Abstract
Curcumin is poorly absorbed driving interest in new preparations. However, little is known about pharmacokinetics and tissue bioavailability between formulations. In this randomized, crossover study we evaluated the relationship between steady-state plasma and rectal tissue curcuminoid concentrations using standard and phosphatidylcholine curcumin extracts. There was no difference in the geometric mean plasma AUCs when adjusted for the 10-fold difference in curcumin dose between the two formulations. Phosphatidylcholine curcumin extract yielded only 20–30% plasma demethoxycurcumin and bisdemethoxycurcumin conjugates compared to standard extract, yet yielded 20-fold greater hexahydrocurcumin. When adjusting for curcumin dose, tissue curcumin concentrations were 5-fold greater for the phosphatidylcholine extract. Improvements in curcuminoid absorption due to phosphatidylcholine are not uniform across the curcuminoids. Furthermore, curcuminoid exposures in the intestinal mucosa are most likely due to luminal exposure rather than plasma disposition. Finally, once-daily dosing is sufficient to maintain detectable curcuminoids at steady-state in both plasma and rectal tissues.
Keywords: Curcumin, Pharmacokinetics, Clinical trial, Steady state, Human
INTRODUCTION
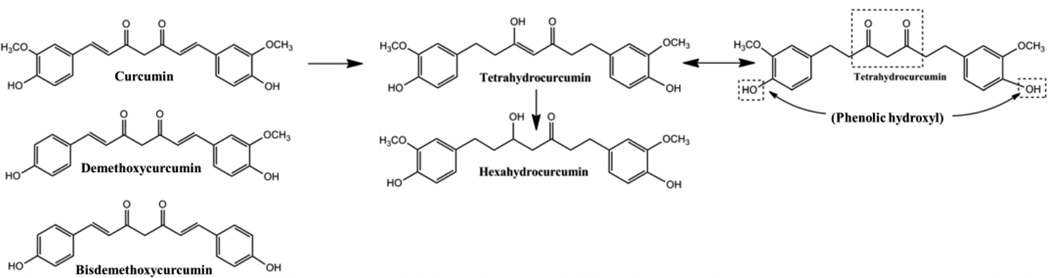
Curcumin is derived from the root of turmeric (curcuma longa [L.]), which is a native southern Asian shrub that is cultivated throughout Asia and Africa and has been used in the medical traditions of China and India for thousands of years.1 Curcumin extracts are typically comprised of three major curcuminoids: curcumin, demethoxycurcumin, bisdemethoxycurcumin (Figure 1). It is this curcuminoid mixture that is found in most commercially available products and has been the subject of much recent scientific and public interest.
Figure 1.
Chemical structures for curcuminoids. Curcumin is metabolized first to tetrahydrocurcumin, then to hexahydrocurcumin. Tetrahydrocurcumin exists in two forms, due to its keto-enol structure. Curcumin glucuronidation forms a major phenolic and minor alcoholic glucuronides, whereas hexahydrocurcumin, the major curcumin reduction metabolite, forms only a phenolic glucuronide. Demethoxycurcumin forms phenolic and alcoholic glucuronides equally, while bisdemethoxycurcumin primarily forms an alcoholic glucuronide. Curcuminoids are also known to be extensively sulfated.
A number of early phase clinical studies provide evidence that curcumin may be beneficial for several inflammatory and malignant disorders including: dyspepsia, osteoarthritis, rheumatoid arthritis, uveitis, orbital pseudotumor, a variety of premalignant or preinvasive malignancies, familial adenomatous polyposis, inflammatory bowel disease, and pancreatic cancer.2 It is thought that curcumin, the most abundant of the curcuminoids found in standard curcumin extracts, is the primary active component responsible for these purported effects, although other curcuminoids may also have important effects.3 However, when administered orally, parent curcuminoids are rarely detected in plasma of human subjects due to poor absorption and rapid metabolism of the low amounts that are absorbed.4 Low oral bioavailability of curcuminoids is suggested by the presence of curcuminoid glucuronide and sulfate conjugates in plasma, as well as conjugates of the primary metabolites formed by curcumin reduction, tetrahydrocurcumin and hexahydrocurcumin.5 However, the low bioavailability of curcumin may not limit its use as a treatment or chemopreventive agent for colorectal cancer as evidenced by the detectable levels of curcumin in colorectal mucosa observed with oral dosing in several studies.6,7
To improve the oral bioavailability of the curcuminoids, new preparations have been developed including nanoparticles, liposomal, phospholipid complexes, and curcumin prodrugs.8–10 For example, approximately 20-fold greater absorption has been observed in both rat and human studies following oral administration of a curcumin phosphatidylcholine complex compared to standard curcumin extract.11,12 However, little is known about how the different pharmacokinetic (PK) properties of these newer formulations affect the delivery of curcuminoids to target tissues such as colorectal mucosa. We hypothesized that the greater absorption of curcumin observed with a phosphatidylcholine complex will result in higher colorectal tissue concentrations compared to standard curcumin extract. Therefore, we evaluated the steady-state plasma and rectal tissue concentrations of curcuminoids for two curcumin formulations to evaluate the relationship between oral absorption, plasma exposure, and rectal tissue concentration.
METHODS
Human Subjects Protection
The study was conducted at the Verne S. Caviness Clinical and Translational Research Center at the University of North Carolina (UNC) at Chapel Hill. The study protocol and informed consent were approved by the UNC institutional review board, and the study was conducted according to the Declaration of Helsinki. The study is registered with the National Library of Medicine at clinicaltrials.gov (NCT01330810). All participants provided written informed consent prior to study enrollment.
Participants and Study Design
This was a blinded, multi-dose, randomized, cross-over study to compare the steady-state plasma area under the curve (AUC) 0–24 between two curcumin formulations in healthy human volunteers. We report summary statistics for additional plasma PK parameters and rectal tissue concentrations for both formulations.
Participants were solicited by advertisement or word of mouth between April 2011 and March 2012. Male and female respondents aged 18 to 65 years with body mass index (BMI) between 18–30 kg/m2 and good general health were eligible. Healthy volunteers were identified by medical history and evaluation of liver and renal function. Exclusion criteria included pregnant or lactating females, history of pancreatic or biliary disease, personal or inherited bleeding disorders or therapeutic anti-coagulation with warfarin, history of bowel resection for any reason, acute illness that would interfere with drug absorption, allergy or hypersensitivity to curcumin, use of any curcumin-containing products within 14 days prior to enrollment, or narcotic or alcohol dependence.
Participants were instructed to take a curcumin preparation once daily by mouth in the morning for six days followed by a seventh dose provided by the research nurse on the morning of their PK visit. Participants were instructed to take each daily dose in the morning on an empty stomach with an eight ounce glass of water. Curcumin phosphatidylcholine extract powder was mixed into a 4 ounce cup of applesauce supplied with each dose, and standard extract pill was taken with the same 4 ounces of applesauce. Immediately prior to the PK visit, participants were fasted overnight for at least 8 hours except for water and instructed to avoid any alcoholic beverages for the preceding 48 hours. Blood was drawn for PK analysis on arrival to the study center in the morning on dosing day seven (0h time point), then participants received the final (7th) dose of curcumin with an eight oz. glass of water. Serial blood samples were collected at time points 0.5, 0.75, 1, 2, 3, 4, 6, 8, 12, 24, and 48 hours after curcumin dosing. Participants received a standardized meal approximately 1 hour after curcumin dosing and were allowed to eat freely 2 hours after the standardized meal. Additionally, rectal mucosal pinch biopsies were obtained at the 1 hour time point (i.e. prior to eating). We used a seven-day washout period before crossing over to the second dosing period when participants received a second curcumin preparation.
Curcumin Formulations and Dose
Two formulations of curcumin were selected for investigation. A standard curcumin extract was formulated into 1 gram tablets using the commercially available C3 extract produced by Sabinsa Corporation. The 1 gram tablet was formulated by Sabinsa according to their method for their commercially available product. We chose to use a 1 gram tablet to reduce the pill burden on participants. Each tablet contained 1000 mg mixed curcuminoids as follows: 730 mg curcumin, 220 mg demethoxycurcumin, and 50 mg bisdemethoxycurcumin. A single daily dose of the standard extract was four tablets providing 4 grams of mixed curcuminoids (2920 mg curcumin). Curcumin phosphatidylcholine complex (Meriva®), was supplied by Indena S.p.A. as powder and was given as a 2 gram dose to be mixed into a 4 oz cup of applesauce. A 2 gram dose of the curcumin phosphatidylcholine extract provided 385 mg of total curcuminoids in the following proportion: 303 mg curcumin, 68 mg demethoxycurcumin, and 14 mg of bisdemethoxycurcumin. For both crossover periods, participants were supplied a 4 oz. cup of apple sauce to be taken with their daily curcumin dose, which is a common co-administrant in PK studies and has been shown to have minimal effect on gastric transit and drug absorption.13–15 All doses were administered from a single lot for each preparation. The manufacturers provided a certificate of analysis that was independently verified by our lab.
A computer generated simple randomization scheme was used to create a list of the two dosing sequences (ie. standard followed by phosphatidylcholine extract, phosphatidylcholine followed by standard extract) by an independent statistician. Participants were assigned to the next dosing sequence by the administering pharmacist using consecutively numbered, sealed, opaque envelopes. Because the standard extract was administered as tablet and the phosphatidylcholine extract was administered as a powder, participants and the research nurses administering the final (7th) dose were aware of the two different formulations but not their identities. All other study personnel, except the pharmacist, were blinded until all analyses were completed.
Curcuminoid Plasma and Tissue Concentrations
Analytic standards for each curcuminoid and metabolite were purchased from Chromadex (curcumin), USP (demethoxycurcumin, bisdemethoxycurcumin), and Cerilliant (tetrahydrocurcumin, hexahydrocurcumin). Whole blood samples were collected in two 3-mL EDTA-lined tubes (K2-EDTA tubes; BD, Franklin Lakes, NJ) and centrifuged at 2400 rpm for 10 min at 4°C. Plasma was collected, frozen, and stored at −80°C until analysis. Plasma concentrations of curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin, and hexahydrocurcumin were quantified using a validated method developed in our lab. Briefly, 100 µL aliquots of the plasma samples were used to determine parent (i.e. unconjugated) or total (i.e. parent + deconjugated) curcuminoids (i.e. curcumin, demethoxycurcumin, bisdemethoxycurcumin) and curcumin reduction metabolites (i.e. tetrahydrocurcumin, hexahydrocurcumin) after a 1 hr incubation at 37°C in the absence or presence of a mixture of β-glucuronidase from bovine liver (480 U; Type B-10, Sigma Aldrich, St. Louis, MO) and sulfatase from H. pomatia (48 U; Type H-1, Sigma Aldrich, St. Louis, MO) in buffered sodium acetate (pH 5.0). Extraction was performed using methyl tert-butyl ether (MTBE) with naringenin as internal standard.
Measurement of plasma samples was carried out on an Agilent HPLC-MS system (1100 series, USA) with a Luna® C18 3 µm analytical column (100 Å, 50 × 2 mm; Phenomenex, Torrance, CA), which was connected with a C18 security guard cartridge (Phenomenex, 4 × 2.0 mm). A linear gradient mobile phase composed of water/0.05% acetic acid as component A and acetonitrile/0.05% acetic acid as component B was used with the following gradient profile: 0 min 27% (B), 2 min 27% (B), 10 min 70% (B), 12 min 27% (B), 15.0 min 27% (B). The limits of detection (LOD) and quantitation (LOQ) were 0.5 and 2.0 ng/mL respectively for all curcuminoids. The method was linear over the range of 2–1000 ng/mL (R2≥0.99), with accuracy >90% and precision <5% for all quality controls and recovery of greater than 85%.
Rectal mucosal biopsies were obtained 1 hour post-dose on Day 7 through a rigid, disposable sigmoidoscope (Welch Allyn KleenSpec Disposable Sigmoidoscope with Obturator) with the participant in the left lateral position using published methods.16,17 Biopsies were taken using a disposable, flexible biopsy forceps (Olympus EndoJaw Alligator Jaw-Step), placed into opaque cryovials, and immediately flash frozen in liquid nitrogen prior to storage at −80°C. Tissue curcuminoid concentrations were quantified using a modified version of our validated plasma method. Rectal tissue samples were weighed, washed using previously published methods7, then homogenized using a micro homogenizer (Pro200 bio-gen series, Proscientific, Oxford, CT) for 10 seconds ×3 and extracted with methanol using hexastol as internal standard. Deconjugation of samples was conducted using β-glucuronidase and sulfatase as described above.
The chromatographic experiments were carried out on a Shimadzu Prominence HPLC system (Shimadzu, Columbia, MD, USA) using an Eclipse XDB-C18 guard column and a Luna C18 (2.1 × 100 mm, 3 um) analytical column (Phenomenex, Torrance, CA) following the chromatographic method developed for plasma. The MS/MS analysis was performed using a triple quadrupole mass spectrometer model API 4000 system from Applied Biosystems/MDS Sciex equipped with Turbo Ion Spray® (TIS) (Applied Biosystems, Foster City, CA, USA). Negative electrospray ionization data were acquired using multiple reaction monitoring (MRM) using the following transitions: 367 → 134 (CC); 307 → 119 (BDC); 337 → 119 (DMC); 371 → 135 (THC); 373 → 179 (HHC). Linearity, LOD, and LOQ were determined using a ten-point calibration curve for each curcuminoid. Curcuminoids were spiked into aliquots of tissue homogenates, prepared as described above with approximately 170 mg ‘blank’ rectal tissue per mL water, at concentrations ranging from 1.9 to 1000 ng/ml. The LOD and LOQ were 0.46 and 1.95 ng/mL respectively for all curcuminoids. The method was linear over this concentration range (R2≥0.99), and curcuminoid tissue concentrations were expressed as ng/mL.
Safety Assessment
All participants were tested for alanine transaminase (ALT), aspartate transaminase (AST), and creatinine both at baseline and follow-up visits, and values above 1.5× the upper limit of normal were considered abnormal. A safety monitoring questionnaire was administered at both visits. Participants were encouraged to fill out a preprinted dairy that included study date, doses taken, alcohol intake, and adverse events and were instructed to call study personnel concerning any adverse events. Adverse events were recorded including dates of onset and resolution, severity, and relationship to drug based on the National Institutes of Health (NIH) Common Terminology Criteria for Adverse Events (CTCAE) v3.0.
Pharmacokinetic and Statistical Analysis
Pharmacokinetic parameters were calculated for each of the curcuminoids and metabolites for each of the participants using non-compartmental methods, Phoenix 64 WinNonlin (v6.3, Pharsight Corp, Mountainview, CA). A constant dosing interval (τ) of 24 h was assumed for the calculation of steady-state AUC0–24 by the linear up/log down trapezoidal method. Data points before Cmax that were below our LOQ were set to 0. For data points after Cmax, the first value below LOQ was set to 1 and consecutive values below the LOQ were set to 0. For our final dataset of 120 PK profiles, we set 49 values to 1, but only 3 of those instances occurred at time points earlier than 24 hours. AUCs are reported as geometric means with 90% confidence intervals (CI); all other PK parameters are reported as geometric means with 95% CI. To determine if there was a difference in plasma exposure between the two formulations, a test of average bioequivalence, using a linear mixed effects model, was conducted using the geometric mean for the ratio of the AUC0–24 for both formulations. Carryover effects were evaluated using ANOVA. A confidence interval for the ratio that fell completely outside of the lower to upper boundary of 0.8 – 1.25 was considered to indicate non-equivalence. Based upon previously reported differences between the two formulations, we expect there to be about a 20-fold difference in AUC11; estimating a 0.7 coefficient of variation and 12 participants in a crossover design, there was 76% power to detect a geometric mean ratio of 2 between the two formulations.
RESULTS
Participants
Twelve of sixteen participants completed both dosing periods and had normal ALT, AST, and creatinine at time of enrollment and exit from the study (Table 1); four participants completed only one cross-over period and were not included in PK analyses. Reasons for dropout included intolerance of biopsy procedure (1) and scheduling conflicts (3). Four participants reported mild stomach upset that was self-limiting, and two participants reported softened stool or mild diarrhea that lasted no longer than 3 days. All participants reported taking all of their doses.
Table 1.
Baseline Participant Characteristics(N=12)
| Characteristic | Mean (SD) or n (%) |
|---|---|
| Age, yrs | 30.8 (12.5) |
| Sex, female | 8 (67%) |
| Race, white | 9 (75%) |
| Ethnicity, non-hispanic | 12 (100%) |
| BMI, kg/m2 | 25.0 (3.0) |
| Missed doses reported | 0 |
For presentation purposes, we define “curcuminoids” to include parent curcuminoids (i.e. curcumin, demethoxycurcumin, bisdemethoxycurcumin) as well as the reduced curcumin metabolites tetrahydrocurcumin and hexahydrocurcumin. Unconjugated curcuminoids were not detected in plasma samples, therefore, the values reported reflect curcuminoid concentrations following deconjugation of plasma samples, referred to as “curcumin conjugates”.
Bioequivalence
Dose-adjusted geometric mean ratios comparing phosphatidylcholine to standard extract favored the phosphatidylcholine extract for all three curcuminoids (curcumin 8.8 [90% CI: 5.0–15.4], demethoxycurcumin 2.9 [1.7–5.2], and bisdemethoxycurcumin 3.0 [1.5–5.9]), and their 90% CI consistently fell above our prespecified upper boundary of 1.25. There were no carry-over effects noted for any of the curcuminoids (p≥0.48).
Plasma PK Parameters
Times to peak curcuminoid concentrations (Tmax) were longer for the standard extract versus the phosphatidylcholine formulation by approximately 2.5 hours for parent curcuminoids and 3.5 hours for reduced metabolites. Although curcumin peak plasma concentrations (Cmax) were similar between the two formulations, peak concentrations of the two reduced metabolites were 1.7- to 2.5-fold higher with the standard formulation: tetrahydrocurcumin (83 vs 147 ng/mL) and hexahydrocurcumin (105 vs 259 ng/mL). The proportion of the AUC0-∞ that was captured in the AUC0–48 was between 85–95% for all curcuminoids (Table 3).
Table 3.
Pharmacokinetic Parameters of Curcuminoid and Curcumin Metabolite Conjugates (N=12)
| Standard Extracta | PC Extractb | |
|---|---|---|
| Curcumin | ||
| Tmax,min | 216 (114–414) | 64 (33–124) |
| Cmax,ng/mL | 57 (32–103) | 71 (42–119) |
| C24, ng/mL | 14 (8–26) | 11 (5–21) |
| AUC0–48, h*ng/mL | 929 (528–1635) | 785 (429–1439) |
| AUC0–48/AUC0-∞ | 0.86 | 0.91 |
| Demethoxycurcumin | ||
| Tmax,min | 180 (120–269) | 55 (38–79) |
| Cmax,ng/mL | 73 (43–122) | 28 (16–51) |
| C24, ng/mL | 9 (5–15) | BLQ |
| AUC0–48, h*ng/mL | 801 (519–1236) | 178 (89–359) |
| AUC0–48/AUC0-∞ | 0.89 | 0.85 |
| Bisdemethoxycurcumin | ||
| Tmax,min | 225 (126–405) | 58 (32–83) |
| Cmax,ng/mL | 58 (32–105) | 24 (13–43) |
| C24, ng/mL | 9 (4–19) | BLQ |
| AUC0–48, h*ng/mL | 706 (409–1218) | 132 (68–253) |
| AUC0–48/AUC0-∞ | 0.95 | 0.86 |
| Tetrahydrocurcumin | ||
| Tmax,min | 295 (186–468) | 93 (42–207) |
| Cmax,ng/mL | 83 (47–148) | 147 (94–231) |
| C24, ng/mL | 28 (14–53) | 30 (18–52) |
| AUC0–48, h*ng/mL | 1662 (902–3064) | 2035 (1316–3466) |
| AUC0–48/AUC0-∞ | 0.84 | 0.89 |
| Hexahydrocurcumin | ||
| Tmax,min | 297 (190–466) | 96 (49–188) |
| Cmax,ng/mL | 105 (58–190) | 259 (148–456) |
| C24, ng/mL | 17 (7–41) | 23 (12–44) |
| AUC0–48, h*ng/mL | 1504 (733–3085) | 2723 (1586–4674) |
| AUC0–48/AUC0-∞ | 0.94 | 0.96 |
All values expressed as geometric mean (95% confidence interval). Comparisons of AUCs were performed as test of bioequivalence reported in Table 2.
4000 mg mixed curcuminoids
400 mg mixed curcuminoids
PC – Phosphatidylcholine, BLQ – below level of quantitation
Geometric mean trough concentrations (24h post-final dose) between standard and phosphatidylcholine formulations were no different for all analytes. For both formulations, geometric mean concentrations were all below 30 ng/mL (Table 3).
Plasma AUC
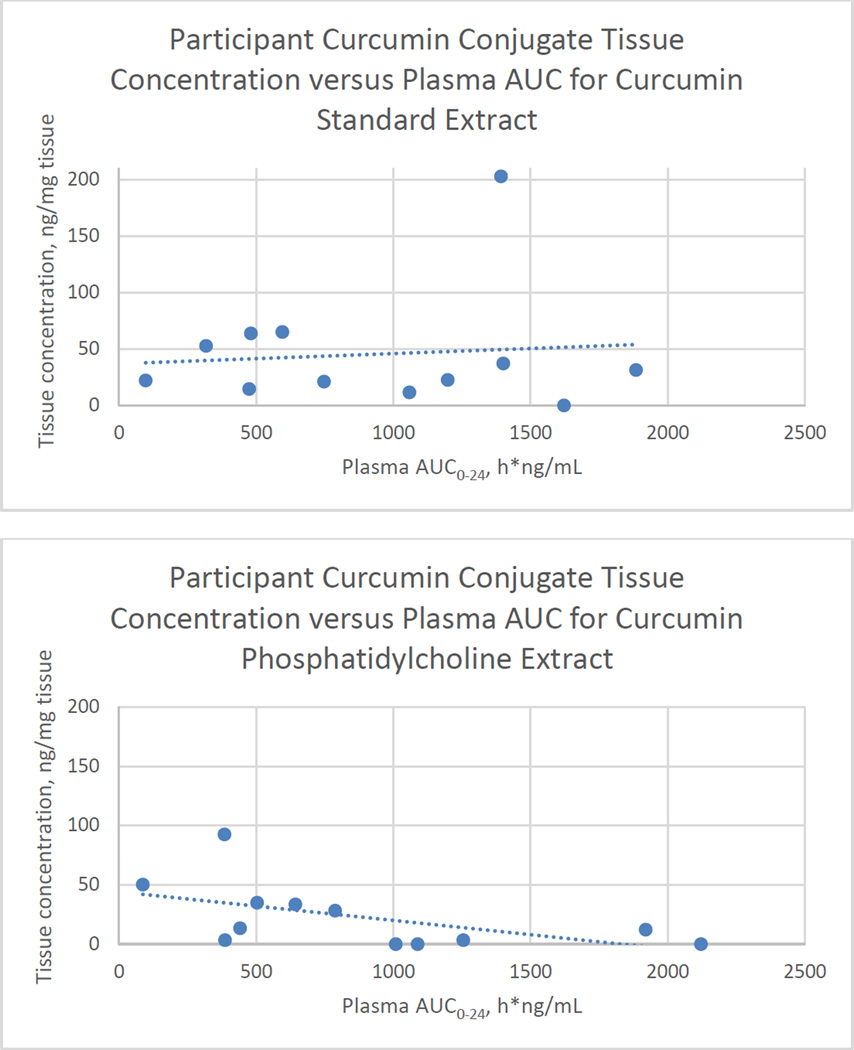
Plasma curcumin AUC0–24 was widely varied among all participants (Figure 2). For participants with plasma curcumin AUC0–24 > 1000 ng*h/mL (high) versus those with AUC0–24 < 1000 ng*h/mL (low), there was no discernible pattern comparing their curcumin AUC0–24 between standard and phosphatidylcholine formulations (ie. participants with low curcumin AUC0–24 after taking standard extract did not appear to uniformly have decreased, increased, or unchanged curcumin AUC0–24 after taking the phosphatidylcholine formulation, data not shown). Trend lines plotting tissue concentration by plasma AUC demonstrated no relationship between tissue and plasma curcumin concentrations for the standard formula but an inverse relationship for the phosphatidylcholine formula (Figure 2).
Figure 2.
Steady-state plasma curcumin area under the curve (AUC0–24) versus tissue curcumin conjugate concentration for individual participants (n=12) by curcumin formulation. Rectal tissue biopsies were obtained 1 hour post-dose after seven consecutive days of curcumin administration. Standard extract dose was 4000 mg mixed curcuminoids; phosphatidylcholine extract dose was 2000 mg, which contains 400 mg mixed curcuminoids. Each point represents a single participant.
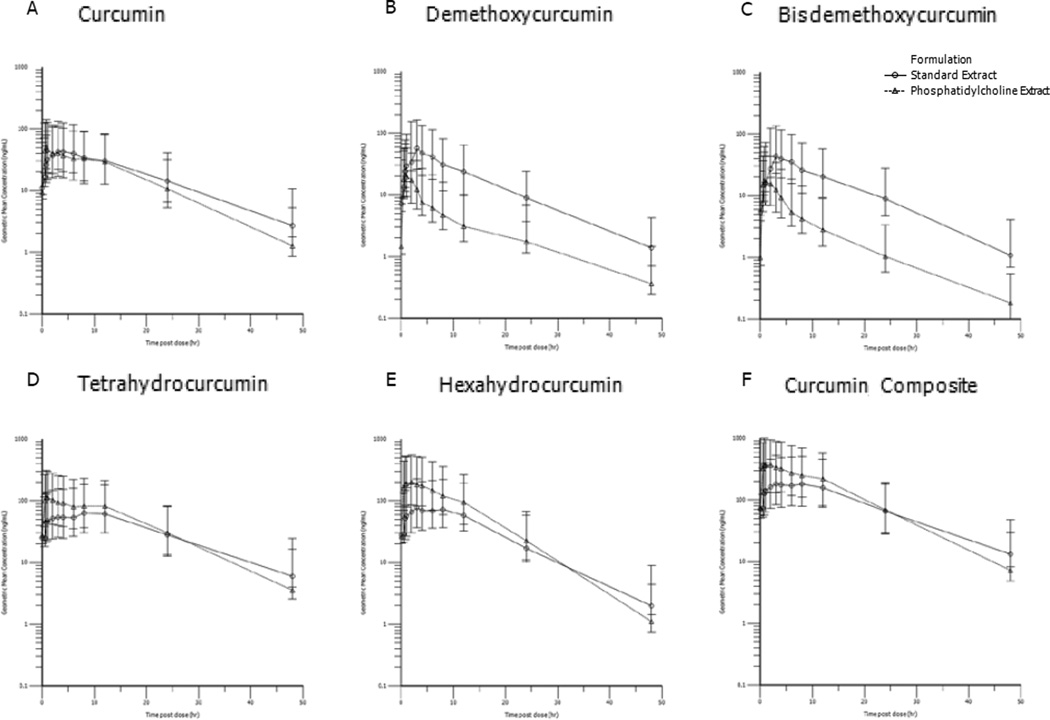
Visual inspection of the terminal elimination phase for curcumin, tetrahydrocurcumin, and hexahydrocurcumin demonstrate longer t1/2 for standard compared to the phosphatidylcholine formulation (Figure 3).
Figure 3.
Geometric mean (95% confidence interval) steady-state plasma concentration versus time for curcuminoid conjugates from standard extract (4 g mixed curcuminoids) and phosphatidylcholine extract (2 g, which is 400 mg mixed curcuminoids). Demethoxycurcumin and bisdemethoxycurcumin are minor constituents of the mixed curcuminoid formulations. Curcumin composite (F) is the composite of curcumin and its reduced metabolites tetrahydrocurcumin and hexahydrocurcumin.
Rectal tissue concentrations
Geometric mean concentrations of curcumin conjugates in rectal mucosa were 363.3 ng/mL for the standard formulation and 177.4 ng/mL for the phosphatidylcholine formulation. Concentrations of demethoxycurcumin and bisdemethoxycurcumin were between 21.0 ng/mL and 28.3 ng/mL for both formulations. Mean parent curcumin concentrations were 7.6 ng/mL (standard) and 2.8 ng/mL (phosphatidylcholine) in rectal mucosa. Parent demethoxycurcumin and bisdemethoxycurcumin were detected but below the level of quantitation. Tetrahydrocurcumin and hexahydrocurcumin were not detected in any of the samples (Table 4).
Table 4.
Geometric mean Rectal Tissue Concentrations of Parent and Curcuminoid Conjugates (N=12)
| Deconjugated curcuminoid | Parent curcuminoid | |||
|---|---|---|---|---|
| Curcuminoida | Standard Extract | PC Extract | Standard Extract | PC Extract |
| Curcumin | 363.3 (213.8–617.3) |
177.4 (72.4–434.8) |
7.6 (2.6–21.7) |
2.8 (0.9–9.1) |
| Demethoxycurcumin | 28.3 (19.6–40.8) |
23.3 (21.0–25.8) |
BLQ | BLQ |
| Bisdemethoxycurcumin | 22.8 (19.4–26.7) |
21.0 (19.9–22.1) |
BLQ | BLQ |
All concentrations are expressed as geometric mean (95% confidience interval) ng/mL.
Tetrahydrocurcumin and hexahydrocurcumin were not detected in any sample.
BLQ – below level of quantitation, PC – Phosphatidylcholine curcumin extract
DISCUSSION
Oral absorption and tissue disposition of curcuminoids from standard curcumin extracts is poor. As a result, numerous curcumin preparations have been developed to increase absorption of curcuminoids. However, few studies have systematically investigated the comparative pharmacokinetics of these preparations. In this study, we compared steady-state plasma and rectal tissue curcuminoid concentrations between standard curcumin and phosphatidylcholine-complexed curcumin preparations. While participants received about 10-fold higher quantities of curcumin when dosed with the standard compared to the phosphatidylcholine preparation, plasma curcumin concentrations, the major curcuminoid in curcumin extracts, were similar (Table 3). Phytosomes, phospholipid-phytochemical complexes, are purported to improve gastrointestinal absorption of poorly water-soluble phytochemicals via the amphipathic properties of the phospholipid18, so it is not surprising to see curcumin plasma AUC0–24 for the phosphatidylcholine preparation was similar to that of the standard extract, even though the curcumin component of the standard extract was nearly 10-fold higher than the phosphatidylcholine extract. Other studies have reported similar improved absorption.11 The curcumin Tmax from the phosphatidylcholine extract was uniformly shorter than from standard extract, so it is likely that phytosomes provide more rapid absorption of curcuminoids. Interestingly, the terminal elimination phases for curcumin, tetrahydrocurcumin, and hexahydrocurcumin appeared to be different between the extracts, suggesting the standard extract may be absorption rate-limited. The dose-adjusted geometric mean ratio of demethoxycurcumin (2.9) and bisdemethoxycurcumin (3.0) were proportionately lower compared to curcumin, suggesting that both demethoxycurcumin and bisdemethoxycurcumin are either more poorly absorbed or more rapidly metabolized than curcumin when taken in the phosphatidylcholine formulation (Table 2). The reason for the disparate plasma concentrations of these curcuminoids between formulations is unclear. Investigators choosing a dose based on purported pharmacodynamic effects from standard powder formulations should bear in mind that improvement in absorption may not be uniform for all curcuminoids with different preparations.
Table 2.
Plasma AUC0–24 and Bioequivalence for Curcuminoid Conjugates(N=12)
| Standard Extract | PC Extract | PC:Standardratioa | |
|---|---|---|---|
| Curcumin | |||
| Dose-adjusted AUC0–24 | 0.25 (0.11–0.39) | 2.21 (0.73–3.69) | 8.8 (5.0–15.4) |
| Actual AUC0–24 | 731.6 (159–1957) | 669.4 (141–3188) | |
| Demethoxycurcumin | |||
| Dose-adjusted AUC0–24 | 0.77 (3.4–1.20) | 2.24 (0.26–4.23) | 2.9 (1.7–5.2) |
| Actual AUC0–24 | 674.5 (184–1343) | 152.5 (29–268) | |
| Bisdemethoxycurcumin | |||
| Dose-adjusted AUC0–24 | 2.97 (1.08–4.86) | 8.89 (4.08–13.70) | 3.0 (1.5–5.9) |
| Actual AUC0–24 | 593.8 (134–1064) | 124.5 (88–259) | |
All values represented as h*ng/mLcurcuminoid, expressed as geometric mean (90% CI), τ=24 hrs.
Test of average bioequivalence - geometric mean ratio (90% CI), using dose-adjusted curcuminoid doses in mg (Standard vs PC extract): curcumin (2920 vs 303), demethoxycurcumin (880 vs 68), bisdemethoxycurcumin (200 vs 14).
AUC – Area under concentration curve, PC – Phosphatidylcholine curcumin extract
The optimal dosing schedule of curcumin extracts has been unclear. Curcuminoids are rapidly metabolized, primarily to conjugates, leading to low or undetectable plasma and tissue concentrations of the parent compounds, whereas high glucuronide and sulfate conjugates are most often detected and reported after enzymatic deconjugation. Pfeiffer, et al.19 have suggested that the conjugates themselves are pharmacologically active, but the precise mechanisms for observed pharmacodynamic effects of curcumin extracts remain unknown. Previous pharmacokinetic studies of curcumin extracts have primarily reported results based on a single dose administration.9,11,20–22 In our study, participants received curcumin extracts for seven consecutive days. Steady-state curcumin conjugate concentrations appear to provide constant plasma exposures when given once daily at the doses used in this study (Figure 3). Peak and AUC0–48 concentrations were higher for phosphatidylcholine compared to standard extract, suggesting the phosphatidylcholine preparation may be an excellent agent to target tetrahydrocurcumin and hexahydrocurcumin concentrations (Table 3).
The concentration-time curves observed for the curcuminoids (Figure 3) may reflect either differences in dissolution profiles or enterohepatic recycling, which can result in their delayed elimination because conjugates can be cleaved in the gut and returned to systemic circulation as unchanged parent. Secondary peaks observed in the plasma concentration-time profiles between 5 and 15 hours post-dose appear well beyond the normal time window associated with formulation dissolution time, gastric emptying, and small intestinal transit, making delayed gastrointestinal transit an unlikely cause for this observation. Although curcuminoids are thought to be rapidly conjugated in the gut, enterohepatic circulation may ensure prolonged systemic and gastrointestinal exposure to curcuminoids and explain their purported pharmacodynamic effects in spite of rapid conjugation.
Rapid absorption of the phosphatidylcholine extract in the upper gastrointestinal tract may be a concern for investigators intending to target gastrointestinal tissues, presuming intestinal exposure is important for pharmacodynamic effect. It is interesting that we did not see detectable levels of tetrahydrocurcumin or hexahydrocurcumin in any tissue samples (Table 4). Tetrahydrocurcumin and hexahydrocurcumin are reduction products of curcumin and were found in high concentration in plasma. Therefore, their absence in rectal mucosa suggest mucosal concentrations are a result of luminal absorption rather than plasma disposition. Although it is possible that rectal mucosa may rapidly clear tetrahydrocurcumin and hexahydrocurcumin, there is currently no physiologic explanation why tetrahydrocurcumin and hexahydrocurcumin would be selectively cleared while other curcuminoids remain, making this a less likely interpretation of our data.
Prior studies suggested a 20-fold difference in PK parameters between the formulations.11 While the two formulations appeared to be non-equivalent, with the phosphatidylcholine extract nominally superior than the standard extract, we consistently found the differences to be less than 20-fold (eg. for AUC0–24: curcumin 8.8, demethoxycurcumin 2.9, bisdemethoxycurcumin 3.0). Notably, these differences were not consistent among the curcuminoids.
Limitations
The phosphatidylcholine extract was taken as a loose powder mixed directly into applesauce while the standard extract was a tablet that was taken concurrently with applesauce. The time to maximum concentration we reported for the phosphatidylcholine powder, therefore, may be shorter than its capsule form, which is how Meriva is commonly supplied.
Participants may not have accurately reported adherence. Because the phosphatidylcholine extract required an additional step to administer (i.e. mix into applesauce) and the powder is known to stain yellow, it is possible there may have been more non-adherence during that dosing period. Since participants were given a single curcumin dose immediately prior to PK estimations, it is possible that our comparisons may not completely reflect steady-state concentrations of the formulations, especially if the doses of the phosphatidylcholine extract were missed immediately preceding the PK visit. However, for both formulations during PK sampling, 9 of 12 participants had a predose trough curcumin plasma concentration between the 12 and 24 hour concentrations for that sampling period, indicating that a dose was most likely taken sometime between 12 and 24 hours prior to the PK sampling. While this does not assure we detected steady-state concentrations, it reduces the risk of differential bias between the two formulations due to non-adherence.
CONCLUSION
Improvements in curcuminoid absorption due to phosphatidylcholine are not uniform across the curcuminoids. Furthermore, curcuminoid exposures in the intestinal mucosa are most likely due to luminal exposure rather than plasma disposition. Finally, once-daily dosing is sufficient to maintain detectable curcuminoids at steady-state in both plasma and rectal tissues.
Acknowledgments
Special thanks to Philip Smith, Julie Dumond, Curtis Johnson, Erick Borg, Benjamin Greener, Kimberly Jeremiah, Danielle Schlafer, Kristine Chambers, and Tresor Medju for clinical research and analytical support.
FUNDING
This work was supported by the National Institutes of Health grants KL2TR00084 (GNA), P50CA106991, P30DK034987, and AT003892-03 (YX, RLH). This work was also supported in part by the Intramural Research Program of the National Institute on Aging (MS, KSSD, RM). Meriva was generously supplied by Indena S.p.A.
Footnotes
No authors are Fellows of the FCP.
Disclosures: There were no conflicts of interest for any authors.
AUTHOR CONTRIBUTIONS
All authors contributed as follows: wrote manuscript (GNA, RM, RLH), designed research (GNA, ADMK, RSS, RLH), performed research (GNA, YX, RM, MS, KSSD), analyzed data (GNA, YX, RM), and contributed new reagents /analytical tools (YX, RM, ADMK, RLH). All authors participated in critical revisions of the manuscript and have given approval of the current manuscript version.
REFERENCES
- 1.ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products. Exeter, UK: Thieme; 2003. [Google Scholar]
- 2.Asher GN, Spelman K. Clinical utility of curcumin extract. Altern Ther Health Med. 2013 Mar-Apr;19(2):20–22. [PubMed] [Google Scholar]
- 3.Sandur SK, Pandey MK, Sung B, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007 Aug;28(8):1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 4.Metzler M, Pfeiffer E, Schulz SI, Dempe JS. Curcumin uptake and metabolism. Biofactors. 2013 Jan-Feb;39(1):14–20. doi: 10.1002/biof.1042. [DOI] [PubMed] [Google Scholar]
- 5.Hoehle SI, Pfeiffer E, Solyom AM, Metzler M. Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J Agric Food Chem. 2006 Feb 8;54(3):756–764. doi: 10.1021/jf058146a. [DOI] [PubMed] [Google Scholar]
- 6.Garcea G, Berry DP, Jones DJ, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol.Biomarkers Prev. 2005;14(1):120–125. [PubMed] [Google Scholar]
- 7.Irving GR, Howells LM, Sale S, et al. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration--a clinical pilot study including assessment of patient acceptability. Cancer Prev Res (Phila) 2013 Feb;6(2):119–128. doi: 10.1158/1940-6207.CAPR-12-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer Lett. 2008 Aug 18;267(1):133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Kanai M, Imaizumi A, Otsuka Y, et al. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother Pharmacol. 2012 Jan;69(1):65–70. doi: 10.1007/s00280-011-1673-1. [DOI] [PubMed] [Google Scholar]
- 10.Storka A, Vcelar B, Klickovic U, et al. Safety, tolerability and pharmacokinetics of liposomal curcumin in healthy humans. Int J Clin Pharmacol Ther. 2015 Jan;53(1):54–65. doi: 10.5414/CP202076. [DOI] [PubMed] [Google Scholar]
- 11.Cuomo J, Appendino G, Dern AS, et al. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod. 2011 Apr 25;74(4):664–669. doi: 10.1021/np1007262. [DOI] [PubMed] [Google Scholar]
- 12.Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007 Jul;60(2):171–177. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 13.Clark AM, Pellock JM, Holmay M, Anders B, Cloyd J. Clinical utility of topiramate extended-release capsules (USL255): Bioequivalence of USL255 sprinkled and intact capsule in healthy adults and an in vitro evaluation of sprinkle delivery via enteral feeding tubes. Epilepsy Behav. 2016 Apr;57(Pt A):105–110. doi: 10.1016/j.yebeh.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 14.Youssef NN, Tron E, Tolia V, Hamer-Maansson JE, Lundborg P, Illueca M. Single-dose pharmacokinetic properties of esomeprazole in children aged 1 – 11 years with endoscopically proven GERD: a randomized, open-label study. Int J Clin Pharmacol Ther. 2014 Nov;52(11):965–972. doi: 10.5414/CP201971. [DOI] [PubMed] [Google Scholar]
- 15.Wytiaz V, Homko C, Duffy F, Schey R, Parkman HP. Foods provoking and alleviating symptoms in gastroparesis: patient experiences. Dig Dis Sci. 2015 Apr;60(4):1052–1058. doi: 10.1007/s10620-015-3651-7. [DOI] [PubMed] [Google Scholar]
- 16.Keku TO, Lund PK, Galanko J, Simmons JG, Woosley JT, Sandler RS. Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2005 Sep;14(9):2076–2081. doi: 10.1158/1055-9965.EPI-05-0239. [DOI] [PubMed] [Google Scholar]
- 17.Martin C, Connelly A, Keku TO, et al. Nonsteroidal anti-inflammatory drugs, apoptosis, and colorectal adenomas. Gastroenterology. 2002 Dec;123(6):1770–1777. doi: 10.1053/gast.2002.37053. [DOI] [PubMed] [Google Scholar]
- 18.Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 2009 Sep;14(3):226–246. [PubMed] [Google Scholar]
- 19.Pfeiffer E, Hoehle SI, Walch SG, Riess A, Solyom AM, Metzler M. Curcuminoids form reactive glucuronides in vitro. J Agric Food Chem. 2007 Jan 24;55(2):538–544. doi: 10.1021/jf0623283. [DOI] [PubMed] [Google Scholar]
- 20.Lao CD, Ruffin MT, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC.Complement Altern.Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki H, Sunagawa Y, Takahashi K, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 2011;34(5):660–665. doi: 10.1248/bpb.34.660. [DOI] [PubMed] [Google Scholar]
- 22.Vareed SK, Kakarala M, Ruffin MT, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008 Jun;17(6):1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]