Introduction
Metronidazole is commonly used to treat intra-abdominal infections in neonates.1,2 The parent drug is converted to 5 metabolites, with 2-hydroxy-metronidazole being the most clinically significant, as it possesses 30–65% of the antimicrobial activity of the parent compound.3,4 In vitro studies have demonstrated that cytochrome P450 2A6 (CYP2A6) is the primary catalyst responsible for metronidazole hydroxylation.5 This enzyme is initially expressed at low levels at birth, with expression increasing over the course of the first year of life to reach adult levels.6 CYP2A6 is known to be a highly polymorphic gene with more than 45 variant alleles that result in inactive to ultra-rapid metabolizer phenotypes. Additionally, certain allelic variants such as CYP2A6*17 have amino acid changes that alter metabolism for some but not other substrates, resulting in different metabolizing phenotypes for the same genotype.7 The role of genetic variation on variable metronidazole metabolism in neonates has not been previously described, nor has the effect of CYP2A6*17 on metronidazole been characterized. As such, the objective of this study was to evaluate the effect of CYP2A6 genetic variation on the pharmacokinetics of metronidazole in a small cohort of preterm neonates.
Methods
This multicenter study was approved by the institutional review boards at each center, and informed consent was obtained from a parent or guardian of each infant prior to enrollment and included permission for genetic analysis. A detailed description of the study design as well as the pharmacokinetic (PK) analysis from the study have been published.8 Briefly, genetic samples for a cohort of 14 neonates were obtained from the leftover cellular components of plasma PK samples from a larger cohort of 24 preterm neonates. Infants were ≤32 weeks gestational age (GA) at birth and <91 days postnatal age (PNA), and required medical treatment with intravenous metronidazole as indicated by microbiological cultures or clinical signs concerning for infection. Metronidazole was administered as a median (range) loading dose of 15.0 mg/kg (13.6–19.0) over 30 minutes followed by a median (range) maintenance dose of 7.5 mg/kg (4.2–15.6) every 12 hours (<14 postnatal days) and 24 hours (≥14 postnatal days). Samples were collected immediately prior to and following the metronidazole infusion, and approximately 1 to 1.5 hours and 3 to 4 hours post-infusion. Samples were also collected at 24–25 hours, 48–49 hours, and 72–73 hours for infants <14 postnatal days and at 12–13 hours, 24–25 hours and 36–37 hours post-infusion for infants ≥14 postnatal days.
Individual metronidazole clearance estimates were from a previously published population PK model that included body weight and postmenstrual age as covariates.8 The metabolic ratio was also calculated as described previously by dividing the hydroxyl-metronidazole steady-state area under the curve from zero to tau (AUC0-τ) by the metronidazole AUC0-τ.8 Following multiple dose administration, the AUC was calculated using the trapezoidal rule with non-compartmental methods in Phoenix WinNonlin (Certara, Cary, NC). The metabolite-to-parent concentration ratio was calculated by dividing the nanomolar concentration of hydroxy-metronidazole by that of metronidazole in each sample. The AUC and concentration values used to calculate the metabolic ratios and metabolite-to-parent concentration ratios were converted to μM*h and μM, respectively, to allow for comparison. A set of descriptive statistics including mean, median, and range was generated for each set of samples for each infant. The median (range) of medians of the group of infants was then determined. Simple linear regression was used to examine expected clearance of metronidazole based on postmenstrual age.
Genomic deoxyribonucleic acid was isolated from these samples and CYP2A6 genotype was determined as previously described.9 The assay included the variant alleles CYP2A6*2, *4, *7, *9, *12, *17, *20, *23–25, *28, *31, and *35, which are prevalent and known to affect the metabolism of nicotine.10
Results
The median (range) gestational age at birth of the cohort was 25.4 weeks (23.1–30.9), median birth weight was 1.2 kg (0.57–1.96), and median postmenstrual age was 30.7 weeks (23.9–35.3) (Table 1). Of the 14 neonates, 2 were heterozygous for the *17 variant allele (CYP2A6*1/*17) and 12 had the wild type genotype, CYP2A6*1/*1. Clearance for metronidazole among the two CYP2A6*1/*17 neonates was 0.044 and 0.031 L/h/kg, compared to the median (range) value for the CYP2A6*1/*1 neonates of 0.041 L/h/kg (0.013–0.101). Body-weight normalized clearance for the CYP2A6*1/*17 subjects did not deviate from the expected clearance predicted by postmenstrual age (Figure 1). The metabolic ratio of the CYP2A6*1/*17 variant infant fell within the range of the wild type CYP2A6*1/*1 infants (Table 2). The median hydroxy-metronidazole-to-metronidazole concentration ratio for the two CYP2A6*1/*17 neonates was 0.106 and 0.236. The median (range) of hydroxy-metronidazole-to-metronidazole concentration ratios in wild type CYP2A6*1/*1 infants was 0.093 (0.008–1.066).
Table 1.
Demographics Stratified by CYP2A6 Genotype
| CYP2A6 *1/*1 n=12 |
CYP2A6 *1/*17 n=2 |
|
|---|---|---|
| Gestational age, weeks | 25.7 (23.1–30.9) | 25.2 (25.1–25.3) |
| Postnatal age, days | 30 (1–66) | 20 (11–29) |
| Postmenstrual age, weeks | 31.6 (23.9–35.3) | 28.1 (26.7–29.4) |
| Birth weight, kg | 1.33 (0.57–1.96) | 0.83 (0.69–0.96) |
| Race | 6 (50) White/Caucasian 4 (33) Black/African American 2 (17) other |
2 (100) Black/African American |
| Sex | 8 (67) female | 2 (100) female |
Median (range) is provided for continuous data and n (%) for categorical data.
Figure 1.
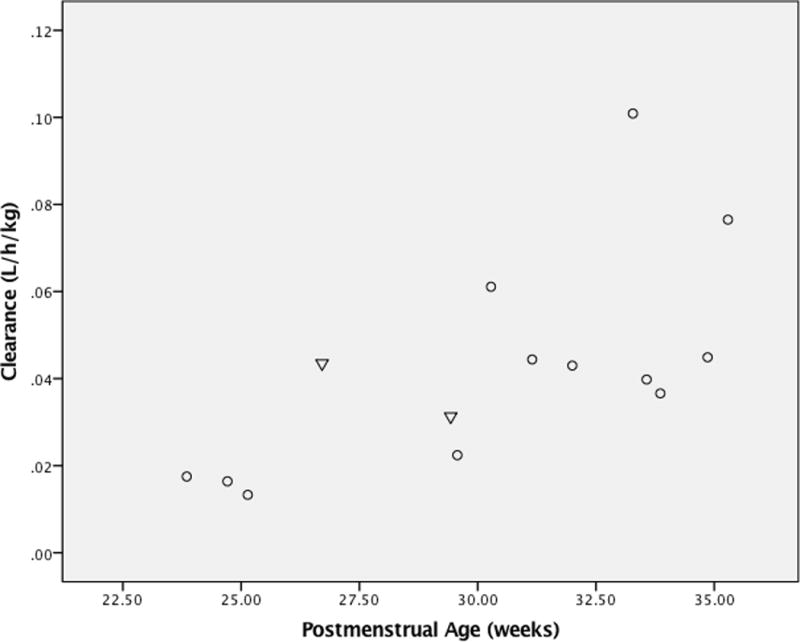
Plasma clearance of metronidazole (L/h/kg) versus postmenstrual age (weeks). Individuals with variant genotype (CYP2A6*1/*17) are shown as triangles and wild type genotype (CYP2A6*1/*1) as circles.
Table 2.
Metronidazole Clearance, Hydroxyl-Metronidazole Area Under the Curve, Metronidazole Area Under the Curve, and Metabolic Ratio for Each Infant
| CYP2A6 Genotype | Postmenstrual Age (weeks) |
Body Weight (kg) |
Clearance (L/hr/kg) |
Hydroxy-metronidazole AUC0-τ (μM*h) |
Metronidazole AUC0-τ (μM*h) |
Metabolic Ratioa |
|---|---|---|---|---|---|---|
| *1/ *1 | 24.71 | 0.61 | 0.02 | 443.49 | 2091.73 | 0.21 |
| *1/ *1 | 25.14 | 0.75 | 0.01 | 26.72 | 2325.45 | 0.01 |
| *1/ *1 | 30.28 | 1.31 | 0.06 | 74.81 | 683.61 | 0.11 |
| *1/ *1 | 23.85 | 0.57 | 0.02 | NE | 2161.85 | — |
| *1/ *1 | 29.57 | 1.34 | 0.02 | 128.24 | 1343.85 | 0.10 |
| *1/ *1 | 33.28 | 1.09 | 0.10 | 363.34 | 385.63 | 0.94 |
| *1/ *1 | 32.00 | 0.93 | 0.04 | 64.12 | 870.58 | 0.07 |
| *1/ *1 | 34.86 | 1.56 | 0.04 | 58.78 | 946.54 | 0.06 |
| *1/ *1 | 35.29 | 1.96 | 0.08 | 154.96 | 584.28 | 0.27 |
| *1/ *1 | 33.57 | 1.76 | 0.04 | NE | NE | — |
| *1/ *1 | 31.15 | 1.35 | 0.04 | NE | NE | — |
| *1/ *1 | 33.86 | 1.64 | 0.04 | 106.87 | 1051.71 | 0.10 |
| *1/ *17 | 26.71 | 0.69 | 0.04 | NE | 858.90 | — |
| *1/ *17 | 29.43 | 0.96 | 0.03 | 101.52 | 1051.71 | 0.10 |
AUC, area under the curve; NE, not estimated.
Metabolic ratio =
Two CYP2A6*1/*1 infants received phenobarbital, an inducer of CYP2A6, as concomitant treatment during the course of the study. The clearance values of these infants were 0.018 and 0.043 L/h/kg, which were within the range (0.013–0.101 L/h/kg) of infants who were not on a CYP2A6 inducer or inhibitor. The two CYP2A6*1/*17 infants did not receive any known inhibitors or inducers of CYP2A6. A one-way analysis of variance showed that there was no statistically significant effect of sex or race on clearance in the entire cohort (p > 0.7). Age was found to be a significant covariate in the population PK model previously published.8
Discussion
The results observed in this small cohort of premature infants suggest that there were no clear trends between CYP2A6*1/*1 and CYP2A6*1/*17 genotype and metronidazole clearance during the first month of life. The clearance values for the two infants with CYP2A6*17 variants were within the range of clearance of metronidazole by wild type infants (*1/*1) of similar postmenstrual age. Additionally, the metabolic ratio and metabolite-to-parent concentration ratio of the *17 variants also fell within the range of wild type values. It is important to note that early in life, the hydroxy-metronidazole AUC0-τ values used in the calculation of the metabolic ratio might not accurately reflect the fraction of metronidazole converted to hydroxy-metronidazole. This is due to the fact that the plasma disposition profile is dependent on both the formation and elimination of hydroxy-metronidazole. If elimination is rate limiting, the amount of plasma metabolite can appear high even though only a fraction of metronidazole is converted into hydroxy-metronidazole. This may be due to the effect of ontogeny and genetic variation on the pathways contributing to the clearance of hydroxy-metronidazole. In future studies, the fraction of metronidazole that has been hydroxylated can be assessed by collecting urine over a dosing interval at steady state or over 5 half-lives after the first dose. Studies in infants and children examining cotinine, the major metabolite of nicotine that is exclusively metabolized by CYP2A6, have demonstrated that genotype becomes a predictor of CYP2A6-mediated cotinine metabolism only after 2 months of age.11
In vitro studies implicate CYP2A6 as the primary CYP catalyzing metronidazole 2-hydroxylation with six-fold greater activity than the next most active enzyme. Although heterologously expressed CYP3A4, CYP3A5, and CYP3A7 also form 2-hydroxy metronidazole, inhibitor studies with human liver microsomes have suggested that CYP3A enzymes do not contribute significantly to metronidazole 2-hydroxylation at therapeutic concentrations.5 However, based on the ontogeny of these proteins, it is possible that they play a more significant role in the metabolism of metronidazole earlier in life due to low levels of CYP2A6, particularly in preterm neonates. For example, CYP3A7 is uniquely expressed at high levels in the fetal liver, with expression peaking in the first postnatal week while CYP3A4 levels increase over the first 6 months of life.12,13 Unlike CYP3A4 and CYP3A7, CYP2A6 is expressed at extremely low levels in fetal liver and does not reach adult levels until after the first year of life.14,15 Given that the clearance of metronidazole in our population of premature infants is lower than values reported in adult populations, it is possible that the ontogeny of metabolizing enzymes and lower levels of expression early in life may contribute to the lack of effect of allelic variation on drug metabolism.16
Another potential explanation for the lack of effect of CYP2A6 genotype on metronidazole PK observed in our cohort is that the protein product of the CYP2A6*1/*17 variant (CYP2A6.17) demonstrates substrate specificity. Using nicotine hydroxylation as a reference for metabolic activity, the CYP2A6*1/*17 infants are expected to be slow metabolizers and the CYP2A6*1/*1 infants normal metabolizers.17,18 However, the CYP2A6.17 enzyme catalyzes near normal rates of coumarin 7-hydroxylation.7 If metronidazole is similar to coumarin as a substrate, there would be no genotype-phenotype discordance in our cohort since the rate of metronidazole clearance would be expected to be similar between the two variants. This is not the case for most variants; for example, CYP2A6*23 has been shown to impair the enzymatic metabolism of both nicotine and coumarin.7 Further studies are needed to determine the activity of CYP2A6 variants as they may affect the biotransformation of metronidazole.
Due to a small sample size, only two genetic variants (CYP2A6*1, CYP2A6*17) were observed in this study cohort. We did not enroll any children of East Asian descent. Some variants such as CYP2A6*4 have a frequency ranging from 0–4% in Caucasian and African Americans to as high as 15–24% in East Asians.19,20 Certain variants are found at substantially higher frequencies in certain populations, such as the polymorphism CYP2A6*17 in African Americans and CYP2A6*7 in East Asians.18, 21 While the results from our study may not be generalizable, they do illustrate the importance of considering the roles of CYP2A6 allelic variants and ontogeny in the biotransformation of medically important substrates such as metronidazole.
Future directions include characterizing the activity of CYP2A6*17 and other variants for metronidazole 2-hydroxylase activity in vitro. Additionally, the effect of ontogeny on metronidazole biotransformation could be further examined in groups of older infants to determine if any genotype-phenotype discordance exists in these populations and, if so, when it might resolve. These studies will be required to further understand the mechanisms and timing of any underlying genotype-phenotype discordance.
Conclusions
In summary, genotype-phenotype relationships for various polymorphic CYP450 enzymes can be obscured early in life by the interplay between ontogeny and genetic variation. While genotype-phenotype concordance has been demonstrated as early as 2 weeks after birth for enzymes such as CYP2D6, other polymorphic enzymes such as CYP2C19 display genotype-phenotype discordance persisting into the first few months of life.22,23 While genotype-phenotype discordance for CYP2A6 would be expected at 1 month of postnatal life, our observation in two infants suggests the possibility of an allele specific pattern of metronidazole metabolism by infants possessing the CYP2A6*17 variant such that this allele does not have impaired activity toward metronidazole.
Acknowledgments
The Best Pharmaceuticals for Children Act – Pediatric Trials Network Steering Committee
Katherine Y. Berezny, Duke Clinical Research Institute, Durham, NC; Gregory L. Kearns, Arkansas Children’s Hospital, Little Rock, AR; Ian M. Paul, Penn State College of Medicine, Hershey, PA; Michael J. Smith, University of Louisville, Louisville, KY; John van den Anker, George Washington University School of Medicine and Health, Washington, DC; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development: David Siegel, MD, Perdita Taylor-Zapata, MD, Anne Zajicek, PharmD, Alice Pagan, BBA.
The EMMES Corporation (Data Coordinating Center): Ravinder Anand, PhD, Traci Clemons, PhD, Gina Simone, BS.
Funding Information
D.G. is funded by grant K23HD083465 from the National Institute of Child Health and Human Development (NICHD) and by the nonprofit Thrasher Research Fund (www.thrasherresearch.org). R.F.T. receives support from Canadian Institutes of Health Research grant TMH109787, an Endowed Chair in Addiction, and the Campbell Family Mental Health Research Institute of the Centre for Addiction and Mental Health. D.K.B. Jr. receives support from the National Institutes of Health (NIH) (award 2K24HD058735-06, National Center for Advancing Translational Sciences award UL1TR001117, NICHD contract HHSN275201000003I, and National Institute of Allergy and Infectious Disease [NIAID] contract HHSN272201500006I); he also receives research support from Cempra Pharmaceuticals (subaward to HHSO100201300009C) for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). M.C.W. receives support for research from the NIH (1R01-HD076676-01A1), the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the NIAID (HHSN272201500006I and HHSN272201300017I), the NICHD (HHSN275201000003I), the Food and Drug Administration (1U01FD004858-01), the Biomedical Advanced Research and Development Authority (HHSO100201300009C), the nonprofit Thrasher Research Fund (www.thrasherresearch.org), and from industry for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The remaining authors have no relevant disclosures to report.
References
- 1.Brook I. Bacteremia due to anaerobic bacteria in newborns. J Perinatol. 1990;10(4):351–356. [PubMed] [Google Scholar]
- 2.Thompson AM, Bizzarro MJ. Necrotizing enterocolitis in newborns: pathogenesis, prevention and management. Drugs. 2008;68(9):1227–1238. doi: 10.2165/00003495-200868090-00004. [DOI] [PubMed] [Google Scholar]
- 3.O’Keefe JP, Troc KA, Thompson KD. Activity of metronidazole and its hydroxy and acid metabolites against clinical isolates of anaerobic bacteria. Antimicrob Agents Chemother. 1982;22(3):426–430. doi: 10.1128/aac.22.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamp KC, Freeman CD, Klutman NE, Lacy MK. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin Pharmacokinet. 1999;36(5):353–373. doi: 10.2165/00003088-199936050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Pearce RE, Cohen-Wolkowiez M, Sampson MR, Kearns GL. The role of human cytochrome P450 enzymes in the formation of 2-hydroxymetronidazole: CYP2A6 is the high affinity (low Km) catalyst. Drug Metab Dispos. 2013;41(9):1686–1694. doi: 10.1124/dmd.113.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008;118(2):250–267. doi: 10.1016/j.pharmthera.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Ho MK, Mwenifumbo JC, Zhao B, Gillam EM, Tyndale RF. A novel CYP2A6 allele, CYP2A6*23, impairs enzyme function in vitro and in vivo and decreases smoking in a population of Black-African descent. Pharmacogenet Genomics. 2008;18(1):67–75. doi: 10.1097/FPC.0b013e3282f3606e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen-Wolkowiez M, Sampson M, Bloom BT, et al. Determining population and developmental pharmacokinetics of metronidazole using plasma and dried blood spot samples from premature infants. Pediatr Infect Dis J. 2013;32(9):956–961. doi: 10.1097/INF.0b013e3182947cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wassenaar CA, Zhou Q, Tyndale RF. CYP2A6 genotyping methods and strategies using real-time and end point PCR platforms. Pharmacogenomics. 2016;17(2):147–62. doi: 10.2217/pgs.15.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Human Cytochrome P450 (CYP) Allele Nomenclature Database: CYP2A6 Allele Nomenclature. Karolinska Institute; website. http://www.cypalleles.ki.se/cyp2a6.htm. Updated January 21, 2014. Accessed May 20, 2016. [Google Scholar]
- 11.Dempsey DA, Sambol NC, Jacob P, 3rd, et al. CYP2A6 genotype but not age determines cotinine half-life in infants and children. Clin Pharmacol Ther. 2013;94(3):400–406. doi: 10.1038/clpt.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H-YL, Lee QP, Rettie AR, Juchau MR. Functional cytochrome P450A3 isoforms in human embryonic tissues: expression during organogenesis. Mol Pharmacol. 1994;46(5):922–928. [PubMed] [Google Scholar]
- 13.Stevens JC, Hines RN, Gu C, et al. Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther. 2003;307(2):573–582. doi: 10.1124/jpet.103.054841. [DOI] [PubMed] [Google Scholar]
- 14.Tateishi T, Nakamura H, Asoh M, et al. A comparison of hepatic cytochrome P450 protein expression between infancy and post-infancy. Life Sci. 1997;61(26):2567–2574. doi: 10.1016/s0024-3205(97)01011-4. [DOI] [PubMed] [Google Scholar]
- 15.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens, and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270(1):414–423. [PubMed] [Google Scholar]
- 16.Sprandel KA, Drusano GL, Hecht DW, Rotschafer JC, Danziger LH, Rodvold KA. Population pharmacokinetic modeling and Monte Carlo simulation of varying doses of intravenous metronidazole. Diagn Microbiol Infect Dis. 2006;55(4):303–309. doi: 10.1016/j.diagmicrobio.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Ho MK, Mwenifumbo JC, Al Koudsi N, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85(6):635–643. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukami T, Nakajima M, Yoshida R, et al. A novel polymorphism of human CYP2A6 gene CYP2A6*17 has an amino acid substitution (V365M) that decreases enzymatic activity in vitro and in vivo. Clin Pharmacol Ther. 2004;76(6):519–527. doi: 10.1016/j.clpt.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14(9):615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Minematsu N, Nakamura H, Furuuchi M, et al. Limitation of cigarette consumption by CYP2A6*4, *7, and *9 polymorphisms. Eur Respir J. 2006;27(2):289–292. doi: 10.1183/09031936.06.00056305. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima M, Fukami T, Yamanaka H, et al. Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharmacol Ther. 2006;80(3):282–297. doi: 10.1016/j.clpt.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Ward RM, Tammara B, Sullivan SE, et al. Single-dose, multiple-dose, and population pharmakokinetics of pantoprazole in neonates and preterm infants with a clinical diagnosis of gastroesophageal reflux disease (GERD) Eur J Clin Pharmacol. 2010;66(6):555–561. doi: 10.1007/s00228-010-0811-8. [DOI] [PubMed] [Google Scholar]
- 23.Leeder JS, Kearns GL. Interpreting pharmacogenetic data in the developing neonate: the challenge of hitting a moving target. Clin Pharmacol Ther. 2012;92(4):434–436. doi: 10.1038/clpt.2012.130. [DOI] [PubMed] [Google Scholar]


