Abstract
Background
Value-based benefit design has been suggested as an effective approach to managing the high cost of pharmaceuticals in health insurance markets. Premera Blue Cross, a large regional health plan, implemented a Value-Based Formulary (VBF) for pharmaceuticals in 2010 that explicitly used cost-effectiveness analysis (CEA) to inform medication copayments.
Objective
To determine the impact of the VBF.
Design
Interrupted time-series of employer-sponsored plans from 2006 to 2013.
Subjects
Intervention group: 5,235 beneficiaries exposed to the VBF. Control group: 11,171 beneficiaries in plans without any changes in pharmacy benefits.
Intervention
The VBF assigned medications with lower value (estimated by CEA) to higher copayment tiers and assigned medications with higher value to lower copayment tiers.
Measures
Primary outcome was medication expenditures from member, health plan, and member plus health plan perspectives. Secondary outcomes were medication utilization, emergency department visits, hospitalizations, office visits, and non-medication expenditures.
Results
In the intervention group after VBF implementation, member medication expenditures increased by $2 per member per month (PMPM) (95% CI, $1 to $3) or 9%, while health plan medication expenditures decreased by $10 PMPM (CI, $18 to $2) or 16%, resulting in a net decrease of $8 PMPM (CI, $15 to $2) or 10%, which translates to a net savings of $1.1 million. Utilization of medications moved into lower copayment tiers increased by 1.95 days’ supply (CI, 1.29 to 2.62) or 17%. Total medication utilization, health services utilization and non-medication expenditures did not change.
Conclusions
Cost-sharing informed by CEA reduced overall medication expenditures without negatively impacting medication utilization, health services utilization or non-medication expenditures.
Keywords: health insurance, pharmaceutical policy, Pharmacoeconomics, pharmacy benefits, program evaluation
INTRODUCTION
Employer-sponsored health plans cover about 149 million Americans and the majority of these plans utilize copayments for prescription drugs.1,2 In the past decade, these plans have increased copayments in order to slow the growth of prescription expenditures.2 More recently, pharmaceutical expenditures have been rapidly growing, partly due to the introduction of new high priced drugs.3 Therefore, health plans may continue to increase cost-sharing to slow expenditure growth for the foreseeable future. However, increasing cost-sharing without considering clinical and economic value may incentivize utilization according to cost and not value.
Some employer groups have attempted to align utilization with value by implementing value-based insurance design (VBID) plans.4-6 These plans have waived or reduced copayments for maintenance medications used to treat chronic conditions.7-19 Although these plans have achieved modest (1.5%-9.4%) increases in medication adherence, the impact on medication and non-medication expenditures has been mixed.9,11,19-23 Studies have found that, waiving or reducing medication copayments is associated with lower member (i.e. out of pocket) medication expenditures and lower member non-medication expenditures and therefore lower total member healthcare expenditures. However, waiving or reducing copayments increases health plan medication expenditures and in some studies is associated with no change in health plan non-medication expenditures.19-23 Therefore total health plan expenditures either increases or does not change. Combining expenditures from both member and health plan perspectives, VBID policies were associated with increased overall (member plus health plan) medication expenditures, while overall non-medication expenditures and grand total healthcare expenditures did not change.6
These results suggest that there may be some design limitations to current VBID plans. One limitation is that these plans have only aligned copayment with value for high value drugs but not for low value drugs. The plans have lowered copayments for high value drugs but have never increased copayments for low value drugs. It has been suggested that in order for VBID plans to be financially sustainable and accessible to a wider patient population, copayment decreases for high value medications may need to be paired with copayment increases for low value medications.24,25 Furthermore, current VBID plans have uniformly reduced copayments for drugs within a therapeutic area despite the fact that not all medications within a therapeutic area have the same value. Aligning each individual medication's copayment to reflect its value may incentivize use of higher value medications.
In 2010, Premera Blue Cross, a large not-for-profit health plan in the Pacific Northwest implemented a value-based formulary (VBF) benefit among its own employees and dependents that explicitly used cost-effectiveness analysis (CEA) to determine drug copayments. The design and implementation of the VBF has been described in detail elsewhere.26 Briefly, Premera pharmacists trained in economic evaluation gather available CEA estimates and when necessary, produce de novo estimates. An external panel of clinical, economic and bioethical experts and lay members uses the incremental cost-effectiveness ratio (ICER) estimates along with information on additional social or ethical values to assign the drug to the appropriate copayment tier. Drugs with higher ICERs are placed on higher copayment tiers to disincentivize use and drugs with lower ICERs are placed on lower copayment tiers to incentivize use. The specific ICER ranges and corresponding copayment tiers and copayment amounts are listed in Table 1.
Although promising and unique, the long-term impact of the VBF must be empirically investigated due to some limitations of this VBF implementation. First, it is unclear whether the available CEA evidence was sufficient to appropriately estimate the value of drugs. Although the quality of CEA studies were assessed based on accepted methodology, studies may, for example, still differ in cost and outcome measurement methods, populations and time horizons, therefore limiting comparability.27 Second, whereas copayment tier assignment is based on population average cost-effectiveness estimates for a drug, actual cost-effectiveness is patient-specific due to heterogeneous treatment effects.28,29 Finally, even if the VBF achieves its intended effect of shifting medication utilization towards higher value medications, total healthcare expenditures may still rise if the increased use is for medications that are higher value, but are not cost-saving (i.e. more health but at higher cost). We used the implementation of the VBF among Premera's employees and their dependents to investigate the impact of a VBF on medication and health services utilization and on medication and non-medication expenditures from member, health plan, and member plus health plan (overall) perspectives.
METHODS
Sample, Data Source and Measurements
The initial sample was drawn from the population of employees and dependents aged 0-64 who were covered under Preferred Provider Organization employer sponsored plans administrated by Premera Blue Cross, the largest private health plan in Washington State. The sample was restricted to include only individuals continuously enrolled at least one year prior to VBF implementation. The intervention group was composed of employees and dependents of Premera in an employer-sponsored plan that implemented the VBF on July 2010. The control group was composed of employees and dependents of five employer sponsored plans administrated by Premera and without any changes in pharmacy benefits over the entire study period. These plans were chosen based on similarity to the intervention group prior to VBF implementation in industry classification, member geography of residence and medication copayment tiers.
The analysis was performed at the individual member level. For each member in our sample, we obtained monthly measures on demographics (age, sex, ZIP code of residence, relationship to employee), prescriptions fills (National Drug Code, generic code number, number of days’ supply, date dispensed, place of purchase (retail or mail order pharmacy)), non-medication services (date of service, place of service, length of hospitalization, procedure, diagnosis, and revenue codes), expenditures (amount paid by member, amount paid by health plan), and plan characteristics (benefit renewal month and medical benefit relativity value). The medical benefit relativity value is an index of medical benefit generosity commonly used in health insurance actuarial analyses that takes into account a large number of plan cost-sharing and utilization characteristics (deductibles, copayments, coinsurance, out-of-pocket maximums, prior authorization, quantity limits, etc.).30-32 The values range between 0 and 1. A value of 0.75 means that a health plan pays 75% of medical expenses and the member pays the remaining 25% for a typical market basket of healthcare interventions.
We used data on individuals’ ZIP code of residence to link to zip code level demographics using the 2009-2013 American Community Surveys and 2010 US Census, including information on median household income, proportion of urban residents, proportion of African American persons, and proportion with bachelor's degree.33-37
Outcomes
We first assessed overall average monthly medication utilization per member. Since the VBF is expected to cause medication switching and since our purpose is to assess the effect of the VBF on medication consumption, and not adherence per se, we measured the per member per month probability of filling a unique medication and the days’ supply of the medication. A unique medication was defined by a unique combination of active ingredient, dosage form, dosage strength, and brand-generic status. This was the basic unit by which copayment tiers, including VBF tiers, were assigned. Therefore copayments are homogenous within a unique combination at a given month for a given plan. We next assessed health services utilization per member per month, as measured by the probability of incurring emergency department (ED) visits, the number of ED visits, the probability of hospitalization, the number of days spent hospitalized, the probability of incurring office visits and the number of office visits. We finally assessed member, health plan, and overall medication and non-medication expenditures per member per month. Based on our sample size, we had 80% power to detect a 2.5% change in overall medication expenditures at p = 0.05.
Since the effect of the VBF on medication utilization may depend on the direction of copayment change and on tier placement, we conducted secondary analyses in which we assessed medication utilization based on two categorization methods: 1) medications moved into lower copayment tiers, higher copayment tiers, or no change in tier in the VBF and 2) medications moved into the preventive tier or into tiers 1-4 in the VBF.
As a falsification test, we assessed the expenditures for vision services (a category of expenditures that is unlikely to be impacted by the VBF policy) from the overall (member plus health plan) perspective. All health plans offered vision benefits and the benefits did not change throughout the period of study.
Study Design and Time Frame
We utilized an interrupted time series design with the interruption coinciding with the policy implementation date occurring on July 2010. This design utilizes characteristics and outcomes of the intervention group in the pre-policy periods and the characteristics and outcomes of control group in the pre-policy and post-policy periods to control for confounding.38,39 We divided our analysis into 3 periods: 3 years before to 1 year before VBF implementation (early pre-VBF period: July 2006 to June 2009), 1 year before VBF implementation (late pre-VBF period: July 2009 to June 2010), and immediately after VBF implementation to 3 years after (post-VBF period: July 2010 to June 2013). The 3 months immediately prior to and after VBF implementation were excluded to avoid measuring potential anticipatory or delayed filling of medications. This left 78 total months of observation. We conducted additional sensitivity analyses that excluded 6, 1 and 0 months immediately prior to and after VBF implementation.
Intervention
On the first month of the late pre-VBF period (July 2009), the intervention group had an increase of $5 in pharmacy copayment in 2 copayment tiers, an increase in the medical deductible from $400 to $500, an increase of $25 in the emergency department copayment, and an increase in the out-of-pocket maximum by $200. The pharmacy benefits in the early pre-VBF, late pre-VBF, and post-VBF periods for the intervention group are described in detail in Table 1. In contrast, there were no changes in the pharmacy benefits for the control group over the entire period. The control group experienced changes in their medical benefits (see Supplemental Digital Content (SDC) Table 1, which provides a description of medical benefits changes). There were no changes to any medical benefits at the time of VBF implementation. We also control for all benefit changes other than the implementation of the VBF policy in our statistical analyses.
Statistical Analyses
Since we aimed to estimate the average effect of the VBF among those exposed, we sought to compare the observed outcomes among VBF members with the expected outcomes for the same group of VBF members had the VBF not been implemented. We obtained the expected estimate by using the contemporaneous observed outcomes in the five control plans that were not exposed to the VBF in our regression models, after adjusting for the covariate distributions in both the groups. We confirmed the similarity of the control group to the intervention group in pre-VBF outcomes trends by examining both the statistical significance and magnitude of the coefficients in our regression models that represented the differential trends in the groups prior to VBF implementation. SDC Figure 1 and SDC Table 2 provide further details about the coefficient tests, the model specifications and how the expected outcomes were calculated.
We generated expected outcomes by using generalized estimating equations (GEEs) with two-part models. For medication and health services utilization, we used binomial distribution with logit link to model probabilities and Poisson distribution with log link to model counts. For medication and non-medication expenditures, we used binomial distribution and logit link to model the probability of incurring expenditures and gamma distribution with log link to model expenditures among those who have incurred expenditures. We assessed overall model fit by using the following goodness-of-fit tests: Pearson's correlation test, Pregibon link test, and a modified Hosmer-Lemeshow test.40,41 We modeled correlations between monthly observations within members using first-order autoregressive correlation structures for probabilities and using robust variance estimators for all other models.42 We generated standard errors and confidence intervals for all estimates using 1000 bootstrap replications.
We adjusted for individual-level characteristics (sex, age, total healthcare expenditure greater than $100,000 in any 12 month period in the pre-VBF period), ZIP code-level characteristics (bachelor's degree, household income, urban residence, African American race, Washington state residence), plan-level characteristics (medical benefits relativity value, benefit renewal month), fixed effects for calendar months (January to December), and study period (early pre-VBF period, late pre-VBF period, and post-VBF periods).43
We describe our data validation process in SDC figure 2. All analyses were done with Stata, version 13.1 (StataCorp). Estimates with P values less than 0.05 were considered statistically significant. This study was approved by the institutional review board at the University of Washington and data privacy board.
RESULTS
Population Characteristics
The intervention group and control group totaled 5,235 members (318,143 member-months) and 11,171 members (660,600 member-months) respectively. In general, the two groups were similar in demographic and socioeconomic characteristics in the pre-policy period although many differences were statistically significant, potentially due to the large sample size. As specified a priori, we adjusted for various demographic and socioeconomic characteristics. Rates of enrollment or attrition (results not shown) did not differ between VBF and control groups in the pre- and post-policy periods.
Changes in mean copayments due to the VBF
Mean copayments increased in the VBF cohort from $21 (SD, $16) to $28 (SD, $25) comparing the late-pre VBF period to the post-VBF period. (Table 3) The VBF assigned 28% of medications (by prescription fill volume in the pre-VBF period) into lower copayment tiers and 4% of medications into higher copayment tiers. (Table 3b) For medications moved into lower copayment tiers, mean copayments decreased from $14 (SD, $11) to $7 (SD, $13). For medications moved into higher copayment tiers, mean copayment increased from $40 (SD, $17) to $79 (SD, $23). For medications with no change in copayment tiers, mean copayment increased from $18 (SD, $15) to $27 (SD, $19).
Table 3b.
Mean copayments of medications by change in tier in the year before and after VBF implementation
| Change in Tier | Number of unique medicationsa, n (%) | Number of prescription claims year before, n (%) | Pre-VBF Copay, mean (SD) | Post-VBF Copay, mean (SD) | Diff, mean (SD) |
|---|---|---|---|---|---|
| No Change in tier | 1,207 (73) | 41,193 (68) | 18 (15) | 27 (19) | 8 (6) |
| Moved into lower tier | 325 (20) | 16,871 (28) | 14 (11) | 7 (13) | −7 (6) |
| Moved into higher tier | 122 (7) | 2,288 (4) | 40 (17) | 79 (23) | 39 (22) |
Changes in Utilization and Expenditures after Adjusting for Secular Trends
The VBF policy had no statistically significant overall impact on medication utilization (see SDC Table 3a for overall medication utilization estimates). Categorizing medications by whether the medications were moved into lower copayment tiers, higher copayment tiers, or no change in tier, we found that only the medications that were moved into lower tiers had a statistically significant change in utilization (SDC Table 3b). These medications had an adjusted 0.02 PMPM (11%; P<0.001) increase in the probability of fill and an adjusted 1.95 day PMPM (17%; P<0.001) increase in days’ supply. Categorizing medications by whether the medications were moved into the preventive tier or into tiers 1-4 in the VBF, we found that only medications that were moved into the preventive tier had a statistically significant change in utilization. These medications had an adjusted 0.02 PMPM (13%; P<0.001) increase in the probability of fill an adjusted 1.68 day PMPM (16%; P<0.001) increase in days’ supply (SDC Table 3c).
The policy impact on health services utilization was generally small. We found no statistically significant changes in probability or quantity of use for ED visits, hospitalization, or office visits (see SDC Table 4 for health services utilization estimates).
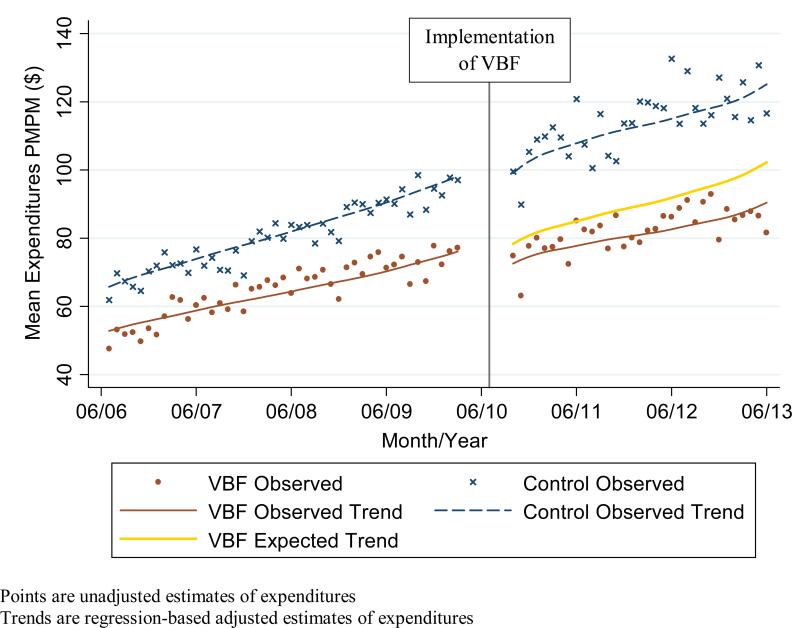
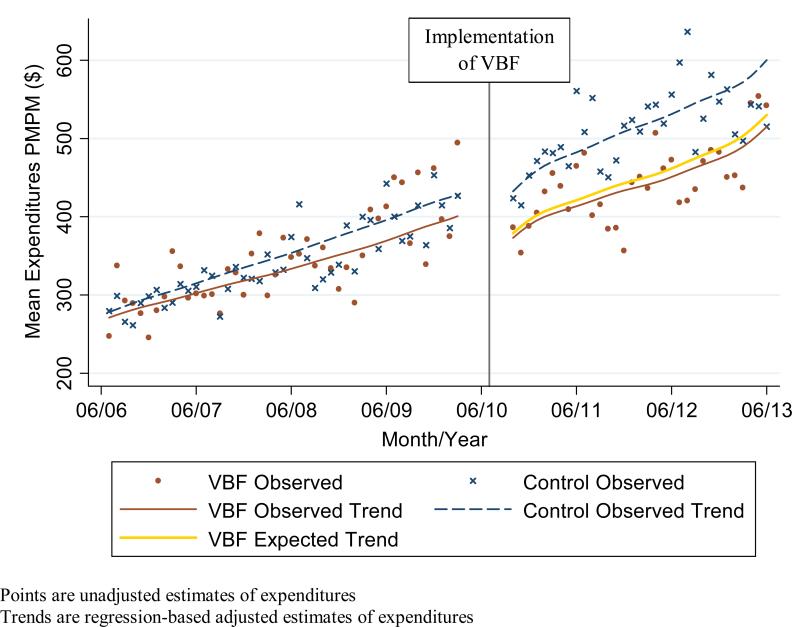
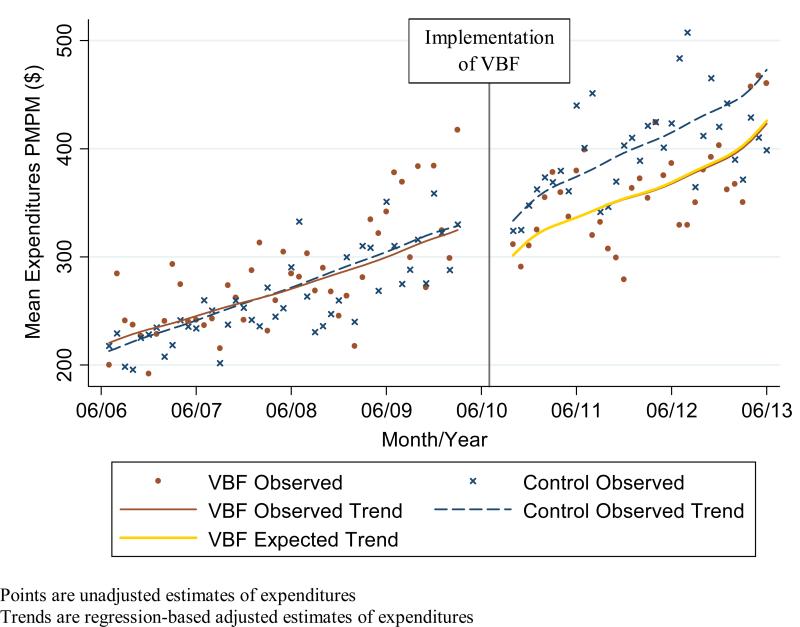
Expenditures in the pre-policy period in the intervention group did not differ statistically in both level and trend from the expenditures in the control group (Figures 1a-c). In the post-policy period, medication and total expenditures in the intervention group seemed to have a decrease in level and trend whereas there was no apparent change in the level or trend of expenditures in the control group. For the adjusted expenditures comparing the observed expenditures in the intervention group with the expected expenditures in the intervention group had the VBF policy not been implemented, we find that member medication expenditures increased significantly by $2 PMPM (9%; P = 0.004) while health plan and overall medication expenditures decreased significantly by $10 PMPM (16%; P = 0.02) and $8 PMPM (10%; P= 0.01) respectively (Table 4). There was no statistically significant impact on member non-medication expenditures ($3 PMPM; 4%; P= 0.20); health plan expenditures ($2 PMPM; 1%; P= 0.91), overall non-medication expenditures ($1 PMPM; 0%; P= 0.95); and overall medication and non-medication expenditures ($9 PMPM; 2%; P= 0.63).
Figure 1a.
Observed and expected medication expenditures Per Member Per Month (PMPM) in intervention (Value-Based Formulary (VBF)) and control groups combining expenditures from member and health plan perspectives
Figure 1c.
Observed and expected overall expenditures (medication and non-medication) Per Member Per Month (PMPM) in intervention (Value-Based Formulary (VBF)) and control groups combining expenditures from member and health plan perspectives
Table 4.
Impact of the Value-based Formulary on medication and non-medication expenditures per member per month
| Expenditures (US $) | Observed Estimatea | Expected Estimateb | VBF Impact | P Value |
|---|---|---|---|---|
| Medication Expenditures | ||||
| Member (95% CI) | 17 (16, 18) | 15 (14, 16) | 2 (1, 3) | 0.004 |
| Health Plan (95% CI) | 64 (56, 72) | 74 (63, 84) | −10 (−18, −2) | 0.02 |
| Overall (95% CI) | 80 (72, 89) | 89 (79, 99) | −8 (−15, −2) | 0.01 |
| Non-Medication Expenditures | ||||
| Member (95% CI) | 61 (60, 64) | 64 (60, 68) | −3 (−6, 1) | 0.20 |
| Health Plan (95% CI) | 293 (267, 320) | 292 (257, 327) | 2 (−35, 38) | 0.91 |
| Overall (95% CI) | 355 (327, 383) | 356 (318, 393) | −1 (−39, 38) | 0.95 |
| Grand Total Expenditures | 436 (406, 465) | 445 (406, 483) | −9 (−49, 30) | 0.63 |
Observed (i.e. factual) estimate: regression-based adjusted estimate of expenditures in the intervention group in the post-VBF period if the VBF had been implemented
Expected (i.e. counterfactual) estimate: regression-based adjusted estimate of expenditures in the intervention group in the post-VBF period if the VBF had not been implemented
Falsification Test and Sensitivity Analyses
The falsification test confirmed that there was no change in vision expenditures due to the VBF in the post-policy period. The sensitivity analyses that excluded 6, 1 and 0 months immediately prior to and after VBF implementation revealed similar results to our primary analyses.
DISCUSSION
This study presents an evaluation of the impact of a more nuanced prescription drug benefit that explicitly used cost-effectiveness evidence to inform medication level copayments.24,26 We found a 10% or $8 PMPM reduction in overall medication expenditure in the Premera cohort. The medication savings equals $1.1 million for the cohort over the three year post-policy time frame. There were no negative impacts on overall medication utilization or health services utilization or non-medication expenditures.
These results are broadly consistent with our first-year findings.26 This study adds a more complete analysis by including member and overall expenditure perspectives, non-medication expenditures, longer duration of follow up, and measures of medication and health services utilization. The additional medication expenditure perspectives indicated that some expenditures were shifted from the health plan to the member; however, there was a net savings in overall medication expenditures. Further, the medication utilization analysis revealed that the VBF increased the utilization of higher value drugs.
These results differ from other VBID policies that found that overall healthcare spending does not change and may actually increase from the health plan perspective.19-23 These previous implementations were limited due to the fact that they did not assess the value of individual medications and adjust copayments based on the individual value estimates. Hence, previous VBID policies have been limited to reducing copayments for specific therapeutic categories.7-19 The formal assessment of the value at the medication level allows for finer assignment of drugs to copayment tiers and allows for copayment increases for low value drugs in addition to copayment decreases for high value drugs. Although copayment increases have resulted in increased member expenditures, net medication expenditures decreased. For a not for profit employer sponsored health plan, such net savings could potentially be returned to the member in the longer term as lower insurance premiums or higher wages.
Policy Implications
The VBF may be a useful framework for both private and public payers interested in innovating in cost-sharing to both incentivize the use of high value drugs and disincentivize the use of low value drugs. After several years of slower growth, pharmaceutical expenditures are again growing rapidly.3 Payers may respond by shifting some of this cost to members through increased cost-sharing. In this context, the VBF may be a nuanced way to cost-share such that patients are shifted towards higher value drugs and negative impacts on overall medication utilization and other health services utilization are minimized.
Beginning January 2017, the Center for Medicare & Medicaid Innovation (CMMI) will carry out demonstration projects to test VBID in Medicare Advantage plans in seven states.44,45 The program will target patients in one of seven chronic disease states. Although the proposed five year studies will further our understanding of the long-term impacts of VBID on the targeted populations, the proposed design is still limited to reducing cost-sharing for high value interventions and does not address low value interventions. As we have shown, by explicitly estimating the value of medications, the VBF incorporated copayment increases for low value drugs with copayment increases for high value drugs, resulting in overall medication savings. Future iterations of VBID should consider an explicit estimate of value in order to inform copayment levels.
Limitations
This study has several limitations. First, our sample was drawn from a working-age population and their dependents and thus our results may not necessarily be generalizable to other populations such as the poor, elderly, or chronically ill. A second limitation is the potential for unobserved confounding. The intervention group was composed of employees of a health plan and their dependents. Hence, although we observed similar pre–policy outcome trends in the VBF and control groups, our estimates of expected trends may be biased if some unobserved confounder affects these trends differentially during the post–policy period. However, an additional analysis restricting the control group to the one plan that was composed of employees and dependents of an actuarial firm (a large proportion of Premera's employees are also actuaries) revealed similar results. Finally, although we find no negative changes in health services utilization, we do not know the impact of the VBF on actual health outcomes. Few studies assessing the impact of health insurance include direct measures of health outcomes. Yet this is an important aspect of understanding the true impact of the health policy changes.46,47
Conclusions
The rise of cost sharing in prescription drug plans has shifted a larger proportion of costs onto plan members. Previous VBIDs has largely resulted in shifting costs back to the health plan. This evaluation of a VBF suggests that it is possible to design value-based benefits in a nuanced way that incentivizes use of higher value medications while reducing overall medication expenditures through copayment increases and decreases without negatively impacting the utilization of medications or health services or non-medication expenditures. Future studies should investigate whether the VBF would have a similar impact in other populations.
Supplementary Material
Figure 1b.
Observed and expected non-medication expenditures Per Member Per Month (PMPM) in intervention (Value-Based Formulary (VBF)) and control groups combining expenditures from member and health plan perspectives
Table 1a.
Pharmacy benefits in the early pre-Value-Based Formulary (VBF), late pre-VBF, and post-VBF periods for the intervention group
| Tier | Early Pre-VBF Copayment | Late Pre-VBF Copayment | Post-VBF Copayment |
|---|---|---|---|
| Preventive | — | — | $0 |
| Tier 1 | $10 | $10 | $20 |
| Tier 2 | $25 | $30 | $40 |
| Tier 3 | $45 | $50 | $65 |
| Tier 4 | — | — | $100 |
Early Pre-VBF period: July 2006-June 2009
Late Pre-VBF period: July 2009-June 2010
Post-VBF period: July 2010-June 2013
Table 1b.
Copayment tier assignment guidelines in the pre-Value-Based Formulary (VBF) and the post-VBF periods for the intervention group
| Tier | Pre-VBF | Post-VBF Typical Case ICER threshold | Post-VBF Special Casea ICER threshold |
|---|---|---|---|
| Preventive | — | Cost-saving and preventive | Cost-saving and preventive |
| Tier 1 | Generic | Cost-saving or < $10,000/QALY | Cost-saving or <$50,000/QALY |
| Tier 2 | Preferred Brand | $10,000-50,000 /QALY | $50,000-150,000 /QALY |
| Tier 3 | Non-Preferred Brand | $50,000-150,000 /QALY | >$150,000 /QALY |
| Tier 4 | — | >$150,000 /QALY, or insufficient evidence to determine ICER | Insufficient evidence to determine ICER |
Special case: drugs that had additional value not reflected by their ICER. These values include ethical issues, disease rarity, unmet clinical needs, regulatory requirements, and other societal considerations.
Table 2.
Sample Characteristics for Intervention and Control Members prior to Value-Based Formulary (VBF) implementation
| Characteristic | VBF Members (n = 5,235) | Control Members (n = 11,171) | P Value |
|---|---|---|---|
| Individual characteristics | |||
| Age, yrs, n (SD) | 31.6 (17.5) | 35.8 (18.5) | <0.001 |
| Charlson score=0, N (%) | 4,422 (84.5) | 9325 (83.5) | 0.08 |
| Charlson score=1, N (%) | 582 (11.1) | 1266 (11.3) | 0.08 |
| Charlson score≥2, N (%) | 231 (4.4) | 580 (5.2) | 0.08 |
| Enrollees per family unit, n (SD) | 3.13 (1.5) | 2.85 (1.6) | <0.001 |
| Female, N (%) | 2,960 (56.5) | 6,378 (57.1) | 0.10 |
| ZIP code characteristics | |||
| African American, % (SD) | 2.9 (3.5) | 4.0 (6.1) | <0.001 |
| Bachelor's degree or higher, % (SD) | 34 (13.6) | 40.8 (17.7) | <0.001 |
| Median household income, $1000 % (SD) | 68.9 (18.5) | 65.1 (20.7) | <0.001 |
| Urban residence, % (SD) | 91.7 (17.0) | 89.8 (21.7) | <0.001 |
| Washington state residence, N (%) | 4,638 (88.6) | 9,533 (85.3) | <0.001 |
| Utilization Characteristics per month | |||
| Number of prescription users, N (%) | 1,785 (34.1) | 4,289 (38.4) | <0.001 |
| Number of prescriptions per member, n (SD) | 0.83 (1.36) | 0.92 (1.34) | <0.001 |
| Number of emergency department visits per member, n (SD) | 0.02 (0.04) | 0.02 (0.03) | 0.26 |
| Number of office visits per member, n (SD) | 0.27 (0.31) | 0.26 (0.27) | 0.06 |
| Number of days in hospital per member, n (SD) | 0.03 (0.14) | 0.02 (0.10) | 0.003 |
Table 3a.
Mean copayments of medications in the year before and after Value-based formulary (VBF) implementation
| Number of unique medicationsa, n | Number of prescription claims year before, n | Pre-VBF Copay, mean (SD) | Post-VBF Copay, mean (SD) | Diff, mean (SD) | |
|---|---|---|---|---|---|
| Overall copayments | 1,654 | 60,352 | 21 (16) | 28 (25) | 7 (13) |
Table 3c.
Mean copayments of medications by VBF tier placement in the year before and after VBF implementation
| VBF Tier | Number of unique medicationsa, n (%) | Number of prescription claims year before, n (%) | Pre-VBF Copay, mean (SD) | Post-VBF Copay, mean (SD) | Diff, mean (SD) |
|---|---|---|---|---|---|
| Preventive | 241 (15) | 14,339 (24) | 9 (3) | 1 (5) | −9 (3) |
| Tier 1 | 850 (51) | 34,815 (58) | 9 (4) | 15 (6) | 6 (5) |
| Tier 2 | 258 (16) | 6,592 (11) | 30 (8) | 39 (8) | 9 (9) |
| Tier 3 | 226 (14) | 3,433 (6) | 46 (8) | 63 (4) | 18 (8) |
| Tier 4 | 79 (5) | 1,173 (2) | 44 (18) | 89 (24) | 44 (26) |
Unique medication: defined as a unique combination of active ingredient, dosage form, dosage strength, and brand-generic status
Acknowledgments
We thank Carol Vogeler, MS (Premera Blue Cross, Spokane, WA) for study coordination. Dan Lee Danielson, MS; Chad Murphy PharmD; and Kathryn Brown PharmD (Premera Blue Cross, Mountlake Terrace, WA) for expert insights into the Value-Based Formulary and health insurance design.
Funding: The authors’ work on this study was supported by funding from the National Institute National Center for Advancing Translational Sciences (TL1-TR000422) and the Agency for Healthcare Research and Quality (R36-11639393).
Footnotes
Conflicts of interest: John Watkins is an employee of Premera Blue Cross. Premera had no role in the design, conduct, and reporting of this study. No other authors have any conflicts of interest.
REFERENCES
- 1.Kaiser Family Foundation and Health Research and Educational Trust [4/22/14];The Uninsured: A Primer - Key Facts about Health Insurance on the Eve of Coverage Expansions. 2013 http://kff.org/report-section/the-uninsured-a-primer-2013-introduction/.
- 2.Kaiser Family Foundation and Health Research and Educational Trust . 2014 employer health benefits survey. KFF; Menlo Park, CA: 2014. [4/5/15]. http://files.kff.org/attachment/2014-employer-health-benefits-survey-full-report. [Google Scholar]
- 3.Martin AB, Hartman M, Benson J, Catlin A, National Health Expenditure Accounts T. National Health Spending In 2014: Faster Growth Driven By Coverage Expansion And Prescription Drug Spending. Health affairs. 2016 Jan 1;35(1):150–160. doi: 10.1377/hlthaff.2015.1194. [DOI] [PubMed] [Google Scholar]
- 4.Fendrick AM, Smith DG, Chernew ME, Shah SN. A benefit-based copay for prescription drugs: patient contribution based on total benefits, not drug acquisition cost. The American journal of managed care. 2001 Sep;7(9):861–867. [PubMed] [Google Scholar]
- 5.Fendrick AM, Chernew ME. Value-based insurance design: a “clinically sensitive” approach to preserve quality of care and contain costs. The American journal of managed care. 2006 Jan;12(1):18–20. [PubMed] [Google Scholar]
- 6.Lee JL, Maciejewski M, Raju S, Shrank WH, Choudhry NK. Value-based insurance design: quality improvement but no cost savings. Health affairs. 2013 Jul;32(7):1251–1257. doi: 10.1377/hlthaff.2012.0902. [DOI] [PubMed] [Google Scholar]
- 7.Kim YA, Loucks A, Yokoyama G, Lightwood J, Rascati K, Serxner SA. Evaluation of value-based insurance design with a large retail employer. The American journal of managed care. 2011 Oct;17(10):682–690. [PubMed] [Google Scholar]
- 8.Choudhry NK, Fischer MA, Avorn J, et al. At Pitney Bowes, value-based insurance design cut copayments and increased drug adherence. Health affairs. 2010 Nov;29(11):1995–2001. doi: 10.1377/hlthaff.2010.0336. [DOI] [PubMed] [Google Scholar]
- 9.Maciejewski ML, Farley JF, Parker J, Wansink D. Copayment reductions generate greater medication adherence in targeted patients. Health affairs. 2010 Nov;29(11):2002–2008. doi: 10.1377/hlthaff.2010.0571. [DOI] [PubMed] [Google Scholar]
- 10.Gibson TB, Wang S, Kelly E, et al. A value-based insurance design program at a large company boosted medication adherence for employees with chronic illnesses. Health affairs. 2011 Jan;30(1):109–117. doi: 10.1377/hlthaff.2010.0510. [DOI] [PubMed] [Google Scholar]
- 11.Chernew ME, Shah MR, Wegh A, et al. Impact of decreasing copayments on medication adherence within a disease management environment. Health affairs. 2008 Jan-Feb;27(1):103–112. doi: 10.1377/hlthaff.27.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Zeng F, An JJ, Scully R, Barrington C, Patel BV, Nichol MB. The impact of value-based benefit design on adherence to diabetes medications: a propensity score-weighted difference in difference evaluation. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2010 Sep-Oct;13(6):846–852. doi: 10.1111/j.1524-4733.2010.00730.x. [DOI] [PubMed] [Google Scholar]
- 13.Choudhry NK, Fischer MA, Avorn JL, et al. The impact of reducing cardiovascular medication copayments on health spending and resource utilization. Journal of the American College of Cardiology. 2012 Oct 30;60(18):1817–1824. doi: 10.1016/j.jacc.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 14.Gibson TB, Wang S, Kelly E, et al. A value-based insurance design program at a large company boosted medication adherence for employees with chronic illnesses. Health Aff. (Millwood) 2011 Jan;30(1):109–117. doi: 10.1377/hlthaff.2010.0510. [DOI] [PubMed] [Google Scholar]
- 15.Gibson TB, Mahoney J, Ranghell K, Cherney BJ, McElwee N. Value-based insurance plus disease management increased medication use and produced savings. Health affairs. 2011 Jan;30(1):100–108. doi: 10.1377/hlthaff.2010.0896. [DOI] [PubMed] [Google Scholar]
- 16.Chernew ME, Juster IA, Shah M, et al. Evidence that value-based insurance can be effective. Health affairs. 2010 Mar-Apr;29(3):530–536. doi: 10.1377/hlthaff.2009.0119. [DOI] [PubMed] [Google Scholar]
- 17.Kelly E, Turner C, Frech-Tamas F, Doyle J, Mauceri E. Value-Based Benefit Design and Healthcare Utilization in Asthma, Hypertension. American Journal of Managed Care. 2009;1(4):217–221. [Google Scholar]
- 18.Maciejewski ML, Wansink D, Lindquist JH, Parker JC, Farley JF. Value-based insurance design program in north Carolina increased medication adherence but was not cost neutral. Health affairs. 2014 Feb;33(2):300–308. doi: 10.1377/hlthaff.2013.0260. [DOI] [PubMed] [Google Scholar]
- 19.Melnick SJ, Motheral BR. Is value-based value wasted? Examining value-based insurance designs through the lens of cost-effectiveness. Journal of managed care pharmacy : JMCP. 2010 Mar;16(2):130–133. doi: 10.18553/jmcp.2010.16.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maciejewski ML, Wansink D, Lindquist JH, Parker JC, Farley JF. Value-based insurance design program in north Carolina increased medication adherence but was not cost neutral. Health affairs. 2014 Feb;33(2):300–308. doi: 10.1377/hlthaff.2013.0260. [DOI] [PubMed] [Google Scholar]
- 21.Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. The New England journal of medicine. 2011 Dec 1;365(22):2088–2097. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- 22.Gibson TB, Mahoney J, Ranghell K, Cherney BJ, McElwee N. Value-based insurance plus disease management increased medication use and produced savings. Health affairs. 2011 Jan;30(1):100–108. doi: 10.1377/hlthaff.2010.0896. [DOI] [PubMed] [Google Scholar]
- 23.Chernew ME, Juster IA, Shah M, et al. Evidence that value-based insurance can be effective. Health affairs. 2010 Mar-Apr;29(3):530–536. doi: 10.1377/hlthaff.2009.0119. [DOI] [PubMed] [Google Scholar]
- 24.Fendrick AM, Smith DG, Chernew ME. Applying value-based insurance design to low-value health services. Health affairs. 2010 Nov;29(11):2017–2021. doi: 10.1377/hlthaff.2010.0878. [DOI] [PubMed] [Google Scholar]
- 25.Davidoff A, Lopert R, Stuart B, Shaffer T, Lloyd JT, Shoemaker JS. Simulated value-based insurance design applied to statin use by Medicare beneficiaries with diabetes. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2012 May;15(3):404–411. doi: 10.1016/j.jval.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan SD, Yeung K, Vogeler C, et al. Design, implementation, and first-year outcomes of a value-based drug formulary. Journal of managed care & specialty pharmacy. 2015 Apr;21(4):269–275. doi: 10.18553/jmcp.2015.21.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein MC, O'Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices--Modeling Studies. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2003 Jan-Feb;6(1):9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 28.Baicker K, Mullainathan S, Schwartzstein J. Behavioral hazard in health insurance. National Bureau of Economic Research bulletin on aging and health. 2013;(1):2–3. [PMC free article] [PubMed] [Google Scholar]
- 29.Basu A, Jena AB, Philipson TJ. The impact of comparative effectiveness research on health and health care spending. Journal of health economics. 2011 Jul;30(4):695–706. doi: 10.1016/j.jhealeco.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milliman Inc. Health Cost Guidelines: Medical Rating Model. 2014 [Google Scholar]
- 31.Milliman Inc. Cost Relativity Analysis Model. 2014 [Google Scholar]
- 32.McDevitt R. Actuarial Value: A Method for Comparing Health Plan Benefits. California Healthcare Foundation; 2008. [Google Scholar]
- 33.U.S. Census Bureau 2009-2013 American Community Survey 5-Year Estimates. 2015 [Google Scholar]
- 34.U.S. Census Bureau 2010 U.S. Census Summary File 1. 2015 [Google Scholar]
- 35.Agency for Healthcare Research and Quality . National Healthcare Quality and Disparities Report and 5th Anniversary Update on the National Quality Strategy. Rockville; p. MD2016. [Google Scholar]
- 36.Briesacher B, Limcangco R, Gaskin D. Racial and ethnic disparities in prescription coverage and medication use. Health care financing review. 2003;25(2):63–76. Winter. [PMC free article] [PubMed] [Google Scholar]
- 37.Cook BL, Manning WG. Measuring racial/ethnic disparities across the distribution of health care expenditures. Health services research. 2009 Oct;44(5 Pt 1):1603–1621. doi: 10.1111/j.1475-6773.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. Journal of clinical pharmacy and therapeutics. 2002 Aug;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 39.Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Academic pediatrics. 2013 Nov-Dec;13(6 Suppl):S38–44. doi: 10.1016/j.acap.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. Wiley; New York: 2000. [Google Scholar]
- 41.Pregibon D. Goodness of Link Tests for Generalized Linear Models. Applied Statistics. 1980;29(1):15–24. [Google Scholar]
- 42.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000 Jun;56(2):645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 43.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care. 2005 Nov;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 44.Center for Medicare and Medicaid Innovation . Announcement of Medicare Advantage Value-Based Insurance Design Model Test. Department of Health and Human Services; 2015. [Google Scholar]
- 45.Ubel PA. Value Promotion in Health Care: The Importance of Symmetry. Jama. 2016 Jan 12;315(2):133–134. doi: 10.1001/jama.2015.18159. [DOI] [PubMed] [Google Scholar]
- 46.Brook RH, Ware JE, Jr., Rogers WH, et al. Does free care improve adults' health? Results from a randomized controlled trial. N Engl J Med. 1983 Dec 8;309(23):1426–1434. doi: 10.1056/NEJM198312083092305. [DOI] [PubMed] [Google Scholar]
- 47.Hsu J, Price M, Huang J, et al. Unintended consequences of caps on Medicare drug benefits. N Engl J Med. 2006 Jun 1;354(22):2349–2359. doi: 10.1056/NEJMsa054436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.