Abstract
Triphenyl phosphate (TPhP) is a flame retardant additive frequently found in consumer products and household dust. We administered 170 μg of TPhP in maternal food from gestational day 8.5 to weaning and evaluated metabolic phenotypes of 3.5 month old male and female rats, and weight-matched males up to 6 months, to assess the development of obesity and type 2 diabetes mellitus (T2DM), respectively. Perinatal TPhP exposure increased body and fat mass in 3.5 month old male and female rats, while leptin and cumulative energy intake were elevated in males and females, respectively. Independent of body mass, perinatal TPhP exposure accelerated T2DM onset in males and increased plasma non-esterified- fasting fatty acids. These observations suggest that perinatal exposure to TPhP exacerbates the development of obesity in male and female UCDavis-T2DM rats and accelerates T2DM onset in male UCD-T2DM rats.
Keywords: triphenyl phosphate, developmental exposure, diabetes mellitus, obesity, leptin, Firemaster 550, and hyperphagia
1. Introduction
Type 2 Diabetes Mellitus (T2DM) is a serious metabolic disease presently affecting at least 29.1 million people in the United States [1]. In 2012, the direct annual cost attributable to T2DM was more than $176 billion, while its indirect costs exceeded $69 billion [1]. T2DM is associated with many serious complications, including cardiovascular disease, blindness, end stage renal failure, and lower-limb amputations [1]. Worldwide, the incidence of T2DM is rising, with most of the increase occurring during the last 20 years [2,3]. Over the past 40 years the prevalence of obesity has increased globally and now more people are obese than are underweight [4]. Interestingly, people today who eat and exercise the same amount as people 20 years ago are still more obese than their counterparts were two decades ago, though the composition of their diet and their other environmental exposures may differ [5]. This provocative finding is consistent with the hypothesis that exposure to environmental pollutants may contribute to the rapid increase in the prevalence of obesity and T2DM that is not explained by dietary factors and reduced physical activity [6–8].
Numerous candidate obesogens are lipophilic pollutants, suggesting adipose tissue is either a target of toxicity or a depot where toxicants are sequestered and from which they are released [9]. Obesogenic chemicals are added to consumer products [10,11] and are dispersed into the human environment where they become ubiquitous [11,12]. Some of these chemicals eventually distribute into human lipids, such as fat and breast milk [13], raising the possibility that they could act as developmental obesogens. Indeed, exposure to the high production volume lipophilic flame retardant mixture, Firemaster 550 (FM 550), from mid gestation to weaning resulted in increased body mass, increased circulating fasting glucose, and impaired glucose tolerance in Wistar rats (Patisaul 2013). Further, exposure to an individual component of the FM 550 technical mixture [12,14], triphenyl phosphate (TPhP), leads to lipid accumulation in mouse bone marrow stromal BMS2 cells at concentrations between 10–40 μM [15].
In addition to the metabolic toxicity associated with TPhP exposure alone or as a substantial component of FM 550 [15–17], TPhP has been found in human breast milk samples from Japan, the Philippines, and Vietnam [13]. Given these prior observations, we hypothesize that developmental exposure to the commercial flame retardant TPhP will produce effects consistent with endocrine disruption, exacerbate the development of obesity and increase the rate of T2DM onset in the UCD-T2DM rat, which is a well characterized animal model of T2DM [18].
2. Materials and Methods
2.1 Rat Husbandry and Diabetes Surveillance
We used the UCD-T2DM rat model of T2DM since this rat model more closely represents the pathophysiology and progression of T2DM in humans than other available rodent models of the disease [18]. UCD-T2DM rats develop progressive hyperglycemia along with hyperinsulinemia resulting from insulin resistance at T2DM onset accompanied by β-cell dysfunction and decompensation [18]. Because the incidence of T2DM among male and female rats is 92% and 43%, respectively, with an average age of onset of diabetes of 6 months in males and 9.5 months in females [18], we only used male rats for the diabetes study (Figure 1). This model has been validated and characterized for the investigation of the pathophysiology of T2DM, as well as T2DM prevention and treatment in numerous peer-reviewed publications [18,20–22]
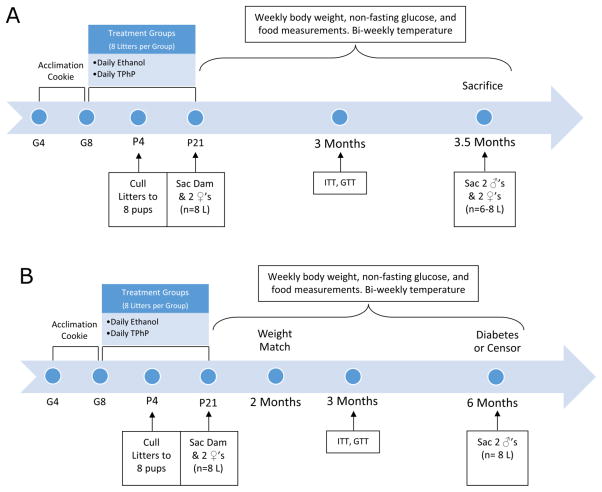
Figure 1. Study groups.
(A) Obesity study design for two female and up to two male rats per litter (L) per treatment which terminated when rats were sacrificed (Sac) at 3.5 months of age (n=6–8 L). (B) Diabetes study design for two weight-matched male rats per litter per treatment which terminated when rats were sacrificed at their onset of T2DM or at 6 months of age (n=8 L). Diabetes was diagnosed with two random plasma glucose measurements of ≥200 mg/dL per ADA guidelines [19]. Gestational Day (G), Postnatal Day (P), Insulin tolerance test (ITT), Glucose tolerance test (GTT)
Animal care and maintenance was conducted in accordance with the applicable portions of the Animal Welfare Act and the U.S. Department of Health and Human Services’ Guide for the Care and Use of Laboratory Animals and approved by the University of California, Davis Institutional Animal Care and Use Committee. Study rats were kept in a climate controlled room at 25°C and 45% 60% average relative humidity on a 12 hour reversed light cycle. All rats were fed Harlan diet 2018 (3.1 kcal/g, 0.57 kcal fat, 1.8 kcal carbohydrates, 0.74 kcal protein; Teklad Diets, Madison WI).
Adult non-pregnant female UCD-T2DM rats (n=16; 3 months old) were paired with males (n=10; 3–4 months old) for a 24 hour period at which point males were removed. This was defined as gestational day zero (G0) if a sperm plug was observed or if the female rats gained at least 30g over the next 7 days. The day of birth was designated postnatal day zero (P0). Pregnant dams were randomly assigned to an exposure group (n=8 per group), and received daily oral TPhP or ethanol vehicle exposure from G8 through weaning (P21) as described in section 2.2 below. Gestational length, litter size were recorded on P0 and the sex of pups was determined and recorded on P4. Body weights of all pups in each litter were obtained periodically from P4–21.
On P4 the litters were culled to 8 pups ensuring up to 4 males and 2 females in each litter by random selection (Figure 1A & 1B). This was done to ensure consistent exposure of pups between litters [13,23]. The time it takes to develop T2DM is accelerated among UCD-T2DM rats with higher body weights on P21. Hence at weaning the largest pups were housed in same sex littermate groups of two females and up to four males as available (Figure 1A & 1B). Urine was collected from the dams using an adapted plastic wrap method outlined by Kurien [24]. Dams were placed in clean cages without bedding for at least 20 minutes then using a pipette up to 500 μl of urine was collected in ethanol rinsed glass vials and placed on ice. At weaning all dams and remaining weanlings were sacrificed (90–330 min post-exposure) by CO2 asphyxiation and rapid decapitation.
Two male rats weighing between 350g–400g on P61, from the TPhP group and the vehicle group were weight- matched across treatments for the diabetes study to eliminate confounding effects of body mass on the association between TPhP and T2DM onset (Figure 1B). This weight range was selected because male UCD-T2DM rats that are between 350–400g at 8 weeks of age develop T2DM at approximately 23 weeks of age [18]. Weight- matched rats were followed until 26 weeks or until they developed T2DM, which was defined as two consecutive weekly non-fasting glucose measurements of ≥200mg/dL [18] in accordance with the American Diabetes Association (ADA) guideline of diagnosing diabetes with a random plasma glucose of 200 mg/dL or higher [19]. The remaining rats were not weight-matched and followed for the 3.5 month obesity study (Figure 1A).
The terminal procedures for rats in both the obesity and diabetes studies were identical. Prior to sacrifice, rats were fasted between 8–12 hours, their body weights recorded, and blood was collected from the tail as above prior to euthanasia. Rats were anesthetized with sodium pentobarbital and euthanized with a 1mL/kg intracardiac injection of saturated potassium chloride. Once cardiac movement had stopped for 30 seconds the rat was decapitated and the hypothalamus, liver, pancreas, heart, mesenteric adipose tissue, quadriceps, kidney, gonadal adipose tissue, inguinal adipose tissue, and brown adipose tissue were collected. All tissues were removed in the order listed above, wet weighed, and snap frozen in liquid nitrogen.
2.2 Exposure
Given that FM550 contains 17% TPhP [16], our oral TPhP dose of 170 μg per day is equivalent to the amount of TPhP present in the 1 mg FM 550 /rat /day dosed over the same developmental period that produced metabolic disruption in a previous study [17]. On average an adult ingests 28 mg of dust per day [25] making oral TPhP administration highly relevant to humans [12,25]. At 28 mg of dust per day, over the course of pregnancy and breastfeeding a woman would ingest a total of 17.5 g of dust [26]. Therefore it would require only 90 μg of TPhP per gram of dust for a woman to receive 21 mg/kg of TPhP. This is the same total exposure as rats in this study and well below 1800 μg of TPhP/g of dust, which to the best of our knowledge is the maximum observed level reported from samples collected in 2007 and 2012 [12,27,28]. Triphenylphosphte (100% purity; Accustandard, New Haven, CT) was weighed to the nearest tenth of a mg, diluted into 100% ethanol (Fisher Scientific, Pittsburg, PA), and vortex mixed to yield a dose solution of 8.5 mg/mL.
Due to their taste aversion to the treat used as a dose vehicle by Patisaul [17], we instead dispensed 20 μL of ethanol with or without TPhP onto a ¼ mini Nilla wafer cookie vehicle (1 quarter equivalent to 1.75 kcal total, 0.65 kcal fat, 1.05 kcal carbohydrates, 0.05 kcal protein; Nabisco, East Hanover, NJ[29] and allowed the ethanol to completely evaporate before administration to UCD-T2DM rats (Figure 1A & 1B). Dams were habituated to eating the ¼ mini Nilla wafer prior to exposure from G4-7 (Figure 1A & 1B), and all rats readily consumed the ¼ mini Nilla wafer within 5 min for all groups by the onset of the exposure period.
2.3 Urine Analysis for the analysis of Diphenylphosphate (DPhP)
Urine analysis for the main and non-volatile metabolite of TPhP, diphenylphosphate (DPhP), was performed with small modifications of a method as previously described [30]. Briefly, DPhP was measured using mixed – mode anion exchange solid phase extraction and a labeled internal standard (deuterated (d10)-DPhP). The modifications included that only 500 μL samples were used for each analysis rather than 1 mL and the samples had to be diluted 200 times with the elution solvent, after the clean-up preparation, due to high levels of DPhP detected (values exceded the upper limits of the quantitation curve). The analysis was performed on a liquid chromatographic system (LC) coupled with tandem mass spectrometry (MS/MS) applying negative electrospray ionization (N-ESI). Recoveries for both DPhP and the labeled analog were 105% ±13% and 122% ± 25%, respectively. For quality assurance purposes, DPhP was evaluated in laboratory blanks and six levels of quality control samples (QCs) during each analysis. DPhP levels in laboratory blanks and materials averaged to 0.3 ng/mL. The method detection limit was calculated by serial dilutions of the lowest calibration standard (0.1 ng/mL) and spiked into 1 mL of pool urine samples that were processed with the whole clean up protocol. The method detection limit (MDL) was set as the lowest amount of analyte that provided a response with: a) at least 5 times the response of the matrix sample; b) both precision (relative standard deviation, RSD) and accuracy (error, %Er) ≤20%. Limit of detection (LOD) was the lowest value detected with RSD >3 So, where So was the standard deviation of the signal received from a urine sample processed and analyzed three times with the same chromatographic method and detected with %Er ≤20%. Those two values for DPhP were 0.2 and 0.1 ng/mL respectively.
2.4 Metabolic and Physical Parameters
Metabolic parameters were evaluated across the lifespan as detailed here and in Figure 1A and 1B. Body weight and food intake per cage were recorded weekly in rats from the obesity and diabetes studies, from P21. Every 2 weeks body temperature was assessed in rats from the obesity and diabetes studies by inserting a thermocouple probe into the rectum (BAT-10, Physitemp, Clifton, NJ). Cumulative energy intake (kcal) was calculated as the difference in food weight divided by the number of rats that had access to it and the number of days they had access, and transformed to kcal [31].
2.4.1 Glucose
Non-fasting blood glucose (AlphaTrak; Abbott Laboratories, Abbott Park, IL) was recorded weekly beginning in P21 aged rats from the obesity and diabetes studies. Tail blood was collected from all rats at 3 months of age fasted for between 8–12 hours to determine fasting blood glucose (AlphaTrak).
At 13 weeks of age, 1 rat/sex/litter/treatment group in both the obesity and diabetes study were randomly assigned to receive an intraperitoneal glucose tolerance test (ipGTT) (Figure 1A & 1B). The rats receiving the ipGTT were administered 1.0 g D-glucose/kg body weight after an overnight 14 hour fast, and blood was collected from the tip of the tail to determine fasting blood glucose [32] at 0, 15, 30, 45, 60, 90, and 120 minutes after the D-glucose bolus. We determined total area under the curve (AUC) using the trapezoidal approach.
At sacrifice, blood was collected from the tail of rats in the obesity and diabetes studies, after fasting for 8–12 hours (Figure 1A & 1B) to determine fasting blood glucose. Fasted plasma collected at sacrifice in the diabetes study (Figure 1B) was tested for glycated albumin (Diazyme; Powey, CA), a glycemic indicator.
2.4.2 Hormones
At 13 weeks of age 1 rat/sex/litter/treatment group in both the obesity and diabetes studies were randomly assigned to receive an intraperitoneal insulin tolerance tests (ipITT) (Figure 1A & 1B). The rats receiving the ipITT were fasted for 4 hours and then 1.0 U Humulin R/kg body weight was administered i.p. and blood was collected from a tail cut to determine fasting blood glucose at 0, 15, 30, 45, 60, 90, and 120 minutes after the Humulin R bolus.
At sacrifice, tail blood was collected from obesity and diabetes studies- rats (Figure 1A & 1B) fasted between 8–12 hours and thyroid stimulating hormone (TSH) (Alpco Diagnostics; Boston, MA), T4 (CalBiotech; Spring Valley, CA) leptin (Meso Scale Discovery; Gaithersburg, MD), and adiponectin (Meso Scale Discovery) were evaluated with blinding to treatment. Tail blood was collected from all rats at 3 months of age (Figure 1A & 1B) and from diabetes study (Figure 1B) rats at the time of sacrifice after fasting for between 8–12 hours to determine plasma insulin by ELISA (Mercodia; Uppsala, Sweden) while blinded to treatment. HOMA-IR, an indicator of insulin resistance, and HOMA-%B, an indicator of pancreatic beta cell function, were calculated using the HOMA2 Calculator, available at https://www.dtu.ox.ac.uk, using fasting blood glucose and specific fasting plasma insulin [33].
2.4.3 Lipids
All lipid measures described in this section were performed by investigators blinded to treatment. Tail blood was collected from rats in the obesity and diabetes studies at 13 weeks of age and at sacrifice, respectively, after fasting for between 8–12 hours (Figure 1A & 1B) to determine total cholesterol, total non-esterified fatty acids (NEFA), and plasma triglycerides by enzyme based colorimetry (Wako Chemicals; Richmond, VA).
Non-esterified Fatty Acid Profiling: Non-esterified fatty acids (NEFAs) were further isolated from fasted plasma, and collected from diabetes study rats, as previously described by Smedes [34] and Gladine [35]. Specifically, plasma aliquots (50 μL) were enriched with a suite of extraction surrogates, including isotopically labeled triglycerides and phospholipids, along with rare cholesteroyl esters and fatty acids as previously reported (Grapov, Adams, Pedersen, Garvey, & Newman, 2012). Lipids were then extracted twice with 10:8:11 cylcohexane/2-propanol/1M ammonium acetate (v/v/v), and the organic extract was dried and reconstituted in 2:1 methanol/toluene (v/v), including pentadeca-(10Z)-enoic acid (Nu-Check Prep Inc.) to track methylation efficiency. Fatty acids were derivatized with 2M (trimethylsilyl) diazomethane (Sigma-Aldrich, St. Louis, MO) in hexanes, reduced to dryness and reconstituted in hexane containing methyl tricosanoate (Nu-Chek Prep)and analyzed by GC-MS on an Agilent 6890/5973N MSD (Agilent Technologies, San Jose, CA) with electron impact ionization using a 30m x 0.25mm x 0.25μm DB-225ms to separate analytes. Quantification was accomplished with ChemStation vE.02.14 (Agilent Technologies, Santa Clara, CA) using internal standard methodologies.
Lipid Mediator Profiling: Non-esterified oxylipins, endocannabinoids, endocannabinoid-like monoacylglycerols and N-acylethanolamines, and nitrated fatty acids were quantified by tandem quadrupole mass spectrometry using slight modifications of previously reported methods [36]. Briefly, analytes were isolated from hypothalami collected from female obesity study rats, as previously described [37] but without ester hydrolysis. Hypothalamus aliquots (~20–25 mg) aliquots were enriched with isotopically labeled surrogates and extracted with 1:10:5 methanol/ethyl acetate/deionized water (v/v/v). Extracts were dried under vacuum, reconstituted in 1:1 MeOH/acetonitrile (v/v) containing internal standards, and filtered at 0.1μm through a PVDF membrane. Analytes were separated on a 2.1 x 150 mm, 1.7μm Acquity UPLC BEH column and detected by negative mode (oxylipins and nitrated fatty acids) and positive mode (endocannabinoids and endocannabinoid-like compounds) electrospray ionization using multiple reaction monitoring on an API 4000 QTrap (Sciex, Framingham, MA, USA). Analytes were quantified against calibration curves and correcting for surrogate losses using Sciex MultiQuant version 3.0.2.
2.5 Semi-quantitative PCR
The RNeasy kit (Qiagen; Hilden, Germany) was used to extract mRNA from aliquots of pulverized mesenteric fat to be used in reverse transcription PCR (Applied Biosystems; Foster City, CA) and semi-quantitative PCR while blinded to treatment. RNA was quantitated by absorbance at 260 nm wavelength and retained with a purity threshold > 1.8 for the 260/280 ratio (NanoQuant, Tecan; Männedorf, Switzerland). Semi-quantitative PCR was performed using SYBR Green primers: Peroxisome proliferator activated receptor gamma (Pparg, F: TGTGAAGGATGCAAGGGTTT, R:CATTCGCCCAAACCTGATGG), CCAAT/enhancer binding protein (C/EBP) alpha (Cebpa, F:TGCGCAAGAGCCGAGATAAA, R:GCGGTCATTGTCACTGGTCA), CCAAT/enhancer binding protein (C/EBP) beta (Cebpb, F:CAAGATGCGCAACCTGGAGA, R:AGCTGCTTGAACAAGTTCCG) in mesenteric fat using beta actin (Actb, F:CTGACAGGATGCAGAAGGAG, R:GATAGAGCCACCAATCCACA) as an endogenous control; the endogenous control did not vary significantly across treatment groups. All primers were designed for Rattus norvegicus by querying the Pubmed gene library with the NCBI Primer-BLAST tool [38]. Primers were designed to span an exon-exon junction with each pair separated by at least one intron in the corresponding genomic DNA. The 2−ddCT method was used to approximate relative transcript fold change between treatment groups [39].
2.6 Statistical Analyses
Relative tissue weights were determined by dividing each tissue weight by body weight. Metabolic parameters were statistically analyzed as follows to determine means ± standard error of the mean (SEM) at a significance threshold of P < 0.05. Analyses of non-fasting blood glucose, HOMA-IR, HOMA-%B, body weight, temperature, tissue weights, relative tissue weights, TSH, adiponectin, insulin, triglycerides, cholesterol, total NEFA, and RNA expression were assessed by modeling the fixed effect of perinatal TPhP and the random effect of litter while stratifying by sex and study group shown in figures 1A and 1B using fixed effects for treatment and litter as a repeated event (PROC MIXED, SAS). All mixed models used LSMEANS to find the mean and SEM and PDIFF to test significance. We also tested for sex interactions at threshold p < 0.1 and if it was significant it was included in the model statement in addition to the sex-stratified models described above. The distribution of the data was checked for normality before T-tests were used to evaluate gestation length, litter size, sex ratio, dam body weight, luciferase E2, cumulative energy intake, and targeted lipids because only one rat per litter was included (PROC TTEST, SAS). ITTs and GTTs were evaluated by stratifying by sex and study group (Fig 1) without random effects because only one rat per litter was included (PROC GLM, SAS). We evaluated whether perinatal TPhP accelerated the onset T2DM by using survival analysis with treatment as a fixed effect and litter as a repeated event (PROC LIFETEST, SAS). This the best method of analyzing differences in the onset of T2DM because survival analysis accounts for the non-normal distribution of time to event data and can account for censoring, that is, ending the observation period before all rats have developed T2DM.
3. Results
3.1 Perinatal TPhP is not overtly toxic
We examined the dams and pups through weaning to determine if any gross maternal or developmental TPhP toxicity was present at this dose. No significant differences were observed in the length of gestation, litter size, sex ratio, or the body weight of the dams or pups at weaning (Table 1), suggesting that exposure to TPhP was not overtly toxic with respect to these parameters.
Table 1.
Gestation and Pre-weaning
| Outcome | Vehicle Control | Treatment (TPhP) |
|---|---|---|
| Gestation Length (days) | 22.9 ± 0.1 | 23.6 ± 0.5 |
| Litter Size | 13.6 ± 0.8 | 13.3 ± 0.8 |
| Sexes per Litter (P4) | ♂6.9 ± 0.7 ♀6.1 ± 0.8 |
♂5.5 ± 1.0 ♀6.5 ± 0.5 |
| Pup BW at weaning (g) | ♂57.2 ± 3.1 ♀54.1 ± 3.5 |
♂57.0 ± 3.6 ♀53.8 ± 4.6 |
| Dam BW at weaning (g) | 330.4 ± 7.9 | 334.0 ± 4.3 |
| Dam TPhP metabolite (DPhP) in urine at weaning (ng/mL) | 2.6 ± 0.6 | 2778.0 ± 2104.0 |
8 Dams per exposure group: gestation length, litter size, sex ratio, or Dam body weight at sacrifice.
3 Dams per exposure group : DPhP. Table depicts means ± SEM
3.2 Perinatal exposure to TPhP increases energy intake and body weight
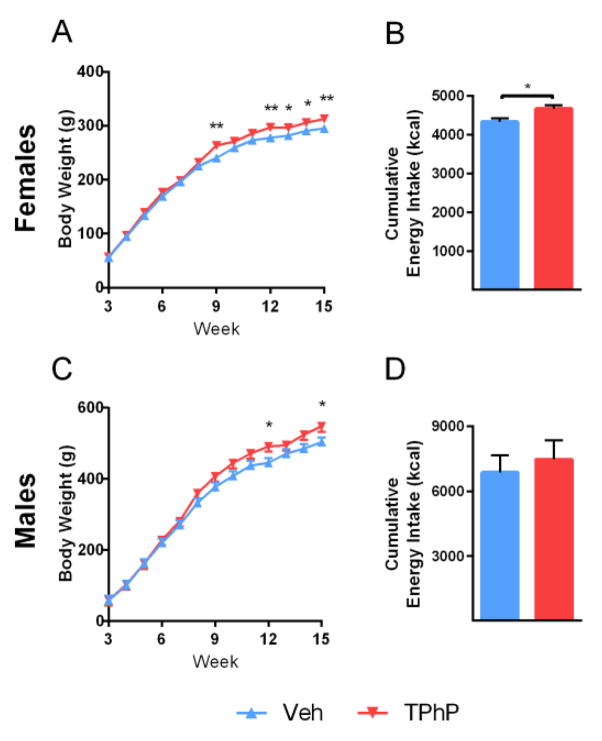
Food intake and body weight were measured weekly in rat offspring and the cumulative energy intake was calculated per rat (Figure 2B & 2D). In both female and male rats, those with perinatal TPhP exposure had significantly higher body weights compared with the vehicle-treated control rats starting at week 9 and week 13, respectively (p < 0.05; Figure 2A & 2C). This effect of TPhP became more marked as the animals aged (treatment x age interaction pinteraction < 0.05, and < 0.01 for females and males, respectively). We also observed that the cumulative energy intake was higher in the female rats exposed to TPhP (7.8%, p < 0.05, Figure 2B) but not in the male rats (Figure 2D).
Figure 2. Weekly physical measurements of male and female UCD-T2DM rats, obesity study.
(A) Female body weight from 3–15 weeks (n=8 TPhP and n=8 Veh exposed litters, treatment x age, pinteraction < 0.05). (B) Female cumulative energy intake (n=8 TPhP and n=8 Veh exposed litters). (C) Male body weight from 3–15 weeks (n=6 TPhP- and n=7 Veh exposed litters, treatment x age pinteraction < 0.01). (D) Male cumulative energy intake (n=6 TPhP and n=7 Veh exposed litters). Figure depicts means ± SEM. *p < 0.05, **p < 0.01
Because core body temperature contributes 60–90% to total energy expenditure [40], we measured core body temperature to evaluate whether perinatal TPhP exposure also modified energy expenditure. Temperature measurements taken biweekly were not significantly different across treatments in the obesity study (Supplemental Figure 1). These data show that both male and female rats perinatally exposed to TPhP had significantly elevated body weight that was at least partially explained by increased caloric intake in female rats.
3.3 Perinatal TPhP exposure increases adult rat fat pad weights
Given that body weight is a non-specific assessment of adiposity, tissues were collected to assess changes in the weight of adipose depots, as well as evidence of overt toxicity, associated with perinatal TPhP exposure. No differences in the weights of tissues collected were observed across treatment groups in female pups sacrificed at weaning (Supplemental Table 1). No difference was seen between vehicle and treatment in both the absolute and relative weights of livers, quadriceps muscle, heart, or pancreas collected at sacrifice in both male and female adult rats in the obesity study (Supplemental Figure 2A and 2B). The kidney weight relative to total body weight was significantly decreased (p < 0.05) in the treated adult male rats in the obesity study however the absolute weight was not significantly different between treatments (Supplemental Figure 2A and 2B). Overall these results suggest there was no overt toxicity to non- adipose tissues of rats exposed to TPhP.
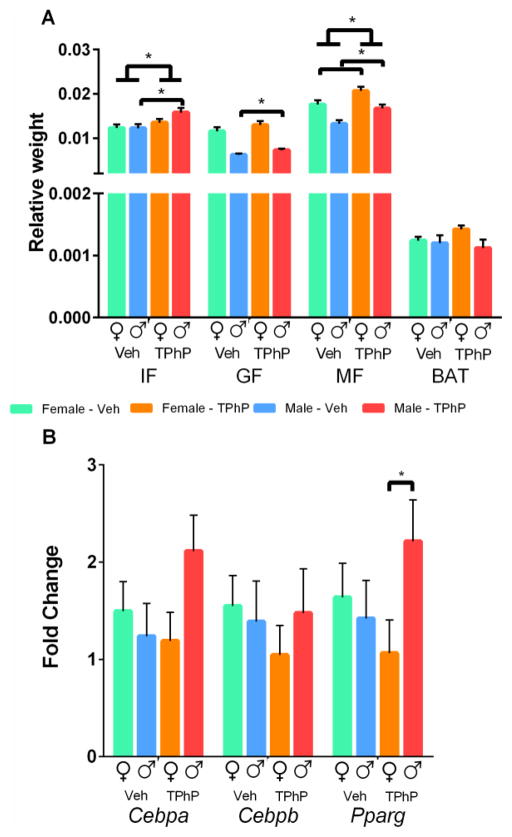
Significant changes were observed in the mean weight of fat depots collected from both male and female adult rats in the obesity study (Figure 3A). In the adult female rats, the mean absolute weights of mesenteric fat and brown adipose depots were significantly increased in the TPhP- exposed group vs. vehicle control (p < 0.05, Supplemental Figure 2A) but after adjusting for body weight only the mesenteric fat remained larger (17%) in the female rats with perinatal TPhP exposure (p < 0.05, Figure 3A). In the adult male rats, the absolute inguinal fat, gonadal fat and mesenteric fat weights were significantly increased in the TPhP exposed group vs. vehicle controls (p < 0.05, Supplemental Figure 2A) and these fat depots remained significantly larger (29%, 17%, and 26% respectively) after adjusting for total body weight (p < 0.05, Figure 3A). Further, independent of sex, both the relative inguinal and mesenteric fat pads were significantly increased in the rats prenatally exposed to TPhP compared with vehicle-treated control animals (p < 0.05, Figure 3A). These results demonstrate that perinatal TPhP exposure increased body adiposity and the size of multiple fat pads in both male and female rats particularly in high risk sites such as inguinal and mesenteric fat pads. Further the data show that males accumulate more fat in these areas than females.
Figure 3. Fat pads and qPCR quantification from male and female UCD-T2DM rats euthanized when 3.5 months old, obesity study.
(A) Weights of individual fat deposits relative to body weight (B). qPCR quantification of master regulators of adipogenesis in mesenteric fat from adult rats.
Inguinal Fat (IF), Gonadal Fat (GF), Mesenteric Fat (MF), Brown Adipose Tissue (BAT). Figure depicts means ± SEM *p < 0.05
3.4 Perinatal TPhP exposure does not alter glucose, insulin, or lipids in early adulthood
Because we have reported that body weight is a determinant of the timing of T2DM onset in this rat model [18], we suspected that increased adiposity of 3 month old rats perinatally exposed to TPhP could lead to abnormalities in glucose and lipid homeostasis. However, fasting lipids (Supplemental Figure 4) and glucose (Figure 4) were not different between treatments in either male or female rats. Further, glucose responses during insulin and glucose tolerance tests were not significantly different between treatments in either male or female rats (Figure 4). No difference was observed in areas under the curve for the glucose tolerance test (Figure 4A & 4B inset). These data suggest that despite increased adiposity in animals exposed to perinatal TPhP, glucose, insulin, and lipids were not altered in early adulthood.
Figure 4. Glucose and insulin tolerance tests performed at 3 months of age on male and female UCD-T2DM rats, obesity study.
(A, C) Plasma glucose excursion in response to intraperitoneal glucose administration (1 g/kg body weight and 75% D-glucose solution) in nondiabetic rats after an overnight fast. Inset shows area under the curve. (B, D) Plasma glucose excursions in response to intraperitoneal insulin administration (0.5 U/kg body weight and 1U/mL insulin solution) in nondiabetic rats after an overnight fast. Inset shows fasting insulin adjusted for body weight. Pinteraction<0.1 for treatment and sex tested on AUC. Figure depicts means ± SEM. (n=6–8L)
3.5 Perinatal TPhP does not alter thyroid hormones
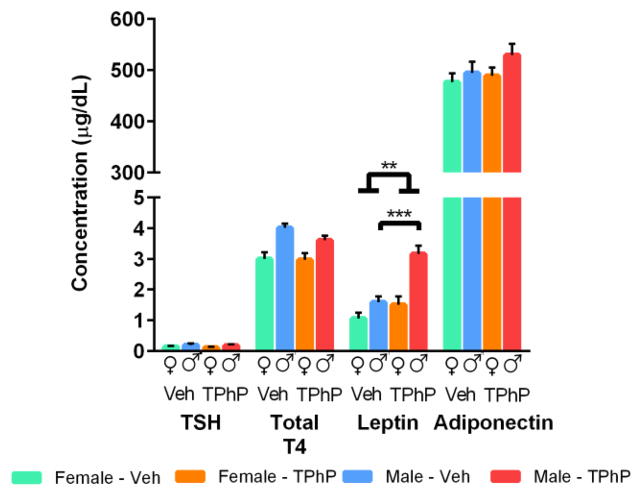
Previous studies have reported that FM 550 is capable of increasing total T4 in rat dams [17]. Therefore we examined whether perinatal TPhP disrupts the thyroid hormone axis of rats in the obesity study. We found that total T4 tended to be lower in the male rats perinatally exposed to TPhP (p = 0.08, Figure 5) and no significant differences were observed in TSH for the male and female rats perinatally exposed to TPhP (Figure 5). These data suggest that increased adiposity by perinatal TPhP exposure is not mediated through disruption of the thyroid hormone axis of adult offspring.
Figure 5. Hormones at sacrifice in adult male and female UCD-T2DM rats, obesity study.
The pinteraction=0.6, 0.006 and 0.21 for Thyroid stimulating hormone, Leptin, and Adiponectin, respectively. (n=8 L TPhP and n=8 L Veh in exposed females and n=6 L TPhP and n=8 L Veh in exposed males). Figure depicts means ± SEM. ** P < 0.01 *** p < 0.001
3.6 Perinatal TPhP exposure increases fasting leptin
Because plasma leptin levels are positively related to adiposity in both rats and humans [41–44], we suspected that perinatal TPhP exposure would be associated with increased fasting leptin in rats of the obesity study. Consistent with this suspicion, there was a doubling of plasma leptin in male rats exposed to TPhP compared with vehicle-treated control male rats while in female rats there was only a 50% increase of plasma leptin concentrations (pinteraction < 0.01). Furthermore, the leptin to adipose ratio, a surrogate for leptin resistance, was 32% higher in the TPhP exposed vs. vehicle control females in the obesity study (p < 0.05). While the leptin to adipose ratio for TPhP exposed vs. vehicle control males in the obesity study, was 39% higher it was not significantly different, consistent with leptin resistance [45,46]. Indeed, independent of sex and body weight there was a significant increase of fasting plasma leptin concentrations (p < 0.01) between the vehicle and TPhP exposed rats in the obesity study (Figure 5). However, adiponectin, a hormone linked to visceral adiposity [45], was not altered by TPhP exposure (Figure 5). These measurements of adipose tissue hormones suggest that the effects of increased adiposity resulting from perinatal TPhP exposure is specific for leptin, and do not appear to influence adiponectin.
3.7 Hypothalamic lipid mediators are unchanged by perinatal TPhP exposure
Lipid mediators, including oxylipins, nitrated fatty acids, endocannabinoids, and endocannabinoid-like compounds, were analyzed among rats of the obesity group as they have been shown to play important roles in inflammatory signaling and energy balance [47,48]. No difference in the concentrations of endocannabinoids, endocannabinoid-like compounds (Supplemental Table 2), oxylipins, or nitrated fatty acids (Supplemental Table 3) was detected in the hypothalamus of female rats in the obesity study. These data suggest that the increase in adiposity seen in female rats in the obesity study is likely not driven by changes in the hypothalamic tone of the lipid mediators measured [49].
3.8 mRNA expression markers of adipogenesis
To assess whether the observed increased fat mass of rats perinatally exposed to TPhP was the result of adipocyte proliferation, we measured changes in the expression of three master regulators of adipogenesis [50]. However, we found no significant differences in the RNA expression of Pparg, Cebpa, or Cebpb across treatment groups in both male and female adult rats in the obesity study (Figure 3B). There was an apparent interaction between treatment and sex for Pparg (pinteraction < 0.1), where among rats with perinatal TPhP exposure, the males have greater Pparg expression than observed in females (p < 0.05), yet this sex difference was absent in the vehicle exposed rats (Figure 3B). There was also an interaction between treatment and sex for Cebpa transcript abundance (pinteraction < 0.1); yet this was driven by a non-significant trend of higher Cebpa in males compared to females among those exposed to TPhP during development (Figure 3B). These data suggest that the increase in adiposity of male rats might be driven by a Pparg mediated increase in adipogenesis.
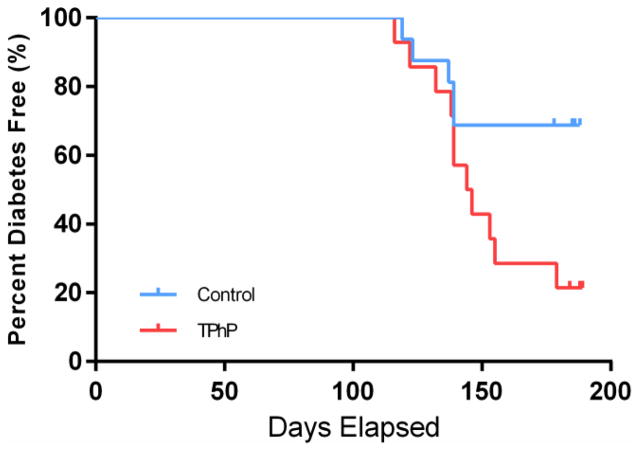
3.9 Perinatal TPhP accelerates onset of T2DM in weight- matched male rats
To examine whether perinatal TPhP exposure accelerates the onset of T2DM, T2DM was diagnosed from two consecutive weekly non-fasting glucose measurements of ≥200mg/dL, according to ADA diagnostic guidelines [19]. We found that perinatal exposure to TPhP accelerated the onset of T2DM in weight-matched male rats (p < 0.05 PROC LIFETEST, Figure 6). Indeed, from the first T2DM case observed to the end of the study, male rats with perinatal TPhP exposure had a higher prevalence of T2DM than control rats independent of body mass. For example, by 26 weeks when the study was censored, 79% of male rats perinatally exposed to TPhP developed T2DM while only 33% of the vehicle treated rats developed T2DM. Consistent with the first case of T2DM of the study being observed in 116 day old rats with perinatal TPhP exposure, no difference in GTT or ITT was observed in the 3 month old weight matched males (Supplemental Figure 5). Because of the much longer latency of T2DM onset in female UCD-T2DM rats, we made an a priori decision to exclude female rats from the diabetes study (Figure 1B) [18], and unfortunately cannot extend our observation of early T2DM onset to female rats here.
Figure 6. Kaplan-Meier plot of T2DM incidence in weight- matched male UCD-T2DM rats, diabetes study.
Weight-matched male rats were developmentally exposed to 170ug of TPhP/day from P4 through GD21. P < 0.05 by log rank test with censoring at 26 weeks of age (LIFETEST).
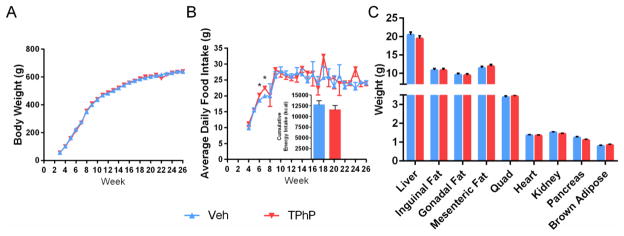
3.10 No effect of perinatal TPhP exposure on adiposity or energy balance in weight-matched male rats
Although male rats were weight-matched between exposure groups for the diabetes study when they were 2 months of age (Figure 1B), because of our earlier observations of adiposity resulting from hyperphagia, we considered whether increased adiposity could explain the earlier onset of T2DM in rats perinatally exposed to TPhP. However, no differences were observed for body weight, cumulative food intake, biweekly body temperature, weights of all tissues collected, or the majority of average daily food intake measurements (Figure 7, Supplemental Figure 1). These results suggest that accelerated onset of T2DM in rats following perinatal exposure to TPhP was independent of adiposity and energy balance.
Figure 7. Physical measurements of weight-matched male UCD-T2DM rats, diabetes study.
(A) Weekly body weight from 3–26 weeks (n=8 TPhP and n=8 Veh exposed litters). (B) Average daily food intake calculated from weekly measurements. Inset shows lifetime food consumption (n=8 L TPhP and n=8 L Veh exposed litters). (C) Absolute weights of all tissues collected at diabetes onset or the 6 month censor date. Figure depicts means ± SEM. *p < 0.05
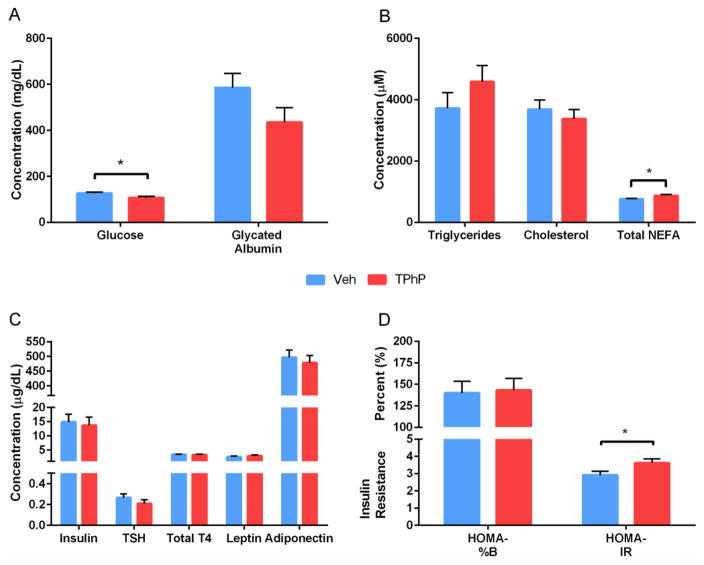
3.11 Non-esterified Fatty Acids are increased by Perinatal TPhP exposure in weight-matched male rats
We conducted preliminary analyses to further characterize the metabolic profile of these rats at the end of the diabetes study. Despite the increased prevalence of diabetes in the TPhP exposed group, fasting blood glucose was significantly higher in the control group compared to the TPhP exposed group (Figure 8A, p < 0.05). While fasting glycated albumin was not significantly altered by treatment, its trend was consistent with the fasting blood glucose (p < 0.1, Figure 8A). No significant treatment differences were observed between TPhP and vehicle exposed rats in the diabetes study for fasting insulin, TSH, total T4, leptin, adiponectin, triglycerides, or cholesterol (Figure 8B–C).
Figure 8. Fasting blood analysis of weight- matched male UCD-T2DM rats at sacrifice, diabetes study.
(A) Glucose and glycated albumin. (B) Triglycerides, Cholesterol, and Non-Esterified Fatty Acids (NEFA), (C) Insulin, Thyroid Stimulating Hormone (TSH), Total T4, Leptin, and, Adiponectin and (D) HOMA-%B and HOMA-IR. Figure depicts means ± SEM. *p < 0.05
Given these seeming paradoxical fasting metabolic parameters among rats with elevated prevalence of T2DM, we evaluated HOMA-IR and HOMA-%B to determine if the relationship between fasting glucose and insulin revealed insulin resistance and beta cell dysfunction, respectively. We found that perinatal exposure to TPhP had no impact on HOMA-%B, indicating beta cells had not yet become impaired at this early stage of T2DM (Figure 8D). However, perinatal TPhP exposure elevated HOMA-IR, consistent with insulin resistance (Figure 8D).
Further consistent with insulin resistance, we found that total NEFA was significantly increased among fasting rats perinatally exposed to TPhP compared to their weight-matched controls in the diabetes study (Figure 8B). To further evaluate changes in total NEFA, we quantified individual fatty acids in weight- matched male rats from the diabetes study. Perinatal TPhP exposure significantly increased plasma concentrations of a number of fatty acids including palmitic acid, cis-9-palmitoleic acid, oleic acid, and cis-vaccenic acid (p < 0.05) while linoleic acid, gamma-linolenic acid, and alpha-linolenic acid along with the stearic acid-palmitic acid and oleic acid-stearic acid ratios were all approaching significance (p < 0.1, Table 2). Because of these results, total saturated fatty acids, total mono-unsaturated fatty acids, and total NEFA were all significantly elevated in adult male rats following perinatal TPhP exposure (Table 2). These elevations in fatty acids after perinatal TPhP exposure are consistent with a diabetic phenotype and have been positively associated with insulin resistance and T2DM independent of body weight [51–53][36].
Table 2.
Non-esterified fatty acid measured in plasma from male rats, diabetes study
| Metabolite (μM) | Vehicle Control | Treatment (TPhP) |
|---|---|---|
| Saturated | ||
| Pentadecylic acid (15:0) | 1.8 ± 0.1 | 1.9 ± 0.1 |
| Palmitic acid (16:0) | 190 ± 10 | 220 ± 10 * |
| Margaric acid (17:0) | 1.33 ± 0.04 | 1.46 ± 0.07 |
| Stearic acid (18:0) | 38 ± 1 | 41 ± 2 |
| Arachidic acid (20:0) | 0.66 ± 0.04 | 0.65 ± 0.03 |
| Mono-Unsaturated | ||
| trans-9-Palmitoleic acid (16:1n7t) | 4.5 ± 0.3 | 4.8 ± 0.3 |
| cis-9-Palmitoleic acid (16:1n7) | 42 ± 4 | 57 ± 6 * |
| Oleic acid (18:1n9) | 124 ± 6 | 146 ± 8 * |
| cis-Vaccenic acid (18:1n7) | 22 ± 1 | 27 ± 1 ** |
| Nonadecylic acid (19:1n7) | 2.6 ± 0.4 | 2.6 ± 0.3 |
| n6-PUFA | ||
| Linoleic acid (18:2n6) | 188 ± 9 | 210 ± 9 |
| Gamma-Linolenic acid (18:3n6) | 2.1 ± 0.1 | 2.5 ± 0.1 |
| Eicosadienoic acid (20:2n6) | 3.4 ± 0.3 | 3.9 ± 0.2 |
| Dishomo-gamma-linolenic acid (20:3n6) | 4.9 ± 0.4 | 5.5 ± 0.5 |
| Arachidonic acid (20:4n6) | 67 ± 3 | 68 ± 3 |
| Docosadienoic acid (22:2n6) | 0.53 ± 0.08 | 0.48 ± 0.06 |
| Adrenic Acid (22:4n6) | 5.7 ± 0.2 | 8 ± 1 |
| Osbond acid (22:5n6) | 2.6 ± 0.2 | 3.4 ± 0.8 |
| n3-PUFA | ||
| Alpha-linolenic acid (18:3n3) | 16 ± 1 | 19 ± 1 |
| Stearidonic acid (20:4n3) | 0.58 ± 0.03 | 0.61 ± 0.04 |
| Eicosapentaenoic acid (20:5n3) | 1.67 ± 0.08 | 2.0 ± 0.2 |
| Docosapentaenoic acid (22:5n3) | 4.9 ± 0.3 | 5.9 ± 0.5 |
| Docosahexaenoic acid (22:6n3) | 42 ± 3 | 43 ± 3 |
| Totals | ||
| Total Saturated Fatty Acids | 230 ± 10 | 260 ± 20 * |
| Total Mono-Unsaturated Fatty Acids | 190 ± 10 | 240 ± 10 * |
| Total n6-PUFA | 270 ± 10 | 300 ± 10 |
| Total n3-PUFA | 65 ± 3 | 70 ± 4 |
| Total NEFA | 760 ± 30 | 870 ± 40 * |
Table depicts means ± SEM.
p < 0.05,
p < 0.01
4. Discussion
In this study, we report that TPhP exposure during gestation and lactation increased obesity phenotypes in male and female UCD-T2DM rats well before the onset of T2DM. Independent of body mass, this perinatal TPhP exposure accelerated the onset and increased the prevalence of T2DM in male UCD-T2DM rats. While mechanisms underlying this accelerated onset of T2DM are not yet understood, consistent with our findings here, the pathophysiology of T2DM has previously been shown to be driven by insulin resistance in this rat model of T2DM [18]. Changes in free fatty acids [54,55] are recognized to contribute to impairments of glucose homeostasis. In particular, palmitic acid, cis-9-palmitoleic acid, oleic acid, and cis-vaccenic acid were increased by perinatal TPhP exposure in our diabetes study, and these fatty acids along with the stearic acid-palmitic acid have been positively linked to insulin resistance, leptin resistance, T2DM, inflammation, and heart disease [51–53,56–59]. This is the first evidence that low-level, developmental exposure to TPhP can increase the risk of developing insulin resistance and T2DM, and that this risk is linked to alterations of circulating fatty acids. Given TPhP is a major component of the second most common flame retardant applied by polyurethane foam [11,60], a common consumer good, our results may have significant public health implications. Future studies of perinatal TPhP exposure should characterize the molecular mechanisms of its pathophysiology during the development and progression of T2DM in both male and female rats with particular attention to elucidating the seeming paradox of lower fasting glucose in the TPhP-treated rats.
The increased body and fat mass in both male and female rats following perinatal exposure to TPhP in the obesity study suggests that TPhP is an environmental obesogen. This increase in body and fat mass appeared to be the result of an energy imbalance resulting from increased cumulative energy intake in the females, with a similar trend being observed in the males. This suggests that increased appetite is the most likely contributor to increased adiposity in female rats exposed to perinatal TPhP. Moreover, the increase in body weight is the result of increased fat deposition, particularly in mesenteric fat, a visceral depot which has been linked to insulin resistance and metabolic syndrome [61,62]. While we did not measure whole body energy expenditure by calorimetry, the majority of thermogenic energy expenditure [40], as assessed by biweekly body temperature measurements, was not significantly altered by perinatal TPhP exposure (Supplemental Figure 1).
A number of hormones and receptors have been implicated in the regulation of food intake and energy metabolism, including leptin [45]. In the obesity study, plasma leptin concentrations were elevated in both male and female rats exposed to TPhP. The greater increase of plasma leptin observed in male compared with female rats is most likely due to obesity- associated leptin resistance in male rats perinatally exposed to TPhP, as indicated by their elevated leptin to adipose ratio [42,63]. Further, along with males rats having higher basal leptin levels compared to females, the significant increase mass of all fat depots suggests a larger capacity for leptin production [43,63,64].
Our results indicated there was also an increase in the adipose expression of Pparg in mesenteric adipose tissue in male rats exposed to TPhP. This is consistent with in vitro experiments that have reported that TPhP activates PPARG [65]. In agreement with the observed phenotypes, exposure to other PPARG agonists, tributyltin or Rosiglitazone, also increased adiposity in offspring [66]. However, we know of no studies evaluating the effects of prenatal PPARG agonists on the risk of developing type-2 diabetes in offspring. If perinatal PPARG agonism has residual anti-diabetic activity, as would be expected by known pharmacological effects of PPARG agonists, this may help explain the paradoxical modest reduction of fasting glucose observed in the diabetic rats perinatal exposed to TPhP in this study. Future studies will need to further evaluate this possibility.
5. Conclusions
This study has demonstrated that developmental exposure to the commercial flame retardant TPhP increases adiposity in both male and female UCD-T2DM rats, most likely due to an increase of energy intake. In addition, perinatal TPhP exposure accelerates the onset of T2DM and impairs fatty acid levels in male UCD-T2DM rats independent of body weight. We have also shown that the UCD-T2DM Rat model is a valuable model for testing the effects developmental exposure to environmental pollutants that can act as obesogens and disrupters of metabolic homeostasis and their consequences on the development of obesity and diabetes.
Supplementary Material
Highlights.
Perinatal TPhP exposure increased body and fat mass in male and female rats.
Perinatal TPhP exposure increased circulating leptin in male rats.
Perinatal TPhP exposure increased cumulative energy intake in female rats.
Independent of body mass, perinatal TPhP exposure accelerated T2DM onset in male rats.
Perinatal TPhP exposure increased plasma non-esterified- fasting fatty acids, including total saturated and monounsaturated fatty acids, in male rats.
Acknowledgments
Grant Support: The NIH (ES023513, DK095980, HL107256, HL121324, U24DK092993, U24DK097154), Office of Environmental Health Hazard Assessment (agreement number 13-E0014-1), the USDA (National Institute of Food and Agriculture, Hatch project 1002182 and Intramural Project 2032-51530-022-00D), and a multi-campus grant from the University of California Office of the President. The USDA is an equal opportunity provider and employer.
Abbreviations
- TPhP
Triphenyl phosphate
- DPhP
Diphenyl Phosphate
- IF
inguinal fat
- GF
gonadal fat
- MF
mesenteric fat
- BAT
brown adipose tissue
- T2DM
Type 2 Diabetes Mellitus
- ADA
American Diabetes Association
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. US Department of Health and Human Services; Atlanta, GA: 2014. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. [Google Scholar]
- 2.Imperial College London. [accessed July 1, 2015];Global Burden of Metabolic Risk Factors of Chronic Diseases. 2013 http://www1.imperial.ac.uk/publichealth/departments/ebs/projects/eresh/majidezzati/healthmetrics/metabolicriskfactors/
- 3.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 4.N.R.F. Collaboration, others. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19· 2 million participants. The Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown RE, Sharma AM, Ardern CI, Mirdamadi P, Mirdamadi P, Kuk JL. Secular differences in the association between caloric intake, macronutrient intake, and physical activity with obesity. Obes Res Clin Pract. 2015 doi: 10.1016/j.orcp.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002;8:185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- 7.Klimentidis YC, Beasley TM, Lin HY, Murati G, Glass GE, Guyton M, Newton W, Jorgensen M, Heymsfield SB, Kemnitz J, Fairbanks L, Allison DB. Canaries in the coal mine: a cross-species analysis of the plurality of obesity epidemics. Proc R Soc B Biol Sci. 2011;278:1626–1632. doi: 10.1098/rspb.2010.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longnecker MP, Daniels JL. Environmental contaminants as etiologic factors for diabetes. Environ Health Perspect. 2001;109:871. doi: 10.1289/ehp.01109s6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Merrill M, Emond C, Kim MJ, Antignac JP, Le Bizec B, Clément K, Birnbaum LS, Barouki R. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect. 2013;121:162–169. doi: 10.1289/ehp.1205485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooke D, Crookes M, Quarterman P, Burns J. Environmental Risk Evaluation Report: Triphenyl Phosphate. 2009 (CAS no. 115-86-6) [Google Scholar]
- 11.Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. Alternate and New Brominated Flame Retardants Detected in U.S. House Dust. Environ Sci Technol. 2008;42:6910–6916. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- 12.Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environ Sci Technol. 2012;46:13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JW, Isobe T, Muto M, Tue NM, Katsura K, Malarvannan G, Sudaryanto A, Chang KH, Prudente M, Viet PH, Takahashi S, Tanabe S. Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere. 2014;116:91–97. doi: 10.1016/j.chemosphere.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Saunders DMV, Higley EB, Hecker M, Mankidy R, Giesy JP. In vitro endocrine disruption and TCDD-like effects of three novel brominated flame retardants: TBPH, TBB, & TBCO. Toxicol Lett. 2013;223:252–259. doi: 10.1016/j.toxlet.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ. Ligand binding and activation of PPARγ by FiremasterR 550: effects on adipogenesis and osteogenesis in vitro. Environ Health Perspect. 2014;122:1225–1232. doi: 10.1289/ehp.1408111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGee SP, Konstantinov A, Stapleton HM, Volz DC. Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: potential role of the aryl hydrocarbon receptor. Toxicol Sci Off J Soc Toxicol. 2013;133:144–156. doi: 10.1093/toxsci/kft020. [DOI] [PubMed] [Google Scholar]
- 17.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM. Accumulation and Endocrine Disrupting Effects of the Flame Retardant Mixture Firemaster® 550 in Rats: An Exploratory Assessment: FM550 IS A CANDIDATE ENDOCRINE DISRUPTOR. J Biochem Mol Toxicol. 2013;27:124–136. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. AJP Regul Integr Comp Physiol. 2008;295:R1782–R1793. doi: 10.1152/ajpregu.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39:S13–S22. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 20.Cummings BP, Bettaieb A, Graham JL, Stanhope KL, Dill R, Morton GJ, Haj FG, Havel PJ. Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic UCD-T2DM rats. Proc Natl Acad Sci U S A. 2011;108:14670–14675. doi: 10.1073/pnas.1107163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings BP, Bettaieb A, Graham JL, Stanhope KL, Kowala M, Haj FG, Chouinard ML, Havel PJ. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153:3620–3632. doi: 10.1210/en.2012-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummings BP, Stanhope KL, Graham JL, Evans JL, Baskin DG, Griffen SC, Havel PJ. Dietary fructose accelerates the development of diabetes in UCD-T2DM rats: amelioration by the antioxidant, alpha-lipoic acid. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1343–1350. doi: 10.1152/ajpregu.00468.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimitsantos E, Escorihuela RM, Fuentes S, Armario A, Nadal R. Litter size affects emotionality in adult male rats. Physiol Behav. 2007;92:708–716. doi: 10.1016/j.physbeh.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 24.Kurien BT, Everds NE, Scofield RH. Experimental animal urine collection: a review. Lab Anim. 2004;38:333–361. doi: 10.1258/0023677041958945. [DOI] [PubMed] [Google Scholar]
- 25.USEPA. Expo Factors Handb 2011. EPA; 2011. Soil and Dust Ingestions - Chapter 5. [Google Scholar]
- 26.CDC. FAQs | Breastfeeding| DNPAO. CDC; 2015. [accessed July 6, 2015]. http://www.cdc.gov/breastfeeding/faq/ [Google Scholar]
- 27.Carignan CC, Heiger-Bernays W, McClean MD, Roberts SC, Stapleton HM, Sjödin A, Webster TF. Flame Retardant Exposure among Collegiate United States Gymnasts. Environ Sci Technol. 2013;47:13848–13856. doi: 10.1021/es4037868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meeker JD, Stapleton HM. House Dust Concentrations of Organophosphate Flame Retardants in Relation to Hormone Levels and Semen Quality Parameters. Environ Health Perspect. 2009;118:318–323. doi: 10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson SA, Boctor SY. Use of food wafers for multiple daily oral treatments in young rats. J Am Assoc Lab Anim Sci JAALAS. 2009;48:292. [PMC free article] [PubMed] [Google Scholar]
- 30.Petropoulou SSE, Petreas M, Park JS. Analytical methodology using ion-pair liquid chromatography–tandem mass spectrometry for the determination of four di-ester metabolites of organophosphate flame retardants in California human urine. J Chromatogr A. 2016;1434:70–80. doi: 10.1016/j.chroma.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 31.La Merrill M, Karey E, Moshier E, Lindtner C, La Frano MR, Newman JW, Buettner C. Perinatal Exposure of Mice to the Pesticide DDT Impairs Energy Expenditure and Metabolism in Adult Female Offspring. PLoS ONE. 2014;9:e103337. doi: 10.1371/journal.pone.0103337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramzy AR, Nausheen S, Chelikani PK. Ileal transposition surgery produces ileal length-dependent changes in food intake, body weight, gut hormones and glucose metabolism in rats. Int J Obes 2005. 2014;38:379–387. doi: 10.1038/ijo.2013.201. [DOI] [PubMed] [Google Scholar]
- 33.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 34.Smedes F. Determination of total lipid using non-chlorinated solvents. The Analyst. 1999;124:1711–1718. doi: 10.1039/a905904k. [DOI] [Google Scholar]
- 35.Gladine C, Newman JW, Durand T, Pedersen TL, Galano JM, Demougeot C, Berdeaux O, Pujos-Guillot E, Mazur A, Comte B. Lipid profiling following intake of the omega 3 fatty acid DHA identifies the peroxidized metabolites F4-neuroprostanes as the best predictors of atherosclerosis prevention. PloS One. 2014;9:e89393. doi: 10.1371/journal.pone.0089393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grapov D, Adams SH, Pedersen TL, Garvey WT, Newman JW. Type 2 diabetes associated changes in the plasma non-esterified fatty acids, oxylipins and endocannabinoids. PloS One. 2012;7:e48852. doi: 10.1371/journal.pone.0048852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picklo MJ, Sr, Newman JW. Antioxidant supplementation and obesity have independent effects on hepatic oxylipin profiles in insulin-resistant, obesity-prone rats. Free Radic Biol Med. 2015;89:182–191. doi: 10.1016/j.freeradbiomed.2015.07.152. [DOI] [PubMed] [Google Scholar]
- 38.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Landsberg L. Core temperature: a forgotten variable in energy expenditure and obesity? Obes Rev Off J Int Assoc Study Obes. 2012;13(Suppl 2):97–104. doi: 10.1111/j.1467-789X.2012.01040.x. [DOI] [PubMed] [Google Scholar]
- 41.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 42.Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996;81:4406–4413. doi: 10.1210/jcem.81.12.8954050. [DOI] [PubMed] [Google Scholar]
- 43.Landt M, Gingerich RL, Havel PJ, Mueller WM, Schoner B, Hale JE, Heiman ML. Radioimmunoassay of rat leptin: sexual dimorphism reversed from humans. Clin Chem. 1998;44:565–570. [PubMed] [Google Scholar]
- 44.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 45.Havel PJ, Bremer AA. Obesity. McGraw Hill; 2010. Endocrine Regulation of Energy Homeostasis: Implications for Obesity and Diabetes. [Google Scholar]
- 46.Vasselli JR, Scarpace PJ, Harris RBS, Banks WA. Dietary Components in the Development of Leptin Resistance. Adv Nutr Int Rev J. 2013;4:164–175. doi: 10.3945/an.112.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balgoma D, Checa A, Sar DG, Snowden S, Wheelock CE. Quantitative metabolic profiling of lipid mediators. Mol Nutr Food Res. 2013;57:1359–1377. doi: 10.1002/mnfr.201200840. [DOI] [PubMed] [Google Scholar]
- 48.Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NKH, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang J, Garcia-Barrio MT, Rudolph TK, Rudolph V, Bonacci G, Baker PRS, Xu HE, Batthyany CI, Chen YE, Hallis TM, Freeman BA. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J Biol Chem. 2010;285:12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taha AY, Gao F, Ramadan E, Cheon Y, Rapoport SI, Kim HW. Upregulated expression of brain enzymatic markers of arachidonic and docosahexaenoic acid metabolism in a rat model of the metabolic syndrome. BMC Neurosci. 2012;13:131. doi: 10.1186/1471-2202-13-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowe CE, O’Rahilly S, Rochford JJ. Adipogenesis at a glance. J Cell Sci. 2011;124:2681–2686. doi: 10.1242/jcs.079699. [DOI] [PubMed] [Google Scholar]
- 51.Choi WS, Kim SH, Chung JH. Relationships of Hair Mineral Concentrations with Insulin Resistance in Metabolic Syndrome. Biol Trace Elem Res. 2014;158:323–329. doi: 10.1007/s12011-014-9946-2. [DOI] [PubMed] [Google Scholar]
- 52.Lankinen MA, Stančáková A, Uusitupa M, Ågren J, Pihlajamäki J, Kuusisto J, Schwab U, Laakso M. Plasma fatty acids as predictors of glycaemia and type 2 diabetes. Diabetologia. 2015;58:2533–2544. doi: 10.1007/s00125-015-3730-5. [DOI] [PubMed] [Google Scholar]
- 53.Van Woudenbergh GJ, Kuijsten A, Van der Kallen CJ, Van Greevenbroek MM, Stehouwer CD, Blaak EE, Feskens EJM. Comparison of fatty acid proportions in serum cholesteryl esters among people with different glucose tolerance status: the CoDAM study. Nutr Metab Cardiovasc Dis NMCD. 2012;22:133–140. doi: 10.1016/j.numecd.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Bergman R, Ader M. Free Fatty Acids and Pathogenesis of Type 2 Diabetes Mellitus. Trends Endocrinol Metab. 2000;11:351–356. doi: 10.1016/S1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- 55.Shrayyef MZ, Gerich JE. Normal Glucose Homeostasis. In: Poretsky L, editor. Princ Diabetes Mellit. Springer; US, Boston, MA: 2010. [accessed April 22, 2015]. pp. 19–35. http://link.springer.com/10.1007/978-0-387-09841-8_2. [Google Scholar]
- 56.Innis SM. Palmitic Acid in Early Human Development. Crit Rev Food Sci Nutr. 2015 doi: 10.1080/10408398.2015.1018045. [DOI] [PubMed] [Google Scholar]
- 57.Mancini A, Imperlini E, Nigro E, Montagnese C, Daniele A, Orrù S, Buono P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules. 2015;20:17339–17361. doi: 10.3390/molecules200917339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanders TAB. Protective effects of dietary PUFA against chronic disease: evidence from epidemiological studies and intervention trials. Proc Nutr Soc. 2014;73:73–79. doi: 10.1017/S0029665113003789. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Folsom AR, Zheng Z-J, Pankow JS, Eckfeldt JH A.S. Investigators, others. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78:91–98. doi: 10.1093/ajcn/78.1.91. [DOI] [PubMed] [Google Scholar]
- 60.Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between Polybrominated Diphenyl Ether (PBDE) Flame Retardants, Phenolic Metabolites, and Thyroid Hormones during Pregnancy. Environ Health Perspect. 2011;119:1454–1459. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang YK, Chen M, Clements RH, Abrams GA, Aprahamian CJ, Harmon CM. Human mesenteric adipose tissue plays unique role versus subcutaneous and omental fat in obesity related diabetes. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2008;22:531–538. doi: 10.1159/000185527. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H, Sairam MR. Sex hormone imbalances and adipose tissue dysfunction impacting on metabolic syndrome; a paradigm for the discovery of novel adipokines. Horm Mol Biol Clin Investig. 2014;17:89–97. doi: 10.1515/hmbci-2014-0002. [DOI] [PubMed] [Google Scholar]
- 63.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–390. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, Garvey WT. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82:1293–1300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- 65.Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol Lett. 2014 doi: 10.1016/j.toxlet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chamorro-García R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect. 2013;121:359–366. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.