Abstract
Increased disease susceptibility during early life has been linked to immune immaturity, regulatory T-cell/TH2 immune biasing and hyporesponsiveness. The contribution of myeloid derived suppressor cells (MDSCs) remains uninvestigated. Here, we assessed peripheral MDSC in HIV-infected and -uninfected children with tuberculosis (TB) disease before, during and after TB treatment, along with matched household contacts (HHCs), HIV-exposed, -infected and -uninfected children without recent TB exposure. Serum analytes and enzymes associated with MDSC accumulation/activation/function were measured by colorimetric- and fluorescence arrays. Peripheral frequencies of cells phenotypically resembling MDSCs were significantly increased in HIV-exposed uninfected (HEU) and M.tb-infected children, but peaked in children with TB disease and remained high following treatment. MDSC in HIV-infected (HI) children were similar to unexposed uninfected controls; however, HAART-mediated MDSC restoration to control levels could not be disregarded. Increased MDSC frequencies in HHC coincided with enhanced indoleamine-pyrrole-2,3-dioxygenase (IDO), whereas increased MDSC in TB cases were linked to heightened IDO and arginase-1. Increased MDSC were paralleled by reduced plasma IP-10 and thrombospondin-2 levels in HEU and significantly increased plasma IL-6 in HI HHC. Current investigations into MDSC-targeted treatment strategies, together with functional analyses of MDSCs, could endorse these cells as novel innate immune regulatory mechanism of infant HIV/TB susceptibility.
Keywords: Myeloid derived suppressor cells (MDSCs), tuberculosis, HIV exposure, children, early life immunity
INTRODUCTION
The global dilemma of childhood mortality is highlighted by an annual death rate of nearly seven million children under the age of five(1). In sub-Saharan Africa, the under-five death rate is 15 times greater when compared with children from developed regions(2). HIV and TB are major contributors fueling child mortality (3). Considering that early life is characterized by immune immaturity and a predominance of a T helper 2 (TH2) immune pattern(4), childhood mortality and morbidity are often attributed to pathogenic infections occurring before the immune system has fully developed(5–7). Estimates indicate that one million children contract TB annually, of which 400,000 succumb to the disease(8). Household exposure to TB confers a particularly high M.tb infection risk, creating a reservoir that sustains the epidemic in adulthood(15). HIV infection further heightens the chance of developing TB disease by 24 fold(9). Although prevention of mother-to-child-transmission (PMTCT) with highly active antiretroviral therapy (HAART) has significantly reduced paediatric HIV, the result being a birth rate of over 2 million HIV-exposed but uninfected (HEU) children each year(16). Importantly, evidence show that HEU children are at greater risk to acquire and succumb to infectious diseases, when compared with children of uninfected mothers. The mechanisms underlying increased susceptibility and dissemination of TB in HEU children, is not fully understood, but it is suggested that immaturity of infant immune cells(11), in-utero exposure to HIV antigens(12), immune activation of the(13), may drive these changes(14).
Another rationale for childhood susceptibility to infections involve the purposeful suppression of immune responses required to ensure safe colonization of beneficial bacteria in the digestive system(15) or the lasting effects of suppressed fetal response to prevent in utero rejection(20). Indeed, several reports demonstrate that fetal T cells are not inherently deficient in antigenic responses; but rather, their function is actively suppressed by regulatory T cells (Tregs) to prevent excessive inflammation and tissue damage following initial pathogenic exposure(20). Considering aforementioned, sub-optimal immunity in children could also be explained by the increased presence of regulatory innate immune cells.
MDSCs represent an innate immune cell population with an immature phenotype, consisting of granulocytic CD15+ G-MDSC and monocytic CD14+ M-MDSC(29). MDSCs have a remarkable(19), including inducing enzymes such as arginase and IDO that downregulate cytokine responses and inhibit T cell proliferation (20). MDSCs occur at low frequencies in healthy adults, but increase during excessive immune stimulation or chronic immune activation, as observed during cancer, sepsis, stress, viral-, fungal- or bacterial infections(21). We have previously demonstrated an increase in peripheral MDSCs in adults with recent M.tb infection and active TB disease, with the associated suppression of protective anti-TB immune responses(22). The immunosuppressive function of MDSCs in adults with these and other pathologies are well described, however, the presence and role of MDSCs in children with M.tb and/or HIV exposure, infection or disease remains undefined. Considering the critical role of MDSCs in host immune suppression and the immune stimulatory conditions associated with M.tb and HIV infections, it is essential to determine the contribution of these cells during early life immunity. We hypothesize that MDSC frequencies are increased in peripheral blood of young children with childhood TB and/or HIV infection, with a corresponding increase in plasma analytes and enzymes associated with MDSC accumulation and immune suppression.
RESULTS
Study Subjects
Cryopreserved peripheral blood samples of 138 children, between the ages of 11 months and four years were selected. None of the participants received TB treatment at the time of enrolment, but treatment was initiated in all newly diagnosed TB cases. All HIV infected children received HAART for the study duration. Study groups consisted of QFN negative HIV unexposed uninfected (HUU; n = 20) and HIV infected (HI; n = 24) community controls without household exposure to a TB case, QFN negative HIV exposed uninfected (HEU; n = 37) children, HIV uninfected household contacts (HHC; n = 22) with recent (baseline) and remote (month 3, month 6) household exposure to a TB case, HIV infected HHC (n = 11) with recent (baseline) and remote (month 3, month 6) household exposure to a TB case, HIV infected TB cases(n = 7) before (baseline), during (month 3) and after (month 6) standard TB treatment, HIV uninfected TB cases (n = 14) before (baseline), during (month 3) and after (month 6) standard TB treatment. Other available demographics and clinical profiles of participants are shown in Table 1. Analyses of PBMC samples from two HIV positive TB cases at baseline and month 6, and one HIV infected community control, had to be excluded due to clumping of cells and poor viability.
Table 1.
Demographic and clinical characteristics of child participants
| Participant Characteristics (n=135) |
TB cases (n=21) |
HHC (n=33) |
HIV Exposed Uninfected (n=37) |
Community controls (n=44) |
Statistical Analyses | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | χ2
statistic |
Significant (Y/N) [P value] |
|
| Male sex | 7 | 33 | 13 | 39 | 21 | 57 | 28 | 64 | 7.70 | No [0.06] |
| BCG vaccinated/scar | 19 | 90 | 31 | 94 | 37 | 100 | 44 | 100 | 6.72 | No [0.08] |
| Ethnicity | 33.16 | Yes [0.00001] | ||||||||
| African | 4 | 19 | 19 | 58 | 34 | 92 | 24 | 55 | ||
| Mixed Ancestry | 17 | 81 | 14 | 42 | 3 | 8 | 19 | 43 | ||
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | ||
| Enrolment QFN positivity | 20 | 97 | 22 | 67 | 0 | 0 | 0 | 0 | 96.34 | Yes [0.00001] |
| Enrolment HIV positive | 7 | 33 | 11 | 33 | 0 | 0 | 24 | 55 | 28.108 | Yes [0.00001] |
| Enrolment HAART* | 7 | 33 | 12 | 36 | 0 | 0 | 1 | 2 | 29.768 | Yes [0.00001] |
| Median | (SD) | Median | (SD) | Median | (SD) | Median | (SD) | |||
| Enrolment weight (kg) | 13 | 7 | 13 | 4 | 6 | 1 | 7 | 3 | Yes [0.0001] | |
| Enrolment age (months) | 30 | 37 | 38 | 20 | 42 | 3 | 17 | 12 | Yes [0.0001] | |
Highly active antiretroviral therapy
Simple linear regression analyses were performed to assess changes in total MDSC frequency relative to infant age and weight. Although a significant, progressive increase in MDSC frequency relative to infant age was observed in HIV uninfected (HU) TB cases, very little of the variance could be explained by the correlation (r2 = 0.1067; p = 0.04). No other statistically significant relationships were indicated for MDSC frequency and infant age and weight (Supporting Information Fig.1 and Fig.2).
Elevated peripheral MDSC frequencies in HIV exposed and M.tb infected children
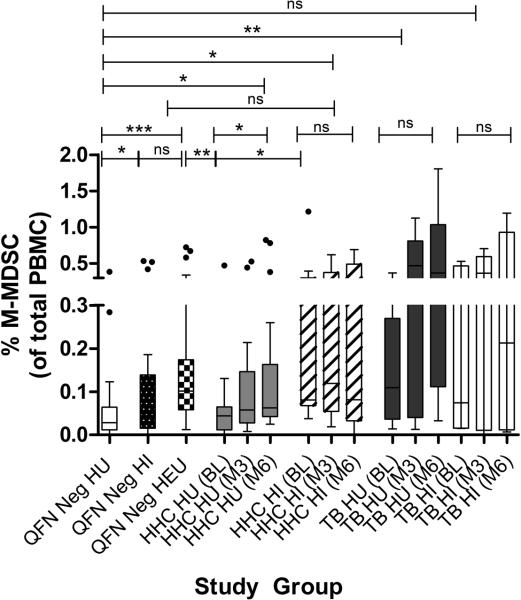
MDSC frequencies are increased in peripheral blood of adults with HIV or TB(26,27). We investigated the frequency of peripheral MDSCs in children with/without HIV and/or TB exposure, infection or disease. Flow cytometric data from QFN negative controls demonstrated that peripheral MDSC frequencies of HIV infected (HI) children matched those of the HIV unexposed uninfected (HU) group (Fig 1a). A significant increase in peripheral MDSC frequencies was however observed in HEU children, when compared with HU and HI controls (Fig 1a). Longitudinal assessments showed a significant increase in peripheral MDSC frequencies in HI and HU HHC with recent (baseline) or remote (month 3-6) TB exposure, relative to that of HU and HI QFN negative controls (Fig 1b-d). Analyses of individuals with active TB disease revealed that peripheral MDSC frequencies peaked in HI and HU TB cases before (baseline), during (month 3) and after (month 6) standard TB treatment, when compared with HU and HI QFN negative controls or HHCs with recent (baseline) or remote (month 3-6) TB exposure (Fig 1b-d). No differences in MDSC frequencies were measured for TB cases during standard TB therapy or for HHC at various time points (Supporting Information Fig.3).
Figure 1.
Higher frequencies of circulating infant MDSC upon HIV exposure and TB infection/disease. (A-D) MDSCs presented as a frequency of total peripheral blood mononuclear cells in (A) QFN negative controls without HIV exposure (n = 20), with HIV infection (n = 23) and HIV exposure uninfected (n = 37); Longitudinal assessments in M.tb infected HHC with (n = 11) and without (n = 22) HIV infection, and TB patients with (n=7) and without ( n = 14) HIV infection at (B) baseline, (C) month 3 and (D) month 6 time points. Each data point represents a single biological sample and the horizontal line in each box represents the median, whiskers = 1.5 interquartile range (IQR) with points beyond 1.5IQR plotted individually. *P≤0.05; ** P ≤0.01;*** P ≤0.001; ns = nonsignificant. Individual samples from each group were evaluated independently.
M-MDSCs are differentially expressed in children with M.tb and HIV
Phenotypically and morphologically, MDSCs are classified as G-MDSC and M-MDSC subsets, each reported to display distinctive immunosuppressive function(28). M-MDSC and G-MDSC frequencies were assessed by flow cytometric analyses of the CD14+ versus CD15+ sub-populations of peripheral MDSCs respectively. M-MDSCs were the predominant subset in all children analysed (Fig. 2). Amongst QFN negative controls, the frequency of M-MDSCs were significantly increased in HI and HEU children relative to the HU group (Fig 2). M-MDSCs were also measured as statically significant increased frequencies in HU HHC at month 6 following remote exposure and in HI HHC at month 3, when compared with HU QFN negative controls (Fig 2). M-MDSC were measured at equally high frequencies in HU TB cases, with significantly higher levels at months 3 and 6, when compared with HU QFN negative controls (Fig 2). No statistically significant differences in M-MDSC frequencies were detected amongst other groups. Since G-MDSCs were measured at particularly low frequencies, with only HU TB cases displaying significant higher levels in comparison to HU QFN negative controls, future analyses of this subset will require fresh samples (Supporting Information Fig.4).
Figure 2.
Higher frequencies of circulating infant M-MDSC upon HIV exposure and TB infection/disease. Frequencies of CD14+ M-MDSCs in QFN negative controls without HIV exposure (n = 20), with HIV infection (n= 23) and HIV exposure uninfected (n = 37); M.tb infected HHC with (n=11) and without (n = 22) HIV infection, and TB patients with (n = 7) and without (n=14) HIV infection. The horizontal line in each box represents the median, whiskers = 1.5 interquartile range (IQR) with points beyond 1.5IQR plotted individually. *P≤0.05; ** P ≤0.01;***P ≤0.001; ns = nonsignificant. Individual samples from each group were evaluated independently.
Altered patterns of MDSC-associated plasma analytes in M.tb and/or HIV exposed/infected children
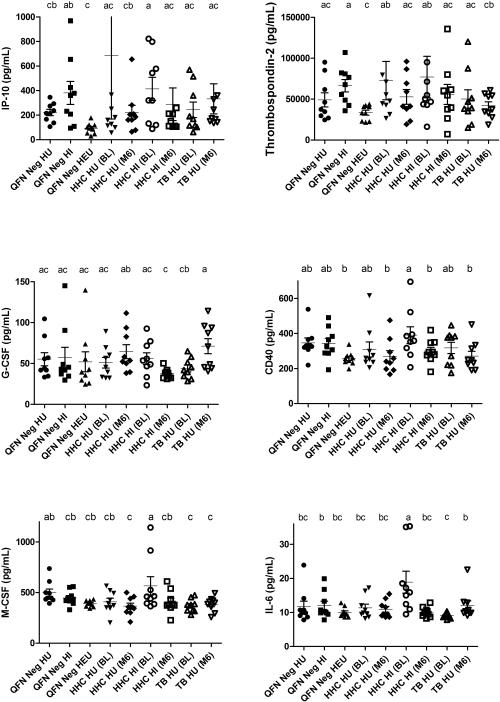
The presence of selected soluble analytes, associated with MDSC accumulation, function and activation was measured in plasma of children from a subset of study groups described above. Several MDSC-associated plasma cyto/chemokine levels remained unchanged or only a trend for statistical variation amongst children from all study groups, infection or disease, including tumour necrosis factor-alpha (TNFa), matrix metalloproteinase-13 (MMP13), vascular endothelial growth factor (VEGF), interleukin-17 (IL-17), IL-2, IL-12, IL-10, IL-1B, IL-1A cluster of differentiation-40 ligand (CD40L), Interferon-gamma (IFN-γ) and receptor for advanced glycation endproducts (RAGE).
Amongst QFN negative controls, HEU children displayed significantly reduced concentrations of plasma IP-10 (p=0.005) and thrombospondin-2 (p=0.002) when compared with HI children which corresponded to a concomitant increase in peripheral MDSC frequencies (Fig 3). When these cytokines were analysed in correlation to MDSC frequency, only IL-6 displayed a significantly positive correlation with MDSC levels (Supporting Information Fig 5).
Figure 3.
Ex-vivo levels of MDSC-associated plasma analytes during M.tb infection and/or HIV infection, exposure and disease. Multiplex cytokine concentrations (pg/mL) of IP-10, G-CSF, M-CSF, IL-6, sCD40 and Thrombospondin-2 from QFN negative controls without HIV exposure (n = 10), with HIV infection (n=10) and HIV exposure uninfected (n = 10); Longitudinal assessments in M.tb infected HHC with (n = 10) and without (n = 10) HIV infection, and TB patients without (n = 9) HIV infection at baseline and month 6 time points. The horizontal line represents the median and whiskers the standard error. Due to multiple comparisons, letters a-d were used to indicate statistical significance, where values with the same letter are not statistically different from each other. P≤0.05 were considered statistically significant. Individual samples from each group were evaluated independently.
HI HHC presented with increased concentrations of plasma IL-6 (p<0.05) when compared with all other groups, however, 6 months later, levels reduced to those measured for other groups (Fig 3). HI HHC also displayed significantly reduced plasma CD40 (p=0.049) and M-CSF (p=0.007) at month 6, however none of these coincided with reduced MDSC frequencies (Fig 3). Children with TB disease presented with significantly increased plasma G-CSF (p= 0.049) concentrations following successful treatment without reflecting a reduction in MDSC frequency (Fig 3). HU TB cases demonstrated significantly lower concentrations of plasma M-CSF (p=0.0009) when compared with HI HHC with recent TB exposure (Fig 3).
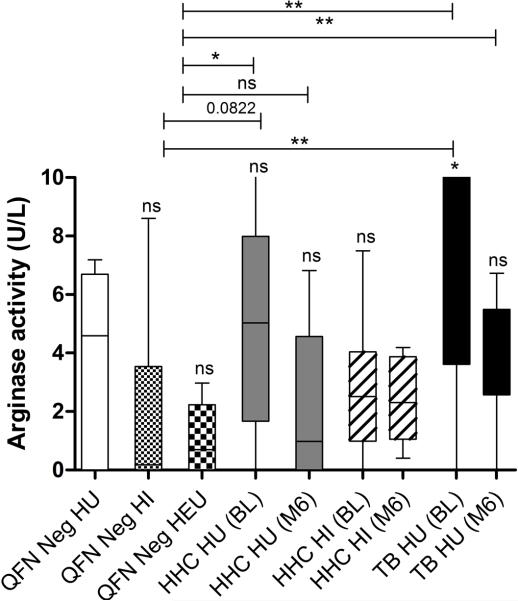
Plasma arginase activity is significantly increased in children with active TB disease
Arginase is widely accepted as one of the most common pathways of T cell suppression (54). In this study we further investigated the relationship of plasma arginase activity along with peripheral MDSC infiltration as a proxy for immunosuppressive potential. Arginase activity was measured at similar levels amongst all QFN negative controls (Fig 4). Only children with active TB disease demonstrated a significant increase in arginase activity relative to QFN negative HU controls (Fig 4). Children with active TB disease and HHC with recent TB exposure, also displayed significantly increased arginase activities relative to that of QFN negative controls, but both groups displayed decreased arginase activities following TB treatment and remote exposure respectively (Fig 4).
Figure 4.
Comparisons of arginase activity (U/L) in ex-vivo plasma of QFN negative controls without HIV exposure (n = 10), with HIV infection (n = 10) and HIV exposure uninfected (n = 10); Longitudinal assessments in M.tb infected HHC with (n = 10) and without (n = 10) HIV infection, and TB patients without (n = 9) HIV infection at baseline and month 6 time points. The horizontal line in each box represents the median, whiskers = 1.5 interquartile range (IQR). *P≤0.05; ** P ≤0.01;***P ≤0.001; ns = nonsignificant. Individual samples from each group were evaluated independently.
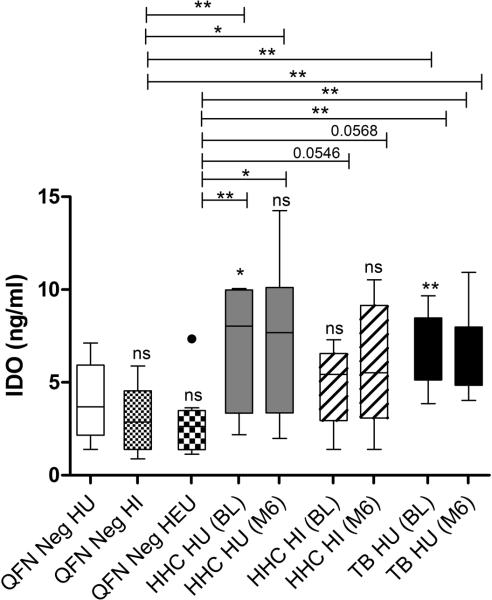
Plasma IDO expression correlates with peripheral MDSC frequencies in M.tb infected children
Human MDSCs inhibition of T cell-based immunity also occur via an IDO-dependent mechanism(20). HU HHC and TB cases displayed significantly increased plasma IDO enzyme levels in comparison to HU, HI and HEU QFN negative controls (Fig 5). IDO levels remained elevated in both groups even after successful TB treatment (month 6) and remote exposure respectively (Fig 5). A trend for increased IDO was also measured in HI HHC when compared with QFN negative HEU (Fig 5).
Figure 5.
Comparisons of IDO concentrations (ng/ml) in ex-vivo plasma of QFN negative controls without HIV exposure (n = 10), with HIV infection (n = 10) and HIV exposure uninfected (n = 10); Longitudinal assessments in M.tb infected HHC with (n = 10) and without (n = 10) HIV infection, and TB patients without (n = 9) HIV infection at baseline and month 6 time points. The horizontal line in each box represents the median, whiskers = 1.5 interquartile range (IQR) with points beyond 1.5IQR plotted individually. *P≤0.05; ** P ≤0.01;***P ≤0.001; ns = nonsignificant. Individual samples from each group were evaluated independently.
DISCUSSION
Efforts to reduce early life mortality and morbidity necessitates a thorough description of all immune components limiting effective immune protection to childhood HIV and M.tb infections. MDSC frequencies are increased and induce immunosuppression in adults during several disease conditions, however information on MDSCs in children are restricted to cord- and peripheral blood compartments of healthy infants(5,26) and children with pulmonary hypertension(27) or solid tumours(42). We demonstrate for the first time that MDSC frequencies are altered in the peripheral blood compartment of young children during HIV and TB exposure and infection. We report significantly higher peripheral frequencies of cells resembling MDSC, in HIV uninfected children born to mothers with HIV infection (HEU), compared to unexposed controls. Increased disease susceptibility of HEU children have been attributed to HIV-specific T cell responses, primed by maternal HIV antigens, the immaturity or immunosuppressive state of infants’ adaptive immune system or excessive immune activation in the HIV infected mother(11,14). Considering the previously reported potent immunosuppressive abilities of MDSCs(29), our findings could suggest an additional description of the vulnerability of the HEU child’s immune system, driven by regulatory innate immune cells. We also show significantly increased peripheral frequencies of cells resembling MDSCs in children with recent and remote household exposure to a TB case, and report the highest peripheral MDSC frequencies in children with active TB disease. These findings suggest a link between MDSC frequency and antigenic load or inflammation intensity, as reported previously (22). Surprisingly, we failed to show a decrease in MDSC frequencies in children with remote TB exposure or upon completion of standard TB treatment, as reported for adults(22). Whether the latter could describe the high rate of relapse/re-infection in infants, remains to be tested. These differences could be attributed to changes in the factors regulating immune polarization in infants versus adults, delayed MDSC kinetics in response to TB treatment, variations in exposure gradients or other factors potentially modulating early stage immunity such as childhood vaccinations(30,31). We also cannot exclude the possibility of unsuccessful TB treatment or the presence of disseminated TB in some children, which requires up to 9 months rather than the standard 6 months of TB treatment, as resolution of baseline symptoms and weight gain are markers of satisfactory treatment response (Table S2). Upon first glance our results also propose that peripheral MDSC frequencies remain unaffected by HIV infection, contrasting many studies reporting a significant increase in MDSCs in HIV infected adults(36). However, given that all HIV infected children in the current study received HAART, it is reasonable to believe that HAART-mediated control of HIV viremia, masked the effect of HIV infection on MDSC frequencies, reflecting former studies indicating HAART-induced restoration of peripheral MDSC frequencies to levels observed in healthy controls(32).
We also assessed peripheral frequencies of M-MDSCs and G-MDSCs, as subset-specific differences have been observed in several disease conditions(33–35). Our findings indicate that cells representing the M-MDSC subset, is the predominant phenotype in PBMCs amongst all study groups. Although significantly increased levels of G-MDSCs were detected in HU TB cases, accurate G-MDSC detection requires real-time evaluation of blood samples. Although the immunosuppressive potential of both M-MDSC and G-MDSC subsets have been described, M-MDSCs are reported as more potent immunosuppressors, inducers of Tregs and predictors of an unfavourable clinical outcome(36–39). Our findings could thus signify an altered immunosuppressive potential of MDSCs in HU children with TB. Even so, the exclusive availability of cryopreserved PBMC samples, could have resulted in an underestimation of peripheral G-MDSCs in our participant groups, as described previously(40).
At this point, we examined factors associated with the development and/or promotion of MDSCs. Our results show that increased peripheral frequencies of cells phenotypically resembling MDSCs, correspond to a concomitant reduction in plasma levels of IP-10 and thrombospondin-2 in HEU children and significantly increased IL-6 in HI HHC. IL-6 has previously been linked to MDSC accumulation and shown to eliminate MDSC following administration of anti-IL-6 antibody(28,49). IP10 is a chemokine mediating trafficking of activated TH1 cells to areas of inflammation(52), whereas thrombospondin-2 is a glycoprotein mediating cell interactions such lung adhesion and tissue remodelling. Interestingly, others failed to show differences in IP-10 cord blood levels amongst HEU and healthy HIV uninfected infants(43), while higher serum thrombospondin-2 were shown for adults with greater cavitary manifestations, subsequently characterizing thrombospondin-2 as a biomarker of TB disease severity(44). Although an inverse relationship between MDSC accumulation and IP-10 production was reported previously(45,46) and others demonstrated that thrombospondin-2 is differentially expressed in a mouse model of transplant activated MDSCs, this is the first report linking plasma levels of these analytes, to peripheral MDSC frequencies in HIV and TB infections. M-CSF is a tumour-derived growth factor involved in monocytic differentiation, promoting in vitro expansion of MDSCs through stimulation of myelopoiesis(65). Counterintuitively, our results show a reduction in plasma M-CSF in children with higher peripheral MDSC frequencies. The documented in vivo effect of M-CSF signalling on MDSCs has however been variable, with some showing that blocking of M-CSF signalling significantly affects MDSC frequencies(15) or not(48), suggesting that the effects are most likely indirect and dependent on other factors in the microenvironment. We further show that significantly higher concentrations of plasma G-CSF in HU TB cases after treatment and reduced plasma CD40 levels in HU HHC with recent versus remote exposure to a TB case, does not reflect changes in peripheral MDSC frequencies. (51)(52)
We show that arginase activity and IDO levels were similar amongst all QFN negative children, but that increased MDSC frequencies in TB cases and HU HHCs, correspond to a concomitant increase in arginase activity and IDO concentrations. Noteworthy, although arginase activity was reduced to levels observed in QFN negative controls following successful treatment or upon remote TB exposure, IDO levels remained high at all time points investigated. High levels of IDO and arginase activity has been described previously during human M.tb infection. Reports of robust IDO expression in pulmonary TB patients(86) and lung lesions of BCG vaccinated non-human primates(55), have resulted in its classification as promising prognostic marker of pulmonary TB(85). Similarly, increased arginase expression in TB granulomas(76), signify a central role for this molecule during TB disease. While our findings remain preliminary and the source of these enzymes could be diverse, we show that both arginase and IDO display different expression kinetics during the course of TB infection, disease and treatment, collectively implying a potential role in MDSC function. MDSC subset-specific distribution of arginase has also been reported, again hinting at a MDSC subset-specific significance during HIV and M.tb infections(57). Nonetheless, only IDO showed significant positive correlation with MDSC frequencies (Supporting Information Fig.6). Future studies evaluating the effect of MDSC-derived IDO and/or arginase deficiency on T cell function or assessment of T cell response following culture in medium conditioned with infant serum, will confirm its functional importance during human M.tb infection.
Study limitations include the lack of access to samples from HI children prior to HAART treatment initiation and samples from mothers, complicating evaluation of childhood HIV infection on MDSC frequencies and the relationship between infant and maternal MDSC levels during gestation. Additionally, plasma samples might be inappropriate for measurement of MDSC-specific analytes, especially considering their low frequency in peripheral blood. Confounding factors include uneven distribution of weight and age between groups, variables with known effects on peripheral MDSC frequencies. Other limitations are the lack of data on the immunosuppressive functionality and clinical implication of increased MDSC frequencies in children. Future studies should assess the functional relevance of MDSCs in children with M.tb and/or HIV infection, either by depletion or maturation of peripheral MDSC subsets, as well as evaluation of the role of MDSC cross-talk with other immune cells.
In summary we show that frequencies of cells with a M-MDSC phenotype, are increased in the PBMC fraction of HEU children and children with M.tb infection, and that MDSC levels are greatest in children with active TB disease. Although no single plasma analyte concentration corresponded to fluctuations in peripheral MDSC frequencies across all groups, increased MDSCs were associated with reduced levels of IP10, thrombospondin and M-CSF. Increased MDSC frequencies in HU recently exposed HHCs and TB cases also corresponded to a concomitant increase in arginase activity and IDO concentrations. Following successful TB treatment or remote TB exposure, IDO levels remained high but arginase activity was significantly reduced. Collectively the results reported here suggest that childhood HIV and M.tb exposure, infection or disease, create an environment where peripheral MDSCs develop and increase, which, considering their reported suppressive potential, may weaken protective responses to either pathogens by manipulating the host cytokine profile and stimulating arginase and IDO induction. We propose that increased MDSC in HEU children might be related to the active in utero induction of HIV specific T-cell responses, whereas MDSCs remain unchanged in HIV infected children receiving HAART due to treatment-associated restriction of viral load. Similarly, increased MDSCs in children with recent M.tb infection and TB disease, is likely attributed to the increased presence of M.tb antigenic load and/or associated immune activation, as suggested previously(63,64). It is likely that the mechanism of MDSC accumulation might differ between HIV and M.tb infections(60,61).
MDSCs’ potential to modulate early life immunity could serve as potential independent biomarker to predict infection progression and severity and also holds promise of potential clinical relevance, as several ongoing clinical trials are currently evaluating novel approaches targeting MDSCs as immunotherapeutic treatment for cancer(62).
MATERIALS AND METHODS
Study setting and design
Two paediatric studies, conducted in the Ravensmead/Uitsig/Site C communities and the Tygerberg Provincial Hospital in the Western Cape Province of South Africa, contributed to the participants for this retrospective sub-study.
Study 1 [Funded by The Norwegian Cooperation for Higher Education (NUFUPRO2007/10183; Thrasher Research Fund (02826) and DMID 1R01AI076199-01A1] was a paediatric household contact (HHC) study which aimed to investigate markers of TB infection in children with recent TB exposure. Adjacent neighbouring households without a known tuberculosis index case, were included in this study. TB cases and HHCs were followed serially at 3 months and 6 months after enrollment. All HIV infected and uninfected children with and without documented TB exposure and disease in this sub-study, were recruited from study 1.
Study 2 [Funded by International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) P1041; National Institute of Allergy and Infectious Diseases (NIAID), U01 AI068632] was a Phase II-III, double-blind, placebo-controlled trial comparing the administration of INH to placebo in the absence of any documented TB exposure. All HIV exposed infected and uninfected children in this sub-study, were enrolled from study 2.
The studies were approved by the Health Research Ethics Committee (HREC) of Stellenbosch University N05/07/129 (NUFU); N08/08/234 (IMPAACTP1041 IGRA); N07/10/254 (THRASHER). Written informed consent was obtained from all parents and caregivers; children older than 7 years gave assent and consent provided for the storage and additional immunological tests.
Children were diagnosed with TB within the last three months of study enrolment through standardized symptom screening(86) at baseline, including chest radiography (anteroposterior and lateral), mycobacterial culture (MGIT; Becton-Dickinson, Franklin Lakes, NJ) of gastric aspirates or sputum as well as positive immunological tests including Quantiferon-TB Gold assays (Cellestis, Ltd., Carnegie, Australia). Two independent experts read chest radiographs, using a standardized pediatric tool(87). All TB cases were followed up after three months and six months of standard TB treatment. Bacteriology at follow-up visits were only performed when clinically indicated (Table S2). Weight gain (or improvement of failure to thrive), the resolution of symptoms suggestive of TB and the improvement of CXR findings in the long term, were considered an appropriate response to therapy. M.tb infection was characterized by interferon-gamma release assay (IGRA) positivity. Infant HHC, had no clinical, radiologic, or microbiologic evidence of active TB, but were classified with recent M.tb infection if they were IGRA positive and living in the same household as an adult, sputum smear–positive TB case. QFN negative community controls had no known recent exposure to an active TB case and had no clinical, radiologic, or microbiologic evidence of active TB and negative IGRA results. HIV exposed children had mothers with confirmed HIV infection at birth. HIV testing and counseling was completed on children with unknown or negative status (Abbott Determine HIV-1/2 rapid test). Positive or indeterminate HIV rapid tests were confirmed with ELISA (children >18 months) or DNA polymerase chain reaction (children ≤18 months). Children infected with HIV had access to combination antiretroviral therapy. In the Western Cape Province, all children follow the SA EPI vaccination schedule and BCG vaccination (Danish strain, 1331, Statens Serum Institute, Copenhagen, Denmark) is routinely administered at or shortly after birth, with a coverage greater than 98%(63).
PBMC isolation
Peripheral blood was collected in heparin-containing tubes and processed within three hours of collection. Blood was diluted with an equal volume of AIM-V media (Thermo Fisher Scientific) and layered over a Ficoll density gradient medium (GE Healthcare) and PBMC isolation performed according to the manufacturers’ instructions (Cellestis, Australia, and Oxford Immunotec, UK). PBMCs were cryopreserved in freezing media containing 90% FCS and 10% DMSO at 1 × 107 PBMC/ml.
Flow cytometry
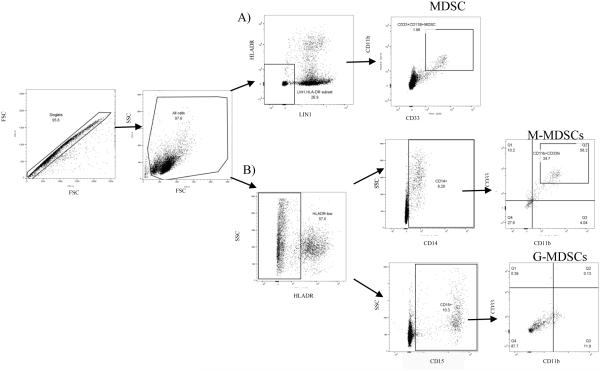
PBMCs were thawed directly following liquid nitrogen retrieval in a water bath at 37 °C and washed with pre-warmed RPMI 1640 (Thermo Fisher Scientific) media (supplemented with 1% L-glutamine and 10% FCS) . Cell quantification and viability was determined by trypan blue exclusion (0.4% solution; Sigma-Aldrich Company Ltd). Cells were resuspended in FACS buffer (1xPBS supplemented with 2% FCS) and antibodies to surface antigens added to cells for 20 min at room temperature. Monoclonal antibodies (eBioscience, San Diego, USA; Becton Dickinson BD Biosciences, Mountain View, CA, USA) to human CD4-PE-Cy7, HLA-DR-APC, CD11b-PerCP, CD33-PE, CD14-Pacific Blue and CD15-HV500, were used to characterize MDSC and subsets, followed by washing in FACS buffer and fixation in 2% paraformaldehyde. Multicolor flow cytometery was employed to acquire and identify cell subsets (FACS CantoII™; BD Biosciences) with appropriate instrument settings and compensation for all antibodies. FMO controls were used to determine positive and negative populations for the MDSC gating strategy. All lymphocyte, monocyte, and granulocyte populations were selected from the single cells gate. From this population, the LIN-1 (CD3, CD14, CD16, CD19, CD20, and CD56-FITC cocktail) and HLA-DR negative and low-staining cells were defined and MDSCs identified as the cells with positive staining for the marker CD11b and high expression of the marker CD33 (Fig 6A), as previously described(22). In order to specifically characterize the various MDSC subsets, M-MDSCs and G-MDSCs were identified according to the most recent standards by Bronte et al., by selecting the HLA-DR negative and low-staining cells from the single cells, followed by selection of cells positive for CD14, CD33 and CD11b (Fig 6B) or CD15, CD33 and CD11b positivity (Fig 6B) respectively(70). Data analysis was performed using FlowJo software (Tree Star Inc.).
Figure 6.
Gating strategy employed to define (A) LIN-1- HLA-DR-/low CD11b+CD33high total MDSCs and the (B) HLA-DR-/low CD14+CD11b+CD33high M-MDSCs and HLA-DR-/low CD15+CD11b+CD33high G-MDSCs subsets. The gating strategy plots are from single experiment.
Plasma cytokine assay
A customized human 21-plex assay was selected (Table S1). The assay was performed as single biological and technical replicates according to manufacturer’s recommendations (R&D systems, Minneapolis, USA) to quantify the levels of cytokines from plasma samples of 9-10 children from selected groups (MAGPIX, Luminex Corporation, Austin, TX, USA).
Arginase assay
Anticoagulated heparinized blood was collected for plasma separation by centrifugation (Eppendorf) at 400 × g for 10 minutes at room temperature. Plasma samples (diluted 1:2 with double-distilled H2O) from 9-10 children from selected groups were used for quantitative colorimetric determination of arginase activity as single biological and technical replicates according to manufacturer’s instructions (Quantichrom, Arginase Assay Kit; BioAssay Systems, Hayward, CA). Briefly, a microplate test kit measured the intensity of urea produced in the arginase reaction at an optical density of 520 nm on a microplate reader (Versamax, Molecular Devices Co., Sunnyvale, CA, USA), and arginase activity (U/L) measured from a urea standard (50 mg/dl).
Indoleamine-pyrrole 2,3-dioxygenase enzyme assay
Plasma IDO levels were measured by means of an enzyme-linked immunosorbent assay (ELISA) according to manufacturer’s instructions (CUSABIO Biotech Co., Wuhan, China). In brief, plasma samples (100 μL) from 9-10 children from selected groups were transferred to a 96 well microplate coated with an IDO capture antibody and incubated for 2 h at 37°C. Horseradish peroxidase (HRP)-labelled monoclonal IDO detection antibody was added and incubated for 1 h at 37°C. Substrate solution was added and incubated at room temperature for 20 min. After addition of stop solution, the optical density of samples were read at wavelengths of 450 and 570 nm (Versamax, Molecular Devices Co., Sunnyvale, USA). A standard curve, provided by the manufacturer, was generated and the sample concentrations determined by 4-parameter logistic curve.
Statistical Analysis
Differences in cell frequencies and enzyme levels between groups was assessed by the Kruskal-Wallis one-way analysis of variance (ANOVA) test and cell frequencies at various time points evaluated by the Wilcoxon rank test, with Dunn post-test correction (GraphPad Prism v.5 software, San Diego, USA). Changes of percentages of cells with age were tested by simple regression analyses (GraphPad Prism v.5 software). Multiplex cytokine assay data were analyzed by mixed model repeated measures ANOVA with Fisher LSD Post-Hoc test ( Statistica 10, StatSoft, Ohio, USA). Chi-square test was used to calculate baseline demographic and clinical categorical data such as gender, ethnicity, QFN positivity, TB treatment, HIV positivity and HAART. P values less than or equal to 0.05 was considered significant.
Supplementary Material
Acknowledgements
The authors thank the study nurses, the TB clinic staff, the study participants and their families for their kind cooperation, the City of Cape Town TB Directorate to conduct the study. Sample collection was supported by the US National Institute of Allergy and Infectious Disease at the National Institutes of Health (R01A076199), the THRASHER Research Fund and the Norwegian Cooperation for Higher Education (NUFU: NUFUPRO-2007/10183). Immunological analyses were supported by the DST/NRF SA Research Chair Initiative (SARChI) for TB Biomarkers.
Abbreviations
- MDSCs
Myeloid derived suppressor cells
- HIV
Human Immunodeficiency Virus
- M.tb
Mycobacterium tuberculosis
- HHCs
household contacts
Footnotes
Conflict of Interest: We have no potential conflicts of interest to report.
REFERENCES
- 1.Danzhen Y, Wardlaw T, Jin Rou N. Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation. The Inter-agency Group for Child Mortality Estimation (IGME); 2012. Levels & Trends in Child Mortality. Report 2012. [Google Scholar]
- 2.Vakili R, Emami Moghadam Z, Khademi G, Vakili S, Saeidi M. Child Mortality at Different World Regions: A Comparison Review. Int J Pediatr. 2015 Aug 1;3(4.2):809–16. [Google Scholar]
- 3.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. The Lancet. 2010 Jun 11;375(9730):1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 4.Prescott SL, Macaubas C, Holt BJ, Smallacombe TB, Loh R, Sly PD, Holt PG. Transplacental Priming of the Human Immune System to Environmental Allergens: Universal Skewing of Initial T Cell Responses Toward the Th2 Cytokine Profile. J Immunol. 1998 May 15;160(10):4730–7. [PubMed] [Google Scholar]
- 5.Gervassi, Horton H. Is Infant Immunity Actively Suppressed or Immature? Virol Res Treat. 2014 Feb;:1. doi: 10.4137/VRT.S12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Härtel C, Adam N, Strunk T, Temming P, Müller-Steinhardt M, Schultz C. Cytokine responses correlate differentially with age in infancy and early childhood. Clin Exp Immunol. 2005 Dec;142(3):446–53. doi: 10.1111/j.1365-2249.2005.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morein B, Blomqvist G, Hu K. Immune responsiveness in the neonatal period. J Comp Pathol. 2007 Jul;137(Suppl 1):S27–31. doi: 10.1016/j.jcpa.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 8.WHO . Global tuberculosis report 2014. WHO; 2014. [Google Scholar]
- 9.Gupta A, Bhosale R, Kinikar A, Gupte N, Bharadwaj R, Kagal A, Joshi S, et al. Maternal tuberculosis: a risk factor for mother-to-child transmission of human immunodeficiency virus. J Infect Dis. 2011 Feb 1;203(3):358–63. doi: 10.1093/jinfdis/jiq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. WHO; 2010. [Google Scholar]
- 11.Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol. 2014 Apr;176(1):11–22. doi: 10.1111/cei.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn L, Meddows-Taylor S, Gray G, Tiemessen C. Human immunodeficiency virus (HIV)-specific cellular immune responses in newborns exposed to HIV in utero. Clin Infect Dis Off Publ Infect Dis Soc Am. 2002 Jan 15;34(2):267–76. doi: 10.1086/338153. [DOI] [PubMed] [Google Scholar]
- 13.Hygino J, Lima PG, Filho RGS, Silva AAL, Saramago CSM, Andrade RM, Andrade DM, et al. Altered immunological reactivity in HIV-1-exposed uninfected neonates. Clin Immunol Orlando Fla. 2008 Jun;127(3):340–7. doi: 10.1016/j.clim.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Kakkar F, Lamarre V, Ducruet T, Boucher M, Valois S, Soudeyns H, Lapointe N. Impact of maternal HIV-1 viremia on lymphocyte subsets among HIV-exposed uninfected infants: protective mechanism or immunodeficiency. BMC Infect Dis. 2014 May 5;14(1):236. doi: 10.1186/1471-2334-14-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013 Dec 5;504(7478):158–62. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghazal P, Dickinson P, Smith CL. Early life response to infection. Curr Opin Infect Dis. 2013 Jun;26(3):213–8. doi: 10.1097/QCO.0b013e32835fb8bf. [DOI] [PubMed] [Google Scholar]
- 17.Burt TD. Fetal Regulatory T Cells and Peripheral Immune Tolerance in utero: Implications for Development and Disease. Am J Reprod Immunol N Y N 1989. 2013 Apr;69(4):346–58. doi: 10.1111/aji.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abastado J-P, Zhi L. In: Myeloid Derived Suppressor Cells: Subsets, Expansion, and Role in Cancer Progression, Tumour Microenvironment and Myelomonocytic Cells. Biswas S, editor. InTech; 2012. [Google Scholar]
- 19.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009 Mar;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol Baltim Md 1950. 2013 Apr 1;190(7):3783–97. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 21.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011 Jul;11(7):802–7. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.du Plessis N, Loebenberg L, Kriel M, von Groote-Bidlingmaier F, Ribechini E, Loxton AG, van Helden PD, et al. Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent Mycobacterium tuberculosis infection suppresses T-cell function. Am J Respir Crit Care Med. 2013 Sep 15;188(6):724–32. doi: 10.1164/rccm.201302-0249OC. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz ML, Lu L, Ramachandran I, Gabrilovich DI. Myeloid-derived suppressor cells in the development of lung cancer. Cancer Immunol Res. 2014 Jan;2(1):50–8. doi: 10.1158/2326-6066.CIR-13-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youn J-I, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of Myeloid-Derived Suppressor Cells in Tumor-Bearing Mice. J Immunol. 2008 Oct 15;181(8):5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raber P, Wyczechowska D, Rodriguez P. Granulocytic and monocytic populations of tumor-infiltrating myeloid-derived suppressor cells (MDSC) suppress T cell proliferation through independent mechanisms. J Immunol. 2012 Jan 5;188:74. Meeting Abstracts 1. 7. [Google Scholar]
- 26.Rieber N, Gille C, Köstlin N, Schäfer I, Spring B, Ost M, Spieles H, et al. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin Exp Immunol. 2013 Oct;174(1):45–52. doi: 10.1111/cei.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeager ME, Nguyen CM, Belchenko DD, Colvin KL, Takatsuki S, Ivy DD, Stenmark KR. Circulating myeloid-derived suppressor cells are increased and activated in pulmonary hypertension. Chest. 2012 Apr;141(4):944–52. doi: 10.1378/chest.11-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg JM, Zidan A-A, Siddiqi R, Zayas M, Brown CD, Forster L, David A, et al. Circulating myeloid-derived suppressor cells in pediatric solid tumor patients. J Clin Oncol. 2012;30 suppl; abstr 9565. [Google Scholar]
- 29.Youn J-I, Gabrilovich DI. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010 Nov 1;40(11):2969–75. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanden Driessche K, Persson A, Marais BJ, Fink PJ, Urdahl KB. Immune Vulnerability of Infants to Tuberculosis. J Immunol Res. 2013 May 13;2013:e781320. doi: 10.1155/2013/781320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakaoka H, Lawson L, Squire SB, Coulter B, Ravn P, Brock I, Hart CA, Cuevas LE. Risk for Tuberculosis among Children. Emerg Infect Dis. 2006 Sep;12(9):1383–8. doi: 10.3201/eid1209.051606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vollbrecht T, Stirner R, Tufman A, Roider J, Huber RM, Bogner JR, Lechner A, et al. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS Lond Engl. 2012 Jul;26(12):F31–37. doi: 10.1097/QAD.0b013e328354b43f. [DOI] [PubMed] [Google Scholar]
- 33.Ning G, She L, Lu L, Liu Y, Zeng Y, Yan Y, Lin C. Analysis of Monocytic and Granulocytic Myeloid-Derived Suppressor Cells Subsets in Patients with Hepatitis C Virus Infection and Their Clinical Significance, Analysis of Monocytic and Granulocytic Myeloid-Derived Suppressor Cells Subsets in Patients with Hepatitis C Virus Infection and Their Clinical Significance. BioMed Res Int BioMed Res Int. 2015 Mar 1;2015(2015):e385378. doi: 10.1155/2015/385378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vetsika E-K, Koinis F, Gioulbasani M, Aggouraki D, Koutoulaki A, Skalidaki E, Mavroudis D, et al. A Circulating Subpopulation of Monocytic Myeloid-Derived Suppressor Cells as an Independent Prognostic/Predictive Factor in Untreated Non-Small Lung Cancer Patients, A Circulating Subpopulation of Monocytic Myeloid-Derived Suppressor Cells as an Independent Prognostic/Predictive Factor in Untreated Non-Small Lung Cancer Patients. J Immunol Res J Immunol Res. 2014 Nov 11;2014(2014):e659294. doi: 10.1155/2014/659294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Höchst B, Mikulec J, Baccega T, Metzger C, Welz M, Peusquens J, Tacke F, et al. Differential Induction of Ly6G and Ly6C Positive Myeloid Derived Suppressor Cells in Chronic Kidney and Liver Inflammation and Fibrosis. PLoS ONE. 2015 Mar 4;10(3):e0119662. doi: 10.1371/journal.pone.0119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Movahedi K, Guilliams M, den Bossche JV, den Bergh RV, Gysemans C, Beschin A, De Baetsellier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell–suppressive activity. Blood. 2008 Apr;111(8):4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 37.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40(1):22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 38.Stromnes IM, Brockenbrough JS, Izeradjene K, Carlson MA, Cuevas C, Simmons RM, Greenberg PD, Hingorani SR. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut. 2014 Nov;63(11):1769–81. doi: 10.1136/gutjnl-2013-306271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, Umansky V. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer. 2015 May 15;136(10):2352–60. doi: 10.1002/ijc.29297. [DOI] [PubMed] [Google Scholar]
- 40.Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods. 2012 Jul 31;381(1–2):14–22. doi: 10.1016/j.jim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sumida K, Wakita D, Narita Y, Masuko K, Terada S, Watanabe K, Satoh T, et al. Anti-IL-6 receptor mAb eliminates myeloid-derived suppressor cells and inhibits tumor growth by enhancing T-cell responses. Eur J Immunol. 2012 Aug;42(8):2060–72. doi: 10.1002/eji.201142335. [DOI] [PubMed] [Google Scholar]
- 42.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol. 1998 Nov;28(11):3696–705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 43.Reikie BA, Adams RC, Leligdowicz A, Ho K, Naidoo S, Ruck CE, de Beer C, et al. Altered Innate Immune Development in HIV-Exposed Uninfected Infants. J Acquir Immune Defic Syndr. 2014 Jul 1;66(3):245–55. doi: 10.1097/QAI.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Groote MA, Nahid P, Jarlsberg L, Johnson JL, Weiner M, Muzanyi G, Janjic N, et al. Elucidating Novel Serum Biomarkers Associated with Pulmonary Tuberculosis Treatment. PLoS ONE. 2013 Apr 18;8(4):e61002. doi: 10.1371/journal.pone.0061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009 May;70(5):325–30. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, Ohlfest JR, Okada H. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011 Apr 1;71(7):2664–74. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lechner MG, Liebertz DJ, Epstein AL. Characterization Of Cytokine-Induced Myeloid-Derived Suppressor Cells From Normal Human Peripheral Blood Mononuclear Cells. J Immunol Baltim Md 1950. 2010 Aug 15;185(4):2273–84. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu XJ, Hu J, Sun L, Xiao Y, Chen ZC, You Y, Zou P, et al. Amplification of functional myeloid-derived suppressor cells during stem cell mobilization induced by granulocyte colony-stimulation-factor. J Huazhong Univ Sci Technolog Med Sci. 2013 Dec 13;33(6):817–21. doi: 10.1007/s11596-013-1204-x. [DOI] [PubMed] [Google Scholar]
- 49.Abrams SI, Waight JD. Identification of a G-CSF-Granulocytic MDSC axis that promotes tumor progression. Oncoimmunology. 2012 Jul 1;1(4):550–1. doi: 10.4161/onci.19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riou C, Perez Peixoto B, Roberts L, Ronacher K, Walzl G, Manca C, Rustomjee R, et al. Effect of Standard Tuberculosis Treatment on Plasma Cytokine Levels in Patients with Active Pulmonary Tuberculosis. PLoS ONE. 2012;7(5):e36886. doi: 10.1371/journal.pone.0036886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen J, Chen X, Wang Z, Zhang G, Chen W. Downregulation of CD40 expression contributes to the accumulation of myeloid-derived suppressor cells in gastric tumors. Oncol Lett. 2014;2:775–80. doi: 10.3892/ol.2014.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan P-Y, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, Divino CM, Chen SH. Immune Stimulatory Receptor CD40 Is Required for T-Cell Suppression and T Regulatory Cell Activation Mediated by Myeloid-Derived Suppressor Cells in Cancer. Cancer Res. 2010;70:99. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Apolo AB, Tomita Y, Lee M-J, Lee S, Frosch A, Steinberg SM, Gulley JL, et al. Effect of cabozantinib on immunosuppressive subsets in metastatic urothelial carcinoma. J Clin Oncol. 2014;32:5s. suppl; abstr 4501. [Google Scholar]
- 54.Blumenthal A, Nagalingam G, Huch JH, Walker L, Guillemin GJ, Smythe GA, Ehrt S, et al. M. tuberculosis Induces Potent Activation of IDO-1, but This Is Not Essential for the Immunological Control of Infection. PLoS ONE. 2012 May 23;7(5):e37314. doi: 10.1371/journal.pone.0037314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehra S, Alvarez X, Didier PJ, Doyle LA, Blanchard JL, Lackner AA, Kaushal D. Granuloma Correlates of Protection Against Tuberculosis and Mechanisms of Immune Modulation by Mycobacterium tuberculosis. J Infect Dis. 2013 Apr 1;207(7):1115–27. doi: 10.1093/infdis/jis778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pessanha Ap, Martimns RA, Mattos-Guaraldi Al, Vianna A, Moreira LO. Arginase-1 expression in granulomas of tuberculosis patients. FEMS Immunol Med Microbiol. 2012 Nov;66(2):265–8. doi: 10.1111/j.1574-695X.2012.01012.x. [DOI] [PubMed] [Google Scholar]
- 57.Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, Liu Y, et al. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J Virol. 2013 Feb;87(3):1477–90. doi: 10.1128/JVI.01759-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2008 Apr 30;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009 Jan;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011 Jan 1;32(1):19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai J, El Gazzar M, Li GY, Moorman JP, Yao ZQ. Myeloid-derived suppressor cells: paradoxical roles in infection and immunity. J Innate Immun. 2015 Jul 2;:116–26. doi: 10.1159/000368233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wesolowski R, Markowitz J, Carson WE. Myeloid derived suppressor cells – a new therapeutic target in the treatment of cancer. J Immunother Cancer. 2013 Jul 15;1:10. doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corrigal J. Western Cape provincial EPI vaccination survey. 2005 [Google Scholar]
- 64.Bronte V, Brandau S, Chen S-H, Colombo MP, Frey AB, Greten TF, Mandruzzato S, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016 Jul 6;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiseman CA, Mandalakas AM, Kirchner HL, Gie RP, Schaaf HS, Walters E, Heseling AC. Novel application of NIH case definitions in a paediatric tuberculosis contact investigation study. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2015 Apr;19(4):446–53. doi: 10.5588/ijtld.14.0585. [DOI] [PubMed] [Google Scholar]
- 66.Marais BJ, Graham SM, Cotton MF, Beyers N. Diagnostic and Management Challenges for Childhood Tuberculosis in the Era of HIV. J Infect Dis. 2007 Aug 15;196(Supplement 1):S76–85. doi: 10.1086/518659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.