Abstract
The lectin homing receptor LECAM-1 (LAM-1, Leu8) and the beta 2 integrins, particularly Mac-1 (CD11b/CD18), participate in leukocyte-endothelial cell interactions in inflammation. LECAM-1 is rapidly shed while Mac-1 expression is dramatically increased upon neutrophil activation, suggesting functionally distinct roles for these molecules. Using intravital video microscopy, we have compared the effect of antibodies against LECAM-1 and CD18 on leukocyte interactions with rabbit mesenteric venules. Anti-LECAM-1 monoclonal antibody and its Fab fragments inhibited initial reversible leukocyte rolling along the vascular wall. Anti-CD18 monoclonal antibody had no effect on rolling but prevented subsequent firm attachment of leukocytes to venular endothelium. These results support a two-step model of leukocyte-endothelial cell interactions: reversible rolling mediated in part by LECAM-1 facilitates leukocyte recruitment by the local microenvironment and precedes activation-dependent firm attachment involving beta 2 integrins.
Full text
PDF

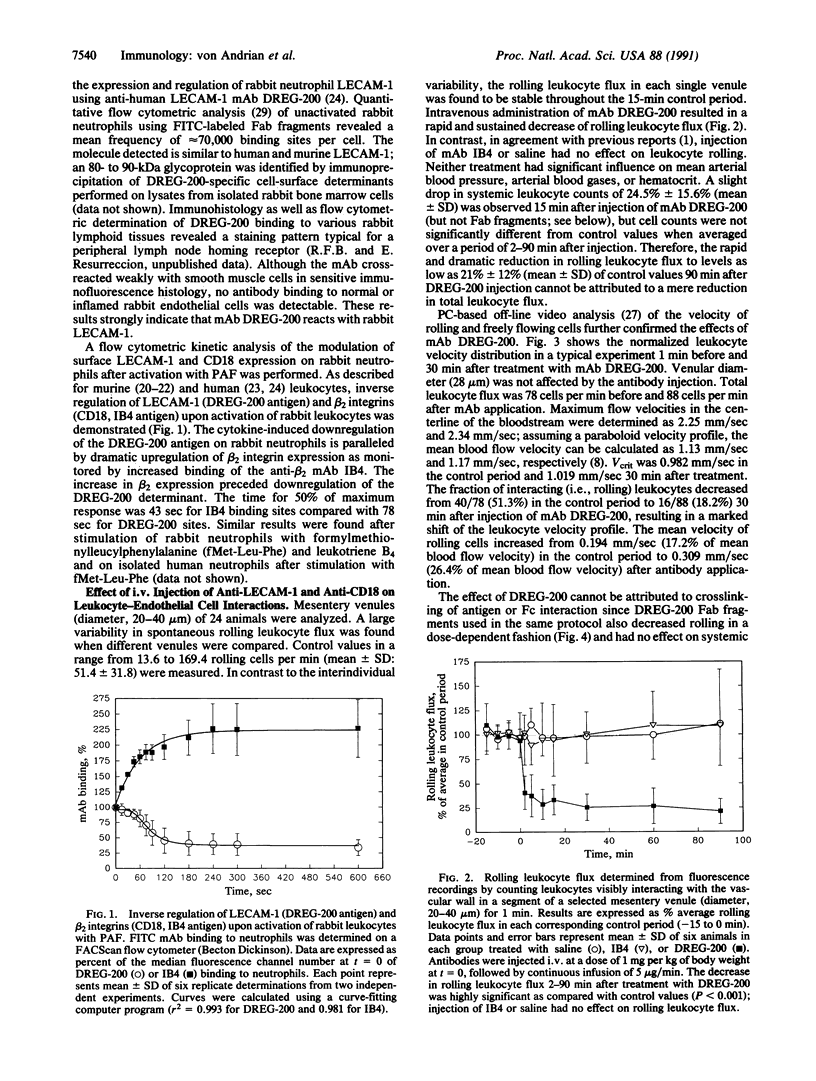
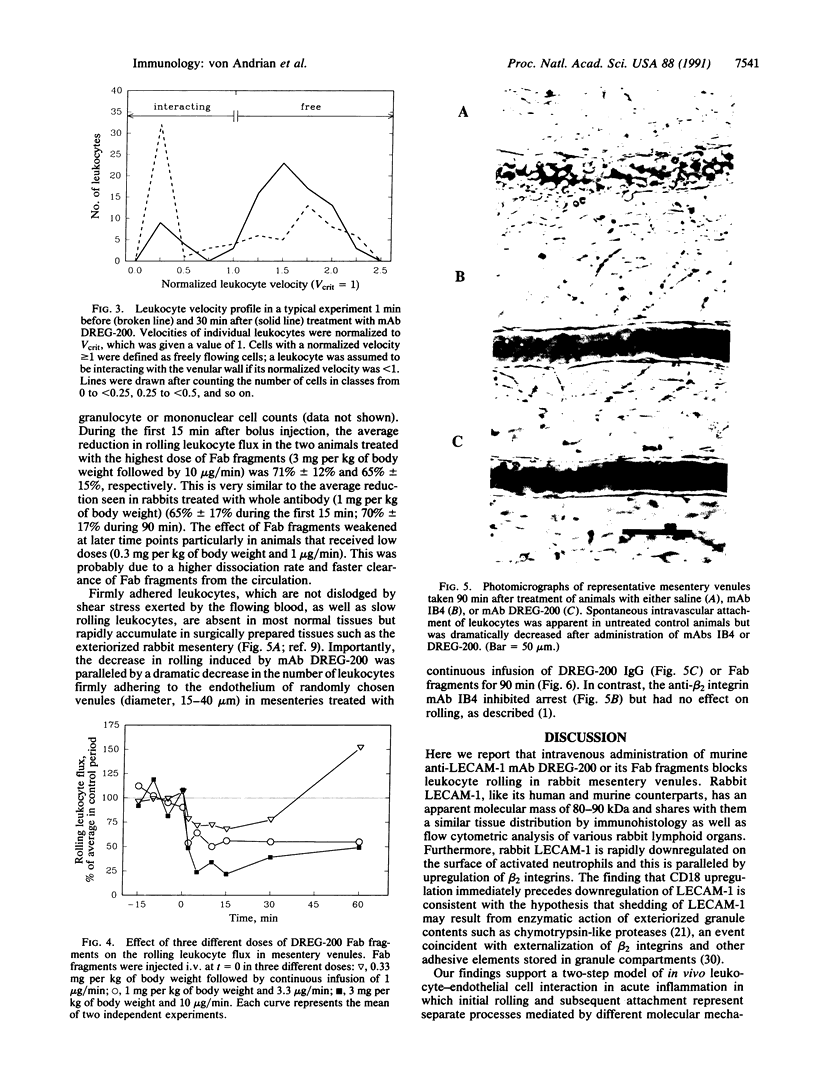
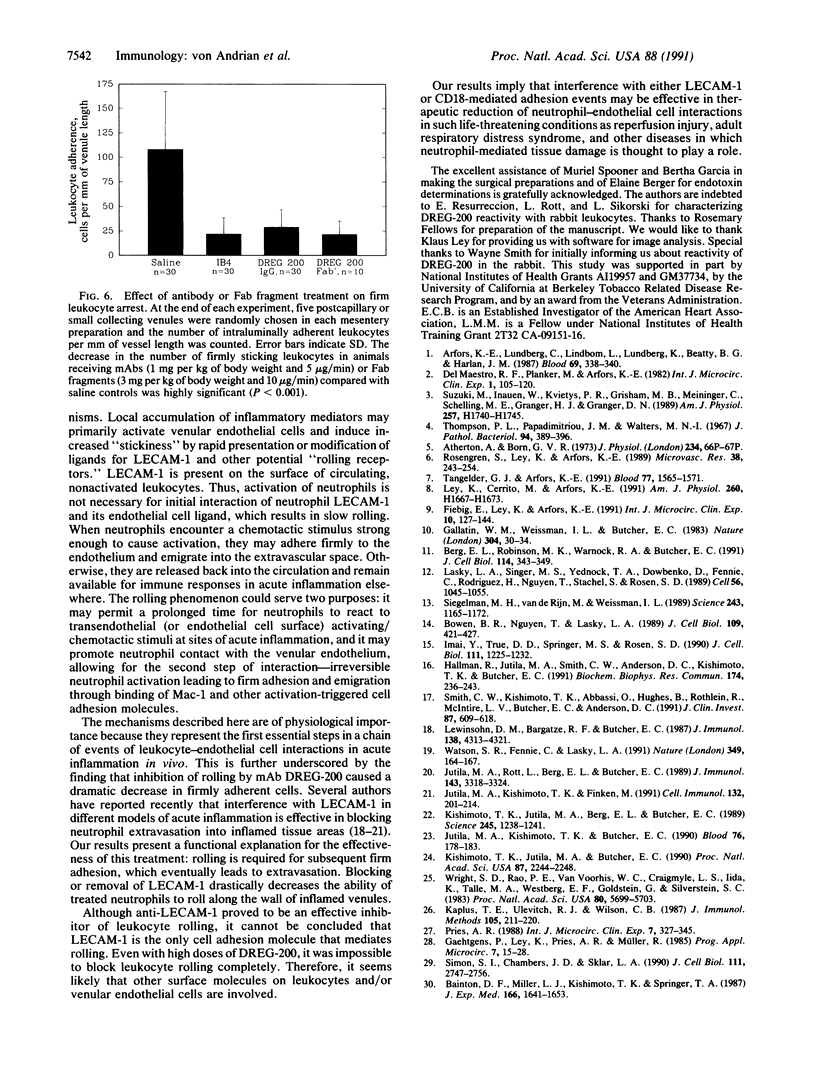
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfors K. E., Lundberg C., Lindbom L., Lundberg K., Beatty P. G., Harlan J. M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987 Jan;69(1):338–340. [PubMed] [Google Scholar]
- Atherton A., Born G. V. Proceedings: Effects of neuraminidase and N-acetyl neuraminic acid on the adhesion of circulating granulocytes and platelets in venules. J Physiol. 1973 Oct;234(2):66P–67P. [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Miller L. J., Kishimoto T. K., Springer T. A. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987 Dec 1;166(6):1641–1653. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg E. L., Robinson M. K., Warnock R. A., Butcher E. C. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991 Jul;114(2):343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen B. R., Nguyen T., Lasky L. A. Characterization of a human homologue of the murine peripheral lymph node homing receptor. J Cell Biol. 1989 Jul;109(1):421–427. doi: 10.1083/jcb.109.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maestro R. F., Planker M., Arfors K. E. Evidence for the participation of superoxide anion radical in altering the adhesive interaction between granulocytes and endothelium, in vivo. Int J Microcirc Clin Exp. 1982;1(2):105–120. [PubMed] [Google Scholar]
- Fiebig E., Ley K., Arfors K. E. Rapid leukocyte accumulation by "spontaneous" rolling and adhesion in the exteriorized rabbit mesentery. Int J Microcirc Clin Exp. 1991 May;10(2):127–144. [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Hallmann R., Jutila M. A., Smith C. W., Anderson D. C., Kishimoto T. K., Butcher E. C. The peripheral lymph node homing receptor, LECAM-1, is involved in CD18-independent adhesion of human neutrophils to endothelium. Biochem Biophys Res Commun. 1991 Jan 15;174(1):236–243. doi: 10.1016/0006-291x(91)90511-5. [DOI] [PubMed] [Google Scholar]
- Imai Y., True D. D., Singer M. S., Rosen S. D. Direct demonstration of the lectin activity of gp90MEL, a lymphocyte homing receptor. J Cell Biol. 1990 Sep;111(3):1225–1232. doi: 10.1083/jcb.111.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutila M. A., Kishimoto T. K., Butcher E. C. Regulation and lectin activity of the human neutrophil peripheral lymph node homing receptor. Blood. 1990 Jul 1;76(1):178–183. [PubMed] [Google Scholar]
- Jutila M. A., Kishimoto T. K., Finken M. Low-dose chymotrypsin treatment inhibits neutrophil migration into sites of inflammation in vivo: effects on Mac-1 and MEL-14 adhesion protein expression and function. Cell Immunol. 1991 Jan;132(1):201–214. doi: 10.1016/0008-8749(91)90019-8. [DOI] [PubMed] [Google Scholar]
- Jutila M. A., Rott L., Berg E. L., Butcher E. C. Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and MAC-1. J Immunol. 1989 Nov 15;143(10):3318–3324. [PubMed] [Google Scholar]
- Karplus T. E., Ulevitch R. J., Wilson C. B. A new method for reduction of endotoxin contamination from protein solutions. J Immunol Methods. 1987 Dec 24;105(2):211–220. doi: 10.1016/0022-1759(87)90268-7. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Butcher E. C. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Yednock T. A., Dowbenko D., Fennie C., Rodriguez H., Nguyen T., Stachel S., Rosen S. D. Cloning of a lymphocyte homing receptor reveals a lectin domain. Cell. 1989 Mar 24;56(6):1045–1055. doi: 10.1016/0092-8674(89)90637-5. [DOI] [PubMed] [Google Scholar]
- Lewinsohn D. M., Bargatze R. F., Butcher E. C. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1987 Jun 15;138(12):4313–4321. [PubMed] [Google Scholar]
- Ley K., Cerrito M., Arfors K. E. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol. 1991 May;260(5 Pt 2):H1667–H1673. doi: 10.1152/ajpheart.1991.260.5.H1667. [DOI] [PubMed] [Google Scholar]
- Pries A. R. A versatile video image analysis system for microcirculatory research. Int J Microcirc Clin Exp. 1988 Nov;7(4):327–345. [PubMed] [Google Scholar]
- Rosengren S., Ley K., Arfors K. E. Dextran sulfate prevents LTB4-induced permeability increase, but not neutrophil emigration, in the hamster cheek pouch. Microvasc Res. 1989 Nov;38(3):243–254. doi: 10.1016/0026-2862(89)90003-4. [DOI] [PubMed] [Google Scholar]
- Siegelman M. H., van de Rijn M., Weissman I. L. Mouse lymph node homing receptor cDNA clone encodes a glycoprotein revealing tandem interaction domains. Science. 1989 Mar 3;243(4895):1165–1172. doi: 10.1126/science.2646713. [DOI] [PubMed] [Google Scholar]
- Simon S. I., Chambers J. D., Sklar L. A. Flow cytometric analysis and modeling of cell-cell adhesive interactions: the neutrophil as a model. J Cell Biol. 1990 Dec;111(6 Pt 1):2747–2756. doi: 10.1083/jcb.111.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Kishimoto T. K., Abbassi O., Hughes B., Rothlein R., McIntire L. V., Butcher E., Anderson D. C., Abbass O. Chemotactic factors regulate lectin adhesion molecule 1 (LECAM-1)-dependent neutrophil adhesion to cytokine-stimulated endothelial cells in vitro. J Clin Invest. 1991 Feb;87(2):609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Inauen W., Kvietys P. R., Grisham M. B., Meininger C., Schelling M. E., Granger H. J., Granger D. N. Superoxide mediates reperfusion-induced leukocyte-endothelial cell interactions. Am J Physiol. 1989 Nov;257(5 Pt 2):H1740–H1745. doi: 10.1152/ajpheart.1989.257.5.H1740. [DOI] [PubMed] [Google Scholar]
- Tangelder G. J., Arfors K. E. Inhibition of leukocyte rolling in venules by protamine and sulfated polysaccharides. Blood. 1991 Apr 1;77(7):1565–1571. [PubMed] [Google Scholar]
- Thompson P. L., Papadimitriou J. M., Walters M. N. Suppression of leucocytic sticking and emigration by chelation of calcium. J Pathol Bacteriol. 1967 Oct;94(2):389–396. doi: 10.1002/path.1700940219. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Fennie C., Lasky L. A. Neutrophil influx into an inflammatory site inhibited by a soluble homing receptor-IgG chimaera. Nature. 1991 Jan 10;349(6305):164–167. doi: 10.1038/349164a0. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]




