Abstract
Acute post-asphyxial encephalopathy around the time of birth remains a major cause of death and disability. The possibility that hypothermia may be able to prevent or lessen asphyxial brain injury is a “dream revisited”. In this review, a historical perspective is provided from the first reported use of therapeutic hypothermia for brain injuries in antiquity, to the present day. The first uncontrolled trials of cooling for resuscitation were reported more than 50 years ago. The seminal insight that led to the modern revival of studies of neuroprotection was that after profound asphyxia, many brain cells show initial recovery from the insult during a short “latent” phase, typically lasting approximately 6 h, only to die hours to days later after a “secondary” deterioration characterized by seizures, cytotoxic edema, and progressive failure of cerebral oxidative metabolism. Studies designed around this conceptual framework showed that mild hypothermia initiated as early as possible before the onset of secondary deterioration, and continued for a sufficient duration to allow the secondary deterioration to resolve, is associated with potent, long-lasting neuroprotection. There is now compelling evidence from randomized controlled trials that mild induced hypothermia significantly improves intact survival and neurodevelopmental outcomes to mid-childhood.
Moderate to severe hypoxic-ischemic encephalopathy (HIE) continues to be a significant cause of acute neurologic injury at birth, occurring in approximately 1 to 2 cases per 1000 term live births in the developed world (1). The risks are approximately ten-fold higher in the developing world (1). The possibility that hypothermia might be able to prevent or lessen asphyxial brain injury has been raised since antiquity, and so should be seen as a “dream revisited” (2).
HISTORICAL OBSERVATIONS
The earliest recommendations for induced hypothermia can be traced back to the Ancient World. The Egyptians, Greeks and Romans first recommended induced cooling for battle-inflicted trauma and a variety of cerebral disturbances (3). The Greek physician Hippocrates observed that infants exposed in the open survived much longer in winter than summer (4). Many centuries later, physiologists such as Claude Bernard and William Edwards first described the effects of hypothermia on the human body (5, 6), and observed that asphyxiated newborn kittens continue to gasp for longer intervals when actively cooled (5). These observations were later confirmed in other animal species (7), and shown to be associated with better functional outcomes after cooling during hypoxia. The rationale for these studies was that hypothermic metabolic suppression during anoxia prolonged survival (8).
These early experimental studies noted above focused entirely on the effects of cooling during severe hypoxia, which is well known to be associated with dose-related, long-lasting neuroprotection (9). The central clinical question is whether cooling after asphyxia or hypoxia-ischemia is beneficial.
Early studies of hypothermia in newborn infants
The apparent protective effects of hypothermia led to small, uncontrolled studies in the 1950s and 1960s in which infants who were not breathing spontaneously at 5 minutes after birth were immersed in cold water until respiration began and then allowed to spontaneously rewarm over many hours (10). Neonatal outcomes were reported to be better than historical controls in over 200 asphyxiated neonates (11).
This empiric approach was overtaken by the development of active resuscitation techniques, case reports of subcutaneous fat necrosis with calcification after cooling (12), and the higher neonatal oxygen requirements and greater mortality rates among premature newborns kept hypothermic after birth (13-16). Because of this evidence, there was a pause in investigations of therapeutic hypothermia for HIE.
In this paper we review the resurgence of animal and human research in to mild post-resuscitation cooling to improve outcomes among infants with HIE.
PRECLINICAL STUDIES
The key advance in understanding the pathogenesis of hypoxic-ischemic brain injury was the clinical and experimental observation in term fetuses, newborns, and adults that injury to the brain is not a single “event” occurring at, or just after, an insult, but rather an evolving process that leads to a significant proportion of cell death well after the initial insult, as recently reviewed (17).
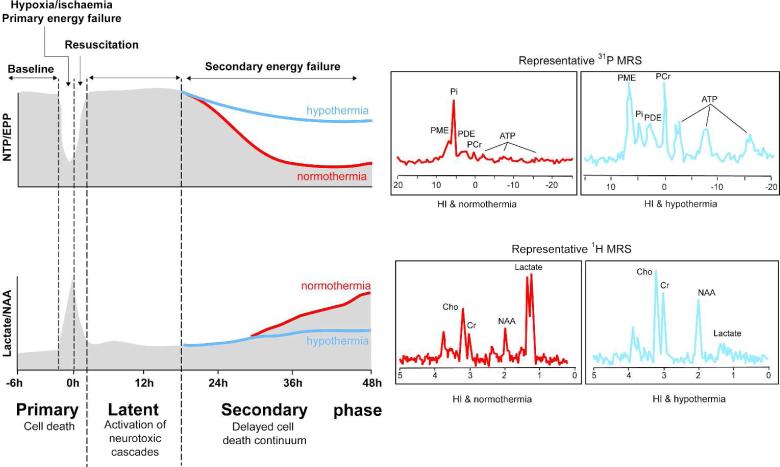
Distinct pathophysiological phases have been identified, as illustrated in Figures 1 and 2. The actual period of hypoxia and ischemia is the primary phase of cell injury. During this phase, there is progressive failure of oxidative metabolism (Figure 1), leading to hypoxic depolarization of cells with cytotoxic edema (Figure 2), failure of reuptake and extracellular accumulation of excitatory amino acids (excitotoxins) (17). Excitotoxins activate their ion channels and promote additional excessive entry of sodium, water, and calcium into the cells. After return of cerebral circulation during resuscitation, cytotoxic edema may transiently resolve over 30 to 60 minutes, with at least partial recovery of cerebral oxidative metabolism in a latent phase (18, 19). This latent phase that lasts approximately 6 h is characterized by continued EEG suppression, with secondary hypoperfusion and suppressed cerebral metabolism (20). This is followed by a secondary phase of deterioration (~6 to 15 h after the insult) that may extend over many days. At term gestation, this secondary phase is marked by delayed onset seizures, secondary cytotoxic edema (Figure 2), accumulation of excitotoxins, failure of cerebral oxidative energy metabolism (Figure 1), and ultimately neuronal death (17).
Figure 1.
Schematic diagram showing the phases of primary and secondary energy failure after hypoxia-ischemia on magnetic resonance spectroscopy (MRS), and the amelioration of secondary energy failure with therapeutic hypothermia started during the latent phase after transient hypoxia-ischemia in a newborn piglet. Upper panel shows phosphorus-31 MRS NTP/EPP peak area ratio at baseline, during and after hypoxia-ischemia. The preservation of high energy phosphates with hypothermia versus normothermia is shown on the diagram (blue line versus red line) and in the representative spectra at 48 h (red normothermia, blue hypothermia). Lower panel shows proton MRS lactate/NAA peak area ratio at baseline, and during and after hypoxia-ischemia. The amelioration of the rise in lactate/NAA with hypothermia versus normothermia is shown on the diagram (blue line versus red line) and in the representative spectra at 48 h (red normothermia, blue hypothermia). NTP: nucleotide tri-phosphate; EPP: exchangeable phosphate pool; NAA: N-acetyl aspartate.
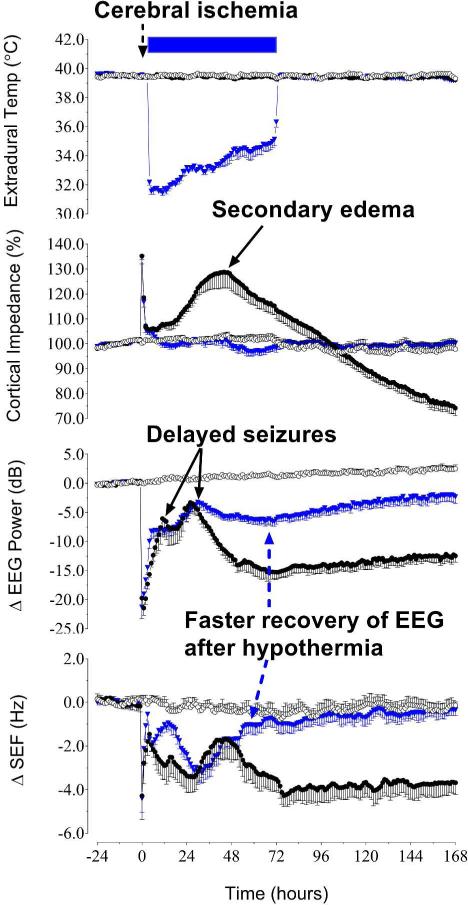
Figure 2.
The effect of hypothermia started 3 h after a 30 minute period of cerebral ischemia in term-equivalent fetal sheep (38). The period of ischemia is shown by the dashed arrow. Cooling is shown by the filled blue bar. The top panel shows changes in extradural temperature (°C) after sham ischemia (open circles), ischemia-normothermia (solid black circles) and ischemia-hypothermia (inverted blue triangles). The lower three panels show changes in cortical impedance (%) a measure of changes in cell swelling (cytotoxic edema), change in electroencephalographic (EEG) power (dB, decibels) and change in the spectral edge frequency of the EEG (SEF, Hz). The hypothermia group shows complete suppression of the secondary rise in impedance, greater recovery of EEG power after resolution of delayed seizures (which occur from approximately 9 to 72 h) and improved SEF that persists after rewarming. Data are mean ± SEM.
The studies discussed in this review strongly suggest that the latent or early recovery phase represents the key window of opportunity for intervention.
Factors Determining Effective Neuroprotection with Hypothermia
Experimentally, the efficacy of neuroprotection with hypothermia is highly dependent on the timing of initiation of cooling, its duration, and its depth.
Cooling during resuscitation and reperfusion
Brief hypothermia, for 1 to 3 h is modestly neuroprotective, provided it is initiated immediately after the insult. For example, after 15 minutes of reversible ischemia in the piglet, mild hypothermia (2° to 3°C) for 1 or 3 h reduced neuronal loss 3 days later (21). Protection was lost if the brief interval of hypothermia was delayed by as little as 15 to 45 minutes after the primary insult (22). Even if such immediate cooling during resuscitation were consistently effective, it is presently impossible to reliably identify the infants requiring resuscitation who will go on to develop HIE.
Immediate or delayed prolonged cooling
A more recent approach has been to continue hypothermia for longer, throughout the secondary phase of deterioration, to suppress delayed encephalopathic processes. Extended cooling for between 5 and 72 h appears to be required for protection (23). In unanesthetized postnatal day(P)21 rats subjected to hypoxia-ischemia, mild hypothermia (2° to 3°C reduction in body temperature) for 72 h immediately after the insult prevented cortical infarction, whereas cooling for 6 h did not (24). In P7 rats a greater reduction in body temperature (by 5°C) for 6 h, starting immediately after hypoxia-ischemia, improved neurobehavioral recovery after 1 and 6 weeks survival (25). Finally, in anesthetized piglets exposed to hypoxia-ischemia, 12 to 48 h of whole body cooling by 3.5 or 5°C or head cooling with mild systemic hypothermia started immediately after hypoxia prevented delayed energy failure (23), reduced neuronal loss (26), and suppressed post-hypoxic seizures (27). Similar results have been reported in adult rodents (28).
It is interesting to contrast the finding of neuroprotection after 6 h of moderate hypothermia in P7 rats (25) but not in older rodents or large animals (29). Potentially this might reflect the more rapid rate of brain development in rodents than larger animals. However, it is important to appreciate that rectal temperatures of healthy nesting neonatal rats are significantly lower than in later life (median of 35.4 °C at P7 vs ~38 °C in adults) (30). Thus, P7 rats effectively receive continuing mild hypothermia after the acute study period.
A Window of Opportunity for Treatment
At present, the window of opportunity for any particular therapy can only be determined empirically. The known characteristics of programmed cell death support that earlier initiation of interventions after hypoxia-ischemia would be more effective than a later start. Specifically, initiation of neuronal degeneration occurs more slowly after a shorter, less severe period of hypoxia-ischemia compared with severe insults (31). However, DNA fragmentation and classic ischemic cell change represent only the terminal events of this cascade and are not suitable to determine reversibility of tissue injury. In vitro studies distinguished latent and active or execution phases during programmed cell death (32). The latent phase is characterized by caspase activation (a large family of enzymes that mediate and amplify apoptosis) in the cytoplasm, while the active phase involves downstream factors that induce DNA fragmentation and chromatin condensation within previously normal nuclei (32). These data suggest that activation of downstream, intranuclear factors corresponds with the transition from latent to execution (“secondary”) phases of programmed neuronal death (32).
Systematic in vivo studies support the central importance of starting treatment in the latent phase. In near-term fetal sheep, moderate hypothermia induced 90 minutes after reperfusion (i.e. in the early latent phase) and continued until 72 h after ischemia prevented secondary cytotoxic edema and improved electroencephalographic recovery (33). There was a concomitant, substantial reduction in parasagittal cortical infarction and improvement in neuronal loss scores in all regions. Only partial protection was observed when initiation of hypothermia was delayed until the onset of secondary seizures, 5.5 h after reperfusion (34). No protection was observed with further delay (until after seizures were established 8.5 h after reperfusion) (35).
Consistent with these findings, in adult gerbils, when the delay before initiating a 24-h period of cooling was increased from 1 to 4 h after ischemia, neuroprotection in the CA1 field of the hippocampus after 6 months of recovery fell from 70 to 12% (36). However, protection was almost completely restored by extending the interval of moderate hypothermia (a reduction in body temperature of up to 5°C) to 48 h or more, even when the start of cooling was delayed until 6 h after reperfusion (37). The optimal duration of hypothermia after delayed initiation of cooling appears to be around 72 h. One study in term-equivalent fetal sheep found no further improvement in EEG or histological protection when hypothermia was extended from 72 to 120 h after cerebral ischemia, and possible reduced protection in some regions (38, 39). These data are consistent with a recent large randomized controlled trial of extended duration of cooling for HIE (40).
Is Neuroprotection Maintained Long Term?
There have been reports that mild hypothermia may only delay rather than prevent neuronal loss after hypoxia-ischemia in the 7 day old rat (41). This most likely reflects an inadequate duration or degree of hypothermia. Subsequent studies both in the 7 day old rat and in adult species confirmed that sufficiently prolonged moderate cooling can be associated with persistent behavioral and histological protection over weeks or months (17, 25). An additional issue may have been rebound hyperthermia in the secondary phase. Even short periods of hyperthermia, 24 h after either global or brief focal ischemia in the adult rat, exacerbated injury (42), and preventing spontaneous delayed pyrexia with antipyretics after post-ischemic hypothermia improved histologic protection (43).
If Some Is Good, Is More Better?
There appears to be a critical depth of brain and body hypothermia of between 32° and 34°C required for effective neuronal rescue. In the fetal sheep undergoing head cooling from 90 minutes after ischemia, neuroprotection was seen only in fetuses that had a sustained fall of the extradural temperature to less than 34°C (normal core temperature in the fetal sheep is 39.5°C) (33). In the adult gerbil, 32°C was more protective than 34°C (44).
There is a potential trade-off between the adverse systemic effects of cooling, which increase below a core temperature of approximately 32 to 34°C (45), and cerebral benefits. In newborn piglets, reducing body temperature by 8°C after hypoxia-ischemia was associated with greater metabolic acidosis, increased blood glucose levels, and greater risk of cardiac arrest and death compared to cooling by 3.5 to 5°C (46). Critically, optimal neuroprotection was seen with cooling by 3.5 to 5°C (26). A similar therapeutic range has been found in adult dogs after cardiac arrest (47).
Cooling the brain with Head or Body Cooling
There are temperature gradients within the brain of newborn animals from a warmer core to a cooler periphery, such that deep brain temperature is approximately 1° to 2°C higher than the surface of the head, 0.7°C higher than core body temperature, and increased during reduced perfusion as occurs after severe asphyxia (48). These gradients reflect thermal conduction and are affected by tissue heat production and local blood flow.
Selectively cooling the head could provide neuroprotection with minimal risk of systemic adverse effects in sick, unstable neonates. Pragmatically, partially selective cerebral cooling can be achieved using a cooling cap applied to the scalp while the body is warmed by an overhead heater to limit systemic hypothermia (49). Mild systemic hypothermia is essential during head cooling to limit the steepness of the intracerebral gradient and potentially over cool the brain periphery while providing cooling of the core. This approach was demonstrated in studies in the piglet to achieve a substantial (median, 5.3°C), sustained decrease in deep intracerebral temperature at the level of the basal ganglia compared with the rectal temperature (50). Although direct brain temperature measurements are not feasible in asphyxiated newborns, head cooling increased the gradient between nasopharyngeal and rectal temperature by nearly 1°C (51).
Alternatively the brain can be cooled by cooling the body. Brain hypothermia induced by whole body cooling results in a more homogenous cooling of the cortex and deeper structures compared to head cooling (52). Temperature gradients across the brain of newborn piglets (2 cm depth below the cortex to the dura) were similar during cooling to gradients under normal body temperature conditions.
Mechanisms of Action of Hypothermia
The precise mechanisms of hypothermic neuroprotection are still unclear. Broadly, cooling suppresses many of the pathways leading to delayed cell death. Hypothermia reduces cellular metabolic demand, excessive accumulation of cytotoxins (e.g., glutamate, (53)) and oxygen free radicals during hypoxia-ischemia and inhibits post-ischemic inflammatory reaction and the intracellular pathways leading to programmed (i.e. apoptosis-like) cell death (17).
Cerebral metabolism, excitotoxins, and free radicals
Hypothermia produces a graded reduction in cerebral metabolism of about 5% for every degree of temperature reduction that delays the onset of anoxic cell depolarization (54). However, the protective effects of hypothermia even during hypoxia-ischemia are not simply the result of reduced metabolism, because cooling improves outcome even when the absolute duration of depolarization is controlled (55). Cooling potently reduces post-depolarization release of numerous toxins including excitatory amino acids, nitric oxide, and other free radicals, as recently reviewed (17). Similarly, cooling begun during reperfusion reduces levels of extracellular excitatory amino acids and nitric oxide production in the piglet (53).
Extracellular levels of excitatory amino acids rapidly return to baseline values after reperfusion (56). Despite normal levels of extracellular glutamate, pathological hyperexcitability of glutamate receptors persists for many hours after hypoxia-ischemia in P10 rats, and neuronal survival is improved by glutamate receptor blockade (57). In preterm fetal sheep transient epileptiform activity was seen in the early post-asphyxial recovery phase, despite a suppressed overall EEG, which correlated with the severity of neuronal loss (58). In preterm fetal sheep, both post-asphyxial moderate cerebral hypothermia and infusion of a glutamate receptor antagonist were partially neuroprotective and associated with marked suppression of epileptiform transient activity in the first 6 h after asphyxia (59, 60). The combination of glutamate receptor antagonist infusion and hypothermia after severe asphyxia showed non-additive neuroprotection, supporting that therapeutic hypothermia is partly protective by attenuating this receptor hyperactivity (61). However, hypothermia was associated with greater neuroprotection than glutamate blockade, suggesting that additional mechanisms are involved.
Supporting this, intra-insult hypothermia did not prevent intracellular accumulation of calcium during cardiac arrest in vivo, or during glutamate exposure in vitro, as reviewed (17). In contrast, in vitro, neuronal degeneration was prevented by cooling initiated after the excitotoxins were washed out (17). These data suggest that hypothermia blocks the intracellular consequences of excitotoxin exposure.
Suppression of inflammatory second messengers
Brain injury leads to induction of the inflammatory cascade with increased release of cytokines and interleukins (62). These compounds are believed to exacerbate delayed injury, either by direct neurotoxicity and induction of apoptosis or by stimulating endothelial cell proinflammatory responses and leukocyte adhesion and infiltration into the ischemic brain. Hypothermia is a potent inhibitor of proliferation, and of superoxide and nitric oxide production by microglia, and reduces microglial activation after transient ischemia in the term-equivalent fetal sheep (17). These data suggest that hypothermic protection against post-ischemic neuronal damage may be, in part, the result of suppression of microglial activation.
Does hypothermia specifically prevent or suppress programmed cell death?
Increasing data suggest that hypothermia has a role in suppressing apoptotic processes, particularly in the developing brain (17). For example, hypothermia begun after severe hypoxia-ischemia in the piglet reduced apoptotic cell death but not necrotic cell death, and hypothermic neuroprotection in the near-term fetal sheep was closely linked with suppression of activated caspase-3 (63). Taken together, these experimental studies suggested that a prolonged duration of mild to moderate cerebral hypothermia could improve long-term outcome, if started as soon as possible within approximately 6 h of hypoxic-ischemic injury. Based on these extremely encouraging data, a number of clinical trials were undertaken.
CLINICAL TRIALS
Pilot studies
Small controlled trials of head cooling with mild systemic hypothermia (51) or whole body cooling (64), reported that cooling asphyxiated newborns was both feasible and without obvious harm. These studies were not powered to evaluate long-term outcome, but there was some suggestion of improved outcomes (65), which was explored in subsequent large trials of efficacy.
Large Randomized Controlled Trials
The encouraging pilot studies supported a series of large trials. A recent Cochrane meta-analysis identified 11 randomized controlled trials involving 1505 infants that compared mild induced hypothermia to normothermia (66). Hypothermia was associated with a substantial reduction in death or moderate or severe neurodevelopmental disability to 18 months of age (relative risk, RR, 0.75; 95% confidence interval (CI) 0.68 to 0.83; number needed to treat 7 (5 to 10); 1344 infants in 8 studies). Cooling was associated with both reduced mortality (RR 0.75; 0.64 to 0.88, from 1468 infants in 11 studies) and reduced risk of neurodevelopmental disability in survivors (RR 0.77; 0.63 to 0.94, from 917 infants in 8 studies). In 6 trials including 1120 infants, hypothermia was associated with a significant increase in normal survival (RR 1.63; 1.12 to 2.7) (67-72).
Improvements were strikingly consistent between these similarly designed trials. All involved initiation of cooling within 6 h of birth (cooling was initiated on average between 4 and 5 h), by either head cooling with mild systemic hypothermia or mild whole body cooling for up to 72 h. For example, in the CoolCap trial, infants with moderate to severe HIE were randomized to head cooling with mild systemic hypothermia (rectal temperature 34-35°C, n=116), or conventional care (n=118) (67). Death or severe disability at 18 months was reduced in infants with moderate amplitude integrated electroencephalographic (aEEG) changes at trial entry (n=172, odds ratio (OR) 0.42; 95% CI 0.22-0.80, p=0.009). However, there was no benefit in infants who had seizures with profound suppression of the aEEG before cooling was started. The improvement in moderately affected infants was primarily related to a more than 50 percent reduction in severe neuromotor disability in survivors and improved continuous BSID-II scores, with no change in early neonatal mortality.
Similarly, in a large multi-center trial of whole body cooling from the National Institute of Child Health and Human Development (NICHD), 208 infants were enrolled based on clinical and laboratory criteria consistent with exposure to severe perinatal hypoxia plus moderate or severe HIE (68). Infants in the experimental group (n=102) were cooled to a rectal temperature of 33.5±0.5 °C for 72 h using a cooling blanket. The incidence of death or moderate-to-severe disability at 18 months was significantly reduced in cooled infants (44%) compared with normothermic infants (62%, RR 0.72; 0.54-0.95, P=0.01).
Subsequent trials had similar results. The Total Body Cooling trial (TOBY) compared body cooling to 33.5±0.5°C for 72 h (n=163) to standard care (n=162) (69). There was no significant effect on death or disability (RR 0.86; 0.68-1.07, P=0.17) at 18 months of age, but survival without neurologic abnormality was improved (RR 1.57; 1.16-2.12, P=0.003). The risk of cerebral palsy was reduced in survivors (RR 0.67; 0.47-0.96, P=0.03), with improvements in the Mental Developmental Index and Psychomotor Developmental Index of the Bayley Scales of Infant Development II.
Similarly, the neo.nEURO.network trial compared term neonates with HIE randomized to either cooling to 33.5±0.05°C with a cooling blanket for 72 h (n=53) followed by slow rewarming or to standard care with normothermia (core temperature 37±0.5°C, n=58). All infants were given prophylactic analgesia with morphine or fentanyl. There was a significant reduction in death or severe disability (51% in the hypothermia group vs 83% in the normothermia group; OR 0.21; 0.09-0.54, P=0.001), and there were fewer clinical seizures in the hypothermia group. It is unknown whether routine sedation contributed to the improvement in this trial.
The China Study Group studied head cooling with systemic hypothermia to a nasopharyngeal temperature of 34±0.2°C and rectal temperature of 34.5–35.0°C for 72 h (n=100) compared to standard care (rectal temperature 36.0–37.5°C, n=94) (70). Cooling was associated with a reduced risk of death or disability (31% after cooling vs 49% after standard care; OR: 0.47; 0.26-0.84, P = 0.01).
Finally, the Infant Cooling Evaluation (ICE) trial evaluated whole-body cooling, induced by turning off the radiant warmer and applying refrigerated gel packs, to achieve a rectal temperature of 33.5±0.5°C for 72 h (n=110) or standard care (37° C, n=111) (72). Cooling was associated with reduced risk of death or major sensorineural disability at 2 years of age (RR, 0.77; 0.62-0.98, P=0.03). This study shows that a very simple albeit more labor intensive method could be used within strict protocols to induce hypothermia in non-tertiary neonatal settings before transport.
An interesting practical observation from several studies was that mild hypothermia delayed recovery of both the aEEG (73), and neurological state (74).
Long-term follow-up
There is now evidence that improved outcomes at 18 to 24 months of age are sustained at school age. The TOBY trial found greater frequency of survival with an IQ score ≥ 85 at 6 to 7 years after treatment with mild hypothermia (52% (75/145), than standard care (39% (52/132); RR 1.31, P=0.04) (75). Normal survival was increased after hypothermia (45% vs. 28%; RR 1.60; 95% CI 1.15-2.22), with reduced risk of both cerebral palsy (21% vs. 36%, P=0.03) and moderate or severe disability (22% vs. 37%, P=0.03). Similarly, the NICHD trial showed a strong trend to reduced risk of either death or an IQ score below 70 at 6 to 7 years of age (47% after hypothermia vs 62% after standard care, P=0.06) (76). These studies support the long-term predictive value of a favorable outcome at 18 months of age.
Systemic effects of hypothermia
The studies discussed support that mild hypothermia is generally safe. Meta-analysis suggests that hypothermia is associated with a significant increase in thrombocytopenia, but no increase in hemorrhagic complications (66). A significant increase in blood pressure has been reported at initiation of cooling, both experimentally (34) and clinically (77). This response is mediated by rapid peripheral vasoconstriction, i.e. centralization of blood flow (78). Hypothermia also slows the atrial pacemaker and intracardiac conduction. Core temperatures less than ~35.5°C are associated with mild but sustained sinus bradycardia, not requiring treatment (66). These changes are consistent with decreased metabolic demand with decreasing temperature. Some infants show markedly prolonged QT duration during cooling (> 98th % corrected for age and heart rate), that resolves with rewarming, but no ventricular arrhythmias (79). Thus, therapies that lengthen the QT interval should be avoided during cooling.
Metabolically, hypothermia has been associated with transient mild hyperglycemia, both in adults (80) and infants (67), but no increase in the rate of hypoglycemia. A similar transient rise in glucose concentrations has been observed in the piglet and near-term fetal sheep (33, 46), and likely reflects hypothermia-induced catecholamine release. Head cooling was associated with scalp edema under the cap, which resolved rapidly before or after removal of the cap (67). Conversely, there was an apparent reduction in the incidence of elevated liver enzymes in the cooled group (38% of cooled infants vs. 53% of controls, p=0.02) (67). Hypothermia has profound anti-inflammatory effects and in older adults seems to increase the risk of infective complications such as pneumonia and bacteremia (81). There is no evidence of increased risk of infection in the newborn studies, but this may reflect prophylactic antibiotic treatment (67, 68).
All the clinical trials to date involved infants ≥ 35-36 weeks gestation. It is unknown whether selected preterm infants with evidence of acute metabolic acidosis on cord blood and clinical encephalopathy would also benefit from hypothermia. Studies in preterm fetal sheep, at an equivalent stage of neural maturation found that head cooling, started 90 minutes after profound asphyxia, reduces white and grey matter loss (58). The NICHD Neonatal Research Network has initiated a randomized trial to determine the efficacy and safety of therapeutic hypothermia in preterm infants 330-356 weeks (NCT01793129).
Conclusions
There is now overwhelming clinical and experimental evidence that mild to moderate post-asphyxial cerebral cooling is associated with long-term improved survival without disability. The key requirements for neuroprotection are that hypothermia be initiated as soon as possible in the latent phase, within the first 6 h, before secondary deterioration, and that it be continued for a sufficient period in relation to the evolution of delayed encephalopathic processes, typically around 72 h. These findings are now supported by large randomized trials of both head cooling combined with mild systemic hypothermia and whole body cooling that found that cooling is safe, at least in the intensive care environment, and associated with a significant improvement in survival without disability. These studies show that as currently applied approximately 15% of infants will have better outcome after cooling compared to standard care (66). Future studies to improve outcomes should now be conducted in the setting of mild induced cooling as the established standard of care.
Acknowledgments
The work reported in this review was supported in part by the Health Research Council of New Zealand, the Lottery Health Board of New Zealand, and the Auckland Medical Research Foundation.
Footnotes
Conflict of interest statement: The authors have no conflicts of interests to disclose or have any competing financial interests.
References
- 1.Lee AC, Kozuki N, Blencowe H, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74(Suppl 1):50–72. doi: 10.1038/pr.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Floyer J. An essay to restore the dipping of infants in their baptism; with a dialogue betwixt a curate and a practitioner, concerning the manner of immersion. Vol. 1722. Holland, London: p. 79. [Google Scholar]
- 3.Celcus AC. On Medicine, Books 1-4. 1-100 AD. Harvard University Press, Loeb Classical Library; pp. 1–512. [Google Scholar]
- 4.Hippocrates De Vetere Medicina. 460–375 BC. Harvard University Press, Loeb Classical Library; pp. 1–432. [Google Scholar]
- 5.Edwards WF. Philadelphia, Haswell, Barrington and Haswell, London: 1832. On the Influence of Physical Agents on Life. pp. 1–489. [Google Scholar]
- 6.Bernard C. Leçons sur la Chaleur Animale. J. B. Baillière; Paris: 1876. pp. 1–471. [Google Scholar]
- 7.Miller JA., Jr. Effects of variations in body temperature upon resistance to asphyxia in the neonatal guinea pig. Cold Spring Harb Symp Quant Biol. 1954;19:152–4. doi: 10.1101/sqb.1954.019.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Brewin EG. Physiology of hypothermia. Int Anesthesiol Clin. 1964;2:803–27. doi: 10.1097/00004311-196408000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Nurse S, Corbett D. Direct measurement of brain temperature during and after intraischemic hypothermia: correlation with behavioral, physiological, and histological endpoints. J Neurosci. 1994;14:7726–34. doi: 10.1523/JNEUROSCI.14-12-07726.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westin B, Miller JA, Jr., Nyberg R, Wedenberg E. Neonatal asphyxia pallida treated with hypothermia alone or with hypothermia and transfusion of oxygenated blood. Surgery. 1959;45:868–79. [PubMed] [Google Scholar]
- 11.Miller JA, Jr., Miller FS. Mechanisms of hypothermic protection against anoxia. Adv Exp Med Biol. 1972;33:571–86. doi: 10.1007/978-1-4684-3228-2_58. [DOI] [PubMed] [Google Scholar]
- 12.Duhn R, Schoen EJ, Siu M. Subcutaneous fat necrosis with extensive calcification after hypothermia in two newborn infants. Pediatrics. 1968;41:661–4. [PubMed] [Google Scholar]
- 13.Silverman WA, Fertig JW, Berger AP. The influence of the thermal environment upon the survival of newly born premature infants. Pediatrics. 1958;22:876–86. [PubMed] [Google Scholar]
- 14.Dunn JM, Miller JA., Jr. Hypothermia combined with positive pressure ventilation in resuscitation of the asphyxiated neonate. Clinical observations in 28 infants. Am J Obstet Gynecol. 1969;104:58–67. doi: 10.1016/s0002-9378(16)34141-2. [DOI] [PubMed] [Google Scholar]
- 15.Buetow KC, Klein SW. Effect of maintenance of “normal” skin temperature on survival of infants of low birth weight. Pediatrics. 1964;34:163–70. [PubMed] [Google Scholar]
- 16.Day RL, Caliguiri L, Kamenski C, Ehrlich F. Body temperature and survival of premature infants. Pediatrics. 1964;34:171–81. [PubMed] [Google Scholar]
- 17.Wassink G, Gunn ER, Drury PP, Bennet L, Gunn AJ. The mechanisms and treatment of asphyxial encephalopathy. Front Neurosci. 2014;8:40. doi: 10.3389/fnins.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azzopardi D, Wyatt JS, Cady EB, et al. Prognosis of newborn infants with hypoxic-ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1989;25:445–51. doi: 10.1203/00006450-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Iwata O, Iwata S, Bainbridge A, et al. Supra- and sub-baseline phosphocreatine recovery in developing brain after transient hypoxia-ischaemia: relation to baseline energetics, insult severity and outcome. Brain. 2008;131:2220–6. doi: 10.1093/brain/awn150. [DOI] [PubMed] [Google Scholar]
- 20.Jensen EC, Bennet L, Hunter CJ, Power GG, Gunn AJ. Post-hypoxic hypoperfusion is associated with suppression of cerebral metabolism and increased tissue oxygenation in near-term fetal sheep. J Physiol. 2006;572:131–9. doi: 10.1113/jphysiol.2005.100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laptook AR, Corbett RJ, Sterett R, Burns DK, Garcia D, Tollefsbol G. Modest hypothermia provides partial neuroprotection when used for immediate resuscitation after brain ischemia. Pediatr Res. 1997;42:17–23. doi: 10.1203/00006450-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Laptook AR, Corbett RJ, Burns DK, Sterett R. A limited interval of delayed modest hypothermia for ischemic brain resuscitation is not beneficial in neonatal swine. Pediatr Res. 1999;46:383–9. doi: 10.1203/00006450-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Thoresen M, Penrice J, Lorek A, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995;37:667–70. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Sirimanne ES, Blumberg RM, Bossano D, et al. The effect of prolonged modification of cerebral temperature on outcome after hypoxic-ischemic brain injury in the infant rat. Pediatr Res. 1996;39:591–7. doi: 10.1203/00006450-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Bona E, Hagberg H, Loberg EM, Bagenholm R, Thoresen M. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatr Res. 1998;43:738–45. doi: 10.1203/00006450-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Alonso-Alconada D, Broad KD, Bainbridge A, et al. Brain cell death is reduced with cooling by 3.5 degrees C to 5 degrees C but increased with cooling by 8.5 degrees C in a piglet asphyxia model. Stroke. 2015;46:275–8. doi: 10.1161/STROKEAHA.114.007330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tooley JR, Satas S, Porter H, Silver IA, Thoresen M. Head cooling with mild systemic hypothermia in anesthetized piglets is neuroprotective. Ann Neurol. 2003;53:65–72. doi: 10.1002/ana.10402. [DOI] [PubMed] [Google Scholar]
- 28.Colbourne F, Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res. 1994;654:265–72. doi: 10.1016/0006-8993(94)90488-x. [DOI] [PubMed] [Google Scholar]
- 29.Colbourne F, Sutherland G, Corbett D. Postischemic hypothermia. A critical appraisal with implications for clinical treatment. Mol Neurobiol. 1997;14:171–201. doi: 10.1007/BF02740655. [DOI] [PubMed] [Google Scholar]
- 30.Wood T, Osredkar D, Puchades M, et al. Treatment temperature and insult severity influence the neuroprotective effects of therapeutic hypothermia. Scientific reports. 2016;6:23430. doi: 10.1038/srep23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beilharz EJ, Williams CE, Dragunow M, Sirimanne ES, Gluckman PD. Mechanisms of delayed cell death following hypoxic-ischemic injury in the immature rat: evidence for apoptosis during selective neuronal loss. Mol Brain Res. 1995;29:1–14. doi: 10.1016/0169-328x(94)00217-3. [DOI] [PubMed] [Google Scholar]
- 32.Samejima K, Tone S, Kottke TJ, et al. Transition from caspase-dependent to caspase-independent mechanisms at the onset of apoptotic execution. J Cell Biol. 1998;143:225–39. doi: 10.1083/jcb.143.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–56. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102:1098–106. doi: 10.1542/peds.102.5.1098. [DOI] [PubMed] [Google Scholar]
- 35.Gunn AJ, Bennet L, Gunning MI, Gluckman PD, Gunn TR. Cerebral hypothermia is not neuroprotective when started after postischemic seizures in fetal sheep. Pediatr Res. 1999;46:274–80. doi: 10.1203/00006450-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–60. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colbourne F, Corbett D, Zhao Z, Yang J, Buchan AM. Prolonged but delayed postischemic hypothermia: a long-term outcome study in the rat middle cerebral artery occlusion model. J Cereb Blood Flow Metab. 2000;20:1702–8. doi: 10.1097/00004647-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Davidson JO, Wassink G, Yuill CA, Zhang FG, Bennet L, Gunn AJ. How long is too long for cerebral cooling after ischemia in fetal sheep? J Cereb Blood Flow Metab. 2015;35:751–8. doi: 10.1038/jcbfm.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson JO, Yuill CA, Zhang FG, Wassink G, Bennet L, Gunn AJ. Extending the duration of hypothermia does not further improve white matter protection after ischemia in term-equivalent fetal sheep. Sci Rep. 2016;6:25178. doi: 10.1038/srep25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shankaran S, Laptook AR, Pappas A, et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. 2014;312:2629–39. doi: 10.1001/jama.2014.16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trescher WH, Ishiwa S, Johnston MV. Brief post-hypoxic-ischemic hypothermia markedly delays neonatal brain injury. Brain Dev. 1997;19:326–38. doi: 10.1016/s0387-7604(97)00027-2. [DOI] [PubMed] [Google Scholar]
- 42.Baena RC, Busto R, Dietrich WD, Globus MY, Ginsberg MD. Hyperthermia delayed by 24 hours aggravates neuronal damage in rat hippocampus following global ischemia. Neurology. 1997;48:768–73. doi: 10.1212/wnl.48.3.768. [DOI] [PubMed] [Google Scholar]
- 43.Coimbra C, Drake M, Boris-Moller F, Wieloch T. Long-lasting neuroprotective effect of postischemic hypothermia and treatment with an anti-inflammatory/antipyretic drug. Evidence for chronic encephalopathic processes following ischemia. Stroke. 1996;27:1578–85. doi: 10.1161/01.str.27.9.1578. [DOI] [PubMed] [Google Scholar]
- 44.Colbourne F, Auer RN, Sutherland GR. Characterization of postischemic behavioral deficits in gerbils with and without hypothermic neuroprotection. Brain Res. 1998;803:69–78. doi: 10.1016/s0006-8993(98)00612-x. [DOI] [PubMed] [Google Scholar]
- 45.Schubert A. Side effects of mild hypothermia. J Neurosurg Anesthesiol. 1995;7:139–47. doi: 10.1097/00008506-199504000-00021. [DOI] [PubMed] [Google Scholar]
- 46.Kerenyi A, Kelen D, Faulkner SD, et al. Systemic effects of whole-body cooling to 35 degrees C, 33.5 degrees C, and 30 degrees C in a piglet model of perinatal asphyxia: implications for therapeutic hypothermia. Pediatr Res. 2012;71:573–82. doi: 10.1038/pr.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinrauch V, Safar P, Tisherman S, Kuboyama K, Radovsky A. Beneficial effect of mild hypothermia and detrimental effect of deep hypothermia after cardiac arrest in dogs. Stroke. 1992;23:1454–62. doi: 10.1161/01.str.23.10.1454. [DOI] [PubMed] [Google Scholar]
- 48.Simbruner G, Nanz S, Fleischhacker E, Derganc M. Brain temperature discriminates between neonates with damaged, hypoperfused, and normal brains. Am J Perinatol. 1994;11:137–43. doi: 10.1055/s-2007-994574. [DOI] [PubMed] [Google Scholar]
- 49.Battin MR, Penrice J, Gunn TR, Gunn AJ. Treatment of term infants with head cooling and mild systemic hypothermia (35.0 degrees C and 34.5 degrees C) after perinatal asphyxia. Pediatrics. 2003;111:244–51. doi: 10.1542/peds.111.2.244. [DOI] [PubMed] [Google Scholar]
- 50.Tooley J, Satas S, Eagle R, Silver IA, Thoresen M. Significant selective head cooling can be maintained long-term after global hypoxia ischemia in newborn piglets. Pediatrics. 2002;109:643–9. doi: 10.1542/peds.109.4.643. [DOI] [PubMed] [Google Scholar]
- 51.Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics. 1998;102:885–92. doi: 10.1542/peds.102.4.885. [DOI] [PubMed] [Google Scholar]
- 52.Laptook AR, Shalak L, Corbett RJ. Differences in brain temperature and cerebral blood flow during selective head versus whole-body cooling. Pediatrics. 2001;108:1103–10. doi: 10.1542/peds.108.5.1103. [DOI] [PubMed] [Google Scholar]
- 53.Thoresen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport. 1997;8:3359–62. doi: 10.1097/00001756-199710200-00033. [DOI] [PubMed] [Google Scholar]
- 54.Nakashima K, Todd MM, Warner DS. The relation between cerebral metabolic rate and ischemic depolarization. A comparison of the effects of hypothermia, pentobarbital, and isoflurane. Anesthesiology. 1995;82:1199–208. doi: 10.1097/00000542-199505000-00015. [DOI] [PubMed] [Google Scholar]
- 55.Bart RD, Takaoka S, Pearlstein RD, Dexter F, Warner DS. Interactions between hypothermia and the latency to ischemic depolarization: implications for neuroprotection. Anesthesiology. 1998;88:1266–73. doi: 10.1097/00000542-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 56.Tan WK, Williams CE, During MJ, et al. Accumulation of cytotoxins during the development of seizures and edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr Res. 1996;39:791–7. doi: 10.1203/00006450-199605000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Jensen FE, Wang C, Stafstrom CE, Liu Z, Geary C, Stevens MC. Acute and chronic increases in excitability in rat hippocampal slices after perinatal hypoxia In vivo. J Neurophysiol. 1998;79:73–81. doi: 10.1152/jn.1998.79.1.73. [DOI] [PubMed] [Google Scholar]
- 58.Bennet L, Roelfsema V, George S, Dean JM, Emerald BS, Gunn AJ. The effect of cerebral hypothermia on white and grey matter injury induced by severe hypoxia in preterm fetal sheep. J Physiol. 2007;578:491–506. doi: 10.1113/jphysiol.2006.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennet L, Dean JM, Wassink G, Gunn AJ. Differential effects of hypothermia on early and late epileptiform events after severe hypoxia in preterm fetal sheep. J Neurophysiol. 2007;97:572–8. doi: 10.1152/jn.00957.2006. [DOI] [PubMed] [Google Scholar]
- 60.Dean JM, George SA, Wassink G, Gunn AJ, Bennet L. Suppression of post hypoxic-ischemic EEG transients with dizocilpine is associated with partial striatal protection in the preterm fetal sheep. Neuropharmacology. 2006;50:491–503. doi: 10.1016/j.neuropharm.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 61.George SA, Barrett RD, Bennet L, Mathai S, Jensen EC, Gunn AJ. Nonadditive neuroprotection with early glutamate receptor blockade and delayed hypothermia after asphyxia in preterm fetal sheep. Stroke. 2012;43:3114–7. doi: 10.1161/STROKEAHA.112.671982. [DOI] [PubMed] [Google Scholar]
- 62.Mallard C, Davidson JO, Tan S, et al. Astrocytes and microglia in acute cerebral injury underlying cerebral palsy associated with preterm birth. Pediatr Res. 2014;75:234–40. doi: 10.1038/pr.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roelfsema V, Bennet L, George S, et al. The window of opportunity for cerebral hypothermia and white matter injury after cerebral ischemia in near-term fetal sheep. J Cereb Blood Flow Metab. 2004;24:877–86. doi: 10.1097/01.WCB.0000123904.17746.92. [DOI] [PubMed] [Google Scholar]
- 64.Shankaran S, Laptook A, Wright LL, et al. Whole-body hypothermia for neonatal encephalopathy: animal observations as a basis for a randomized, controlled pilot study in term infants. Pediatrics. 2002;110:377–85. doi: 10.1542/peds.110.2.377. [DOI] [PubMed] [Google Scholar]
- 65.Battin MR, Dezoete JA, Gunn TR, Gluckman PD, Gunn AJ. Neurodevelopmental outcome of infants treated with head cooling and mild hypothermia after perinatal asphyxia. Pediatrics. 2001;107:480–4. doi: 10.1542/peds.107.3.480. [DOI] [PubMed] [Google Scholar]
- 66.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 68.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 69.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 70.Zhou WH, Cheng GQ, Shao XM, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: A multicenter randomized controlled trial in China. J Pediatr. 2010;157:367–72. 72.e1–3. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 71.Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126:e771–8. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 72.Jacobs SE, Morley CJ, Inder TE, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 73.Thoresen M, Hellstrom-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126:e131–9. doi: 10.1542/peds.2009-2938. [DOI] [PubMed] [Google Scholar]
- 74.Gunn AJ, Wyatt JS, Whitelaw A, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr. 2008;152:55–8. doi: 10.1016/j.jpeds.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 75.Azzopardi D, Strohm B, Marlow N, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371:140–9. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 76.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–92. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thoresen M, Whitelaw A. Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic-ischaemic encephalopathy. Pediatrics. 2000;106:92–9. doi: 10.1542/peds.106.1.92. [DOI] [PubMed] [Google Scholar]
- 78.Gordon CJ, Heath JE. Integration and central processing in temperature regulation. Annu Rev Physiol. 1986;48:595–612. doi: 10.1146/annurev.ph.48.030186.003115. [DOI] [PubMed] [Google Scholar]
- 79.Gunn TR, Wilson NJ, Aftimos S, Gunn AJ. Brain hypothermia and QT interval. Pediatrics. 1999;103:1079. doi: 10.1542/peds.103.5.1079. [DOI] [PubMed] [Google Scholar]
- 80.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 81.Todd MM, Hindman BJ, Clarke WR, Torner JC. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 2005;352:135–45. doi: 10.1056/NEJMoa040975. [DOI] [PubMed] [Google Scholar]