Abstract
Rumex palustris responds to complete submergence with upward movement of the younger petioles. This so-called hyponastic response, in combination with stimulated petiole elongation, brings the leaf blade above the water surface and restores contact with the atmosphere. We made a detailed study of this differential growth process, encompassing the complete range of the known signal transduction pathway: from the cellular localization of differential growth, to the hormonal regulation, and the possible involvement of a cell wall loosening protein (expansin) as a downstream target. We show that hyponastic growth is caused by differential cell elongation across the petiole base, with cells on the abaxial (lower) surface elongating faster than cells on the adaxial (upper) surface. Pharmacological studies and endogenous hormone measurements revealed that ethylene, auxin, abscisic acid (ABA), and gibberellin regulate different and sometimes overlapping stages of hyponastic growth. Initiation of hyponastic growth and (maintenance of) the maximum petiole angle are regulated by ethylene, ABA, and auxin, whereas the speed of the response is influenced by ethylene, ABA, and gibberellin. We found that a submergence-induced differential redistribution of endogenous indole-3-acetic acid in the petiole base could play a role in maintenance of the response, but not in the onset of hyponastic growth. Since submergence does not induce a differential expression of expansins across the petiole base, it is unlikely that this cell wall loosening protein is the downstream target for the hormones that regulate the differential cell elongation leading to submergence-induced hyponastic growth in R. palustris.
Changes in the orientation of plant organs in response to environmental factors are widespread in the plant kingdom. These nastic responses (Palmer, 1985) can be divided into epinastic (downward) and hyponastic (upward) curvatures and are caused by differential growth, with, respectively, the adaxial (upper) or the abaxial (lower) side of the organ growing more rapidly (Kang, 1979). One of the environmental factors inducing hyponastic leaf movement is flooding (Ranunculus repens and Caltha palustris, Ridge, 1987; Leontodon taraxacoides, Grimoldi et al., 1999; Paspalum dilatatum, Insausti et al., 2001; Rumex palustris, Cox et al., 2003). In the semiaquatic species R. palustris, hyponastic growth upon complete submergence, together with stimulated petiole elongation, contributes to increased survival by enabling the leaves to reach the water surface so that gas exchange with the atmosphere is restored (for review, see Voesenek et al., 2003).
The gaseous hormone ethylene accumulates in plants during submergence (for review, see Peeters et al., 2002) and has been implicated as an important factor in hyponastic growth of R. palustris (Voesenek and Blom, 1989; Banga et al., 1997). Ethylene also plays a role in other differential growth processes like petiole epinasty (for review, see Kang, 1979), shoot gravitropism (Kaufman et al., 1995; Friedman et al., 2003), the seedling apical hook (Ecker, 1995), and shading-induced hyponasty (Pierik et al., 2003; Vandenbussche et al., 2003).
Auxin is an important regulator of differential growth processes. Early studies led to the hypothesis that a lateral translocation of indole-3-acetic acid (IAA) takes place in a plant organ in response to environmental cues (e.g. gravity and light). The resulting asymmetrical distribution of IAA is the basis for differential cell elongation leading to nastic or tropic responses (Went and Thimann, 1937). More recently, molecular and genetic studies have largely supported this hypothesis (for review, see Friml and Palme, 2002). Auxin exerts its effect on growth via the regulation of multiple cellular processes (for review, see Blancaflor and Masson, 2003). Examples are differential expression of an auxin-induced K+-channel involved in osmotic regulation of growth (Fuchs et al., 2003), and auxin-induced activation of plasma membrane H+-ATPases leading to apoplast acidification (for review, see Becker and Hedrich, 2002) and expansin activation (Cosgrove, 2000). Expansins are cell wall proteins that loosen the cell wall in a pH-dependent manner, thereby allowing turgor-driven cell enlargement (McQueen-Mason and Cosgrove, 1994). Close correlations have been observed between expansin gene expression and extension growth, suggesting expansins as key endogenous regulators of plant cell enlargement (Cosgrove, 2000). Zhang and Hasenstein (2000) observed a differential distribution of expansins in graviresponding maize (Zea mays) roots, indicating the involvement of these cell wall loosening proteins in differential growth processes.
The plant hormones abscisic acid (ABA) and GA influence leaf orientation in opposite ways. Application of GA induced leaf hyponasty of Pittosporum eugenioides, whereas leaves treated with ABA showed epinasty (Dwyer et al., 1995). The role of GA in inducing more upright organ orientation has also been described in a number of other studies (Palmer, 1964; Blake et al., 1980; Clúa et al., 1996). In tobacco, ethylene-induced hyponastic petiole movement requires GA (Pierik, 2004). Recently, it has been shown that ethylene-mediated enhancement of apical hook formation depends on GA in Arabidopsis seedlings (Vriezen et al., 2004).
We made a detailed study of submergence-induced hyponastic growth in R. palustris petioles. Our studies encompassed the complete range of the known signal transduction pathway, from the cellular localization of differential growth, to the hormonal regulation, and the possible involvement of the cell wall loosening protein expansin as a downstream target. Our objectives were to determine: (1) the region of the petiole responsible for submergence-induced hyponastic growth; (2) the role of the plant hormones ethylene, auxin, GA, and ABA; and (3) whether expansin is a downstream target involved in hyponastic growth. We localized submergence-induced hyponastic growth to a small abaxial region of the petiole base. The plant hormones ethylene, auxin, ABA, and GA are involved in different and sometimes overlapping stages of this differential growth process, but they do not exert their effect via the establishment of a differential expansin expression across the petiole base. A model for the regulation of hyponastic growth is proposed.
RESULTS
Submergence-Induced Hyponastic Growth
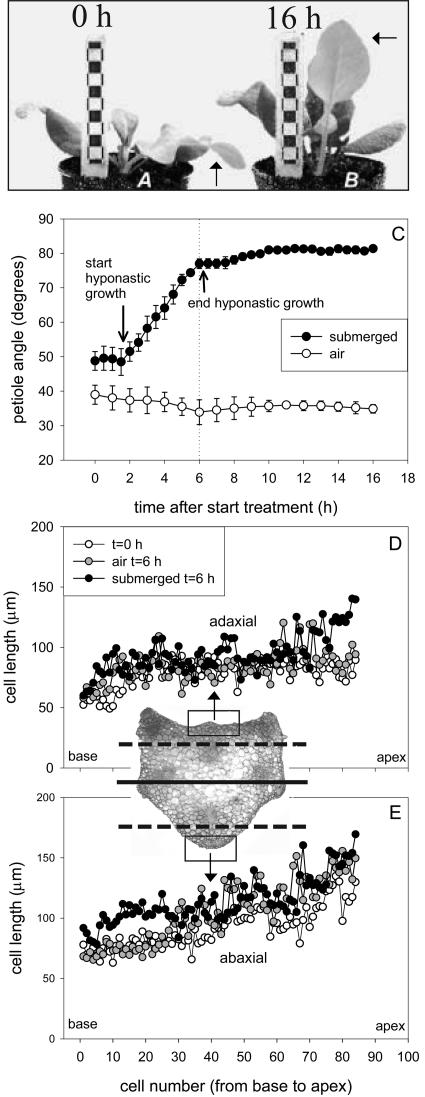
Figure 1 shows the orientation of the third oldest petiole of a 27-d-old R. palustris plant after 0 h (A) and 16 h (B) of submergence. The submergence treatment induced an almost vertical orientation of the petiole. The kinetics of the submergence-induced increase in petiole angle are shown in Figure 1C. After a lag-phase of 2.1 h (se 0.5 h) the angle of submerged petioles started to increase, reaching a maximum angle of 81° (se 1°) after 6.4 h (se 0.4 h). The petiole angle of plants in air decreased slightly in time. Since the angle of intact air-grown plants was very consistent in all experiments, it is only shown in Figure 1C. The difference of approximately 10° between submerged and air-grown petioles immediately after the onset of submergence (at t = 0 h) was caused by buoyancy.
Figure 1.
A and B, Orientation of the third oldest petiole (third oldest leaf is indicated by arrows) of a 27-d-old R. palustris plant after 0 and 16 h of submergence. C, Hyponastic growth kinetics of the third oldest petiole under water (4 replicate plants; bars represent ses). Lag-phase, 2.1 h (se 0.5 h); end hyponastic growth, 6.4 h (se 0.4 h). D and E, Epidermal cell length along the adaxial (upper; D) and abaxial (lower; E) surface of the petiole, from the base to the apex. Plots are means of 4 replicate plants. The insert shows a transverse section through the petiole, and the boxes in the section indicate the collenchyma strands on which the epidermal cell length was measured. The lines in the insert are explained in Figures 4 and 6. Mean se never exceeded 15% in D and E.
Localization Hyponastic Growth
We set out to localize the petiole region that was responsible for the upward movement of the petiole upon submergence. After 6 h of submergence the largest difference in angle was observed between plants in air and under water (Fig. 1C). Therefore, epidermal cell lengths were measured at this time point, along the entire length of an ab- and adaxial collenchyma strand (see boxes, Fig. 1 insert). In this study, we limited our measurements to the epidermis, since this structure is thought to be rate limiting for elongation (for review, see Kutschera, 2001). We observed differential growth at the base of submerged petioles (Fig. 1, D and E). In the basal region of the petiole, cells on the abaxial (lower) surface of plants submerged for 6 h were larger than those on the same surface of air-grown plants, whereas there was no difference in cell length between these two treatments on the basal adaxial (upper) surface. This differential growth took place only across a basal region comprising approximately one-third of the petiole, and there were no significant differences in cell length in the more apical regions. Differential cell length distribution in a similar basal petiole region was also observed after 4 h of submergence (data not shown).
We calculated if the observed differential cell elongation in the epidermis of the petiole base could account for the change in petiole angle of approximately 30° after 6 h of submergence. The petiole was represented by parts of two circles, with the abaxial surface belonging to the outer circle and the adaxial surface to the inner circle. In this model, the difference in length between the ab- and adaxial petiole surface determines the angle of the petiole, given that the diameter of the petiole remains constant (data not shown). From the data in Figure 1, D and E, it was calculated that the difference in length between the ab- and adaxial surface increased 1.54-fold in submerged plants compared to air-grown plants. According to the model, this 1.54-fold increase will result in an identical increase in petiole angle after 6 h of submergence. Given that the initial petiole angle was 49°, this 1.54-fold increase was sufficient to explain the observed petiole angle of 77° after 6 h of submergence (Fig. 1C).
Hormonal Regulation of Submergence-Induced Hyponastic Growth
The hyponastic response was divided into three phases: (1) initiation, the length of the lag-phase; (2) speed of the response, once the angle has started to change; and (3) the maximum petiole angle that was reached, and maintenance of this maximum petiole angle. The effect of the hormones ethylene, auxin, ABA, and GA on these three phases is described below.
Ethylene
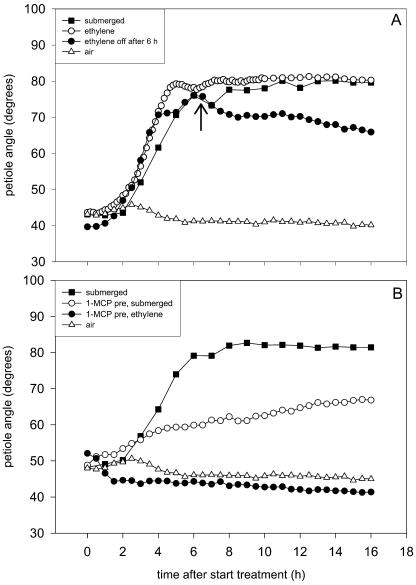
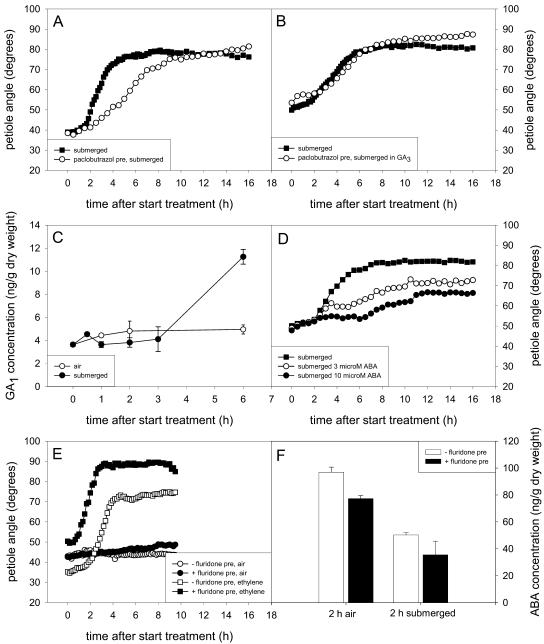
The gaseous hormone ethylene accumulates in R. palustris upon submergence and has been shown to be an important regulator of nondifferential elongation growth in this species (for review, see Voesenek et al., 2003). Since there were indications that this hormone is also involved in the regulation of hyponastic growth (Voesenek and Blom, 1989; Banga et al., 1997), we examined its role in this differential growth process under water. Ethylene treatment of plants in air did induce hyponastic growth, with a lag-phase similar to that of submerged petioles (Fig. 2A). The maximum petiole angle was 80° for both treatments, but in ethylene-treated plants the angle increased slightly faster than upon submergence, and the hyponastic response was completed somewhat earlier (P < 0.01). To test whether ethylene was also required for maintenance of an upright petiole angle, the ethylene addition was switched off when the maximum angle was reached (after 6 h). This resulted in a gradual decrease in angle over the next 10 h and a lower angle at the end of the experiment compared to plants treated continuously with ethylene (P < 0.01).
Figure 2.
Hyponastic growth of the third oldest petiole of R. palustris plants (27-d-old) that were treated with ethylene or submerged (A), or pretreated with 1-MCP and subsequently submerged or treated with ethylene (B). The arrow in A represents the time when the ethylene treatment was stopped. Plots are means of three to eight replicate plants. Mean se never exceeded 6% in A and 5% in B. Lag-phase and end hyponastic growth, (A) 1.6 h (se 0.2 h) and 4.5 h (se 0.1 h) for ethylene, 2.1 h (0.2 h) and 5.5 h (se 0.2 h) for submerged.
Pretreatment with the ethylene perception inhibitor 1-methylcyclopropene (1-MCP) abolished the distinct kinetics of hyponastic growth in submerged plants (Fig. 2B). Although these petioles did show an increase in angle, it was smaller than in submerged plants without 1-MCP (P < 0.01) and no distinct lag-phase was observed. In contrast, plants pretreated with 1-MCP and subsequently exposed to ethylene did not show any hyponastic growth (Fig. 2B).
Auxin
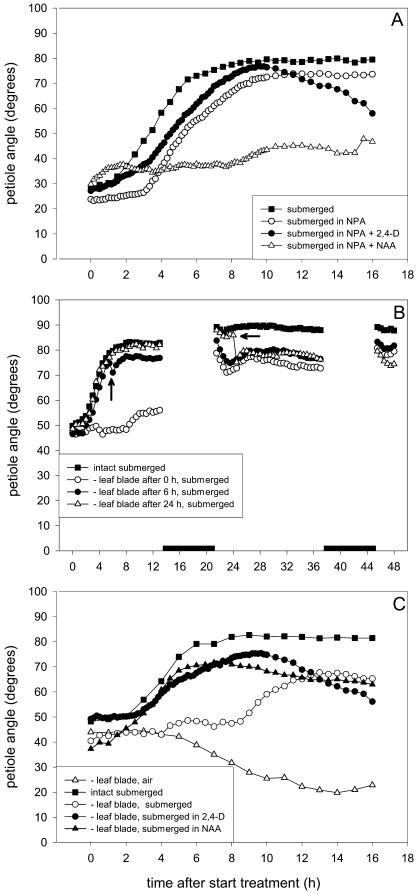
Auxin and the polar auxin transport (PAT) pathway play an important role in a number of differential growth processes, like gravitropism and phototropism (for review, see Friml and Palme, 2002). Therefore, we examined if auxin and PAT are also involved in submergence-induced hyponastic growth in R. palustris. Firstly, plants were pretreated with naphthylphtalamic acid (NPA), an inhibitor of the auxin efflux carrier (Fig. 3A). This pretreatment almost doubled the lag-phase of hyponastic growth under water (P < 0.001), indicating that a functioning PAT pathway is required for proper initiation of the hyponastic response. NPA pretreatment did not affect the maximum petiole angle or maintenance of this angle. The effect of another inhibitor of the auxin efflux carrier, 2,3,5-triiodobenzoic acid, was similar, but weaker. Inhibition of the auxin influx carriers with 1-naphthoxyacetic acid had no effect (data not shown). The NPA-induced delay in the lag-phase of hyponastic growth was almost completely rescued by the synthetic auxin 2,4-dichlorophenoxy acetic acid (2,4-D). In contrast, 1-naphthaleneacetic acid (NAA) completely abolished hyponastic growth (Fig. 3A).
Figure 3.
Hyponastic growth of the third oldest petiole of submerged 27-d-old R. palustris. A, Intact plants treated with NPA and the synthetic auxins NAA or 2,4-D. B, Debladed leaves after 0, 6, or 24 h into the submergence treatment. C, Debladed leaves at the start of the experiment and subsequently submerged in 2,4-D or NAA. Plots are means of three to eight replicate plants. Mean se never exceeded 21% in A, 4% in B, and 20% in C. The black boxes in B represent the night period, and the arrows indicate leaf blade removal. Lag-phase and end hyponastic growth, (A) 1.7 h (se 0.4 h) and 7.2 h (se 0.3 h) for submerged, 3.2 h (0.1 h) and 8.5 h (se 0.5 h) for NPA, 1.6 h (se 0.3 h) and 8.6 h (se 0.1 h) for NPA + 2,4-D; (C) 2.4 h (se 0.04 h) and 6.3 h (se 0.7 h) for submerged, 2.8 h (0.2 h) and 7.5 h (se 1.1 h) for 2,4-D, 1.2 h (0.2 h) and 6.1 h (se 0.4 h) for NAA, lag-phase of debladed (−leaf blade) 8.6 h (se 0.2 h).
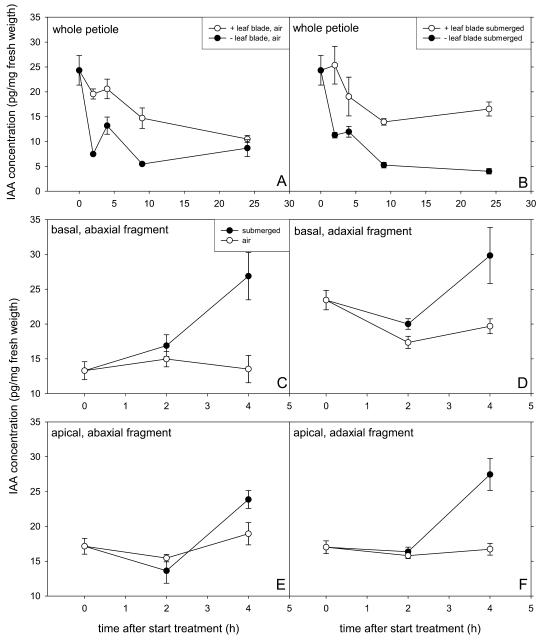
Secondly, we tested if the kinetics of hyponastic growth were also affected by deblading at a number of times during submergence. This treatment is thought to reduce the endogenous auxin concentration in the petiole since the leaf blade, which is an important auxin source, is removed (Ljung et al., 2001). We checked if in R. palustris deblading does indeed lower the auxin content of the petiole. Figure 4, A and B, show that removal of the leaf blade at the start of the experiment caused a strong decrease in IAA concentration in petioles in air and under submerged conditions (P < 0.01), an effect that was apparent within 2 h of blade excision and sustained for up to 24 h in submerged plants. The effect of this drop in petiolar IAA concentration on the kinetics of hyponastic growth under water is shown in Figure 3, B and C. Deblading at the start of submergence strongly delayed the onset of hyponastic growth (P < 0.01). Additionally, the maximum petiole angle was approximately 10° lower than in intact submerged plants (Fig. 3B). Deblading after 6 h of submergence, when hyponastic growth was almost completed, or after 24 h, when the maximum petiole angle was maintained, also resulted in a decrease in maximum petiole angle of approximately 10° (Fig. 3B). Thus, IAA supplied from the leaf blade is important for both initiation of hyponastic growth and for the petiole to reach and maintain its maximum angle in submerged R. palustris plants. Additionally, deblading at the start of the experiment resulted in a drop in petiole angle of air-grown plants after 16 h (Fig. 3C), indicating that IAA from the leaf blade is important for maintenance of the petiole angle in air.
Figure 4.
Endogenous IAA concentration (picogram per milligram fresh weight) of different parts of the third oldest petiole of R. palustris (27-d-old). A and B, Whole petiole of intact or debladed leaves (leaf blade removed at t = 0 h). C to F, Ab- and adaxial fragments of the base and the remaining apical part, cut as shown in the insert in Figure 1 (dashed lines). Data are means of four to eight replicate plants (bars represent ses).
The delay in the onset of hyponastic growth resulting from deblading could be rescued by submergence in 2,4-D or NAA (Fig. 3C). Gibberellic acid 3 (GA3), or the ethylene precursor 1-amino-cyclopropane-1-carboxylic acid (ACC), could not rescue the longer lag-phase of submerged debladed petioles, nor could exposing the plants to ethylene (data not shown).
Endogenous IAA Concentration in the Petiole
An asymmetrical distribution of IAA across a plant organ has been shown to be the basis for differential cell elongation leading to nastic or tropic responses (Went and Thimann, 1937; for review, see Friml and Palme, 2002). Therefore, we examined if the differential cell elongation in the petiole base of submerged R. palustris plants (Fig. 1, D and E) correlated with a submergence-induced, asymmetrical redistribution of endogenous IAA in this region after 4 h of treatment, a time-point when the speed of hyponastic growth was at its fastest. Firstly, we analyzed ab- and adaxial halves of the petiole base of submerged plants (data not shown) and observed a slight decrease in IAA concentration in both halves similar to that observed in intact petioles shown in Figure 4B. However, it was possible that an asymmetrical redistribution of IAA was only detectable in the outer cell layers and the relatively large sample size of the halved petioles could have masked the detection of such a redistribution. Therefore, we also measured the IAA concentration in small strips of the ab- and adaxial surface of the petiole base. We found that in these small fragments of both petiole surfaces, the IAA concentration was elevated after 4 h of submergence (P < 0.001; Fig. 4, C and D). Since the concentration of intact or halved petioles did not increase and even showed a slight decrease, we propose that this localized increase in the outer cell layers was brought about by a redistribution of IAA toward this region. This redistribution of IAA after 4 h of submergence was slightly asymmetric, with the difference between submerged and air-grown petioles in the abaxial basal segment being approximately 100%, whereas it was only approximately 50% in the basal adaxial segment. Apical ab- and adaxial fragments also showed an increase in IAA concentration between 2 and 4 h of submergence (Fig. 4, E and F), suggesting that redistribution of auxin also took place in this apical petiole region.
GA
We tested the role of GA in submergence-induced hyponastic growth of R. palustris, since this hormone has been implicated in leaf movements in a number of other species (e.g. Palmer, 1964; Blake et al., 1980; Clúa et al., 1996). Using the GA biosynthesis inhibitor paclobutrazol we found that GA modulates the speed of the hyponastic response (Fig. 5A). Pretreatment with paclobutrazol resulted in a slower change of the petiole angle during submergence. Although the lag-phase of hyponastic growth and the maximum petiole angle were not affected, paclobutrazol treated plants reached their maximum angle later than submerged plants without paclobutrazol (P < 0.05). This slower hyponastic growth could be rescued completely by GA3 addition (Fig. 5B). We also examined if the regulatory role of GA on the speed of hyponastic growth was exerted via the GA already present in the petiole, or whether it depended on a submergence-induced increase in the concentration of this hormone. Figure 5C shows that in R. palustris, the GA1 concentration of submerged petioles was higher than that of petioles in air after 6 h of treatment (P < 0.05). Since the hyponastic response under water is already initiated when the GA1 concentration starts to increase, it seems likely that the influence of GA on the hyponastic response is exerted via the levels of GA that are already present in the petiole and does not depend on an increase in petiolar GA concentration.
Figure 5.
Hyponastic growth of the third oldest petiole of 27-d-old submerged R. palustris plants (pre)treated with (A) paclobutrazol, (B) paclobutrazol and GA3, (D) ABA, and (E) fluridone. Plants in E were not submerged, but treated with ethylene. Mean se never exceeded 10%; bars in C and F represent ses. Lag-phase and end hyponastic growth, (A) 1.5 h (se 0.01 h) and 4.3 h (0.3 h) for submerged, 2.0 h (0.3 h) and 6.9 h (0.2 h) for paclobutrazol; (B) 1.6 h (0.2 h) and 6.2 h (0.5 h) for submerged, 2.3 h (0.2 h) and 7.4 h (0.3 h) for paclobutrazol + GA3; (E) 2.0 h (0.01 h) and 3.7 h (0.01 h) for −fluridone pretreatment + ethylene, 0.7 h (0.04 h) and 3.0 h (0.3 h) for +fluridone pretreatment + ethylene. Endogenous GA1 (C) or ABA (F) concentrations of the third oldest petiole of air-grown and submerged plants are also shown. Plots are means of three to four replicate plants.
ABA
ABA is involved in the regulation of epinastic leaf movement in P. eugenioides (Dwyer et al., 1995) and it is a negative regulator of nondifferential elongation growth in submerged R. palustris (Voesenek et al., 2003) and deepwater rice (Hoffmann-Benning and Kende, 1992). Therefore, we examined if ABA plays a similar negative role in submergence-induced hyponastic growth in R. palustris. To test this, we added ABA to the submergence water and found that this partially inhibited hyponastic growth (Fig. 5D). Although the petiole angle during the 1st h of submergence was not affected, the increase in angle slowed down after approximately 4.5 h when submerged in 3 μm ABA, and even earlier when submerged in 10 μm ABA (P < 0.05).
We also reduced the endogenous ABA concentration using fluridone, an inhibitor of ABA biosynthesis (Fig. 5F) and subsequently examined the effect on hyponastic growth using a saturating concentration of ethylene as a proxy for submergence (Fig. 5E). Fluridone treatment reduced the endogenous ABA concentration by 20% to 30% (Fig. 5F). It has been shown for Arabidopsis that a similar decrease in ABA concentration can lead to disruption of ABA responses (Leon-Kloosterziel et al., 1996). In our study, a clear stimulatory effect of fluridone on ethylene-induced hyponastic growth was observed. In fluridone-treated plants the lag-phase for hyponastic growth was reduced from 2 h (se 0.01 h) to 0.7 h (se 0.04 h; P < 0.01). Additionally, the maximum petiole angle of 89° (se 0.2°) was higher than that of ethylene-treated plants that had not received a fluridone pretreatment (74°; se 3°; P < 0.05). Similar results were obtained when fluridone pretreated plants were submerged (data not shown). Furthermore, a reduction in endogenous ABA concentration was observed in petioles after 2 h of submergence (P < 0.05; Fig. 5F; see also Voesenek et al., 2003; Benschop, 2004). We conclude that ABA negatively affects the speed and maximum petiole angle of hyponastic growth and that lowered ABA concentrations upon submergence, in combination with ethylene, stimulate hyponastic growth by reducing the length of the lag-phase and increasing the maximum petiole angle.
Hormonal Regulation of the Gravitropic Set-Point Angle
The gravitropic set-point angle (GSA) is the angle with respect to the gravity vector that an organ maintains. For each plant organ, this GSA is determined by its developmental stage and by environmental conditions (Digby and Firn, 1995). In R. palustris the GSA is under hormonal control, since it was affected by the hormone pretreatments and the inhibitor applications described above (Table I). 1-MCP pretreatment did not affect the petiole angle at the start of the experiment. Inhibition of polar auxin transport with NPA lowered the GSA and this effect could be rescued by both the synthetic auxins NAA and 2,4-D. Additionally, the petiole angle of debladed plants in air decreased during the experiment (Fig. 3C), indicating that leaf-blade derived IAA is important for maintenance of the GSA of plants in air. There was no significant effect of inhibition of GA biosynthesis by paclobutrazol, but subsequent treatment with GA3 resulted in a higher initial petiole angle (Table I). Paclobutrazol pretreatment did, however, lower the GSA of the leaf blade (data not shown). Fluridone pretreatment (inhibition of ABA biosynthesis) strongly increased the initial petiole angle.
Table I.
Effect of hormone/inhibitor pretreatment on initial petiole angle of R. palustris at the start of the experiment
| Petiole Angle at Start Experiment (Degrees)
| ||
|---|---|---|
| No Pretreatment | Pretreatment | |
| 52 (2)a | 52 (3)a | 1-MCP |
| 39 (3)a | 24 (5)b | NPA |
| 39 (3)a | 30 (9)a | NPA + NAA |
| 39 (3)a | 27 (11)a | NPA + 2,4-D |
| 41 (4)a | 38 (9)a | Paclobutrazol |
| 41 (4)a | 54 (2)b | Paclobutrazol + GA3 |
| 35 (2)a | 50 (3)b | Fluridone |
The initial petiole angle (at t = 0 of the experiment) is compared for plants that received a hormone/inhibitor pretreatment and plants that were not pretreated. Plants were 27-d-old and data are means of four replicate plants (se between brackets). Means with different letters (shown as footnotes) are significantly different (P < 0.05).
RpEXPA1 Expression in the Petiole Base
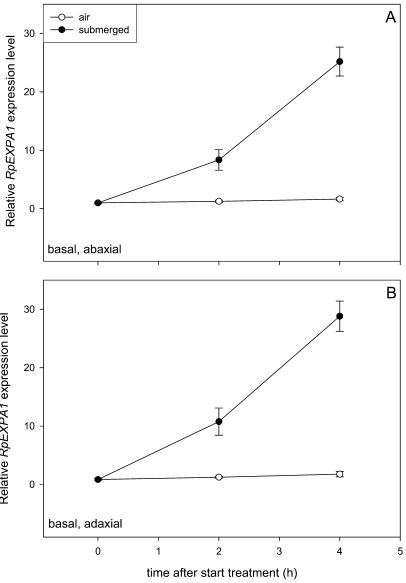
Expression of the cell wall loosening gene Rumex palustris EXPANSIN A 1 (RpEXPA1) has previously been reported to increase upon submergence and the kinetics of this increase match the timing of nondifferential stimulated petiole elongation in this species (Vriezen et al., 2000; Vreeburg, 2004). Here, we examined if RpEXPA1 also acts as a downstream target gene in hyponastic growth underwater, by determining if the differential cell elongation in the petiole base was correlated with a differential expression of this gene in ab- and adaxial halves of this region. Figure 6 shows that submergence increased RpEXPA1 transcript levels in the petiole base in time and compared to plants in air (P < 0.01). However, the increase was similar for the ab- and adaxial halves of the petiole base. A similar increase in RpEXPA1 transcript levels was observed in the apical ab- and adaxial halves of the petiole (data not shown). Transcript levels of a number of other expansins (RpEXPA8, 10, 15, and 18) also did not show differences between the ab- and adaxial basal halves of the petiole upon submergence (data not shown).
Figure 6.
Relative RpEXPA1 expression level in ab- and adaxial halves of the base of the third oldest petiole of R. palustris plants (27-d-old; five replicate plants). 18S was used as an internal standard and values are expressed as a ratio to the value of RpEXPA1 at t = 0 for the basal abaxial segment (set to 1). The petiole base was cut as shown in the insert in Figure 1 (solid line). Bars represent ses.
DISCUSSION
Hyponastic Growth Is Localized at the Abaxial Surface of the Petiole Base
Submergence of R. palustris resulted in differential growth across the petiole base, leading to hyponastic petiole movement (Fig. 1). The differential cell elongation in this region was calculated to be sufficient for the observed change in petiole angle. Our results are consistent with the existing literature on hyponastic growth responses, in which upward curvature is attributed to more rapid growth on the abaxial side of the organ (e.g. Hayes and Lippincott, 1976; Kang, 1979). Note that in R. palustris the increased cell elongation only took place in the base of the petiole and not along the entire abaxial petiole surface.
Ethylene Stimulates the Onset and Speed of Hyponastic Growth and Is Required for Maintenance of the Maximum Petiole Angle
The gaseous hormone ethylene accumulates in the plant upon submergence (for review, see Voesenek et al., 2003) and we show here that it plays an important stimulatory role in the onset, speed, and maintenance of hyponastic growth of R. palustris petioles upon submergence (Fig. 2). Ethylene sensing during submergence is important for hyponastic growth to occur. Blocking the ethylene receptors by pretreatment with 1-MCP completely abolished hyponastic growth in ethylene-treated plants (Fig. 2B). However, submerged plants that were pretreated with 1-MCP did not show a complete inhibition. This difference between ethylene-treated and submerged plants indicates the existence of ethylene-independent partial hyponastic growth in R. palustris underwater. The signal causing this differential growth is yet unknown.
Auxin Transported from the Leaf Blade Is Required for the Onset of Hyponastic Growth and for the Maximum Petiole Angle and Its Maintenance
The auxin efflux inhibitor NPA blocks cycling of the auxin efflux carriers, thereby inhibiting polar transport of auxin (Geldner et al., 2001). Treatment of submerged plants with NPA resulted in a doubling of the lag-phase of hyponastic growth (Fig. 3A). Thus, polar transport of auxin is important for correct timing of the initiation of hyponastic growth in submerged plants. The NPA-induced delayed lag-phase could only be overcome by the synthetic auxin 2,4-D and not by NAA (Fig. 3A). This illustrates the different affinities of these synthetic auxins for the influx and efflux carriers. 2,4-D enters the cell via the influx carrier and diffuses out, not using the efflux carrier (Delbarre et al., 1996). Therefore, treatment with 2,4-D bypassed the inhibitory effect of NPA on the efflux carrier and allowed for hyponastic growth. Although 2,4-D does not show cell-to-cell transport, the submergence solution probably provided an ample supply of the synthetic auxin to responsive cells. Ottenschläger et al. (2003) reported that the endogenous auxin transport mechanism can compensate for a surplus of exogenous auxin and this mechanism probably prevented accumulation of 2,4-D in the cells. In contrast, NAA is taken up readily by diffusion and requires the efflux carrier to leave the cell (Delbarre et al., 1996). Consequently, in NPA-treated plants submerged in NAA, this synthetic auxin will accumulate to high levels in the cells and disturb hyponastic growth. Our results are consistent with those of Parry et al. (2001), who found that the inhibitory effect of 1-naphthoxyacetic acid on the influx carriers could only be overcome by NAA and not by 2,4-D.
Deblading is thought to reduce the endogenous auxin concentration in the petiole, since the (younger) leaf blades are important auxin production sites (Ljung et al., 2001). In R. palustris the leaf blade is indeed an auxin source for the petiole, because deblading reduced endogenous petiolar IAA concentrations (Fig. 4, A and B). We have shown that auxin from the leaf blade is important for a correct timing of the initiation of submergence-induced hyponastic growth in the petiole (Fig. 3, B and C), since deblading resulted in a dramatic delay in the onset of hyponastic growth underwater. However, the angle of debladed petioles did eventually start to increase. This was not caused by IAA biosynthesis in the petiole, or IAA import from elsewhere in the plant, since IAA levels in submerged debladed petioles were still low after 4, 9, and 24 h (Fig. 4B). This is in contrast with studies employing decapitation in maize, where the coleoptile stump could take over auxin production (for review, see Iino, 1995; Haga and Iino, 1998). It is possible, however, that in our study, by a yet unknown mechanism, the petiole tissue eventually became more sensitive to the IAA still present.
The prolonged lag-phase of hyponastic growth caused by deblading at the start of submergence could be rescued by both the synthetic auxins 2,4-D and NAA (Fig. 3C), but not by ethylene, the ethylene precursor ACC, or GA3 (data not shown). This indicates that the effect of deblading is exerted via auxin, and most probably not via another factor. We have also shown that deblading when the hyponastic response is already completed (after 6 or 24 h of submergence) caused a 10° decline in the maximum petiole angle (Fig. 3B). To summarize, we have shown that leaf-blade derived IAA is needed not only for a correct timing of the onset of hyponastic growth, but also for the maximum petiole angle to be reached and maintained.
Submergence Induces a Slightly Asymmetrical Lateral Redistribution of Auxin in the Petiole
Differential growth processes often involve relocation of IAA in the responding organ, resulting in an asymmetrical auxin distribution that causes cells on one side of the organ to elongate faster than cells on the other side (Philippar et al., 1999; Friml and Palme, 2002; Fuchs et al., 2003). Since auxin was shown to be important for the onset and maintenance of hyponastic growth in R. palustris (Fig. 3), we investigated if a redistribution of auxin took place in the petiole upon submergence. The IAA levels of intact petioles decreased gradually during submergence, as did those of intact petioles in air (P < 0.01; Fig. 4, A and B). This was probably the result of maturation of the petiole tissue (also described for Arabidopsis leaves by Ljung et al., 2001). We observed a similar decrease in ab- and adaxial petiole halves (data not shown). In spite of this overall decrease in petiolar IAA concentration in intact petioles or petiole halves, we did find a very localized asymmetrical increase of the auxin concentration after 4 h of submergence, with more IAA accumulating in the abaxial outer cell layers than in the adaxial outer cell layers of the petiole base (P < 0.01; Fig. 4, C–F). This indicates that submergence caused a slightly asymmetrical, lateral redistribution of endogenous IAA to the outer cell layers of the petiole base. Recently, a redistribution of endogenous IAA to the outer cell layers was proposed by Morelli and Ruberti (2000, 2002) to function in shading-induced stem elongation of seedlings. The redistribution of auxin after 4 h of submergence observed in this study took place too late to be involved in the onset of hyponastic growth (which takes place 2 h after submergence; Fig. 1C). However, it could play a role in maintenance of the differential upright petiole orientation. We hypothesize that an asymmetry in the concentration of, and/or sensitivity to, yet unknown growth-regulating factors across the petiole base plays a role in regulating the onset of hyponastic growth upon submergence. Alternatively, it is possible that the asymmetrical redistribution of IAA already took place after 2 h of submergence, corresponding to the onset of hyponastic growth, but that it was too localized to detect at that time point.
GA Positively Regulates the Speed of Hyponastic Growth
Our experiments have shown that GA plays a stimulatory role in hyponastic growth. More specifically, GA influences the speed of this differential growth process. Inhibition of GA biosynthesis by paclobutrazol caused hyponasty to take place slower, without affecting the lag-phase of the response (Fig. 5A). This slower response could be rescued by the addition of GA3 to the submerged plants (Fig. 5B). The incomplete inhibition of hyponastic growth by paclobutrazol was not the result of insufficient application of the inhibitor, since a more severe pretreatment did not cause a stronger inhibition (data not shown). In a study on the role of GA in petiole elongation upon submergence, Rijnders et al. (1997) also observed only a partial inhibition by paclobutrazol. Submergence elevated the concentration of the active GA1 between 3 and 6 h (Fig. 5C), too late to be involved in regulating the onset of hyponastic growth. Therefore, we conclude that the GA already present in the petiole positively regulates the speed of the upward petiole movement under water. It is likely that the increase in GA1 concentration between 3 and 6 h after submergence plays a role in the process of nondifferential petiole elongation, as described previously by Rijnders et al. (1997).
In Arabidopsis, GA mediates destabilization of DELLA proteins, which are nuclear growth repressors (Fu and Harberd, 2003). It is possible that in submerged R. palustris petioles, paclobutrazol treatment results in slower destabilization of DELLA proteins and, thus, in slower growth of localized cells responsible for hyponastic growth. The function of the growth-repressing DELLA proteins is also influenced by ethylene and auxin in Arabidopsis (Achard et al., 2003; Fu and Harberd, 2003).
ABA Negatively Regulates Initiation, Speed, and the Maximum Petiole Angle of Submergence-Induced Hyponastic Growth
Submergence-induced hyponastic growth is negatively regulated by ABA (Fig. 5, D and E). This is consistent with results from Dwyer et al. (1995) who found that ABA promoted epinastic (downward) growth in P. eugenioides. In R. palustris, externally applied ABA reduced the maximum leaf angle reached underwater and affected the speed of the response (Fig. 5D), whereas the ABA biosynthesis inhibitor fluridone exaggerated the response to ethylene (Fig. 5E), thereby shortening the lag-phase of the response and inducing a higher maximum petiole angle. Furthermore, a reduction in endogenous ABA concentration was observed in submerged petioles (Fig. 5F). In summary, our results indicate that submergence down-regulates ABA levels and thus facilitates hyponastic growth.
Hormonal Regulation of the GSA Corresponds to the Role These Hormones Play in Submergence-Induced Hyponastic Growth
The influence of the hormone (inhibitors) on GSA (Table I) of the petiole reflected the effect of these substances on submergence-induced hyponastic growth, with ethylene, auxin, and GA being positive regulators and ABA a negative regulator of petiole angle.
RpEXPA1 Is Probably Not the Downstream Target for Regulation of Differential Growth
We regarded the cell wall loosening proteins expansins as possible candidates for the as-yet unknown growth-regulating factors that are expressed differentially across the petiole base leading to asymmetric cell elongation in this region and thus upward movement of the petiole. In R. palustris, nondifferential petiole elongation upon submergence or ethylene treatment coincides with up-regulation of an expansin transcript, RpEXPA1 (Vriezen et al., 2000; Vreeburg, 2004). Submergence also up-regulates the abundance of the size class containing the RpEXPA1 protein (Vreeburg, 2004). Although we found a similar submergence-induced up-regulation of the RpEXPA1 gene in this study, this expansin was not differentially up-regulated across the petiole base in response to submergence (Fig. 6). A number of other R. palustris expansin transcripts (RpEXPA8, 10, 15, and 18) also did not show differential expression (data not shown). Therefore, we conclude that the expansins we tested are probably not the target proteins that are differentially expressed in submerged R. palustris petioles, and that the up-regulation that took place upon submergence plays a role in the process of nondifferential petiole elongation previously described by Vriezen et al. (2000) and Vreeburg (2004). However, we cannot exclude that the nondifferential up-regulation of expansins upon submergence plays a role in the regulation of hyponastic growth since it does coincide with the kinetics of this growth process. The R. palustris expansin gene family consists of at least 19 members (A.J.M. Peeters, unpublished data), and it remains possible that one of the expansins that we have not examined in this study is important in the differential regulation of hyponastic growth. Additionally, other cell wall-loosening enzymes (for review, see Cosgrove, 1997), aquaporins (Siefritz et al., 2004), or K+-channels (Fuchs et al., 2003) could be differentially expressed, resulting in submergence-induced hyponastic growth of R. palustris petioles.
Model for Hyponastic Growth
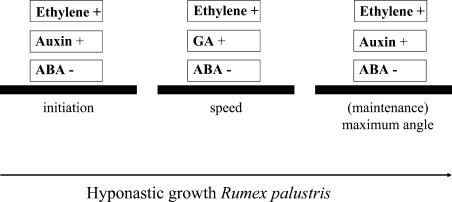
We propose a model for the involvement of the plant hormones ethylene, auxin, GA, and ABA in the different stages of submergence-induced hyponastic growth of R. palustris petioles (Fig. 7). Submergence results in accumulation of ethylene in the plant tissue (for review, see Peeters et al., 2002), acting as an important positive signal for initiation, speed, and maintenance of hyponastic growth. Auxin is also an important positive regulator of initiation and (maintenance) of the maximum petiole angle. Submergence results in a slightly asymmetrical, lateral redistribution of IAA to the outer cell layers of the petiole, possibly via ethylene. This redistribution of IAA is too late to be involved in the onset of hyponastic growth, but probably plays a role in maintenance of this differential growth response. We hypothesize that an asymmetry in growth-regulating factors develops across the petiole base to regulate the onset of hyponastic growth underwater. At this point, it is not clear what growth-regulating factors create this asymmetry, or how this is brought about. We do know that the expansin genes tested are not expressed differentially in response to submergence. Possible candidates are an asymmetric distribution of auxin sensitivity, or a differential distribution of cell wall loosening factors other than the expansins we already examined. The hormones ABA and GA are also involved in the regulation of the hyponastic growth response. Submergence decreases the concentration of the growth inhibitor ABA and this decline positively regulates the initiation, speed, and maximum angle of hyponastic growth. In contrast, GA positively influences the speed of upward petiole movement.
Figure 7.
Model describing the involvement of the plant hormones ethylene, auxin, ABA, and GA in the different stages of submergence-induced hyponastic growth of R. palustris petioles. Stimulation is indicated by “+” and inhibition by “–.”
MATERIALS AND METHODS
Plant Material and Growth Conditions
Rumex palustris (Sm.) plants were grown as described in Cox et al. (2003). The third oldest petiole of 27-d-old plants was studied and all experiments started between 8 am and 11 am.
Petiole Angle Measurements
In intact plants, the angle between the adaxial surface of the petiole and the horizontal was measured using time-lapse photography as described by Cox et al. (2003). Unless stated otherwise, experiments were made in continuous light to make photography possible. To acclimatize, plants were placed singly in open glass cuvettes (18.5 × 24.5 × 25.5 cm) in the camera system on the day before the experiment.
Submergence Treatment
To achieve submergence, tap water (20°C) was gently pumped into the cuvette until a water depth of 20 cm (from the soil surface) was reached. Control plants rested on a moist irrigation mat in the cuvette and were not submerged.
Cell Length Measurements
Epidermal imprints (Schnyder et al., 1990; Allard and Nelson, 1991) along the ab- and adaxial petiole surface were obtained using polyvinylformaldehyde/formvar (Brunschwig, Amsterdam). Cell lengths in one file of collenchyma (see boxes, Fig. 1 insert) stretching from the petiole base to the apex were measured using an IBAS image analysis system (Zeiss Vision, Oberkochen, Germany). Imprints were microscopically examined (objective 10×, projective 1.25×) and scanned with a Panasonic (Tokyo) b/w CCD camera (type WWWC-CD50; frame size 768 × 512 pixels; 256 gray levels; pixel size, 0.8017 μm × 0.8403 μm).
Ethylene and 1-MCP Treatment
Ethylene in a concentration of 5 μL L−1 or air (70% relative humidity) was flushed through glass cuvettes (15.0 × 17.5 × 29.0 cm) at 75 L h−1. This ethylene concentration is known to saturate ethylene-induced petiole elongation in R. palustris (Voesenek and Blom, 1989). The ethylene concentration inside the cuvettes was checked regularly on a gas chromatograph (GC955, Synspec, Groningen, Netherlands) and remained constant.
The gaseous ethylene perception inhibitor 1-MCP was released from Ethylbloc (Floralife, Walterboro, SC) containing 0.14% 1-MCP by dissolving it in water in an airtight container at 40°C for 12 min. 1-MCP gas was then collected from the headspace with a syringe and injected into an airtight cuvette (for 1 μL L−1, 1.6 g ethylbloc/m3) for a 3-h pretreatment. Longer pretreatment did not affect the results.
Auxin (Inhibitor) Treatment and Removal of the Leaf Blade
Intact plants were pretreated with 125 μL 1.10−3 m NPA (17 and 2 h before submergence) or NAA (2 h before submergence), or 125 μL 1.10−4 m 2,4-D (2 h before submergence) per plant, by brushing a pretreatment solution on the third oldest petiole and the basal part of the third leaf blade. Ethanol concentration in the pretreatment solution never exceeded 3% and the pH was set to 7.5 to 8.0. Tween 20 (0.1%) was added to ensure even distribution of the solution over the plant tissue. A pretreatment solution without the synthetic auxins/inhibitor, but containing 3% ethanol and 0.1% Tween, was used as a control. Pretreated plants were submerged in a solution containing 2.5.10−5 m NPA (0.2% ethanol) with or without NAA or 2,4-D in the concentrations mentioned above. All chemicals were obtained from Duchefa, Haarlem, The Netherlands.
To reduce the endogenous auxin concentration in the petiole, the leaf blade of the third oldest petiole was removed using scissors, and silicone vacuum grease was applied to the wound surface. No acclimatisation period was inserted after removal of the leaf blade, since it did not influence the response. Debladed plants were submerged in tap water (20°C) with or without the synthetic auxins NAA (1.10−5 m) or 2,4-D (5.10−6 m). Ethanol concentration in the submergence solutions never exceeded 0.03% and the pH was set to 7.5 to 8.0.
Analysis of Endogenous IAA Content
We measured the endogenous IAA concentration of different parts of the third oldest petiole in three separate experiments: (1) in single intact petiole from debladed or intact leaf, (2) in ab- and adaxial petiole halves of intact leaves, and (3) in ab- and adaxial petiole fragments of intact leaves (pooled per 10 plants). In the latter harvest, the petiole was divided into two parts: the most basal 2.5 mm and the remaining apical part (8–10 mm). Of both parts, ab- and adaxial fragments (containing the epidermis, the collenchyma bundle and some cortex cells, comprising 20%–30% of the total fresh weight; dashed lines, Fig. 1 insert) were cut along the entire length with a razor blade under a dissection microscope. The start of treatment for the replications of this harvest was spaced out in time (interval 20 min) to allow quick harvesting and freezing of the plant material. Upon harvesting, the total fresh weight was determined and the sample was immediately frozen in liquid nitrogen and stored at −80°C. Extraction and purification of the samples and measurement of the endogenous IAA concentration by gas chromatography (GC)-selected reaction monitoring-mass spectrometry (MS) was performed essentially as described in Edlund et al. (1995). Calculation of isotope dilution factors was based on the addition of 1 ng [13C6] IAA/sample.
Paclobutrazol/GA Treatment
Intact plants were pretreated with 5 mL 1.10−4 m paclobutrazol (72 and 65 h before submergence) or GA3 (19 and 1 h before submergence). The pretreatment solution (also containing 0.2% ethanol and 0.1% Tween 20, pH 7.5–8.0) was applied to the third oldest petiole, the basal part of the third leaf blade, and to the soil. A pretreatment solution without hormone/inhibitor, but containing 0.2% ethanol and 0.1% Tween was used as a control. Pretreated plants were gently lowered into tap water or a solution containing 1.10−4 m GA3 (0.06% ethanol). All chemicals were obtained from Duchefa, Haarlem, The Netherlands. Measurement of endogenous GA1 concentration was performed by GC-MS as described in Benschop (2004).
ABA/Fluridone Treatment
Intact plants were submerged in tap water containing 3.10−6 m or 1.10−5 m ABA (Sigma, Zwijndrecht, The Netherlands). Ethanol concentration in the submergence solution never exceeded 0.01% and the pH was set to 7.5 to 8.0.
Plants were pretreated with fluridone (Duchefa) once, 63 h before the start of the experiment, by administering 10 mL of a 1.10−4 m (0.1% acetone) pretreatment solution to the soil. Control plants were pretreated in a similar fashion with a solution of tap water containing 0.1% acetone. Measurement of endogenous ABA concentration was performed with GC-MS-selected reaction monitoring as described by Benschop (2004).
RNA Extraction and Real-Time RT-PCR Measurement of RpEXPA1 mRNA
The petiole was divided into two parts: the most basal 2.5 mm and the remaining apical part (8–10 mm). Both parts were cut in half longitudinally (solid line, Fig. 1 insert), giving an abaxial and an adaxial half (pooled per 21 plants). These halves were immediately transferred to liquid nitrogen and stored at −80°C. The start of treatment for the replications was spaced out in time (interval 15 min) to allow quick harvesting and freezing of the plant material. RNA extraction and real-time RT-PCR monitoring of RpEXPA1 mRNA took place as described in Vreeburg (2004). The nomenclature of expansins was adjusted to Kende et al. (2004).
Statistical Analysis
The lag-phase for the start and finish of hyponastic growth was calculated as described in Cox et al. (2003). Data were compared using a two-way ANOVA (Figs. 1, B and C, 4, and 6), a one-way ANOVA, and a Bonferroni's posthoc test (Figs. 3 and 5D) or an independent samples t test (Table I; Figs. 1A, 2, 4, C–F, 5, A–C, E, and F) using the program SPSS version 10 (SPSS, Chicago).
Acknowledgments
We thank Henri Groeneveld (Department of Plant Ecophysiology, Utrecht University, The Netherlands) for providing the cross-section of an R. palustris petiole and Yvonne de Jong-van Berkel, Rob Welschen, and Ingabritt Carlsson (Umeå Plant Science Centre, Sweden) for technical assistance. Maarten Terlou (Image Analysis Department, Faculty of Biology, Utrecht University) developed the image analysis macro and Ronald van Trigt (Faculty of Pharmacy, Utrecht University) and Wim Huibers (Department of Plant Ecophysiology, Utrecht University) designed the computerized digital camera system. Frank Millenaar, Danny Tholen, and Jordi Bou (Department of Plant Ecophysiology, Utrecht University) developed the method to calculate the change in angle caused by differential growth.
This work was supported by the Dutch Science Foundation (PIONIER grant no. 800.84.470).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.049197.
References
- Achard P, Vriezen WH, Van Der Straeten D, Harberd NP (2003) Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15: 2816–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard G, Nelson CJ (1991) Photosynthate partitioning in basal zones of tall fescue leaf blades. Plant Physiol 95: 663–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga M, Bögemann GM, Blom CWPM, Voesenek LACJ (1997) Flooding resistance of Rumex species strongly depends on their response to ethylene: rapid shoot elongation or foliar senescence. Physiol Plant 99: 415–422 [Google Scholar]
- Becker D, Hedrich R (2002) Channeling auxin action: modulation of ion transport by indole-3-acetic acid. Plant Mol Biol 49: 349–356 [PubMed] [Google Scholar]
- Benschop JJ (2004) The role of abscisic acid in ethylene-induced elongation. PhD thesis. Utrecht University, Utrecht, The Netherlands
- Blake TJ, Pharis RP, Reid DM (1980) Ethylene, gibberellins, auxin and the apical control of branch angle in a conifer, Cupressus arizonica. Planta 148: 64–68 [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Masson PH (2003) Plant gravitropism. Unraveling the ups and downs of a complex process. Plant Physiol 133: 1677–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clúa A, Bottini R, Brocchim GN, Bogino J, Luna V, Montaldi ER (1996) Growth habit of Lotus tenuis shoots and the influence of photosynthetic photon flux density, sucrose and endogenous levels of gibberellins A1 and A3. Physiol Plant 98: 381–388 [Google Scholar]
- Cosgrove DJ (1997) Cellular mechanisms underlying growth asymmetry during stem gravitropism. Planta 203: S130–S135 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (2000) Loosening of plant cell walls by expansins. Nature 407: 321–326 [DOI] [PubMed] [Google Scholar]
- Cox MCH, Millenaar FF, van Berkel YEMD, Peeters AJM, Voesenek LACJ (2003) Plant movement: submergence-induced petiole elongation in Rumex palustris depends on hyponastic growth. Plant Physiol 132: 282–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre A, Muller P, Imhoff V, Guern J (1996) Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 198: 532. [DOI] [PubMed] [Google Scholar]
- Digby J, Firn RD (1995) The gravitropic set-point angle (GSA): the identification of an important developmentally controlled variable governing plant architecture. Plant Cell Environ 18: 1434–1440 [DOI] [PubMed] [Google Scholar]
- Dwyer PJ, Bannister P, Jameson PE (1995) Effects of three plant growth regulators on growth, morphology, water relations, and frost resistance in lemonwood (Pittosporum eugenioides A. Cunn). NZ J Bot 33: 415–424 [Google Scholar]
- Ecker JR (1995) The ethylene signal transduction pathway in plants. Science 268: 667–675 [DOI] [PubMed] [Google Scholar]
- Edlund A, Eklöf S, Sundberg B, Moritz T, Sandberg G (1995) A microscale technique for gas chromatography-mass spectrometry measurements of picogram amounts of indole-3-acetic acid in plant tissue. Plant Physiol 108: 1043–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H, Meir S, Halevy AH, Philosoph-Hadas S (2003) Inhibition of the gravitropic bending response of flowering shoots by salicylic acid. Plant Sci 165: 905–911 [DOI] [PubMed] [Google Scholar]
- Friml J, Palme K (2002) Polar auxin transport-old questions and new concepts. Plant Mol Biol 49: 273–284 [PubMed] [Google Scholar]
- Fu X, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421: 740–743 [DOI] [PubMed] [Google Scholar]
- Fuchs I, Phillipar K, Ljung K, Sandberg G, Hedrich R (2003) Blue light regulates an auxin-induced K+-channel gene in the maize coleoptile. Proc Natl Acad Sci USA 100: 11795–11800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof Y, Jürgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Grimoldi AA, Insausti P, Roitman GG, Soriano A (1999) Responses to flooding intensity in Leontodon taraxacoides. New Phytol 141: 119–128 [Google Scholar]
- Haga K, Iino M (1998) Auxin-growth relationships in maize coleoptiles and pea internodes and control by auxin of the tissue sensitivity to auxin. Plant Physiol 117: 1473–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AB, Lippincott JA (1976) Growth and gravitational response in the development of leaf blade hyponasty. Am J Bot 63: 383–387 [Google Scholar]
- Hoffmann-Benning S, Kende H (1992) On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol 99: 1156–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M (1995) Gravitropism and phototropism of maize coleoptiles: evaluation of the Cholodny-Went theory through effects of auxin application and decapitation. Plant Cell Physiol 36: 361–367 [Google Scholar]
- Insausti P, Grimoldi AA, Chaneton EJ, Vasellati V (2001) Flooding induces a suite of adaptive plastic responses in the grass Paspalum dilatatum. New Phytol 152: 291–299 [Google Scholar]
- Kang BG (1979) Epinasty. In W Haupt, ME Feinleib, eds, Encyclopedia of Plant Physiology, New Series, Vol 7., Physiology of Movements. Springer-Verlag, Berlin, pp 647–667
- Kaufman PB, Wu L, Brock TG, Kim D (1995) Hormones and the orientation of growth. In PJ Davies, ed, Plant Hormones. Kluwer Academic Press, Dordrecht, The Netherlands, pp 547–571
- Kende H, Bradford KJ, Brummell DA, Cho HT, Cosgrove DJ, Flemming AJ, Gehring C, McQueen-Mason S, Rose JKC, Voesenek LACJ (2004) Nomenclature for members of the expansin superfamily of genes and proteins. Plant Mol Biol (in press) [DOI] [PubMed]
- Kutschera U (2001) Stem elongation and cell wall proteins in flowering plants. Plant Biol 3: 466–480 [Google Scholar]
- Leon-Kloosterziel KM, Alvares Gil M, Ruijs GJ, Jacobsen SE, Olzewski NE, Schwartz SH, Zeevaart JAD, Koornneef M (1996) Isolation and characterization of abscisic acid-deficient mutants at two new loci. Plant J 10: 655–661 [DOI] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28: 465–474 [DOI] [PubMed] [Google Scholar]
- McQueen-Mason S, Cosgrove DJ (1994) Disruption of hydrogen bonding between wall polymers by proteins that induce plant wall extension. Proc Natl Acad Sci USA 91: 6574–6578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli G, Ruberti I (2000) Shade avoidance responses. Driving auxin along lateral routes. Plant Physiol 122: 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli G, Ruberti I (2002) Light and shade in the photocontrol of Arabidopsis growth. Trends Plant Sci 7: 399–404 [DOI] [PubMed] [Google Scholar]
- Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100: 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JH (1964) Comparative study on the effects of applied indoleacetic acid and horizontal orientation of the primary shoot, upon internode extension and petiole orientation in Helianthus annuus, and the modifying influence of gibberellic acid. Planta 61: 283–297 [Google Scholar]
- Palmer JH (1985) Epinasty, hyponasty, and related topics. In RP Pharis, DM Reid, eds, Encyclopedia of Plant Physiology, New Series, Volume 11, Hormonal Regulation of Development III, Role of Environmental Factors. Springer-Verlag, Berlin, pp 139–168
- Parry G, Delbarre A, Marchant A, Swarup R, Napier R, Perrot-Rechenmann C, Bennett MJ (2001) Novel auxin transport inhibitors phenocopy the auxin influx carrier mutation aux1. Plant J 25: 399–406 [DOI] [PubMed] [Google Scholar]
- Peeters AJM, Cox MCH, Benschop JJ, Vreeburg RAM, Bou J, Voesenek LACJ (2002) Submergence research using Rumex palustris as a model; looking back and going forward. J Exp Bot 53: 391–398 [DOI] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Lüthen H, Hoth S, Bauer K, Haga K, Thiel G, Ljung K, Sandberg G, Böttger M, et al (1999) Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA 96: 12186–12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Cuppens MLC, Voesenek LACJ, Visser EJW (2004) Interactions between ethylene and gibberellins in phytochrome-mediated shade avoidance responses in tobacco. Plant Physiol 136: 2928–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Visser EJW, De Kroon H, Voesenek LACJ (2003) Ethylene is required in tobacco to successfully compete with proximate neighbours. Plant Cell Environ 26: 1229–1234 [Google Scholar]
- Ridge I (1987) Ethylene and growth control in amphibious species. In RMM Crawford, ed, Plant Life in Aquatic and Amphibious Habitats. Blackwell Scientific Publications, Oxford, pp 53–76
- Rijnders JGHM, Yang Y, Kamiya Y, Takahashi N, Barendse GWM, Blom CWPM, Voesenek LACJ (1997) Ethylene enhances gibberellin levels and petiole sensitivity in flooding-tolerant Rumex palustris but not in flooding-intolerant R. acetosa. Planta 203: 20–25 [Google Scholar]
- Schnyder H, Seo S, Rademacher IF, Kühbauch W (1990) Spatial distribution of growth rates and of epidermal cell lengths in the elongation zone during leaf development in Lolium perenne L. Planta 181: 423–431 [DOI] [PubMed] [Google Scholar]
- Siefritz F, Otto B, Bienert GP, van der Krol A, Kaldenhoff R (2004) The plasma membrane aquaporin NtAQP1 is a key component of the leaf unfolding mechanism in tobacco. Plant J 37: 147–155 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Vriezen WH, Smalle J, Laarhoven LJJ, Harren FJM, Van Der Straeten D (2003) Ethylene and auxin control the Arabidopsis response to decreased light intensity. Plant Physiol 133: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Benschop JJ, Bou J, Cox MCH, Groeneveld HW, Millenaar FF, Vreeburg RAM, Peeters AJM (2003) Interaction between plant hormones regulates submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris. Ann Bot (Lond) 91: 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Blom CWPM (1989) Growth responses of Rumex species in relation to submergence and ethylene. Plant Cell Environ 12: 433–439 [Google Scholar]
- Vreeburg RAM (2004) Expansins in submergence-induced petiole elongation of Rumex palustris: kinetics and regulation. PhD thesis. Utrecht University, Utrecht, The Netherlands
- Vriezen WH, Achard P, Harberd NP, Van der Straeten D (2004) Ethylene-mediated enhancement of apical hook formation in etiolated Arabidopsis thaliana seedlings is gibberellin dependent. Plant J 37: 505–516 [DOI] [PubMed] [Google Scholar]
- Vriezen WH, De Graaf B, Mariani C, Voesenek LACJ (2000) Submergence induces expansin gene expression in flooding tolerant Rumex palustris and not in flooding intolerant R. acetosa. Planta 210: 956–963 [DOI] [PubMed] [Google Scholar]
- Went FW, Thimann KV (1937) Phytohormones. MacMillan, New York, London
- Zhang N, Hasenstein KH (2000) Distribution of expansins in graviresponding maize roots. Plant Cell Physiol 41: 1305–1312 [DOI] [PubMed] [Google Scholar]