Abstract
The success of epilepsy surgery is highly dependent on correctly identifying the entire epileptogenic region. Current state-of-the-art for localizing the extent of surgically amenable areas involves combining high resolution three-dimensional magnetic resonance imaging (MRI) with electroencephalography (EEG) and magnetoencephalography (MEG) source modeling of interictal epileptiform activity. Coupling these techniques with newer quantitative structural MRI techniques, such as cortical thickness measurements, however, may improve the extent to which the abnormal epileptogenic region can be visualized. In this review we assess the utility of EEG, MEG and quantitative structural MRI methods for the evaluation of patients with epilepsy and introduce a novel method for the co-localization of a structural MRI measurement to MEG and EEG source modeling. When combined, these techniques may better identify the extent of abnormal structural and functional areas in patients with medically intractable epilepsy.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-016-0506-7) contains supplementary material, which is available to authorized users.
Keywords: MEG, EEG, MRI, Epilepsy, Imaging, Focal cortical dysplasia
Introduction
Epilepsy is a chronic medical condition which can detrimentally impact daily living and quality of life. It affects almost 70 million people worldwide, with up to one third of these cases being refractory to medications [1, 2]. The mortality rate for medically refractory epilepsy is five times that of the general population, underscoring the importance of improving the detection and treatment of this condition [2]. With respect to studies of this cohort, the International League Against Epilepsy (ILAE) task force recently created a working definition for refractory epilepsy, which better enables comparison between research findings, as previously the majority used varying definitions, with the most common being failure of two anti-epileptic drugs (AEDs) [3].
For medically refractory patients, non-pharmacologic treatment options include dietary treatment, resective surgery, transection, neurostimulation, and laser ablation. Unfortunately, most of these treatment options do not have well defined outcomes, or have a low rate of favorable outcomes. For those with temporal lobe epilepsy, resective surgery is by far the best option when looking at all temporal-lobe epilepsy patients, including those with mesial temporal sclerosis (MTS) as well as other causes such as cortical malformations [4]. Several different studies have assessed the outcomes of patients with temporal [4, 5] as well as extratemporal epilepsy [6–8] who have undergone surgery. These studies show that those with MTS have the best outcomes followed by those with temporal and then extratemporal lobe epilepsy (see Table 1). There are, however, no studies that compare extratemporal lobe surgery to medical management. For patients with frontal lobe epilepsy, which is the second most common following temporal lobe onset, further medical management is often considered prior to surgery, though surgery still shows improved outcomes for a number of individuals (27% seizure free in one study [8]). While the lobe affected influences seizure freedom rates, these reported outcomes may be due to the extent of the resection. One recent review of the literature concluded that complete resection of the anatomic and physiologic epileptogenic zone is the only predictor of postoperative seizure freedom [9]. Given the lack of treatment options with proven odds of a positive outcome in pharmacoresistent epilepsy, some of these patients undergo epilepsy surgery even when the standard presurgical evaluation provides relatively imprecise localizing information.
Table 1.
Seizure-free outcomes (Engle Class I) at 1 to 5 years based on pathology location/type
| Type of study | Length of follow-up | MTS | Exclusively temporal pathology (non-MTS) | Extratemporal | |
|---|---|---|---|---|---|
| Wiebe et al. [4] | RCT | 1 year | 85% (all types of TLE)* | ||
| Engel et al. [5] | RCT | 2 years | 73% (all types of TLE)* | ||
| Sisodiya et al. [6] | Meta-analysis | 1 year 2 years |
42% 35% |
34% 38% |
|
| Fauser et al. [7] | Retrospective cohort | 2 years | 52-67%** | ||
| Tellez-Zenteno et al. [8] | Meta-analysis | 5 years | 66% | 46% (occipital) 27% (frontal) |
|
MTS mesial temporal sclerosis, TLE temporal lobe epilepsy, RCT randomized controlled trial
*Differentiation was not made between patients with MTS and other temporal pathologies
**Separate outcomes for temporal and extratemporal were not provided. 49% of patients had temporal (non-HC) pathology, 26% extratemporal (frontal > occipital > parietal > insular), 23% multilobar, and 3% hemispheric
Since the literature suggests that more definitive localization of the structural and functional epileptogenic zones should theoretically improve epilepsy surgery outcomes [10], and recent advances in diagnostic and therapeutic tools may provide the keys for this [11], studies are needed to determine if this can be achieved in practice. Localizing the epileptogenic zone relies on accurate characterization of the anatomic region involved with imaging and electrophysiologic tools. Patients with complete resection of the anatomic and physiologic epileptogenic zone are most likely to become seizure free [12]. Patients who have complete resection of either the anatomic or physiologic lesion alone achieve seizure freedom up to 50% of the time. In patients where the resection of both the anatomic and functional epileptogenic zones is incomplete, seizure freedom is unlikely [12]. Therefore, utilization of newer neuroimaging modalities and evaluation with a multimodal approach may provide a more complete characterization of the epileptogenic zone.
In this review, we present the advantages and limitations for the available techniques used for the localization of structural and functional epileptogenic regions. In addition, we introduce ideas for future improvement of current techniques, including a novel method for the identification of the extent and concordance of structural and functional abnormalities.
Current Advantages and Limitations in the Detection of Epileptogenic Regions (Using Structural MRI)
Detecting lesions with MRI is paramount for the successful removal of the patient’s epileptogenic region. The most common structurally identifiable seizure etiology in children and the second most common in adult surgical patients is cortical malformations, particularly focal cortical dysplasias (FCDs) [13–16]. Ideally, FCDs would be identified pre-surgically with visual inspection of MRI. A study by Colombo et al. [10] however, found only 61% of histologically verified FCDs were identified by visual inspection of MRI. As imaging technology improves more of these lesions are being detected, though there are still a sizable number of patients with FCDs that are not routinely identified by current MRI technology. This may be due to the fact that there are several characteristics of FCDs that are more subtle to recognize by visual analysis including: abnormal thickness of the cortex, blurring of the boundaries between gray and white matter, gray or white matter signal abnormalities, and subtle gyral pattern changes [17]. In addition, resecting the entire epileptogenic zone becomes even more difficult in these MRI-negative patients. Similarly, other studies have shown that up to 50-80% of FCDs, later confirmed by postoperative histologic studies, escape routine visual inspection with high resolution MRI [18, 19]. Finally, incomplete resection of the lesion due to poor visualization of the malformation’s boundaries may contribute to poor surgical outcomes in these patients [6, 20]. This stresses the importance of using additional tools, such as quantitative morphometric analysis techniques, to identify the presence and extent of the structural and functional abnormality.
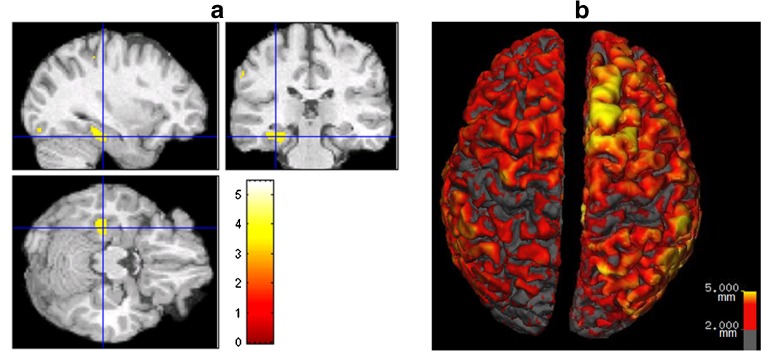
Quantitative morphometric analyses of MRI images are relatively recently developed techniques which have been shown to be useful in detecting structural lesions in some patients with MRI-negative scans. A number of different analysis methods exist including: voxel-based morphometry (VBM), cortical morphometry, and sulcal curvature analysis (Table 2). While each has limitations, they all have been shown to improve the sensitivity of detecting subtle lesions in individual epilepsy patients [19, 21–28]. Most of these studies use VBM to compare an individual patient’s MRI to a group of normal controls. The process involves coregistration of the patient’s brain to an average template and then calculating statistical differences between the individual brain and the control brains. Figure 1a shows an example of one patient from the Epilepsy Center at Rush University Medical Center, who was initially characterized as non-lesional. When VBM was used to compare this patient to a group of healthy controls, a statistically significant area of abnormality was revealed in the left temporal lobe (image in Fig. 1a is neurologically oriented). This example along with the studies referenced above demonstrate the utility of VBM as an additional tool for the assessment of FCDs [22–24, 27]. Bonilha et al. [22] reported that, in 64% of patients (7 out of 11), VBM analysis identified abnormalities in gray matter that extended beyond the area of visually detected FCDs. In a validation study, however, Mehta et al. [29] revealed that several commonly used VBM methods had low sensitivity and could not delineate the spatial extent of lesions when compared to expert visual tracing analysis. This may be due to the fact that the patient’s brains are morphologically distorted to fit the template, which has led to the implementation of other techniques that may improve these deficiencies.
Table 2.
Techniques, uses and limitations of different quantitative morphometric methods and sample studies using them
| Technique | Uses and limitations | Studies using this technique | Study findings |
|---|---|---|---|
| Voxel-based Morphometry (VBM) | Coregistration of the patient's brain to an average template and then calculating statistical differences between the individual brain and the control brains Primarily used to detect differences in gray- matter concentration/density May miss small, spatially restricted lesions due to voxel-wise averaging and volume- based averaging |
Bonilha et al. [22] Colliot et al. [23, 42] Kassubek et al. [24] Wilke et al. [27] Bruggemann et al. [44] Mehta et al. [29] |
VBM identified abnormalities in gray matter that extend beyond the area defined as a FCD by MRI VBM identified most of the FCDs, but VBM may be unable to detect more subtle lesions without strong signal intensities VBM is a useful screening tool for FCDs VBM can be used to detect cortical malformations with a high degree of accuracy. VBM may be helpful in detecting subtle dysplasia in MRI negative patients but it is ineffective for precise delineation of lesion margins Several VBM techniques had low sensitivity when compared to expert visual tracing analysis |
| Surface-based Morphometry (SBM) | Surface-based coregistration methods align specific cortical sulci and gyri across brains. This allows a more precise matching and comparison of anatomical structures across subjects when compared to VBM. | Bessen et al. [21] Hong et al. [25] Thesen et al. [19] |
Used proprietary software to detect small FCDs Used proprietary software to perform analysis which aids in detecting FCDs in MRI negative patients Cortical thickness measurements with FreeSurfer software, in epilepsy patients, can be helpful in identifying more subtle lesions on MRI |
| Sulcal curvature analysis | Measurement of sulcal patterns, orientation and depth. | Ronan et al. [26] Kim et al. [43] |
Used FreeSurfer to analyze curvature and found it helpful to identify subtle FCDs Used specific proprietary software to evaluate sulcal patterns in TLE patients verses controls |
TLE temporal lobe epilepsy, FCD focal cortical dysplasia
Fig. 1.
(a). Neurologically-oriented MRI images in coronal, axial and sagittal planes showing a color map of significantly (threshold of p = 0.001) different voxels in one patient with left temporal lobe epilepsy (age 27), who was initially considered non-lesional, compared to a group of 30 healthy controls (mean age 25). The colors represent the t values shown on the color bar. (b). 3D view of the cortical thickness analysis, using FreeSurfer, on a different patient initially deemed non-lesional. Increased cortical thickness was found in the superior and medial surface of the right superior frontal gyrus (yellow area)
FreeSurfer software is a powerful tool for examining the thickness of the cortical mantel that improves upon the short-comings of the VBM technique mentioned above. An advantage of FreeSurfer is that it has been validated against postmortem tissue measurements [30, 31] as well as manual MRI measurements [32, 33]. Only one published study has used FreeSurfer for the measurement of cortical thickness in the detection of epileptogenic cortical malformations in individual epilepsy patients [19]. With this technique, Thesen et al. [19] showed 100% sensitivity and 84% specificity for the identification of cortical malformations when using cortical thickness measurements alone. In addition, they reported that all patients who went to surgery were seizure free after a median follow-up period of 1.7 years and all had abnormal pathology of the resected tissue. Figure 1b shows an example of a patient, from the Epilepsy Center at Rush University Medical Center, who was initially deemed non-lesional. Cortical thickness analysis using the FreeSurfer software, however, revealed increased cortical thickness measurements in the right superior frontal gyrus. The full extent of the cortical thickness abnormality can be identified, using the software’s three-dimensional (3D) viewing, and resected in its entirety, if feasible.
EEG and MEG
Noninvasive recording of cerebral electric activity is currently done by directly sampling from the scalp using EEG or with magnetosensors close to the scalp using MEG. EEG and MEG each have unique advantages and disadvantages for the 3D localization of abnormal electrical activity.
EEG is the oldest and most utilized method for assessing the location of patients’ ictal and interictal epileptiform activity. Using the most common set-up, standard 10-20 system and additional temporal lobe coverage, EEG uses approximately 25 sampling electrodes to assess electrical brain activity. Additional electrodes can be added easily to EEG if desired, which can be helpful in improving localization. EEG is also relatively inexpensive, particularly when compared to MEG. Since the electrodes are affixed directly to the scalp, EEG is less sensitive to movement artifact than other techniques that require a fixed position such as MEG. This ultimately allows for better localization of the ictal onset zone, especially when there is movement during a seizure. Both EEG and MEG have very high temporal resolution, making them advantageous over other modalities such as MRI or PET. The biggest disadvantage of scalp EEG is low spatial resolution, requiring the involvement of large areas of brain tissue (lower limit of 6 centimeters [34]) to detect a spike. Finally, while EEG samples overlying cortex well, it can incorrectly localize tangential activity deep within a sulcus which is in sharp contrast to MEG.
MEG is a useful non-invasive diagnostic tool for identifying the location of interictal activity in patients with intractable epilepsy. Modern day MEG offers whole head coverage with groups of sensors positioned in over 100 locations. This coverage, when combined with the superimposition on MRI, called magnetic source imaging (MSI), can improve the localization of interictal discharges [35]. Besides offering superior head coverage, another advantage of MEG is that it has optimal sensitivity for generators of epileptiform activity with a tangential orientation to the skull. This characteristic complements EEG well, since EEG is more sensitive to radially oriented sources. MEG’s ability to detect epileptiform discharges parallel to the skull allows the identification of such activity deep within a sulcus, where scalp EEG detection is more difficult. Therefore, because of these characteristics mentioned above, MEG can be especially helpful in regions of cortical malformations with unique cortical orientations.
MEG has been found to be useful in locating areas of epileptogenicity in FCDs [36]. It is especially useful in pinpointing small FCDs that were not initially found on MRI [37, 38]. Wilenius et al. [37] concluded that this may be the case because FCDs seem to be preferentially located at the bottom of deep sulci at an angle tangential to the surface.
While extracranial EEG is utilized for all epilepsy patients, for individuals with more difficult to localize foci, MEG is often helpful in planning for surgery and for reducing the amount of intracranial EEG needed. Zhang et al. [28] reviewed several studies and concluded that MEG is statistically equivalent to intracranial EEG in localizing the seizure focus.
Future Directions: Combining Structural and Functional Methods
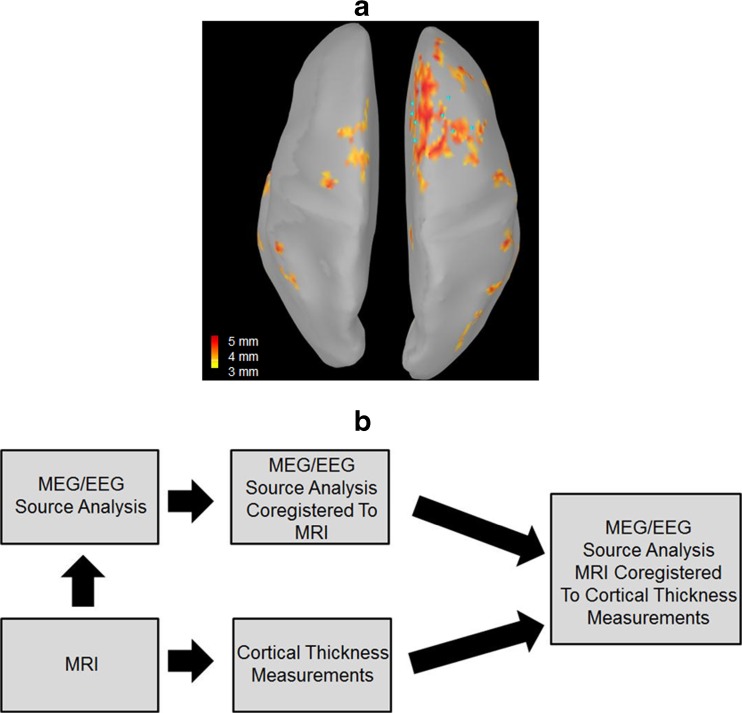
Studies have shown that coregistration of multimodal techniques improves the detection of epileptogenic abnormalities and ultimately the surgical outcomes in patients with epilepsy [39–41]. To our knowledge, no studies have examined the feasibility of superimposing functional 3D source analysis data onto quantitative structural analysis results (for example, cortical thickness measurements derived from FreeSurfer). Figure 2 shows an example of how this looks in a patient with intractable frontal lobe epilepsy. To generate this image, 3D dipoles were generated from MEG and EEG interictal data and then coregistered to the patients MRI. This rendering was then coregistered to the cortical thickness maps generated by FreeSurfer software suite and displayed in 3D. The dark red areas are regions of abnormal cortical thickness and the light blue dots are the patient’s MEG/EEG interictal dipole source modeling locations. Just by visualizing this figure one can appreciate the advantage of a visual display of both functional and structural data together in one image. In the future, techniques like this may better guide grid implantation and resection during surgery in a small subset of surgical patients. Studies are needed, however, to validate if this provides advantages over current methods.
Fig. 2.
(a). 3D view, from the patient shown in Fig. 1b, of the region of abnormal cortical thickness (shown in dark red) with superimposed MEG/EEG source modeling (light blue dots) of the patient’s interictal epileptiform activity. (b). Diagram showing the steps taken to generate the image in a
Conclusion
In this review we assess the progress that has been made in identifying abnormalities in intractable epilepsy, particularly those that are difficult to detect like FCDs. As it currently stands, MEG provides a complimentary method to assess sources that may be difficult to detect with EEG. In addition, quantitative MRI techniques may improve the discovery of difficult to visualize cortical abnormalities. Future work entails combining structural and functional data into one image to best visualize surgical planning. Future studies will be needed to evaluate the sensitivity and specificity of the combination of these methods with surgical outcome data and if the addition of other modalities may improve the accuracy even further.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 1224 kb)
Acknowledgments
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-016-0506-7) contains supplementary material, which is available to authorized users.
References
- 1.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling MR, Feldman H, Kinman J, Liporace JD, O'Connor MJ. Seizure control and mortality in epilepsy. Ann Neurology. 1999;46:45–50. doi: 10.1002/1531-8249(199907)46:1<45::AID-ANA8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Kwan P, Arzimanoqlou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshe SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2000;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 4.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized controlled trial of surgery for temporal lobe epilepsy. New England Journal of Medicine. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 5.Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling M, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K. Early surgical therapy for drug-resistant temporal lobe epilepsy. JAMA. 2012;307:922–930. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sisodiya SM. Surgery for malformations of cortical development causing epilepsy. Brain. 2000;123:1075–1091. doi: 10.1093/brain/123.6.1075. [DOI] [PubMed] [Google Scholar]
- 7.Fauser S, Essang C, Altenmuller D, Staack AM, Steinhoff J, Strobl K, Bast T, Schubert-Bast S, Stephani U, Wiegand G, Prinz M, Brandt A, Zentner J, Schulze-Bonhage A. Long-term seizure outcome in 211 patients with focal cortical dysplasia. Epilepsia. 2015;56:66–76. doi: 10.1111/epi.12876. [DOI] [PubMed] [Google Scholar]
- 8.Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 9.Perry M, Duchowny M. Surgical versus medical treatment for refractory epilepsy: Outcomes beyond seizure control. Epilepsia. 2013;54:2060–2070. doi: 10.1111/epi.12427. [DOI] [PubMed] [Google Scholar]
- 10.Columbo N, Tassi L, Calli C, Citterio A, Russo GL, Scialfa G, Spreafico R. Focal cortical dysplasia: MR imaging, histopathologic, and clinical correlations in surgically treated patients with epilepsy. Am J Neuroradiol. 2003;24:724–733. [PMC free article] [PubMed] [Google Scholar]
- 11.Bernasconi A, Antel SB, Collins DL, Bernasconi N, Olivier A, Dubeau F, Pike GB, Andermann F, Arnold D. Texture analysis and morphological processing of magnetic resonance imaging assist detection of focal cortical dysplasia in extra-temporal partial epilepsy. Annals of Neurology. 2001;49(6):770–775. doi: 10.1002/ana.1013. [DOI] [PubMed] [Google Scholar]
- 12.Perry MS, Dunvoyer C, Dean P, Bhatia S, Ragheb J, Miller I, Resnick T, Jayakar P, Duchowny M. Predictors of seizure freedom after incomplete resection in children. Neurology. 2010;75:1448–1453. doi: 10.1212/WNL.0b013e3181f88114. [DOI] [PubMed] [Google Scholar]
- 13.Colombo N, Salamon N, Raybaud C, Ozkara C, Barkovich J. Imaging of malformations of cortical development. Epileptic Disorders. 2009;11:194–205. doi: 10.1684/epd.2009.0262. [DOI] [PubMed] [Google Scholar]
- 14.Harvey AS, Cross JH, Shinnar S, Mathern GW. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49:146–155. doi: 10.1111/j.1528-1167.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 15.Lerner JT1, Salamon N, Hauptman JS, Velasco TR, Hemb M, Wu JY, Sankar R, Donald Shields W, Engel J Jr, Fried I, Cepeda C, Andre VM, Levine MS,Miyata H, Yong WH, Vinters HV, Mathern GW. Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: a critical review and the UCLA experience. Epilepsia. 2009;50:1310-35. [DOI] [PubMed]
- 16.Raymond AA, Fish DR, Sisodiya SM, Alsanjari N, Stevens JM, Shorvon SD. Abnormalities of gyration, heterotopias, tuberous sclerosis, focal cortical dysplasia, microdysgenesis, dysembryoplastic neuroepithelial tumour and dysgenesis of the archicortex in epilepsy. Clinical, EEG and neuroimaging features in 100 adult patients. Brain. 1995;118:629–660. doi: 10.1093/brain/118.3.629. [DOI] [PubMed] [Google Scholar]
- 17.Columbo N, Salamon N, Raybaud C, Ozkara C, Barkovich AJ. Imaging of malformations of cortical development. Epileptic Disord. 2009;11:194–205. doi: 10.1684/epd.2009.0262. [DOI] [PubMed] [Google Scholar]
- 18.Besson P, Andermann F, Dubeau F, Bernasconi A. Small focal cortical dysplasia lesions are located at the bottom of a deep sulcus. Brain. 2008;131:3246–3255. doi: 10.1093/brain/awn224. [DOI] [PubMed] [Google Scholar]
- 19.Thesen T, Quinn BT, Carlson C, Devinsky O, DuBois J, McDonald CR, French J, Leventer R, Felsovalyi O, Halgren E, Kuzniecky R. Detection of epileptogenic cortical malformations with surface-based MRI morphometry. PLoS ONE. 2011;6:e16430. doi: 10.1371/journal.pone.0016430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmini A, Andermann F, Olivier A, Tampieri D, Robitaille Y, Andermann E, et al. Focal neuronal migration disorders and intractable partial epilepsy: a study of 30 patients. Ann Neurol. 1991;30:741–9. doi: 10.1002/ana.410300602. [DOI] [PubMed] [Google Scholar]
- 21.Besson P, Bernasconi N, Colliot O, Evans A, Bernasconi A. Surface-based texture and morphological analysis detects subtle cortical dysplasia. Med Image Comput Comput Assist Interv. 2008;11:645–652. doi: 10.1007/978-3-540-85988-8_77. [DOI] [PubMed] [Google Scholar]
- 22.Bonilha L, Montenegro MA, Rorden C, Castellano G, Guerreiro MM, Cendes F, Lui LM. Voxel-based morphometry reveals excess gray matter concentration in patients with focal cortical dysplasia. Epilepsia. 2006;47:908–915. doi: 10.1111/j.1528-1167.2006.00548.x. [DOI] [PubMed] [Google Scholar]
- 23.Colliot O, Mansi T, Bernasconi N, Naessens V, Klironomos D, Bernasconi A. Segmentation of focal cortical dysplasia lesions on MRI using level set evolution. Neuroimage. 2006;32:1621–1630. doi: 10.1016/j.neuroimage.2006.04.225. [DOI] [PubMed] [Google Scholar]
- 24.Kassubek J, Huppertz HJ, Spreer J, Schulze-Bonhage A. Detection and localization of focal cortical dysplasia by voxel-based 3-D MRI analysis. Epilepsia. 2002;43:596–602. doi: 10.1046/j.1528-1157.2002.41401.x. [DOI] [PubMed] [Google Scholar]
- 25.Hong SJ, Kim H, Schrader D, Bernasconi N, Bernhardt BC, Bernasconi A. Automated detection of cortical dysplasia type II in MRI-negative epilepsy. Neurology. 2014;83:48–55. doi: 10.1212/WNL.0000000000000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ronan L, Scanlon C, Murphy K, Maguire S, Delanty N, Doherty C, Fitzsimons M. Cortical curvature analysis in MRI-negative temporal lobe epilepsy: A surrogate marker for malformations of cortical development. Epilepsia. 2011;52:28–34. doi: 10.1111/j.1528-1167.2010.02895.x. [DOI] [PubMed] [Google Scholar]
- 27.Wilke M, Kassubek J, Ziyeh S, Schulze-Bonhage A, Huppertz HJ. Automated detection of gray matter malformations using optimized voxel-based morphometry: a systematic approach. NeuroImage. 2003;20:330–343. doi: 10.1016/S1053-8119(03)00296-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Liu W, Chen H, Xia H, Zhou Z, Mei S, Liu Q, Li Y. Multimodal neuroimaging in presurgical evaluation of drug-resistant epilepsy. Neuroimage Clinical. 2013;4:35–44. doi: 10.1016/j.nicl.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta S, Grabowski TJ, Damasio H. Evaluation of voxel-based morphometry for focal lesion detection in individuals. Neuroimage. 2003;20:1438–1454. doi: 10.1016/S1053-8119(03)00377-X. [DOI] [PubMed] [Google Scholar]
- 30.Cardinale F, Chinnici G, Bramerio M, Mai R, Sarori I, Cossu M, Lo Russo G, Castana L, Columbo N, Caborni C, De Moni E, Ferrigno G. Validation of Freesurfer-estimated brain cortical thickness: comparison with histologic measurements. Neuroinformatics. 2014;12:535–542. doi: 10.1007/s12021-014-9229-2. [DOI] [PubMed] [Google Scholar]
- 31.Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/WNL.58.5.695. [DOI] [PubMed] [Google Scholar]
- 32.Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 33.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. 2004. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 34.Ebersole JS. Defining epileptogenic foci: past, present, future. J Clin Neurophysiol. 1997;14:470–483. doi: 10.1097/00004691-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Ebersole JS, Squires KC, Eliashiv SD, Smith JR. Application of magnetic source imaging in evaluation of candidates for epilepsy surgery. Neuroimaging Clin N Am. 1995;5:267–288. [PubMed] [Google Scholar]
- 36.Bast T, Oezkan O, Rona S, Stippich C, Seitz A, Rupp A, FauserS ZJ, Rating D, Scherg M. EEG and MEG source analysis of single and averaged interictal spikes reveals intrinsic epileptogenicity in focal cortical dysplasia. Epilepsia. 2004;45:621–631. doi: 10.1111/j.0013-9580.2004.56503.x. [DOI] [PubMed] [Google Scholar]
- 37.Wilenius J, Medvedovsky M, Metsahonkala L, Makela JP, Paetau A, Valanne L, Paetau R. Interictal MEG reveals focal cortical dysplasias: Special focus on patients with no visible MRI lesions. Epilepsy Research. 2013;105:337–348. doi: 10.1016/j.eplepsyres.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Wu XT, Rampp S, Buchfelder M, Kuwert T, Blumcke I, Dorfler A, Zhou D, Stefan H. Interictal magnetoencephalography used in magnetic resonance imaging-negative patients with epilepsy. Acta Neurologica Scandinavica. 2013;127:274–280. doi: 10.1111/j.1600-0404.2012.01712.x. [DOI] [PubMed] [Google Scholar]
- 39.Chandra SP, Bal CS, Jain S, Joshua SP, Gaikwad S, Garg A, Ansari A, Nehra A, Sarkar C, Tripathi M. Intraoperative coregistration of magnetic resonance imaging, positron emission tomography, and electrocorticographic data for neocortical lesional epilepsies ma improve the localization of the epileptogenic focus: a pilot study. World Neurosurg. 2014;82:110–117. doi: 10.1016/j.wneu.2013.02.057. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez S, Donaire A, Seres E, Setoain X, Falcon C, Bargallo N, Sanmarti F, Maestro I, Rumia J, Boget T, Aparicio J, Carreno M. PET/MRI and PET/MRI/SISCOM coregistration in the presurgical evaluation of refractory focal epilepsy. Epilepsy Res. 2015;111:1–9. doi: 10.1016/j.eplepsyres.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Storti SF, Formaggio E, Franchini E, Bongiovanni LG, Cerini R, Fiaschi A, Michel CM, Manganotti P. A multimodal imaging approach to the evaluation of post-traumatic epilepsy. MAGMA. 2012;25:345–360. doi: 10.1007/s10334-012-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colliot O, Bernasconi N, Khalili N, Antel SB, Naessens V, Bernasconi A. Individual voxel-based analysis of gray matter in focal cortical dysplasia. Neuroimage. 2006;29:162–171. doi: 10.1016/j.neuroimage.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Kim H, Bernasconi N, Bernhardt N, Colliot O, Bernasconi A. Basal temporal sulcal morphology in healthy controls and patients with temporal lobe epilepsy. Neurology 2008:2159-2165. [DOI] [PubMed]
- 44.Bruggemann JM, Wilke M, Som SS, Bye AME, Bleasel A, Lawson JA. Voxel-based morphometry in the detection of dysplasia and neoplasia in childhood epilepsy: combined grey/white matter analysis augments detection. Epilepsy Res 2007;77(2–3):93–101. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)