to exert a proinflammatory effect on endothelial cells, causing reduced nitric oxide bioavailability, and vascular smooth muscle cells, increasing chemokine and cytokine expression.(66, 67) Serum uric acid levels have also been suggested to be predictive of cardiovascular disease due to its sensitivity for underlying inflammation and remodeling within the arterial vessel wall.(68, 69) Lastly, serum uric acid has been shown to be linearly related with the activity of xanthine oxidase. Increased xanthine oxidase enzyme activity may be associated with a corresponding increased production of free oxygen radicals, potentially activating the atherosclerotic process.(70) This is further supported by studies showing that xanthine oxidase inhibition is associated with improved endothelial function, cardiovascular risk, and plaque progression in pre-clinical and clinical studies.(71–75).(76) Thus, xanthine oxidase activity and increased oxygen free radicals may play a key role in initiation and progression of atherosclerosis, even independent of uric acid. Our findings from the current study demonstrating an association between increased inflammatory markers, uric acid levels and impaired endothelial dysfunction are thus, consistent with these observations.(60)
Limitations
The findings are based upon cross-sectional data at initial presentation. A temporal association between uric acid and development of endothelial dysfunction cannot be inferred with certainty. Our data regarding outcomes in these patients were obtained via a questionnaire-based method, which inherently could be biased and cause an overall underestimation of the number of events in each group. Additionally, we do not have data describing the length of antihypertensive therapy in these patients, and this could have a potential effect on endothelial function. To best address this, we have adjusted for use of antihypertensive medications in our analysis. Lastly, menopausal status was assessed by questionnaire and thus hormonal levels are not available.
Conclusion
Increased serum uric acid levels are associated with coronary microvascular endothelial dysfunction and increased inflammatory markers in postmenopausal women. Detection of markers of inflammation including uric acid may be useful in understanding the mechanism and predicting progression of patients with nonobstructive coronary artery disease and atherosclerosis. Moreover, further investigation is necessary to explore the utility of lowering uric acid levels in risk factor modification and attenuation of non-obstructive coronary disease and atherosclerosis.
Table 1.
Baseline characteristics in 229 postmenopausal women undergoing coronary blood flow (CBF) response to intracoronary acetylcholine
| Variable | CMD (n=133) | No CMD (n=88) |
|---|---|---|
| Age (years) | 57 (IQR 51–65) | 58 (53–67) |
| Diabetes | 6.0% (8) | 3.4% (14) |
| Hypertension | 50.0% (66) | 46.1% (41) |
| Hyperlipidemia | 63.6% (84) | 60.9% (53) |
| Body mass index (kg/m2) |
28.1 (IQR 24.2,31.9) | 27.2 (IQR 23.8,31.6) |
| Neutrophils (×10^9) | 3.4(2.8,4.3) | 3.8 (3.0,4.7) |
| hsCRP (mg/L) | 0.6 (0.3,2.0) | 0.4(0.2,1.6) |
| Uric acid (mg/dL) | 4.7(4.1, 5.7) | 4.3 (3.5,5.5) |
| Antihypertensive use | 20.0% (27) | 21.3%(19) |
| Follow-up duration (years) |
6.2 (3.8, 10.1) | 6.5 (3.2,11.5) |
CMD = coronary microvascular dysfunction; hsCRP = high sensitivity C-reactive protein
Perspectives.
In postmenopausal women with nonobstructive women, we find that uric acid levels are higher in those with elevated inflammatory markers and coronary microvascular dysfunction. Moreover, serum uric acid levels appeared to be associated with increased major adverse cardiovascular events. Further investigation is necessary to explore the role of uric acid and uric acid lowering therapies in management of cardiovascular risk in postmenopausal women.
Novelty and Significance.
- What is New
- Coronary microvascular dysfunction and endothelial dysfunction are associated with increased serum uric acid levels.
- Higher serum uric acid levels were significantly associated with increased hsCRP and major adverse cardiovascular events in patients with CED
- What is Relevant
- Elevated uric acid levels may be a maker of increased cardiovascular risk secondary to underlying coronary microvascular dysfunction and increased inflammation.
- Summary
- Increased serum uric acid levels are associated with coronary microvascular dysfunction and increased inflammatory markers.
- Increased serum uric acid levels are associated with increased major adverse cardiovascular events in postmenopausal women.
- Measurement of uric acid and uric acid lowering therapy may have a role in management of postmenopausal women’s cardiovascular risk.
Acknowledgments
Sources of Funding and Acknowledgments: This work was supported by the National Institute of Health (NIH Grants HL-92954 and AG-31750) and the Mayo Foundation
Conflicts of Interest and Relevant Relationships with Industry:
Dr. Amir Lerman is a member of the advisory board of Itamar Medical, a company that produces EndoPAT, a device for noninvasive endothelial function detection. This device was not used in this study. Dr. Lilach O Lerman is his spouse.
Footnotes
There are no relevant disclosures.
References
- 1.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. The New England Journal of Medicine. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 2.Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–1501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]
- 3.Brand FN, McGee DL, Kannel WB, Stokes J, 3rd, Castelli WP. Hyperuricemia as a risk factor of coronary heart disease: The Framingham Study. American Journal of Epidemiology. 1985;121:11–18. doi: 10.1093/oxfordjournals.aje.a113972. [DOI] [PubMed] [Google Scholar]
- 4.Freedman DS, Williamson DF, Gunter EW, Byers T. Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I Epidemiologic Follow-up Study. American Journal of Epidemiology. 1995;141:637–644. doi: 10.1093/oxfordjournals.aje.a117479. [DOI] [PubMed] [Google Scholar]
- 5.Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women--the Third National Health and Nutrition Examination Survey. Arthritis Research & Therapy. 2008;10:R116. doi: 10.1186/ar2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wingrove CS, Walton C, Stevenson JC. The effect of menopause on serum uric acid levels in non-obese healthy women. Metabolism: Clinical and Experimental. 1998;47:435–438. doi: 10.1016/s0026-0495(98)90056-7. [DOI] [PubMed] [Google Scholar]
- 7.Calvo RY, Araneta MR, Kritz-Silverstein D, Laughlin GA, Barrett-Connor E. Relation of serum uric acid to severity and progression of coronary artery calcium in postmenopausal White and Filipino women (from the Rancho Bernardo study) The American Journal of Cardiology. 2014;113:1153–1158. doi: 10.1016/j.amjcard.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Sciacqua A, Perticone M, Tassone EJ, et al. Uric acid is an independent predictor of cardiovascular events in post-menopausal women. International Journal of Cardiology. 2015;197:271–275. doi: 10.1016/j.ijcard.2015.06.069. [DOI] [PubMed] [Google Scholar]
- 9.Coutinho Tde A, Turner ST, Peyser PA, Bielak LF, Sheedy PF, 2nd, Kullo IJ. Associations of serum uric acid with markers of inflammation, metabolic syndrome, and subclinical coronary atherosclerosis. American Journal of Hypertension. 2007;20:83–89. doi: 10.1016/j.amjhyper.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Hebert LA, Rovin B. Uric acid and cardiovascular risk. The New England Journal of Medicine. 2009;360:540. author reply -1. [PubMed] [Google Scholar]
- 11.Jin YL, Zhu T, Xu L, et al. Uric acid levels, even in the normal range, are associated with increased cardiovascular risk: the Guangzhou Biobank Cohort Study. International Journal of Cardiology. 2013;168:2238–2241. doi: 10.1016/j.ijcard.2013.01.214. [DOI] [PubMed] [Google Scholar]
- 12.Mazza A, Zamboni S, Rizzato E, et al. Serum uric acid shows a J-shaped trend with coronary mortality in non-insulin-dependent diabetic elderly people. The Cardiovascular Study in the Elderly (CASTEL) Acta Diabetologica. 2007;44:99–105. doi: 10.1007/s00592-007-0249-3. [DOI] [PubMed] [Google Scholar]
- 13.Ioachimescu AG, Brennan DM, Hoar BM, Hazen SL, Hoogwerf BJ. Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: a preventive cardiology information system (PreCIS) database cohort study. Arthritis and Rheumatism. 2008;58:623–630. doi: 10.1002/art.23121. [DOI] [PubMed] [Google Scholar]
- 14.Leiba A, Vinker S, Dinour D, Holtzman EJ, Shani M. Uric acid levels within the normal range predict increased risk of hypertension: a cohort study. Journal of the American Society of Hypertension : JASH. 2015;9:600–609. doi: 10.1016/j.jash.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Niskanen LK, Laaksonen DE, Nyyssonen K, et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Archives of Internal Medicine. 2004;164:1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 16.Spoon DB, Lerman A, Rule AD, et al. The association of serum uric acid levels with outcomes following percutaneous coronary intervention. Journal of Interventional Cardiology. 2010;23:277–283. doi: 10.1111/j.1540-8183.2010.00555.x. [DOI] [PubMed] [Google Scholar]
- 17.Bhagat K. Endothelial dysfunction in cardiovascular disease. Hospital Medicine. 1998;59:434–435. [PubMed] [Google Scholar]
- 18.Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Global Cardiology Science & Practice. 2014;2014:291–308. doi: 10.5339/gcsp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuzawa Y, Li J, Aoki T, et al. Predictive value of endothelial function by noninvasive peripheral arterial tonometry for coronary artery disease. Coronary Artery Disease. 2015;26:231–238. doi: 10.1097/MCA.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuzawa Y, Lerman A. Endothelial dysfunction and coronary artery disease: assessment, prognosis, and treatment. Coronary Artery Disease. 2014;25:713–724. doi: 10.1097/MCA.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad M, Reriani M, Khosla S, et al. Coronary microvascular endothelial dysfunction is an independent predictor of development of osteoporosis in postmenopausal women. Vascular Health and Risk Management. 2014;10:533–538. doi: 10.2147/VHRM.S63580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassar A, Morgenthaler TI, Rihal CS, et al. Coronary endothelial function in patients with obstructive sleep apnea. Coronary Artery Disease. 2014;25:16–22. doi: 10.1097/MCA.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi BJ, Prasad A, Gulati R, et al. Coronary endothelial dysfunction in patients with early coronary artery disease is associated with the increase in intravascular lipid core plaque. European Heart Journal. 2013;34:2047–2054. doi: 10.1093/eurheartj/eht132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nanchen D, Stott DJ, Gussekloo J, et al. Resting heart rate and incident heart failure and cardiovascular mortality in older adults: role of inflammation and endothelial dysfunction: the PROSPER study. European Journal of Heart Failure. 2013;15:581–588. doi: 10.1093/eurjhf/hfs195. [DOI] [PubMed] [Google Scholar]
- 25.de Jager J, Dekker JM, Kooy A, et al. Endothelial dysfunction and low-grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: the Hoorn Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:1086–1093. doi: 10.1161/01.ATV.0000215951.36219.a4. [DOI] [PubMed] [Google Scholar]
- 26.Sakr SA, Abbas TM, Amer MZ, et al. Microvascular angina. The possible role of inflammation, uric acid, and endothelial dysfunction. International Heart Journal. 2009;50:407–419. doi: 10.1536/ihj.50.407. [DOI] [PubMed] [Google Scholar]
- 27.Zhan Y, Dong Y, Tang Z, Zhang F, Hu D, Yu J. Serum Uric Acid, Gender, and Low Ankle Brachial Index in Adults With High Cardiovascular Risk. Angiology. 2015;66:687–691. doi: 10.1177/0003319714566228. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Fan Y, Li J, et al. Serum uric acid as a predictor for cardiovascular and all-cause mortality in women versus men. International Journal of Cardiology. 2015;185:125–128. doi: 10.1016/j.ijcard.2015.03.121. [DOI] [PubMed] [Google Scholar]
- 29.Strasak AM, Kelleher CC, Brant LJ, et al. Serum uric acid is an independent predictor for all major forms of cardiovascular death in 28,613 elderly women: a prospective 21-year follow-up study. International Journal of Cardiology. 2008;125:232–239. doi: 10.1016/j.ijcard.2007.11.094. [DOI] [PubMed] [Google Scholar]
- 30.Prasad M, McBane R, Reriani M, Lerman LO, Lerman A. Coronary endothelial dysfunction is associated with increased risk of venous thromboembolism. Thrombosis Research. 2016;139:17–21. doi: 10.1016/j.thromres.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 31.Reriani M, Flammer AJ, Li J, et al. Microvascular endothelial dysfunction predicts the development of erectile dysfunction in men with coronary atherosclerosis without critical stenoses. Coronary Artery Disease. 2014;25:552–557. doi: 10.1097/MCA.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widmer RJ, Chung WY, Herrmann J, Jordan KL, Lerman LO, Lerman A. The association between circulating microRNA levels and coronary endothelial function. PloS One. 2014;9:e109650. doi: 10.1371/journal.pone.0109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maniu CV, Higano ST, Lerman A. Assessing coronary endothelial dysfunction. Circulation. 2002;106:e48. doi: 10.1161/01.cir.0000030083.02775.d7. discussion e. [DOI] [PubMed] [Google Scholar]
- 34.Gossl M, Modder UI, Gulati R, et al. Coronary endothelial dysfunction in humans is associated with coronary retention of osteogenic endothelial progenitor cells. European Heart Journal. 2010;31:2909–2914. doi: 10.1093/eurheartj/ehq373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gossl M, Yoon MH, Choi BJ, et al. Accelerated coronary plaque progression and endothelial dysfunction: serial volumetric evaluation by IVUS. JACC. Cardiovascular Imaging. 2014;7:103–104. doi: 10.1016/j.jcmg.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai XM, Wei L, Ma LL, et al. Serum uric acid and its relationship with cardiovascular risk profile in Chinese patients with early-onset coronary artery disease. Clinical Rheumatology. 2015;34:1605–1611. doi: 10.1007/s10067-015-2878-1. [DOI] [PubMed] [Google Scholar]
- 37.Quinones Galvan A, Natali A, Baldi S, et al. Effect of insulin on uric acid excretion in humans. The American Journal of Physiology. 1995;268:E1–E5. doi: 10.1152/ajpendo.1995.268.1.E1. [DOI] [PubMed] [Google Scholar]
- 38.Acikgoz N, Ermis N, Yagmur J, et al. Uric acid level and its association with carotid intima-media thickness in patients with cardiac syndrome X. Medical principles and practice : International Journal of the Kuwait University, Health Science Centre. 2012;21:115–119. doi: 10.1159/000332583. [DOI] [PubMed] [Google Scholar]
- 39.Ho WJ, Tsai WP, Yu KH, et al. Association between endothelial dysfunction and hyperuricaemia. Rheumatology. 2010;49:1929–1934. doi: 10.1093/rheumatology/keq184. [DOI] [PubMed] [Google Scholar]
- 40.Maruhashi T, Nakashima A, Soga J, et al. Hyperuricemia is independently associated with endothelial dysfunction in postmenopausal women but not in premenopausal women. BMJ Open. 2013;3:e003659. doi: 10.1136/bmjopen-2013-003659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato M, Hisatome I, Tomikura Y, et al. Status of endothelial dependent vasodilation in patients with hyperuricemia. The American Journal of Cardiology. 2005;96:1576–1578. doi: 10.1016/j.amjcard.2005.07.068. [DOI] [PubMed] [Google Scholar]
- 42.Zoccali C, Maio R, Mallamaci F, Sesti G, Perticone F. Uric acid and endothelial dysfunction in essential hypertension. Journal of the American Society of Nephrology : JASN. 2006;17:1466–1471. doi: 10.1681/ASN.2005090949. [DOI] [PubMed] [Google Scholar]
- 43.The smart way to better health: CEO leadership school explores smartphones and mHealth. Caring : National Association for Home Care Magazine. 2012;31:8–11. [PubMed] [Google Scholar]
- 44.Larsen KS, Pottegard A, Lindegaard HM, Hallas J. Effect of Allopurinol on Cardiovascular Outcomes in Hyperuricemic Patients: A Cohort Study. The American Journal of Medicine. 2016;129:299–306. doi: 10.1016/j.amjmed.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Bellomo G. The relationship between uric acid, allopurinol, cardiovascular events, and kidney disease progression: a step forward. American journal of kidney diseases : The Official Journal of the National Kidney Foundation. 2015;65:525–527. doi: 10.1053/j.ajkd.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Guedes M, Esperanca A, Pereira AC, Rego C. What is the effect on cardiovascular events of reducing hyperuricemia with allopurinol? An evidence-based review. Revista portuguesa de cardiologia : orgao oficial da Sociedade Portuguesa de Cardiologia = Portuguese journal of cardiology : An Official Journal of the Portuguese Society of Cardiology. 2014;33:727–732. doi: 10.1016/j.repc.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Chen JH, Lan JL, Cheng CF, et al. Effect of Urate-Lowering Therapy on All-Cause and Cardiovascular Mortality in Hyperuricemic Patients without Gout: A Case-Matched Cohort Study. PloS One. 2015;10:e0145193. doi: 10.1371/journal.pone.0145193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solomon DH, Liu CC, Kuo IH, Zak A, Kim SC. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: a cohort study using electronic medical records linked with Medicare claims. Ann Rheum Dis. 2016;75:1674–1679. doi: 10.1136/annrheumdis-2015-207984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imazio M, Gaita F. Colchicine for cardiovascular medicine. Future Cardiology. 2016;12:9–16. doi: 10.2217/fca.15.59. [DOI] [PubMed] [Google Scholar]
- 50.Liu PJ, Ma F, Lou HP, Zhu YN, Chen Y. Relationship between serum uric acid levels and metabolic syndrome in Chinese postmenopausal women. Climacteric : The Journal of the International Menopause Society. 2014;17:148–154. doi: 10.3109/13697137.2013.818969. [DOI] [PubMed] [Google Scholar]
- 51.Park JS, Kang S, Ahn CW, Cha BS, Kim KR, Lee HC. Relationships between serum uric acid, adiponectin and arterial stiffness in postmenopausal women. Maturitas. 2012;73:344–348. doi: 10.1016/j.maturitas.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Liu ZM, Ho SC. The association of serum C-reactive protein, uric acid and magnesium with insulin resistance in Chinese postmenopausal women with prediabetes or early untreated diabetes. Maturitas. 2011;70:176–181. doi: 10.1016/j.maturitas.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Simon JA, Lin F, Vittinghoff E, Bittner V. The relation of postmenopausal hormone therapy to serum uric acid and the risk of coronary heart disease events: the Heart and Estrogen-Progestin Replacement Study (HERS) Annals of Epidemiology. 2006;16:138–145. doi: 10.1016/j.annepidem.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Lieberman EH, Gerhard MD, Uehata A, et al. Estrogen improves endothelium-dependent, flow-mediated vasodilation in postmenopausal women. Annals of Internal Medicine. 1994;121:936–941. doi: 10.7326/0003-4819-121-12-199412150-00005. [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto M, Akishita M, Eto M, et al. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–3435. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 56.Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. British Medical Journal. 1973;1:449–451. doi: 10.1136/bmj.1.5851.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson RJ, Kang DH, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 58.Meisinger C, Koenig W, Baumert J, Doring A. Uric acid levels are associated with all-cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: the MONICA/KORA cohort study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:1186–1192. doi: 10.1161/ATVBAHA.107.160184. [DOI] [PubMed] [Google Scholar]
- 59.Bandukwala F, Huang M, Zaltzman JS, Nash MM, Prasad GV. Association of uric acid with inflammation, progressive renal allograft dysfunction and post-transplant cardiovascular risk. The American Journal of Cardiology. 2009;103:867–871. doi: 10.1016/j.amjcard.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 60.Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Seminars in Nephrology. 2005;25:39–42. doi: 10.1016/j.semnephrol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 61.Whayne TF., Jr Coronary atherosclerosis, low-density lipoproteins and markers of thrombosis, inflammation and endothelial dysfunction. The International Journal of Angiology : Official Publication of the International College of Angiology, Inc. 2007;16:12–16. doi: 10.1055/s-0031-1278237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marroquin OC, Kip KE, Mulukutla SR, et al. Inflammation, endothelial cell activation, and coronary microvascular dysfunction in women with chest pain and no obstructive coronary artery disease. American Heart Journal. 2005;150:109–115. doi: 10.1016/j.ahj.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Matsubara T, Ishibashi T, Hori T, et al. Association between coronary endothelial dysfunction and local inflammation of atherosclerotic coronary arteries. Molecular and Cellular Biochemistry. 2003;249:67–73. [PubMed] [Google Scholar]
- 64.Ruggiero C, Cherubini A, Ble A, et al. Uric acid and inflammatory markers. European Heart Journal. 2006;27:1174–1181. doi: 10.1093/eurheartj/ehi879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 66.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. Journal of the American Society of Nephrology : JASN. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 67.Park JH, Jin YM, Hwang S, Cho DH, Kang DH, Jo I. Uric acid attenuates nitric oxide production by decreasing the interaction between endothelial nitric oxide synthase and calmodulin in human umbilical vein endothelial cells: a mechanism for uric acid-induced cardiovascular disease development. Nitric oxide : Biology and Chemistry / Official Journal of the Nitric Oxide Society. 2013;32:36–42. doi: 10.1016/j.niox.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–2825. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 69.Tamakoshi K, Yatsuya H, Kondo T, et al. The metabolic syndrome is associated with elevated circulating C-reactive protein in healthy reference range, a systemic low-grade inflammatory state. International journal of obesity and related metabolic disorders : Journal of the International Association for the Study of Obesity. 2003;27:443–449. doi: 10.1038/sj.ijo.0802260. [DOI] [PubMed] [Google Scholar]
- 70.Doehner W, Jankowska EA, Springer J, Lainscak M, Anker SD. Uric acid and xanthine oxidase in heart failure - Emerging data and therapeutic implications. International Journal of Cardiology. 2016;213:15–19. doi: 10.1016/j.ijcard.2015.08.089. [DOI] [PubMed] [Google Scholar]
- 71.Baldus S, Koster R, Chumley P, et al. Oxypurinol improves coronary and peripheral endothelial function in patients with coronary artery disease. Free Radical Biology & Medicine. 2005;39:1184–1190. doi: 10.1016/j.freeradbiomed.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doehner W, Schoene N, Rauchhaus M, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 73.Soucy KG, Lim HK, Attarzadeh DO, et al. Dietary inhibition of xanthine oxidase attenuates radiation-induced endothelial dysfunction in rat aorta. Journal of Applied Physiology. 2010;108:1250–1258. doi: 10.1152/japplphysiol.00946.2009. [DOI] [PubMed] [Google Scholar]
- 74.Mercuro G, Vitale C, Cerquetani E, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. The American Journal of Cardiology. 2004;94:932–935. doi: 10.1016/j.amjcard.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 75.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 76.Nomura J, Busso N, Ives A, et al. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Scientific Reports. 2014;4:4554. doi: 10.1038/srep04554. [DOI] [PMC free article] [PubMed] [Google Scholar]