Abstract
Background
HIV is associated with elevated markers of vascular remodeling that may contribute to arterial fibrosis and stiffening, and changes in pulse pressure (PP). These changes may, in turn, deleteriously affect autoregulation of cerebral blood flow and neurocognitive function.
Methods
To evaluate these mechanisms, we studied markers of vascular remodeling, PP, and neurocognitive function among older (≥50 years of age) HIV-infected (HIV+; n = 72) and HIV-seronegative (HIV-; n = 36) adults. Participants completed standardized neurobehavioral and neuromedical assessments. Neurocognitive functioning was evaluated using a well-validated comprehensive battery. Three plasma biomarkers of vascular remodeling (i.e., angiopoietin 2, Tie-2, and vascular endothelial growth factor; VEGF) were collected.
Results
HIV+ and HIV- participants had similar levels of plasma Ang-2 (p = .48), Tie-2 (p = .27), VEGF (p = .18), and PP (p = .98). In a multivariable regression model, HIV interacted with Tie-2 (β = .41, p < .01) and VEGF (β = −.43, p = .01) on neurocognitive function, such that lower Tie-2 and higher VEGF values were associated with worse neurocognitive function for HIV+ participants. Greater Tie-2 values were associated with increased PP (r = .31, p < .01). In turn, PP demonstrated a quadratic association with neurocognitive function (β = −.33, p = .01), such that lower and higher, relative to mean sample, PP values were associated with worse neurocognitive function.
Conclusions
These findings indicate that vascular remodeling and altered cerebral blood flow autoregulation contribute to neurocognitive function. Furthermore, HIV moderates the association between vascular remodeling and neurocognitive function but not the association between PP and neurocognitive function.
Keywords: aging, arterial stiffness, cognition, pulse pressure
Introduction
HIV-associated neurocognitive impairment (NCI) remains highly prevalent in the current era of combination antiretroviral therapy (cART),1 although the severity of impairment tends to be milder than in the pre-cART era.1,2 Even mild forms of NCI can be associated with poor everyday functioning.3,4 The burden of HIV-associated NCI is projected to increase as persons living with HIV (HIV+) age.5 In addition, older age-related diseases that affect the general population, such as cardiovascular disease (CVD), are increasingly observed in HIV+ persons6–8 and are anticipated to increase in the future.7 With the increased incidence and prevalence of CVD, there is a growing body of evidence demonstrating the detrimental effect of CVD risk factors on neurocognitive function among HIV+ persons.9–11
HIV disease, which is characterized by chronic inflammation, may confer risk for increased arterial stiffness.12–15 Arterial stiffening is a complex process involving structural and functional changes in the arterial wall that occurs with normal aging16 and is accelerated by chronic inflammatory conditions such as diabetes mellitus and hypertension.17,18 Arterial remodeling is influenced by multiple biological pathways, including vascular endothelial growth factor (VEGF) and angiopoietin pathways.19 VEGF and angiopoietins are considered to work in concert during vascular remodeling, such that VEGF is expressed during the earliest stages of vascular remodeling and the angiopoietin pathway plays a larger role in vessel maturation.20 With increased arterial stiffening, changes in blood pressure (BP) occur such that systolic blood pressure (SBP) increases, diastolic blood pressure (DBP) decreases, and pulse pressure (PP)–defined as the difference between SBP and DBP readings–increases.16 PP is a surrogate marker of arterial stiffness, and elevation of PP is an independent risk factor for future cardiovascular events and cerebrovascular disease.21–25 Markers of arterial stiffness are associated with neurocognitive performance and decline in relatively healthy, middle-aged adults.26,27 On the other end of the spectrum, poor cerebral perfusion related to low PP is associated with increased risk of NCI.28
The neurologic consequences of vascular remodeling and arterial stiffness in the context of chronic HIV disease remain unclear and warrants investigation. The first aim of this study is to examine the relation of vascular remodeling with neurocognitive function. Angiogenic growth factors were examined because of their role in vascular remodeling.19,29 Second, we aim to investigate the association of vascular remodeling with PP. We hypothesize that biomarkers associated with vascular remodeling will be related to greater PP. Lastly, we aim to evaluate the association between neurocognitive function and PP. We hypothesize that PP will have a quadratic relationship with neurocognitive function, such that lower and higher PP values (relative to sample mean PP) will be associated with worse neurocognitive function.
Methods
Participants and Procedure
The present study included 72 HIV+ and 36 HIV- community-dwelling, older (i.e., aged 50 and above) adults who participated in the California HIV/AIDS Research Program Successfully Aging Seniors with HIV study at the UCSD HIV Neurobehavioral Research Program. The study protocol was approved by the UCSD Institutional Review Board, and all participants provided written informed consent to participate. Inclusion criteria were 1) being at least 50 years of age, 2) being on cART (for HIV+ participants), and 3) having an undetectable plasma HIV viral load (<48 copies/ml for HIV+ participants). Exclusion criteria included a history of non-HIV related neurologic disorders or any other known condition that might account for impaired neurocognitive function (e.g., seizure disorder). Each participant underwent standardized neuropsychological, neuromedical, and psychiatric assessments.
Neurocognitive Assessment
Participants completed a comprehensive neurocognitive test battery (administration time: 2 to 2.5 hours) that assesses seven neurocognitive domains consistent with Frascati recommendations for neuroAIDS research.30 The seven neurocognitive domains were speed of information processing (SIP), learning, memory, executive functioning, verbal fluency, working memory, and fine motor functioning (see Heaton et al. 20101 for details on the specific test battery). Raw scores from the neurocognitive tasks were converted to demographically adjusted T-scores (Mean = 50, SD = 10 in healthy subjects) using the best available normative standards, which correct for the effects of age, education, sex and ethnicity, as appropriate.31–33 T-scores were further corrected for exposure to previous neurocognitive assessment, as needed. The demographically adjusted T-scores were averaged to derive a global T-score, the main outcome of interest in statistical analyses.
Neuromedical Assessment
Medical characterization of study participants included measurement of vital signs, anthropometrics, medical comorbidities, and current prescription medications. Blood was collected by venipuncture and aliquots were stored at −80C until assayed. BP was measured in the seated position with an automated sphygmomanometer. PP was calculated as the difference between SBP and DBP. Body mass index (BMI) was calculated from measured height and weight. Medical comorbidities and prescribed and over-the-counter medications were determined by interview.
For the HIV+ group, the following HIV disease characteristics were collected: AIDS diagnosis, estimated duration of HIV infection, current and nadir CD4+ T-cell counts, and duration of cART. HIV RNA level was measured in plasma by RT-PCR (Abbott Diagnostics; lower limit of quantitation 48 copies/mL).
Biomarkers Related to Vascular Remodeling
Vascular remodeling-related biomarkers were measured in plasma by immunoassay: Angiopoietin-2 (Ang-2) (R&D Systems, Minneapolis, MN), VEGF and endothelium-specific receptor tyrosine kinase (Tie-2) (Meso Scale Discovery, Rockville, MD). Assays were performed in duplicate, and assays for the samples with coefficients of variation greater than 20% or outliers that were more than 4 standard deviations from the mean were repeated. Assays for 10% of the samples were also repeated to ensure operator and batch consistency.
Psychiatric Assessments
Study participants underwent a comprehensive psychiatric research evaluation. The Composite International Diagnostic Interview (CIDI),34 a computer-assisted clinical interview was administered. The CIDI assesses the presence of lifetime and current affective disorders [e.g., major depressive disorder (MDD)] and substance use disorders using diagnostic criteria based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.35 The Medical Outcome Study 36 Item Short-Form version 1.0 (MOS: SF-36)36 was used to assess physical functioning and emotional well-being.
Statistical Analyses
Comparison of demographic, medical, biomarker, psychiatric, and neuropsychological data across HIV+ and HIV− groups was performed with two-tailed t-test or Pearson’s chi-squared test, as appropriate. Biomarkers were log10 transformed to reduce skewness as is standard practice. Pearson’s correlations for continuous variables and t-tests for categorical variables were conducted between the dependent variables and explanatory variables (listed in Tables 1 and 2) to identify covariates to include in subsequent multivariable linear regression models (critical α = .10).
Table 1.
Demographic and clinical characteristics of sample (N = 108)
| All (N = 108) | HIV+ (n = 72) | HIV− (n = 36) | p-value | |
|---|---|---|---|---|
| Descriptive demographics | ||||
| Age, mean (SD) | 58.5 (6.3) | 58.2 (6.5) | 59.0 (5.9) | .53 |
| Education, mean (SD) | 14.5 (2.6) | 14.6 (2.6) | 14.3 (2.7) | .57 |
| Male, n (%) | 83 (76.9%) | 61 (84.7%) | 22 (61.1%) | .006 |
| Non-Hispanic White, n (%) | 84 (77.8%) | 61 (84.7%) | 23 (63.9%) | .01 |
| Vital Signs and Anthropometrics | ||||
| Pulse pressure, mean (SD) | 58.8 (17.8) | 58.8 (17.6) | 58.7 (18.5) | .98 |
| SBP, mean (SD) | 134.4 (20.9) | 133.4 (20.7) | 136.4 (21.7) | .49 |
| DBP, mean (SD) | 75.6 (11.1) | 74.6 (10.0) | 77.7 (12.8) | .17 |
| MAP, mean (SD) | 95.0 (12.5) | 94.0 (11.8) | 97.0 (13.7) | .23 |
| Pulse, mean (SD) | 65.2 (10.9) | 65.5 (11.5) | 64.7 (9.8) | .70 |
| BMI, mean (SD)a | 27.4 (5.7) | 27.5 (5.8) | 27.3 (5.4) | .87 |
| Medical comorbidites | ||||
| Hyperlipidemia, n (%) | 54 (50.0%) | 43 (59.7%) | 11 (30.6%) | .004 |
| Hypertension, n (%) | 45 (41.7%) | 32 (44.4%) | 13 (36.1%) | .41 |
| Ever smoker, n (%) | 45 (41.7%) | 30 (41.7%) | 15 (41.7%) | 1.00 |
| Current smoker, n (%) | 37 (34.3%) | 27 (37.5%) | 10 (27.8%) | .32 |
| Diabetes mellitus, n (%) | 27 (25.0%) | 20 (27.8%) | 7 (19.4%) | .35 |
| Hepatitis C Virus, n (%) | 22 (20.4%) | 16 (22.2%) | 6 (16.7%) | .50 |
| Current Medications | ||||
| NSAID, n (%) | 33 (30.6%) | 25 (34.7%) | 8 (22.2%) | .18 |
| Antihypertensive drug, n (%) | 34 (31.5%) | 26 (36.1%) | 8 (22.2%) | .14 |
| Lipid-lowering drug, n (%) | 40 (37.0%) | 32 (44.4%) | 8 (22.2%) | .02 |
| Antidepressant drug, n (%) | 35 (32.4%) | 32 (44.4%) | 3 (8.3%) | <.001 |
| Psychiatric characteristics/diagnoses | ||||
| Current MDD, n (%)a | 9 (8.4%) | 8 (11.3%) | 1 (2.8%) | .13 |
| LT MDD, n (%) | 50 (46.3%) | 40 (55.6%) | 10 (27.8%) | .006 |
| LT alcohol use disorder, n (%) | 52 (48.6%) | 36 (50.0%) | 16 (45.7%) | .68 |
| LT cannabis use disorder, n (%) | 30 (28.0%) | 23 (31.9%) | 7 (20.0%) | .20 |
| LT meth use disorder n (%) | 29 (27.1%) | 21 (29.2%) | 8 (22.9%) | .49 |
| LT cocaine use disorder, n (%) | 23 (21.5%) | 17 (23.6%) | 6 (17.1%) | .44 |
| MOS physical health, mean (SD) | 70.3 (22.7) | 65.2 (23.2) | 80.3 (18.1) | <.001 |
| MOS mental health, mean (SD) | 70.7 (23.1) | 65.2 (22.9) | 81.7 (19.5) | <.001 |
| Biomarkers of vascular injury and remodeling | ||||
| Ang-2 (pg/mL), median [IQR] | 4073 [2865, 5359] | 4030 [3002, 5443] | 4144 [2110, 5427] | .48 |
| Tie-2 (pg/mL), median [IQR] | 7307 [6088, 8484] | 7568 [6184, 8386] | 7041 [5540, 8529] | .27 |
| VEGF (pg/mL), median [IQR] | 115 [80, 178] | 126 [87, 206] | 107 [73, 159] | .18 |
Note: Ang-2 = angiopoietin-2; BMI = body mass index; DBP = diastolic blood pressure; LT = lifetime; MAP = mean arterial pressure; MDD = major depressive disorder; meth = methamphetamine; MOS = Medical Outcome Study; NSAID = nonsteroidal anti-inflammatory drug; SBP = systolic blood pressure; Tie-2 = endothelium-specific receptor tyrosine kinase; VEGF = vascular endothelial growth factor
n = 107
Table 2.
HIV+ group characteristics
| HIV disease-related characteristic | HIV+ (n = 72) |
|---|---|
| AIDS, n (%)a | 43 (60.6%) |
| Duration of HIV infection (years), mean (SD)a | 17.3 (8.1) |
| Current CD4+ T cell count, median [IQR]b | 654 [487 – 843] |
| Nadir CD4+ T cell count, median [IQR] | 180 [49 – 308] |
| Duration of exposure to ARVs (years), mean (SD)a | 11.9 (7.1) |
| Undetectable plasma HIV RNA, n (%) | 70 (100.0%) |
| Prescribed protease inhibitor, n (%) | 36 (50.0%) |
Note: AIDS = acquired immunodeficiency syndrome; ARV = antiretroviral; CD4 = cluster of differentiation 4
n = 71;
n = 70
Three multivariable linear regression tests were conducted to examine the association between: 1) biomarkers of vascular remodeling (independent variables; IV) and neurocognitive function (dependent variable; DV), 2) biomarkers of vascular remodeling (IV) and PP (DV), and 3) PP (IV) and neurocognitive function (DV). Given our objective to examine whether or not HIV moderates these relations, all multivariable linear regression analyses tested for HIV by IV interactions. Prior to final model selection, the variable inflation factor was used to check for multicollinearity among predictor variables, and model selection was re-run to include only one of the correlated variables at a time. To achieve parsimonious models, variables were only retained in the final models if they had p values < .10. All statistical tests were performed with JMP 11.0.0 (SAS, 2013).
Results
Participants
The sample consisted of 108 participants who were predominantly middle-aged [mean 58.5 (SD 6.3) years], non-Hispanic white (77.8%) men (76.9%) with some college education [mean 14.5 (SD 2.6) years]. Demographic, medical, and psychiatric characteristics of the sample are presented in Table 1. In general, HIV serostatus groups were largely comparable (i.e., p values for group differences > .05) across many characteristics, including age, education, vital signs, anthropometrics, medical comorbidities, medical prescriptions, and proportion of current MDD and lifetime substance use disorders. About 44 vs. 36% of HIV+ and HIV- groups were hypertensive, respectively, and of whom 81% vs. 62% were taking anti-hypertensive medication(s). The HIV+ group had more non-Hispanic white participants (84.7% vs. 63.9%, p = .01), men (84.7% vs. 61.1%, p = .006), hyperlipidemia (59.7% vs. 30.6%, p = .004), and lifetime MDD (55.6% vs. 27.8%, p = .006).
Among the HIV+ participants (n = 72), the mean estimated duration of HIV infection was 17.3 years, the mean duration of exposure to cART was 11.9 years, the median current CD4+ T cell count was 654 cells/mm3, and the median nadir CD4+ T cell count was 180 cells/mm3. HIV disease-related characteristics of the HIV+ sample are presented in Table 2.
HIV+ participants had worse neurocognitive function [global T-score = 45.8 (SD 7.0)] than HIV- participants [global T-score = 49.9 (SD 6.4)] (p = .004). HIV+ and HIV- participants had similar levels of plasma VEGF, Ang-2, and Tie-2 (p values for group differences > .05; Table 1). PP values did not differ between the HIV+ [mean (SD) = 58.8 (17.6)] and HIV- [mean (SD) = 58.7 (18.5)] participants (p = .98).
Vascular Remodeling and Neurocognitive Function
At the univariable level, global T-scores did not have statistically significant correlations with the biomarkers of vascular remodeling: Ang-2 (r = −.03, p = .77), Tie-2 (r < .01, p = .99), and VEGF (r = −.10, p = .32). Multivariable linear regression analyses were conducted to test for potential interacting effects of HIV and biomarkers of vascular remodeling on neurocognitive function. Of the covariates listed in Tables 1 and 2, pulse, diabetes, antidepressant prescription, current MDD, lifetime MDD, and the MOS physical health composite were associated with global T-scores (p’s < .10). The best fitting model of neurocognitive function (Model adjusted R2 = .13, p < .01; Table 4, Model 1) identified a statistically significant interaction between HIV and Tie-2 (β = .32, p = .03), such that lower Tie-2 values were associated with lower global T-scores (i.e., worse neurocognitive function) for HIV+ participants. For HIV- participants, higher Tie-2 values were associated with lower global T-scores. The interaction between HIV and VEGF was retained in the final model (β = −.33, p = .05). Neither the interaction term between HIV and Ang-2 nor the main effect of Ang-2 met statistical significance (p’s > .10) and were not retained in the final model.
Table 4.
Multivariable linear regression models (N = 108)
| Adj R2 | F | β | p-value | |
|---|---|---|---|---|
| Model 1. Association between vascular injury (independent variable) and neurocognitive functioning (dependent variable) | ||||
| Overall model: | .13 | 3.53 | <.01 | |
| HIV serostatus [ref: HIV-] | −.28 | <.01 | ||
| HIV * Tie-2 interaction | .32 | .03 | ||
| HIV * VEGF interaction | −.33 | .05 | ||
| Tie-2 | −.22 | .15 | ||
| VEGF | .24 | .16 | ||
| Diabetes mellitus | −.21 | .03 | ||
| Model 2. Association between vascular injury (independent variable) and pulse pressure (dependent variable) | ||||
| Overall model: | .24 | 11.44 | <.01 | |
| BMI | .32 | <.01 | ||
| Tie-2 | .31 | <.01 | ||
| Age | .22 | .01 | ||
| Model 3. Association between pulse pressure (independent variable) and neurocognitive functioning (dependent variable) | ||||
| Overall model: | .22 | 4.57 | <.01 | |
| HIV serostatus [ref: HIV-] | −.29 | <.01 | ||
| Pulse pressure | .41 | <.01 | ||
| Pulse pressure2 | −.33 | .01 | ||
| HIV * Tie-2 interaction | .41 | <.01 | ||
| HIV * VEGF interaction | −.43 | .01 | ||
| Tie-2 | −.38 | .02 | ||
| VEGF | .38 | .03 | ||
| Diabetes mellitus | −.19 | .04 | ||
Note: BMI = body mass index; Tie-2 = endothelium-specific receptor tyrosine kinase (log-transformed); Pulse pressure2 = quadratic term; VEGF = vascular endothelial growth factor (log-transformed)
Vascular Remodeling and Pulse Pressure
To examine empirically whether vascular remodeling was related to elevated PP, correlational analyses were performed in the entire sample (Table 3). Higher PP correlated with higher values of Tie-2 (r = .31, p < .01) but not VEGF (r = .10, p = .32) or Ang-2 (r = .03, p = .76). Multivariable linear regression analysis was performed to test for interacting effects of HIV and biomarkers of vascular remodeling on PP. Of the covariates listed in Tables 1 and 2, age, BMI, dyslipidemia, hypertension, current smoker status, and NSAID, antihypertensive, and lipid-lowering drug use were associated with PP (p’s < .10). In the best fitting model (Model adjusted R2 = .24, p < .01; Table 4, Model 2), Tie-2 (β = .31, p < .01), age (β = .22, p = .01), and BMI (β = .32, p < .01) were statistically significant predictors of PP. The interactions between HIV and biomarkers of vascular remodeling were not statistically significant, nor were the main effects of Ang-2 and VEGF (p’s > .10).
Table 3.
Correlations between vascular biomarkers and vital signs (N = 108)
| Ang-2 | Tie-2 | VEGF | |
|---|---|---|---|
| Tie-2 | 0.11 | ||
| VEGF | 0.28* | 0.29* | |
| SBP | −0.06 | 0.36** | 0.09 |
| DBP | −0.16 | 0.19 | 0.00 |
| MAP | −0.13 | 0.31* | 0.05 |
| Pulse pressure | 0.03 | 0.31* | 0.10 |
Note: Ang-2 = angiopoietin-2; DBP = diastolic blood pressure; MAP = mean arterial pressure; SBP = systolic blood pressure; Tie-2 = endothelium-specific receptor tyrosine kinase; VEGF = vascular endothelial growth factor
p<.01,
p<.001,
p<.0001
Pulse Pressure and Neurocognitive Function
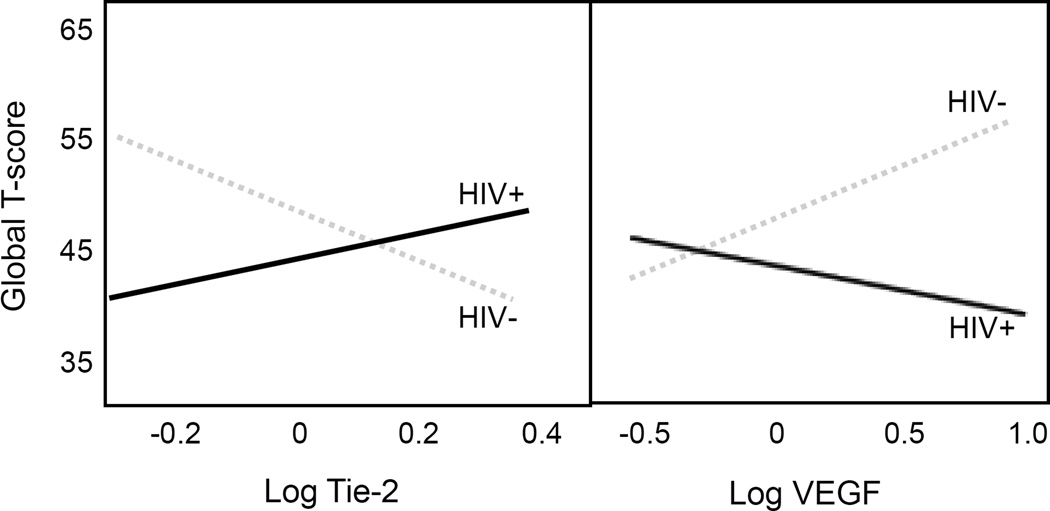
Multivariable linear regression with polynomial terms was used to determine the association between PP and neurocognitive function. The best model identified that both the linear (β = .41, p < .01) and quadratic (β = −.33, p = .01) terms for PP were statistically significant in the model for neurocognitive function (Model adjusted R2 = .22, p < .001; Table 4, Model 3). HIV interacted with Tie-2 (β = .41, p < .01) and with VEGF (β = −.43, p = .01) in relation to neurocognitive function. As illustrated in Figure 1, lower Tie-2 and higher VEGF values were associated with worse neurocognitive function for HIV+ participants. For HIV- participants, higher Tie-2 and lower VEGF values were associated with worse neurocognitive function. Diabetes mellitus was associated with worse neurocognitive function in the multivariable model (β = −.19, p = .04). The interaction between HIV and PP was not statistically significant (p < .10) and thus not retained in the final model.
Figure 1.
HIV interacts with biomarkers of vascular remodeling on neurocognitive function
To explore whether other vital signs (SBP, DBP, and MAP) demonstrate a similar association with neurocognitive function, additional multivariable linear regression models were conducted. Only SBP had a significant quadratic association (β = −.24, p = .03) with neurocognitive function (Model adjusted R2 = .17, p = .001). The other vital signs (DBP and MAP) did not have significant associations (linear or a quadratic) with neurocognitive function (p’s > .05).
It should be noted that although age was univariably associated with PP, it was not included in model 3 described above because we used demographically adjusted T-scores in our analyses. In post-hoc analyses using non-age adjusted global T-scores, age and both the linear and quadratic terms of PP were independently associated with neurocognitive function (data not presented).
In post-hoc analyses, we examined the associations between vascular remodeling, PP, and neurocognitive function at the domain level by conducting multivariable linear regression analyses. Statistically significant models were obtained for fine motor functioning (Model adjusted R2 = .28, p < .001), SIP (Model adjusted R2 = .23, p = .001), executive functioning (Model adjusted R2 = .19, p = .01), and learning (Model adjusted R2 = .19, p = .01). The interaction of HIV and Tie-2 on neurocognitive function was statistically significant (p’s < .05) in the models for fine motor functioning, SIP, and learning. The interaction of HIV and VEGF on neurocognitive function was statistically significant (p’s < .05) in the models for fine motor functioning, SIP, and learning. Lastly, a statistically significant quadratic association between PP and neurocognitive function (p’s < .05) was observed in the models for fine motor functioning, SIP, and executive functioning.
Discussion
In our cohort of older HIV+ and HIV- adults, markers of vascular remodeling and arterial stiffness were associated with neurocognitive function. HIV interacted with biomarkers of vascular remodeling (i.e., Tie-2 and VEGF) on neurocognitive function. For HIV+ adults, lower Tie-2 values and higher VEGF values were associated with worse neurocognitive function. Vascular remodeling, as measured by higher Tie-2 values, was associated with greater PP values. In turn, PP had a quadratic relationship with neurocognitive function. Relative to the sample mean, lower and higher values of PP were associated with worse neurocognitive function. Together, these findings indicate that both vascular remodeling and altered cerebral blood flow autoregulation contribute to neurocognitive function. These effects appear to be driven by the neurocognitive domains of fine motor functioning, SIP, executive functioning, and learning.
Our investigation explored the association of angiogenic growth factors with both PP and neurocognitive function given their crucial role in vascular remodeling.19,29 A complex interplay of the angiopoietins, Tie-2, VEGF, and other pro- or antiangiogenic factors contribute to angiogenesis and vascular remodeling.37 Angiopoietins bind to the endothelial tyrosine kinase receptor Tie-2 to exert context-dependent biological functions,37 and circulating levels of Ang-2 and Tie-2 have been associated with CVD risk factors (e.g., hypertension, diabetes mellitus, and abdominal obesity).38 Ang-2 has previously been found to have a positive association with PP.37 Our study did not find an association between PP and Ang-2; however, we found a positive association between PP and Tie-2. Given the cross-sectional design of this study, we are unable to infer whether this association reflects a dysregulation of vascular endothelial growth factors that contributes to microvascular rarefaction (i.e., a deficiency in mature small vessels)37 or PP-induced vascular remodeling.37 Future research, particularly studies employing longitudinal designs, may tease apart the temporal association between vascular remodeling, PP, and cerebrovascular disease in the context of HIV disease.
The interactions between vascular remodeling (i.e., Tie-2 and VEGF) and HIV on neurocognitive function suggest that HIV is interacting with the aging brain to affect neurological function. VEGF is generally considered to have neuroprotective effects,39 whereas upregulated levels of Tie-2 may reflect pathological angiogenesis.40 For HIV- adults, we found that higher Tie-2 and lower VEGF values were associated with worse neurocognitive function. Counterintuitively, lower Tie-2 values and higher VEGF values were associated with worse neurocognitive function for HIV+ adults. One potential interpretation of these counterintuitive associations is that the neuroprotective effect of VEGF is attenuated within our HIV+ sample, given that they demonstrate good immunologic and virologic status. Emerging evidence indicates that the neuroprotective effect of VEGF may be most robust among adults with greater risk factors for Alzheimer’s disease (AD).39 It is possible that our HIV serostatus groups differ in regard to AD risk factors that, in turn, may be influencing the effect of VEGF on neurocognitive function. Alternatively, the association between lower Tie-2 values and worse neurocognitive function observed among the HIV+ sample may reflect pathological angiogenesis. Pathological angiogenesis shares many cellular and molecular processes with physiological angiogenesis (e.g., sprouting of new blood vessels and recruitment of inflammatory cells to sites of inflammation); however pathological angiogenesis is characterized by a highly disorganized vascular network.40 Given that HIV is characterized by chronic inflammation, HIV+ adults may be particularly vulnerable to pathological angiogenic processes.
Despite not finding an interacting effect of HIV and PP on neurocognitive function, our results show an association between PP and neurocognitive function that holds in the overall sample. Arterial stiffness may lead to neurocognitive decline due to augmented pressure pulses that penetrate and cause damage to the smaller blood vessels of the brain.41 Previous research indicates that cerebrovascular disease may be a key underpinning in HIV-associated NCI.42 We found a quadratic association between PP and neurocognitive function. This is consistent with literature demonstrating a U-shaped relationship between PP and risk of AD and dementia, whereby both lower and higher end of the PP spectrum confers risk.28 Alternatively, the association between PP and neurocognitive function may reflect normal brain arterial aging. A recent histopathologic study showed that with age, the arteries of the brain undergo degenerative changes characterized by arterial thickening, even in the absence of atherosclerosis.43 These degenerative changes are hypothesized to be the downstream effect of mechanical forces of blood flow.43 Thus, it is possible that PP is indexing arterial stiffening that may be occurring in the periphery and brain.
Our sample of older HIV+ adults did not demonstrate different levels of arterial stiffness relative to older HIV- adults. This finding is in agreement with studies finding no differences in arterial stiffness by HIV serostatus (e.g.,12), although there have been reports of increased arterial stiffness in the context of HIV.44 Divergent results among studies may be related to differences in cohort characteristics. For example, among HIV+ persons, commonly reported determinants of arterial stiffness are low nadir CD4 T-cell counts (e.g., <350 cells per microliter), age, hypertension, and high cholesterol levels.44–52 Given our HIV+ sample demonstrated good immunologic and virologic status, potential effects of HIV-related characteristics on PP may be greatly diminished. Discerning differences in PP among HIV+ adults on suppressive cART as compared with HIV- adults requires careful selection of the appropriate HIV- comparison group.53 Our HIV+ and HIV- samples were largely comparable across many characteristics (e.g., age and prevalence of medical comorbidities and lifetime substance use disorders). Thus, our failure to detect differences in PP may indicate that HIV+ persons do not have greater arterial stiffening when compared to an HIV- sample with a comparable prevalence of comorbidities.
Consistent with previous studies involving older HIV+ adults,10,54 we found that diabetes mellitus emerged as an independent predictor of neurocognitive function. Diabetes mellitus has shown an association with NCI in HIV+ adults older than 55 years.10 Likely mechanisms for the effect of diabetes mellitus on neurocognitive function may include direct damage to the brain from hyperglycemia, brain exposure to higher levels of glucose given disruption of the blood-brain barrier by HIV,55 and/or increased risk for cerebral atherosclerosis. Imaging studies demonstrate an association between diabetes mellitus and morphological changes in the brain that are predominantly subcortical, which is similar to the subcortical effects of HIV.56–58
Limitations of the study include its cross-sectional design and the high likelihood of selection bias, given that the parent study aimed to investigate “successful aging” with HIV and thus may have biased our sample toward a group of HIV+ patients demonstrating good immunologic and virologic profiles. Given our small sample size, we did not test whether the effect of vascular remodeling on neurocognitive function is mediated by PP given the likelihood of Type II error. Our study collected resting BP rather than ambulatory BP, which may potentially demonstrate a different association with neurocognitive function or revealed differing BP profile between HIV+ and HIV- individuals. Our study utilized a proxy measure of arterial stiffness, whereas pulse wave analysis is a more direct and non-invasive method of assessing large artery or aortic stiffness.59 Although vascular remodeling and PP were found to have associations with neurocognitive function, the clinical utility of these markers depends on the availability of efficacious treatments to reduce pathological angiogenesis and arterial stiffness and whether treatment-induced reductions of these processes translate to improved neurocognitive outcomes.59
The etiology of HIV-associated NCI in the cART era is multifactorial and may be related to both direct and indirect consequences of HIV, the immune response, and comorbid factors,60 such as subclinical CVD. Delineating the relative contribution of CVD risk factors, such as vascular remodeling and PP, in the pathogenesis of HIV-associated NCI may allow for the identification of adjunct therapies aimed at improving health outcomes for persons aging with HIV/AIDS.
Acknowledgments
Source of Funding: Ms. Montoya was supported by NIMH Diversity Supplement R01MH099987. The study was more broadly supported by the California HIV/AIDS Research Program IDEA Award ID10-SD-057 (PI: Moore, David J.); the HIV Neurobehavioral Research Center (HNRC) Award P30MH062512; and NIH K24 M097873 (PI: Letendre, Scott).
Appendix
* The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Footnotes
Conflicts of Interest: For the remaining authors, none were declared.
References
- 1.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 4.Andrade ASA, Deutsch R, Celano SA, et al. Relationships Among Neurocognitive Status, Medication Adherence Measured by Pharmacy Refill Records, and Virologic Suppression in HIV-Infected Persons. J Acquir Immune Defic Syndr. 2013;62:282–292. doi: 10.1097/QAI.0b013e31827ed678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cysique LA, Bain MP, Brew BJ, Murray JM. The burden of HIV-associated neurocognitive impairment in Australia and its estimates for the future. Sex Health. 2011;8:541–550. doi: 10.1071/SH11003. [DOI] [PubMed] [Google Scholar]
- 6.Dube MP, Lipshultz SE, Fichtenbaum CJ, et al. Effects of HIV infection and antiretroviral therapy on the heart and vasculature. Circulation. 2008;118:e36–e40. doi: 10.1161/CIRCULATIONAHA.107.189625. [DOI] [PubMed] [Google Scholar]
- 7.Giannarelli C, Klein RS, Badimon JJ. Cardiovascular implications of HIV-induced dyslipidemia. Atherosclerosis. 2011;219:384–389. doi: 10.1016/j.atherosclerosis.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Penney AT, Iudicello JE, Riggs PK, et al. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS. 2013;27:5–16. doi: 10.1089/apc.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker JT, Kingsley L, Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73:1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78:485–492. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sattler FR, He J, Letendre S, et al. Abdominal obesity contributes to neurocognitive impairment in HIV-infected patients with increased inflammation and immune activation. J Acquir Immune Defic Syndr. 2015;68:281–288. doi: 10.1097/QAI.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papita A, Albu A, Fodor D, Itu C, Carstina D. Arterial stiffness and carotid intima-media thickness in HIV infected patients. Med Ultrason. 2011;13:127–134. [PubMed] [Google Scholar]
- 13.Echeverria P, Bonjoch A, Molto J, et al. Pulse wave velocity as index of arterial stiffness in HIV-infected patients compared with a healthy population. J Acquir Immune Defic Syndr. 2014;65:50–56. doi: 10.1097/QAI.0b013e3182a97c17. [DOI] [PubMed] [Google Scholar]
- 14.van Wijk JP, de Koning EJ, Cabezas MC, et al. Functional and structural markers of atherosclerosis in human immunodeficiency virus-infected patients. J Am Coll Cardiol. 2006;47:1117–1123. doi: 10.1016/j.jacc.2005.09.073. [DOI] [PubMed] [Google Scholar]
- 15.Chan W, Dart AM. Vascular stiffness and aging in HIV. Sex Health. 2011;8:474–484. doi: 10.1071/SH10160. [DOI] [PubMed] [Google Scholar]
- 16.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 17.Kumeda Y, Inaba M, Goto H, et al. Increased thickness of the arterial intima-media detected by ultrasonography in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:1489–1497. doi: 10.1002/art.10269. [DOI] [PubMed] [Google Scholar]
- 18.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 19.Zachariah JP, Xanthakis V, Larson MG, et al. Circulating vascular growth factors and central hemodynamic load in the community. Hypertension. 2012;59:773–779. doi: 10.1161/HYPERTENSIONAHA.111.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang ZG, Zhang L, Tsang W, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Blacher J, Staessen JA, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160:1085–1089. doi: 10.1001/archinte.160.8.1085. [DOI] [PubMed] [Google Scholar]
- 22.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 23.Glynn RJ, Chae CU, Guralnik JM, Taylor JO, Hennekens CH. Pulse pressure and mortality in older people. Arch Intern Med. 2000;160:2765–2772. doi: 10.1001/archinte.160.18.2765. [DOI] [PubMed] [Google Scholar]
- 24.Sesso HD, Stampfer MJ, Rosner B, et al. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in Men. Hypertension. 2000;36:801–807. doi: 10.1161/01.hyp.36.5.801. [DOI] [PubMed] [Google Scholar]
- 25.Nation DA, Delano-Wood L, Bangen KJ, et al. Antemortem pulse pressure elevation predicts cerebrovascular disease in autopsy-confirmed Alzheimer's disease. J Alzheimers Dis. 2012;30:595–603. doi: 10.3233/JAD-2012-111697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pase MP, Pipingas A, Kras M, et al. Healthy middle-aged individuals are vulnerable to cognitive deficits as a result of increased arterial stiffness. J Hypertens. 2010;28:1724–1729. doi: 10.1097/HJH.0b013e32833b1ee7. [DOI] [PubMed] [Google Scholar]
- 27.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 28.Qiu C, Winblad B, Viitanen M, Fratiglioni L. Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older: a community-based, longitudinal study. Stroke. 2003;34:594–599. doi: 10.1161/01.STR.0000060127.96986.F4. [DOI] [PubMed] [Google Scholar]
- 29.Marketou ME, Kontaraki JE, Tsakountakis NA, et al. Arterial stiffness in hypertensives in relation to expression of angiopoietin-1 and 2 genes in peripheral monocytes. J Hum Hypertens. 2010;24:306–311. doi: 10.1038/jhh.2009.95. [DOI] [PubMed] [Google Scholar]
- 30.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherner M, Suarez P, Lazzaretto D, et al. Demographically corrected norms for the Brief Visuospatial Memory Test-revised and Hopkins Verbal Learning Test-revised in monolingual Spanish speakers from the U.S.-Mexico border region. Arch Clin Neuropsychol. 2007;22:343–353. doi: 10.1016/j.acn.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 33.Demograhic effects and use of demographically corrected norms with the WAIS-III and WMS-III. In: Heaton RK, Taylor MJ, Manly JJ, editors; Tulsky DS, Heaton RK, Chelune G, et al., editors. Clinical Interpretation of the WAIS-III and WMS-III. San Diego, CA: Academic Press; 2002. [Google Scholar]
- 34.World Health Organization. Composite International Diagnostic Interview (CIDI, version 2.1) Geneva: World Health Organization; 1998. [Google Scholar]
- 35.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 36.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 37.Lieb W, Zachariah JP, Xanthakis V, et al. Clinical and genetic correlates of circulating angiopoietin-2 and soluble Tie-2 in the community. Circ Cardiovasc Genet. 2010;3:300–306. doi: 10.1161/CIRCGENETICS.109.914556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel JV, Abraheem A, Chackathayil J, et al. Circulating biomarkers of angiogenesis as indicators of left ventricular systolic dysfunction amongst patients with coronary artery disease. J Intern Med. 2009;265:562–567. doi: 10.1111/j.1365-2796.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 39.Hohman TJ, Bell SP, Jefferson AL Alzheimer's Disease Neuroimaging I. The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: exploring interactions with biomarkers of Alzheimer disease. JAMA Neurol. 2015;72:520–529. doi: 10.1001/jamaneurol.2014.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18–26. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 41.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 42.Soontornniyomkij V, Umlauf A, Chung SA, et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS. 2014;28:1297–1306. doi: 10.1097/QAD.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutierrez J, Honig L, Elkind MS, et al. Brain arterial aging and its relationship to Alzheimer dementia. Neurology. 2016;86:1507–1515. doi: 10.1212/WNL.0000000000002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seaberg EC, Benning L, Sharrett AR, et al. Association between human immunodeficiency virus infection and stiffness of the common carotid artery. Stroke. 2010;41:2163–2170. doi: 10.1161/STROKEAHA.110.583856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng Y, Ye YC, Luo L, et al. Premature atherosclerosis in patients with acquired immunodeficiency syndrome. Chin Med J (Engl) 2010;123:3396–3399. [PubMed] [Google Scholar]
- 46.Ferraioli G, Tinelli C, Maggi P, et al. Arterial stiffness evaluation in HIV-positive patients: a multicenter matched control study. AJR Am J Roentgenol. 2011;197:1258–1262. doi: 10.2214/AJR.11.6712. [DOI] [PubMed] [Google Scholar]
- 47.Strategies for Management of Antiretroviral Therapy Study G. El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 48.Lekakis J, Ikonomidis I, Palios J, et al. Association of highly active antiretroviral therapy with increased arterial stiffness in patients infected with human immunodeficiency virus. Am J Hypertens. 2009;22:828–834. doi: 10.1038/ajh.2009.90. [DOI] [PubMed] [Google Scholar]
- 49.van Vonderen MG, Smulders YM, Stehouwer CD, et al. Carotid intima-media thickness and arterial stiffness in HIV-infected patients: the role of HIV, antiretroviral therapy, and lipodystrophy. J Acquir Immune Defic Syndr. 2009;50:153–161. doi: 10.1097/QAI.0b013e31819367cd. [DOI] [PubMed] [Google Scholar]
- 50.Monteiro P, Miranda-Filho DB, Bandeira F, et al. Is arterial stiffness in HIV-infected individuals associated with HIV-related factors? Braz J Med Biol Res. 2012;45:818–826. doi: 10.1590/S0100-879X2012007500116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho JE, Deeks SG, Hecht FM, et al. Initiation of antiretroviral therapy at higher nadir CD4+ T-cell counts is associated with reduced arterial stiffness in HIV-infected individuals. AIDS. 2010;24:1897–1905. doi: 10.1097/QAD.0b013e32833bee44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong C, Althoff K, Gange SJ. Identifying the appropriate comparison group for HIV-infected individuals. Curr Opin HIV AIDS. 2014;9:379–385. doi: 10.1097/COH.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valcour VG, Shikuma CM, Shiramizu BT, et al. Diabetes, insulin resistance, and dementia among HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;38:31–36. doi: 10.1097/00126334-200501010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leibson CL, Rocca WA, Hanson VA, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 56.Akisaki T, Sakurai T, Takata T, et al. Cognitive dysfunction associates with white matter hyperintensities and subcortical atrophy on magnetic resonance imaging of the elderly diabetes mellitus Japanese elderly diabetes intervention trial (J-EDIT) Diabetes Metab Res Rev. 2006;22:376–384. doi: 10.1002/dmrr.632. [DOI] [PubMed] [Google Scholar]
- 57.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt R, Launer LJ, Nilsson LG, et al. Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes. 2004;53:687–692. doi: 10.2337/diabetes.53.3.687. [DOI] [PubMed] [Google Scholar]
- 59.Pase MP, Herbert A, Grima NA, Pipingas A, O'Rourke MF. Arterial stiffness as a cause of cognitive decline and dementia: a systematic review and meta-analysis. Intern Med J. 2012;42:808–815. doi: 10.1111/j.1445-5994.2011.02645.x. [DOI] [PubMed] [Google Scholar]
- 60.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep. 2011;8:54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]