Abstract
The most common sex chromosome aneuploidy, Klinefelter syndrome (KS), is associated with primary gonadal failure and increased morbidity and mortality from cardiometabolic disorders in adulthood. Children with KS also have a high prevalence of metabolic syndrome (MetS). To assess the relationship of gonadal and cardiometabolic function in children with KS, we evaluated serum hormones (gonadotropins, inhibin B (INHB), anti-mullerian hormone (AMH), total testosterone (TT)), and features of MetS (waist circumference, fasting lipid panel, fasting blood glucose (FBG), and blood pressure) in 93 prepubertal boys with KS age 4–12 years (mean 7.7 ± 2.5 years). The cohort was grouped by age and tanner stage, and biomarkers were compared to normal ranges. 80% of this prepubertal cohort had ≥1 feature of metabolic syndrome (MetS) and 11% had ≥3 features of MetS. Risk of MetS was independent of age and body mass index. Sertoli cell dysfunction was common with 18% having an INHB below the normal range. A low INHB was associated with higher FBG, triglycerides, and LDL and lower HDL (p<0.05). An INHB <50 ng/dl yielded a sensitivity of 83% and a specificity of 79% for having ≥3 features of MetS. INHB and AMH positively correlated with each other (p<0.001), and high AMH was protective of MetS. TT was below the lower limit of normal in 49% of subjects, with mean values significantly lower than expected (3.3 ng/dl versus 4.9 ng/dl, p<0.0001), however no convincing relationship between TT and MetS was seen. In conclusion, gonadal and cardiometabolic dysfunction are prevalent in prepubertal boys with KS. Although the relationship of testosterone deficiency and MetS is well known, this study is the first to report an association between impaired sertoli cell function and cardiometabolic risk.
Keywords: Klinefelter syndrome; 47, XXY; hypogonadism; gonadal function; inhibin B; anti-mullerian hormone; cardiometabolic; metabolic syndrome; sex chromosome aneuploidy
INTRODUCTION
Klinefelter syndrome (KS), defined by a supernumerary X chromosome in males, occurs in ~1/650 male births (Bojesen et al., 2003). It is the most common sex chromosome aneuploidy and the leading cause of primary hypogonadism in males (Bojesen, et al., 2003). While the large majority of children with KS are not diagnosed, advances in prenatal screening are shifting this landscape. With the increasing diagnosis rates of KS in infants and children, there is a great need for research on the natural history of KS in childhood in order to develop an evidence base for management.
Adult males with KS have hypergonadotropic hypogonadism and an increased prevalence of disorders related to insulin resistance, with up to half of men with KS exhibiting the metabolic syndrome (MetS) (Pacenza et al., 2012, Salzano et al., 2016). Testosterone deficiency is associated with MetS and related disorders in multiple populations, including KS (Bojesen et al., 2006b, Traish et al., 2011). While it is uncertain whether hypogonadism is present in infants and prepubertal boys with KS, recent studies have found evidence of abnormal body composition and features of MetS in young boys with KS (Aksglaede et al., 2008, Bardsley et al., 2011, Fennoy, 2011). Assessment of testicular function in childhood is challenging, as the hypothalamic-pituitary-gonadal axis is quiescent until puberty (Grinspon et al., 2012). However, biomarkers of sertoli cell function, inhibin B (INHB) and anti-mullerian hormone (AMH), can aid in the identification of testicular dysfunction, as sertoli cell function is independent of the hypothalamic-pituitary-gonadal axis at this age (Grinspon, et al., 2012). Previous studies of sertoli cell biomarkers in prepubertal boys with KS are limited by small sample sizes and focus on either young infants or older, peripubertal children (Bastida et al., 2007, Wikstrom et al., 2004).
In this study, we investigated testicular function in boys with KS age 4–12 years of age. We hypothesized that biomarkers of sertoli cell function would be abnormal, providing evidence of early testicular dysfunction. Furthermore, we examined the relationship between testicular function and cardiometabolic risk.
MATERIALS AND METHODS
Study Design
Boys with KS were recruited to participate in a study that included assessment with auxologic measurements, physical examination, bone age, and fasting blood test (NCT00348946). The protocol was approved by the local institutional review board. At least one parent provided written consent and children provided assent if appropriate.
Setting and Participants
The study took place at Thomas Jefferson University from 2005–2011. Participants were recruited from local endocrinology clinics and advertisements primarily through family support networks for children affected by KS. Eligible participants were 4–12 years old with a diagnosis of KS or KS variants, including supernumerary sex chromosome aneuploidy and mosaicism with less than 50% of cells 46,XY. Exclusion criteria included treatment with androgen within the previous year or major systemic disease. A total of 93 boys participated in this study. To allow comparison to prepubertal laboratory normal values, we divided the subjects into two groups: younger than 9.5 years (n=60) and ≥ 9.5 years (n=33). All subjects in the younger group were clinically prepubertal, with testicular volumes 2 mL or less and tanner stage 1 pubic hair.
Physical Examination
Measurements of height, weight, body-mass index (BMI), arm span, upper-to-lower (U:L) segment ratio, waist circumference, and waist-to-hip ratio were obtained by one of two examiners (KK, JR) and converted to standard deviation scores (SDS) using age- and gender-specific norms (Hall et al., 1989). Blood pressure (BP) was obtained in the seated position by auscultation. Body fat was assessed by the electronic skin fold caliper and calculator Skyndex System I (Skyndex, Albuquerque, NM). Skin fold measurements were taken from the right triceps and calf, after which the instrument calculated the percent body fat using the Slaughter Lohman formula for boys (% body fat = 0.735 (triceps + calf) + 1.0) (Slaughter et al., 1988). Body fat percentage was converted to SDS using age-and gender-specific normative data.(Laurson et al., 2011) Muscle tone and muscle mass were subjectively assessed to be normal, decreased or significantly decreased upon examination by an experienced clinician (JR). Bone age of the left hand was interpreted by a single pediatric endocrinologist (JR) according to the methods of Greulich and Pyle (Greulich et al., 1959).
Testicular volume was measured by palpation using the Prader orchidometer and converted to SDS using published normative data (Hall, et al., 1989). If testes were different volumes, the average was used in analysis. Pubic hair and breast tissue were assessed according to Tanner staging (Hall, et al., 1989). Stretched penile length (SPL) was measured to the nearest 0.25 cm, and compared to published normative data (Hall, et al., 1989).
Laboratory Testing
Blood was obtained in a fasting state in the morning. Serum for luteinizing hormone (LH), follicle stimulating hormone (FSH), inhibin B (INHB), and anti-mullerian hormone (AMH) was processed and frozen until the time of study completion when all samples were measured simultaneously. Normal age- and pubertal-dependent ranges were determined from a control population of 304 boys age 4.0–13.0 without any endocrine or metabolic disorder. The FSH immunoassay (TRFIA AutoDelfia Wallac, PerkinElmer, Courtaboeuf, France) had a minimum quantifiable value of 0.033 IU/L, interassay coefficient of variation (CV) of 2.1–4.8%, and intra-assay CV of <5%. The LH immunoassay (TRFIA AutoDelfia Wallac, PerkinElmer, Courtaboeuf, France) had a minimum quantifiable value of 0.024 IU/L, interassay CV of 1.3–3.8%, and intra-assay CV of <5%. The AMH enzyme-linked immunosorbent assay (AnshLabs, Webster, TX) had a quantitative limit of 0.2 pmol/L, interassay CV of <9% at concentrations of 7–110 pmol/L (1–15 ng/mL), intra-assay CV of <4% above 5 pmol/L (0.7 ng/mL). The INHB enzyme-linked immunosorbent assay (AnshLabs, Webster, TX) had no cross-reactivity with activins or inhibin A, with a quantitative limit of 2.2 pg/ml, and had an interassay CV of 9.7% and 4.8% at a concentration of 40 pg/mL and 93 pg/mL respectively; and an intra-assay CV of 4.6% and 2.8% at a mean concentration of 53 pg/mL and 96 pg/mL respectively. Total testosterone (TT) concentration was analyzed at Esoterix Laboratories, Inc by high pressure liquid chromatography tandem mass spectrometry (Calabasas Hills, CA), with inter and intra-assay CV of <10%. Hormone concentrations below the limit of detection were assigned the lower limit of quantitation for the assay.
Additional laboratory assessments including serum feasting blood glucose (FBG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol, and triglycerides (TG) were measured using commercial assays. Features of MetS were defined as follows: waist circumference >75%ile for age, triglycerides >100 mg/dl, HDL <50 mg/dl, fasting FBG >110 mg/dl, and systolic or diastolic BP >90%ile for age and height (de Ferranti et al., 2004). The presence of three or more of these features was consistent with a diagnosis of MetS. As there are no universally accepted criteria for MetS in children, we used the de Ferranti criteria to allow comparison to the only other study of MetS in children with KS (Bardsley, et al., 2011, de Ferranti, et al., 2004). Homeostasis model assessment (HOMA) was calculated as a measure of insulin resistance as follows: [fasting BG (mmol L) × fasting insulin (μU mL)] 22.5. Insulin resistance was defined as HOMA ≥ 2.5.
Statistical Analyses
Demographic, anthropometric, cardiometabolic, and gonadal function data were summarized with descriptive statistics. T-test or Wilcoxon rank sum test was used to compare gonadal function biomarkers of the sample to the normative mean or median. The younger and older groups and groups with abnormal and normal gonadal function were compared using either two-sided t-tests or Wilcoxon rank sum tests. Pearson or Spearman correlation and linear regression coefficients were calculated to test the association between continuous variables. Logistical regression was used to analyze the relationship between INHB and ≥3 MetS features. As this is an exploratory analysis, no adjustments were made for multiple comparisons, and all results were considered statistically significant at a p-value ≤ 0.05. Statistical analysis was performed using GraphPad Prism version 6.00 for Mac, GraphPad Software, La Jolla California USA and SAS version 9.4, SAS Institute, Cary North Carolina, USA.
RESULTS
The mean age of the 93 subjects was 7.7 ± 2.5 years with a range of 4.0 to 12.9 years. Bone age was six months delayed (7.1 ± 2.6 years). Karyotype, ethnicity, and age at diagnosis are presented in Table 1. The indication for prenatal testing was advanced maternal age in all but one. Reasons for diagnostic testing in the first two years of life included gross motor delays and hypotonia (n=3), micropenis (n=1), dysmorphic features (n=1) and other (n=1). Reasons for diagnostic testing in childhood included developmental delays (n=19), behavior and/or psychiatric problems (n=7), and other (n=3). Those with previous exposure to androgen therapy (23%) had received testosterone at various ages, dosages, and durations, and did not differ with respect to physical, cardiometabolic, or gonadal function outcomes. The subjects with 48,XXYY and 48,XXXY (n=2) were both in the older group and did not differ from the 47,XXY group with respect to gonadal and cardiometabolic function.
Table 1.
Subject Characteristics (n=93)
| Karyotype | |
| 47,XXY | 88 (95%) |
| 47,XXY/46,XY | 2 (2%) |
| 48,XXYY | 1 (1%) |
| 48,XXXY | 1 (1%) |
| 46,XX+SRYtrans | 1 (1%) |
|
| |
| Race/Ethnicity | |
| Caucasian | 67 (72%) |
| African American | 12 (13%) |
| Hispanic | 8 (9%) |
| Asian/Pacific Islander | 5 (5%) |
| Other | 1 (1%) |
|
| |
| Diagnosis ascertainment | |
| Prenatal | 58 (62%) |
| Birth – 2 yrs | 6 (6%) |
| 2–12 yrs | 29 (31%) |
Physical Features
Standard Deviation Scores (SDS) for anthropometric variables are presented in Table 2. Interestingly, although tall stature is regarded as a cardinal feature of KS, only a minority of boys (10%) had height ≥ 2SDS in childhood. Height and calculated mid-parental height for the group were both 0.6 SDS. Upper to lower segment ratio (U:L) was more disproportionate (relatively longer extremities) in the younger group when compared to the older group (p<0.05); this was the only anthropometric variable that significantly differed between the younger and older participants. When evaluated as continuous variables, none of the anthropometric variables correlated with age. Decreased muscle mass was present in 63/93 (68%) and at least mild hypotonia was nearly universal (97%).
Table 2.
Anthropometric data and cardiometabolic profiles
| 4–9.5 yrs | 9.5–12.9 yrs | All ages | |
|---|---|---|---|
|
| |||
| Number | 63 | 30 | 93 |
|
| |||
| Height SDS | 0.67 ± 1.0 | 0.41 ± 1.1 | 0.59 ± 1.0 |
| ≥ 2SDS (%) | 6/63 (9.5%) | 3/30 (10%) | 9/93 (9.7%) |
|
| |||
| U:L segment SDS | −1.2 ± 2.5 | −0.29 ± 1.5 | −0.81 ± 2.4* |
| ≤ 2SDS (%) | 26/63 (41%) | 4/30 (13%) | 30/93 (32%) |
|
| |||
| Arm Span SDS | 0.41 ± 1.0 | −0.09 ± 1.3 | 0.24 ± 1.1 |
| ≥ 2SDS (%) | 3/63 (4.8%) | 1/30 (3.3%) | 4/93 (4.3%) |
|
| |||
| Weight SDS | 0.72 ± 1.1 | 0.49 ± 1.2 | 0.64 ± 1.1 |
| ≥ 2SDS (%) | 6/63 (9.5%) | 3/30 (10%) | 9/93 (9.7%) |
|
| |||
| Head circumference SDS | 0.08 ± 1.3 | 0.62 ± 1.0 | 0.26 ± 1.2 |
| ≥ 2SDS (%) | 4/63 (6.3%) | 3/30 (10%) | 7/93 (7.5%) |
| ≤ 2SDS (%) | 2/63 (3.2%) | 0/30 (0%) | 2/93 (2.2%) |
|
| |||
| BMI SDS | 0.54 ± 1.1 | 0.4 ± 1.3 | 0.49 ± 1.2 |
| ≥ 2SDS (%) | 3/63 (4.8%) | 2/30 (6.7%) | 5/93 (5.4%) |
|
| |||
| Waist to hip ratio SDS | 1.8 ± 0.86 | 1.8 ± 1.0 | 1.8 ± 0.92 |
| ≥ 2SDS (%) | 29/63 (46%) | 13/30 (43%) | 42/93 (45%) |
|
| |||
| Percent body fat SDS | 1.2 ± 0.9 | 0.9 ± 0.9 | 1.1 ± 0.9 |
| ≥ 2SDS (%) | 11/63 (17%) | 0/30 (63%) | 11/93 (12%) |
|
| |||
| Total cholesterol mean ± SD | 164 ± 25 | 164 ± 39 | 164 ± 30 |
| > 170 mg/dl | 24/63 (38%) | 11/30 (37%) | 35/93 (38%) |
|
| |||
| LDL mean ± SD | 104 ± 22 | 105 ± 35 | 104 ± 26 |
| > 110 mg/dl | 21/63 (33%) | 11/30 (37%) | 32/93 (34%) |
|
| |||
| HDL mean ± SD | 47 ± 10 | 43 ± 11 | 45 ± 10 |
| < 50 mg/dl | 43/63 (68%) | 20/30 (67%) | 63/93 (68%) |
|
| |||
| Triglycerides mean ± SD | 68 ± 46 | 78 ± 47 | 71 ± 47 |
| > 100 mg/dl | 8/63 (13%) | 7/30 (23%) | 15/93 (16%) |
|
| |||
| FBG mean ± SD | 85 ± 8.1 | 85 ± 6.6 | 85 ± 7.6 |
| > 110 mg/dl | 0 | 0 | 0 |
|
| |||
| HOMA-IR mean ± SD | 1.7 ± 1.1 | 3.0 ± 1.6 | 2.1 ± 1.4* |
| ≥2.5 | 3/18 (17%) | 6/9 (67%) | 9/27 (33%) |
|
| |||
| MetS | |||
| 0 features | 14/63 (22%) | 6/30 (20%) | 20/93 (22%) |
| 1 feature | 27/63 (43%) | 11/30 (37%) | 38/93 (41%) |
| 2 features | 16/63 (25%) | 9/30 (30%) | 25/93 (27%) |
| ≥ 3 features | 6/63(10%) | 4/30 (13%) | 10/93 (11%) |
denotes a significant difference between younger and older groups (p < 0.05)
SDS=standard deviation score, U:L=upper to lower segment ratio, BMI=body mass index, FBG=fasting blood glucose, HDL=high density lipoprotein, Tg=triglycerides, LDL=low density lipoprotein; HOMA-IR= Homeostatic model assessment insulin resistance, MetS=Metabolic syndrome
Cardiometabolic Assessment
Despite a normal BMI percentile in 95% of subjects, the waist-to-hip ratio was above the 95th percentile for age in 45%, and the mean percent body fat was +1.1 ± 0.9 SDS. Mean values for fasting lipid panel and blood glucose are presented in Table 2. Dyslipidemia was prevalent with 18% of the group having both elevated triglycerides and low HDL. There was no significant difference between the younger and older groups. Fasting insulin was obtained in a subgroup (n=27) and calculated HOMA-IR was elevated in a third of these subjects. HOMA-IR weakly correlated with age (r=0.43, p=0.254), but was not related to weight or BMI. At least one feature of MetS was present in 79%, while full MetS criteria were met in 11% (Table 2).
Gonadal Function
One child had a history of cryptorchidism. SPL was small for age, particularly in the younger group where 17% met the standard definition of micropenis (<2.5 SDS). SPL was greater in those with a history of receiving testosterone therapy (SDS −1.4 ± 0.9 vs −1.8±0.7, p=0.04). Mean testicular volume was 0.9 mL (range 0.25 mL to 2 mL) in the younger group and 1.9 mL (range 0.5–4 mL) the older group. No boys in the younger group had pubic hair or gynecomastia. Pubic hair was present in 40% of boys in the older group with the average age of those with Tanner 2 pubic hair of 10.8 years (range 10.0–12.9). Gynecomastia (Tanner 2) was present in 20% of the older group at an average age of 10.9 years (range 9.5–12.9).
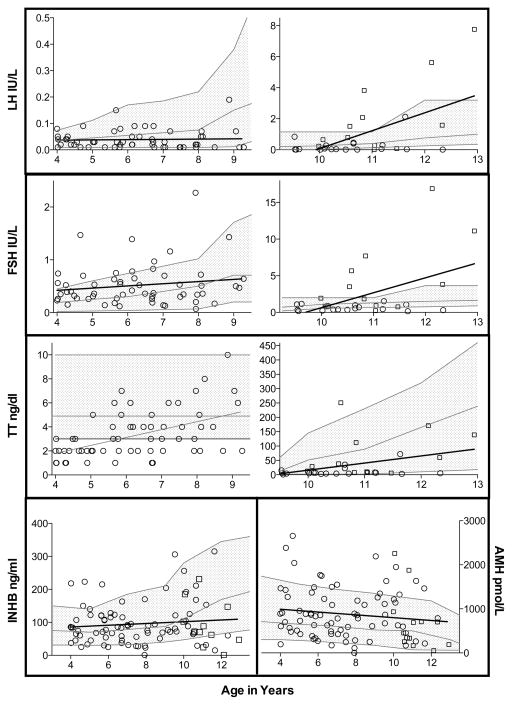
Gonadal function biomarkers compared to laboratory normative data by age and pubertal stage are summarized in Table 3 and Figure 1. LH, FSH and TT all positively correlated with age. Unexpectedly, LH concentrations were significantly lower than referenced medians in boys with Tanner 1 pubic hair, normal in Tanner 2, and then elevated by Tanner 3. FSH was elevated in 20% of all subjects, and 50% of boys with Tanner 2 or 3 pubic hair. Median FSH was at or above laboratory median values for all ages and pubertal stages but only reached significance in the youngest group. TT was below the lower limit of normal (≤3 ng/dl) in 49% of all subjects, with mean values significantly lower than expected in the younger group (mean TT 3.3 ng/dl v. 4.9 ng/dl, p<0.0001) and failed to rise as expected in Tanner 3. Of the four subjects with Tanner 3 pubic hair, two had elevated LH and FSH with low TT (Fig. 2).
Table 3.
Gonadal Function Biomakers by Age and Tanner Stage.
| 4–9.5 yrs (n=60) | 9.5–12.9 yrs | |||
|---|---|---|---|---|
|
| ||||
| T1 (n=18) | T2 (n=8) | T3 (n=4) | ||
|
| ||||
| LH IU/L Median [IQR] | 0.03* [0.01–0.05] | 0.04*[0.01–0.32] | 1.2 [0.37–1.96] | 2.9 [0.03–7.3] |
| Normal median [5–95%] | 0.06 [0–0.22] | 0.7 [0.07–2.15] | 1.0 [0.3–2.85] | 1.4 [0.7–3.8] |
| >95th%ile N (%) | 2 (3.3%) | 2 (11%) | 3 (38%) | 2 (50%) |
|
| ||||
| FSH IU/L Median [IQR] | 0.39* [0.27–0.64] | 0.5[0.34–0.95] | 2.7 [1.1–5.2] | 5.9^ [0.37–15.5] |
| Normal median [5–95%] | 0.33 [0.02–1.02] | 0.5 [0.2–2.3] | 1.4 [0.3–3.1] | 1.5 [0.7–3.8] |
| >95th%ile N (%) | 13 (22%) | 0 (0%) | 4 (50%) | 2 (50%) |
|
| ||||
| TT ng/dl Mean ± SD | 3.3* ± 1.9 | 11.8 ± 17 | 65 ± 83 | 75^ [18–138] |
| Normal mean [5–95%] | 4.9 [3–10] | 4.9 [3–10] | 42 [18–150] | 190 [100–320] |
| <5th%ile N (%) | 28 (47%) | 3 (17%) | 3 (38%) | 2 (50%) |
|
| ||||
| INHB ng/mL Median [IQR] | 77 [45–115] | 113 [63–197] | 95 [70–175] | 36^[18–51] |
| Normal median [5–95%] | 74 [30–185] | 110 [45–220] | 160 [70–300] | 170 [90–315] |
| <5th%ile N (%) | 8 (13%) | 3 (17%) | 2 (25%) | 4 (100%) |
|
| ||||
| AMH pmol/L Median [IQR] | 783* [428–1259] | 826* [489–1296] | 694* [421–1169] | 176 [136–318] |
| Normal median [5–95%] | 653 [200–1740] | 540 [150–1400] | 188 [70–820] | 70 [30–500] |
| >95th%ile N (%) | 7 (12%) | 5 (28%) | 2 (25%) | 1 (25%) |
|
| ||||
| Testicular volume z-score | −1.68 ± 1.0* | −0.46 ± 1.4 | −0.46 ± 1.4 | −0.5 ± 1.0 |
| SDS mean ± SD (Range) | (−3.31–0.86) | (−2.71–2.17) | (−1.19– 3.8) | (−1.81–0.5) |
| <2 SDS (%) | 21 (35%) | 2 (11%) | 0 (0%) | 0 (0%) |
| Volume (mL) mean ± SD | 0.9 ± 0.4 | 1.6 ± 0.9 | 2.3 ± 1.2 | 2.5 ± 1.3 |
|
| ||||
| SPL z-score | −1.9 ±0.7* | −1.59 ±0.5 | −.1.18 ±0.8 | −0.77 ±1.6 |
| SDS mean ± SD (Range) | (−3.37– −0.24) | (−2.69 – −0.5) | (−2.34–0.09) | (−2.17–1.5) |
| <2 SDS (%) | 21 (35%) | 3 (17%) | 1 (13%) | 1 (25%) |
| Length (cm) mean ± SD | 4.0 ± 0.8 | 4.4 ± 0.6 | 5.0 ± 1.1 | 5.8 ± 2.6 |
Group median (or mean) is significantly different than the control group, Wilcoxin signed rank test or t-test p-value <0.05.
Group median (or mean) is outside the normal range for healthy controls
SDS=standard deviation score, IQR=interquartile range, LH=luteinizing hormone, FSH=follicle stimulating hormone, TT=total testosterone, INHB=Inhibin B, AMH=anti-mullerian hormone, SPL=stretched penile length
Figure 1.
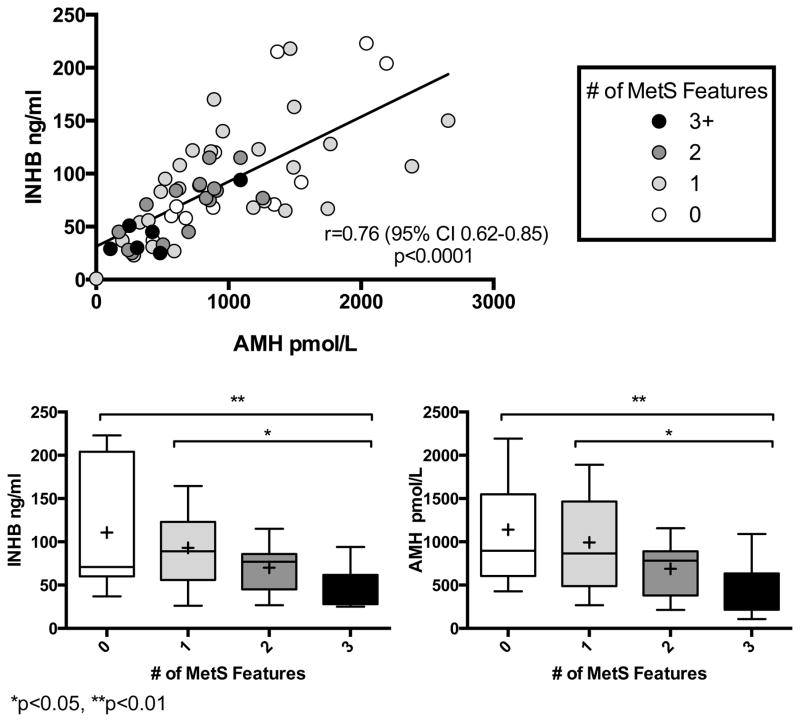
Figure 2.
Median values for INHB were not significantly different than expected, but INHB was low (<5%ile for age) in 18% of subjects (Table 3). Boys with low INHB had shorter penile length (z-score −2.3 vs −1.9, p=0.12) and smaller testicular volume (z-score −2.7 vs −1.5, p=0.06) but these differences did not reach statistical significance. There were no differences in anthropometric measurements between those with low or normal INHB. Although INHB concentrations did not correlate with age, the percentage of subjects who had low INHB increased with age and pubertal development. All four of the subjects with Tanner 3 pubic hair had low INHB.
AMH and INHB strongly correlated with each other (r=0.71, p<0.0001), and both were independent of FSH and LH. AMH weakly correlated inversely with serum TT (r=−0.22, p=0.04), while INHB did not. AMH was elevated in 16% of the group overall, most commonly in subjects ~9–11 years of age. Those with elevated AMH had higher INHB (p<0.0001) than subjects with normal or low AMH, while LH, FSH and TT did not differ. Individuals with elevated AMH did not differ in anthropometric data compared to those with normal AMH.
The Relationship Between Gonadal and Cardiometabolic Function
INHB
Boys with low INHB had greater cardiometabolic risk markers than boys with normal INHB, including higher FBG, lower HDL, higher fasting triglycerides and higher LDL (all p<0.05). In the younger group, where pubertal variability is minimized, these differences remained significant for all except LDL (Table 4). In a logistic regression model in the younger group, low INHB was significantly associated with the probability of meeting ≥3 MetS criteria (p=0.047). An INHB cutoff of ≤50 ng/dl yields a sensitivity of 83.3% and specificity of 79.2% for meeting full criteria for MetS in boys <9.5 years of age.
Table 4.
Characteristics of boys age 4–9.5 yrs with low INHB or high AMH.
| Low INHB N=8 |
Normal INHB N=51 |
P-value | High AMH N=7 |
Normal AMH N=51 |
P-value | |
|---|---|---|---|---|---|---|
| Age (mean ± SD) | 7.1 ± 1.2 | 6.2 ± 1.5 | 0.12 | 6.0 ± 1.7 | 6.3 ± 1.5 | 0.64 |
| Bone Age (mean ± SD) | 6.7 ± 1.5 | 5.7 ± 1.7 | 0.84 | 5.8 ± 2.2 | 5.8 ± 1.7 | 0.93 |
| Bone Age Delay (mean ± SD) | −0.4 ± 1.2 | −0.5 ± 0.9 | 0.76 | −0.3 ± 0.7 | −0.5 ± 0.9 | 0.53 |
| Height SDS (mean ± SD) | 0.6 ± 1.1 | 0.7 ± 1.0 | 0.91 | 0.4 ± 0.9 | 0.7 ± 1.1 | 0.52 |
| BMI SDS (mean ± SD) | 0.0 ± 1.4 | 0.6 ±1.1 | 0.17 | 0.0 ±1.7 | 0.7 ± 1.0 | 0.13 |
| Waist-to-hip ratio (mean ± SD) | 0.99 ± 0.02 | 0.99 ± 0.04 | 0.71 | 0.99 ± 0.03 | 0.99 ± 0.04 | 0.66 |
| Body fat % z-score (mean ± SD) | 1.0 ± 1.0 | 1.2 ± 0.9 | 0.67 | 0.9 ± 1.3 | 1.2 ± 0.9 | 0.52 |
| SPL z-score (mean ± SD) | −2.1 ± 0.6 | −1.8 ± 0.7 | 0.33 | −1.4 ± 0.8 | −1.9 ± 0.7 | 0.11 |
| Testicular volume z-score (mean ± SD) | −2.3 ± 0.6 | −1.6 ± 1.0 | 0.08 | −1.6 ± 1.2 | −1.6 ± 1.0 | 0.93 |
| FSH IU/L median (IQR) | 0.34 (0.22–0.49) | 0.40 (0.27–0.64) | 0.44 | 0.65 (0.15–1.4) | 0.37 (0.27–0.56) | 0.32 |
| LH IU/L median (IQR) | 0.015 (0.01–0.03) | 0.03 (0.01–0.05) | 0.11 | 0.05 (0.01–0.09) | 0.03 (0.01–0.05) | 0.48 |
| TT ng/dl median (IQR) | 3.5 (2.3–5.5) | 3.0 (2.0–4.0) | 0.36 | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 0.91 |
| AMH pmol/L median (IQR) | 277 (142–468) | 866 (568–1345) | <0.01 | 2040 (1748–2384) | 698 (425–956) | --- |
| INHB ng/dl median (IQR) | 26 (24–29) | 84 (65–120) | --- | 128 (92–204) | 74 (45–106) | <0.01 |
| FBG mg/dl median (IQR) | 93 (87–98) | 85 (80–87) | <0.01 | 82 (75–85) | 85 (81–90) | 0.07 |
| HDL mg/dl median (IQR) | 36 (33–43) | 47 (43–54) | <0.01 | 52 (51–56) | 44 (39–50) | <0.01 |
| Tg mg/dl median (IQR) | 77 (67–109) | 50 (39–77) | <0.01 | 43 (32–62) | 53 (41–86) | 0.09 |
| LDL mg/dl median (IQR) | 111 (103–124) | 103 (84–116) | 0.12 | 119 (103–128) | 103 (84–115) | 0.06 |
SD=standard deviation, IQR=interquartile range, BMI=body mass index, SPL=stretched penile length, FSH=follicle simulating hormone, LH=luteinizing hormone, TT=total testosterone, AMH=anti-mullerian hormone, INHB=inhibin B, FBG=fasting blood glucose, HDL=high density lipoprotein, Tg=triglycerides, LDL=low density lipoprotein
AMH
In the younger group, subjects with AMH above the upper limit of normal had more favorable cardiometabolic phenotype (Table 4), even when adjusted for INHB in multivariate analysis. When the older boys were included, however, only higher HDL and lower BMI SDS were noted and these did not reach statistical significance. Figure 2 illustrates the overall trend of the relationship between low Sertoli cell biomarkers (INHB and AMH) and increased MetS features.
TT
Participants with low serum TT had no statistically significant differences in physical and cardiometabolic features when compared to boys with normal TT. In the younger group alone, those with lower TT had a shorter height SDS (p=0.09), greater bone age delay (p=0.05), and a greater waist to hip ratio z-score (p=0.11), though these differences did not meet statistical significance.
DISCUSSION
In the largest cohort of pre- and peripubertal boys with KS reported to date we have found evidence of prepubertal cardiometabolic and gonadal dysfunction, as well as an association between gonadal function and cardiometabolic risk.
Cardiometabolic Risk
Cardiometabolic disorders, including MetS and type 2 diabetes, are common in men with KS and contribute to significant morbidity and mortality (Bojesen et al., 2010, Bojesen et al., 2006a, Jiang-Feng et al., 2012, Swerdlow et al., 2005). We now report 1 in 9 prepubertal boys with KS have 3 or more features of MetS. This is in agreement with two recent studies, one reporting a similar prevalence of MetS. (Bardsley, et al., 2011) using the same criteria for defining MS and the other reporting a high body fat percentage in children with KS (Aksglaede, et al., 2008). While elevated waist circumference and dyslipidemia were prevalent in this sample, elevated BP and impaired FBG were quite rare. Importantly, the risk of MetS features appears to be independent of age and BMI, emphasizing the need for anticipatory guidance and early screening regardless of BMI. The high prevalence of MetS without obesity in prepubertal boys with KS calls for further study of the mechanisms involved in insulin resistance and interventions to prevent or treat the metabolic risk in this population. Even though not obese by BMI measurement, waist-to-hip ratio and body fat percentage were increased, indicating abnormal body composition with increased adiposity.
Gonadal Function
FSH was elevated in 20% of subjects, while LH was lower than expected. Elevated FSH may represent gonadal insufficiency, though FSH did not correlate with INHB or AMH, biomarkers of sertoli cell function. Overall, gonadotropins are challenging to interpret in prepubertal children as the hypothalamic-pituitary-gonadal axis is largely quiescent and not responsive to the state of gonadal function (Grinspon, et al., 2012).
To our knowledge, this is the first study to utilize TT by LC/MS, a highly sensitive, precise, and accurate assay, in prepubertal boys with KS and we report here that TT was below the expected range for age in nearly half the prepubertal participants. This finding is similar to that reported in 55 boys with KS age 2–14.6 years, where 66% had TT concentrations in the lowest quartile (Zeger et al., 2008). This further supports the hypothesis that androgen deficiency is present prior to puberty in boys with KS, though we recognize the challenges of quantifying serum testosterone in the low ranges in which prepubertal concentrations fall. Due to the low serum concentrations of testosterone and the variability in TT assays, measuring TT in prepubertal boys for clinical purposes remains of limited utility.
AMH and INHB are products of sertoli cells and are both excellent markers for gonadal function in prepubertal boys (Grinspon, et al., 2012). Previous reports from small studies of prepubertal males with KS have largely suggested these biomarkers are normal (Aksglaede et al., 2011, Christiansen et al., 2003, Lahlou et al., 2004, Zeger, et al., 2008), however around 20% of infants had INHB concentrations below the lower limit of normal (Cabrol et al., 2011). In the large cohort described here, there were a subset of individuals in whom INHB and/or AMH were abnormal. In addition, we found a very strong correlation between INHB and AMH concentrations, which has been reported in other prepubertal populations with testicular dysfunction, such as cryptorchidism (Cortes et al., 2015).
INHB is reflective of sertoli cell mass and is largely independent of gonadotropin stimulation in the prepubertal period (Grinspon, et al., 2012). In the current study, the median concentration of INHB was within the normal reference range for age, however 18% of participants had an INHB below this normal range, while very few had an INHB above the normal range. Based on knowledge of about testicular physiology, low prepubertal INHB concentrations in KS likely represent poor testis function. Testicular volume was normal for age in several boys with low INHB and INHB concentrations did not correlate with LH, FSH, or TT in this cohort. Therefore, neither physical examination nor routinely available gonadotropin or androgen measurements can serve as a substitute for serum INHB in quantifying sertoli cell function.
AMH is another product of sertoli cells, however the regulation of AMH in normal prepubertal boys is complex (Matuszczak et al., 2013). AMH production and secretion requires both FSH stimulation of functioning sertoli cells and low intratesticular testosterone concentrations (Grinspon, et al., 2012, Grinspon et al., 2010). Therefore, testicular insufficiency affecting both sertoli and leydig cells would have opposing effects on serum AMH. We found median AMH concentrations to be higher than laboratory norms, particularly in the 9–11 year old boys. This suggests there may be a failure to mount appropriate intratesticular testosterone concentrations at the beginning of puberty in boys with KS. A delayed pubertal decline in serum AMH has been reported in boys with KS, and also hypothesized to be due to a lack of appropriate pubertal rise in intratesticular testosterone (Aksglaede, et al., 2011, Wikstrom, et al., 2004). Interestingly, although AMH did weakly correlate inversely with TT, individuals with high AMH did not exhibit signs of androgen deficiency such as shorter phallic length. Alternatively, higher AMH could represent better functioning sertoli cells, which is congruent with our finding of a strong correlation between AMH and INHB. Due to the complex regulation of AMH, this biomarker may not be optimal as a measure of testicular function in boys with KS.
The Relationship of Gonadal and Cardiometabolic Function
Male hypogonadism is strongly associated with central adiposity, insulin resistance, MetS, type 2 diabetes, and stroke in multiple populations, although it remains unclear in many of these studies if low TT is causative or a biomarker of poor metabolic health (Holmegard et al., 2016, Moriarty-Kelsey et al., 2010, Traish, 2014, Zitzmann, 2009). Historically, many clinical features of KS, including cardiometabolic dysfunction, have been attributed to long-standing hypogonadism. Therefore, a fundamental question is whether hypogonadism in childhood has an impact on the cardiometabolic phenotype. Using INHB and AMH as a proxy for overall testicular function, our data demonstrate a strong inverse relationship between testicular function and cardiometabolic risk, even in these young boys. We are unable to determine causality with this cross-sectional dataset. However due to the nearly universal testicular dysfunction in KS and the positive effects of testosterone treatment on metabolic risk factors in other populations, one hypothesis is that subtle prepubertal hypogonadism contributes to an unfavorable cardiometabolic profile (Aversa et al., 2010, Pacenza, et al., 2012, Traish et al., 2014). Alternative hypotheses for this relationship include the proposition that the extra X chromosome results in both gonadal and cardiometabolic dysfunction, or less likely, that cardiometabolic function causes testicular dysfunction. Longitudinal and interventional studies are needed to further delineate this relationship.
Boys with KS were found to have features of MetS independent of age or BMI. Obtaining INHB in prepubertal boys with KS may be clinically useful in risk stratifying for cardiometabolic dysfunction, particularly if longitudinal studies prove INHB to be predictive of future cardiometabolic dysfunction. Boys with prepubertal INHB < 50 ng/ml are at the highest risk for meeting MetS criteria. Future investigations for prevention interventions could include trials of early androgen supplementation and/or physical exercise programs, particularly in boys found to have poor gonadal function.
The strengths of this study include the large sample size of prepubertal boys and the sensitive and simultaneously run hormone assays. Ascertainment and selection biases are frequent limitations when studying conditions like KS, but it is important over half of our sample had an incidental diagnosis of KS. Although we did not correct for multiple comparisons since the intent of the study was exploratory, we presented the overall trends that were consistent throughout the dataset. When appropriate, potential confounding variables, such as age and tanner stage, were controlled for by evaluating the younger and older groups independently and adjustment in multivariate analyses.
Conclusion
In the largest cohort of pre- and peripubertal boys with KS to date we report a high prevalence of cardiometabolic risk factors including increased waist circumference, dyslipidemia, and insulin resistance independent of age and BMI. We have also found evidence of prepubertal gonadal dysfunction with significantly lower serum TT, a high prevalence of low INHB, and a high prevalence of elevated AMH. Of most interest, however, is the finding that prepubertal gonadal dysfunction is associated with increased markers of cardiometabolic risk. These findings require validation in larger longitudinal cohorts to determine the full clinical significance.
Acknowledgments
The authors thank the participants and their families for their involvement in this study. This study was sponsored by NIH/NINDS RO1NS050597 awarded to JR. The study was supported in part by NIH/NIDDK 2T32DK63687011A1 and the Institute for Research in Endocrinology and Metabolism, Paris, France.
Grants/Fellowships supporting the writing of the paper:
National Institute of Neurological Disorders and Stroke (NINDS) NS050597
National Institute Diabetes and Digestive and Kidney Diseases (NIDDK) 2T32DK63687011A1
Footnotes
Disclosure Summary: The authors have nothing to disclose.
Clinical Trial Registration Number: NCT00348946
Author’s Contributions
SD & JR analyzed the data and wrote the paper, JR designed the study and performed the research, MB & KK contributed to data acquisition and paper revisions, NL, MCT, and LP contributed to the analysis and interpretation of the data, PZ contributed to data interpretation and critically revising the paper. All authors approve the submitted version.
References
- Aksglaede L, Christiansen P, Sorensen K, Boas M, Linneberg A, Main KM, Andersson AM, Skakkebaek NE, Juul A. Serum concentrations of Anti-Mullerian Hormone (AMH) in 95 patients with Klinefelter syndrome with or without cryptorchidism. Acta Paediatr. 2011;100:839–45. doi: 10.1111/j.1651-2227.2011.02148.x. [DOI] [PubMed] [Google Scholar]
- Aksglaede L, Molgaard C, Skakkebaek NE, Juul A. Normal bone mineral content but unfavourable muscle/fat ratio in Klinefelter syndrome. Arch Dis Child. 2008;93:30–4. doi: 10.1136/adc.2007.120675. [DOI] [PubMed] [Google Scholar]
- Aversa A, Bruzziches R, Francomano D, Rosano G, Isidori AM, Lenzi A, Spera G. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7:3495–503. doi: 10.1111/j.1743-6109.2010.01931.x. [DOI] [PubMed] [Google Scholar]
- Bardsley MZ, Falkner B, Kowal K, Ross JL. Insulin resistance and metabolic syndrome in prepubertal boys with Klinefelter syndrome. Acta Paediatr. 2011;100:866–70. doi: 10.1111/j.1651-2227.2011.02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastida MG, Rey RA, Bergada I, Bedecarras P, Andreone L, del Rey G, Boywitt A, Ropelato MG, Cassinelli H, Arcari A, Campo S, Gottlieb S. Establishment of testicular endocrine function impairment during childhood and puberty in boys with Klinefelter syndrome. Clin Endocrinol (Oxf) 2007;67:863–70. doi: 10.1111/j.1365-2265.2007.02977.x. [DOI] [PubMed] [Google Scholar]
- Bojesen A, Host C, Gravholt CH. Klinefelter’s syndrome, type 2 diabetes and the metabolic syndrome: the impact of body composition. Mol Hum Reprod. 2010;16:396–401. doi: 10.1093/molehr/gaq016. [DOI] [PubMed] [Google Scholar]
- Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab. 2006a;91:1254–60. doi: 10.1210/jc.2005-0697. [DOI] [PubMed] [Google Scholar]
- Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88:622–6. doi: 10.1210/jc.2002-021491. [DOI] [PubMed] [Google Scholar]
- Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, Bennett P, Laurberg P, Frystyk J, Flyvbjerg A, Christiansen JS, Gravholt CH. The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006b;29:1591–8. doi: 10.2337/dc06-0145. [DOI] [PubMed] [Google Scholar]
- Cabrol S, Ross JL, Fennoy I, Bouvattier C, Roger M, Lahlou N. Assessment of Leydig and Sertoli cell functions in infants with nonmosaic Klinefelter syndrome: insulin-like peptide 3 levels are normal and positively correlated with LH levels. J Clin Endocrinol Metab. 2011;96:E746–53. doi: 10.1210/jc.2010-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen P, Andersson AM, Skakkebaek NE. Longitudinal studies of inhibin B levels in boys and young adults with Klinefelter syndrome. J Clin Endocrinol Metab. 2003;88:888–91. doi: 10.1210/jc.2002-021379. [DOI] [PubMed] [Google Scholar]
- Cortes D, Clasen-Linde E, Hutson JM, Li R, Thorup J. The Sertoli cell hormones inhibin-B and anti Mullerian hormone have different patterns of secretion in prepubertal cryptorchid boys. J Pediatr Surg. 2015 doi: 10.1016/j.jpedsurg.2015.08.059. [DOI] [PubMed] [Google Scholar]
- de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–7. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- Fennoy I. Testosterone and the child (0–12 years) with Klinefelter syndrome (47XXY): a review. Acta Paediatr. 2011;100:846–50. doi: 10.1111/j.1651-2227.2011.02184.x. [DOI] [PubMed] [Google Scholar]
- Greulich W, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2. Stanford, CA: Standford University Press; 1959. [Google Scholar]
- Grinspon RP, Loreti N, Braslavsky D, Bedecarras P, Ambao V, Gottlieb S, Bergada I, Campo SM, Rey RA. Sertoli cell markers in the diagnosis of paediatric male hypogonadism. J Pediatr Endocrinol Metab. 2012;25:3–11. doi: 10.1515/jpem-2011-0453. [DOI] [PubMed] [Google Scholar]
- Grinspon RP, Rey RA. Anti-mullerian hormone and sertoli cell function in paediatric male hypogonadism. Horm Res Paediatr. 2010;73:81–92. doi: 10.1159/000277140. [DOI] [PubMed] [Google Scholar]
- Hall J, Froster-Iskanius U, JEA . Handbook of Normal Physical Measurements. Oxford: Oxford University Press; 1989. [Google Scholar]
- Holmegard HN, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A, Benn M. Sex Hormones and Ischemic Stroke: A Prospective Cohort Study and Meta-Analyses. J Clin Endocrinol Metab. 2016;101:69–78. doi: 10.1210/jc.2015-2687. [DOI] [PubMed] [Google Scholar]
- Jiang-Feng M, Hong-Li X, Xue-Yan W, Min N, Shuang-Yu L, Hong-Ding X, Liang-Ming L. Prevalence and risk factors of diabetes in patients with Klinefelter syndrome: a longitudinal observational study. Fertil Steril. 2012;98:1331–5. doi: 10.1016/j.fertnstert.2012.07.1122. [DOI] [PubMed] [Google Scholar]
- Lahlou N, Fennoy I, Carel JC, Roger M. Inhibin B and anti-Mullerian hormone, but not testosterone levels, are normal in infants with nonmosaic Klinefelter syndrome. J Clin Endocrinol Metab. 2004;89:1864–8. doi: 10.1210/jc.2003-031624. [DOI] [PubMed] [Google Scholar]
- Laurson KR, Eisenmann JC, Welk GJ. Body fat percentile curves for U.S. children and adolescents. Am J Prev Med. 2011;41:S87–92. doi: 10.1016/j.amepre.2011.06.044. [DOI] [PubMed] [Google Scholar]
- Matuszczak E, Hermanowicz A, Komarowska M, Debek W. Serum AMH in Physiology and Pathology of Male Gonads. Int J Endocrinol. 2013;2013:128907. doi: 10.1155/2013/128907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty-Kelsey M, Harwood JE, Travers SH, Zeitler PS, Nadeau KJ. Testosterone, obesity and insulin resistance in young males: evidence for an association between gonadal dysfunction and insulin resistance during puberty. J Pediatr Endocrinol Metab. 2010;23:1281–7. doi: 10.1515/jpem.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacenza N, Pasqualini T, Gottlieb S, Knoblovits P, Costanzo PR, Stewart Usher J, Rey RA, Martinez MP, Aszpis S. Clinical Presentation of Klinefelter’s Syndrome: Differences According to Age. Int J Endocrinol. 2012;2012:324835. doi: 10.1155/2012/324835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzano A, Arcopinto M, Marra AM, Bobbio E, Esposito D, Accardo G, Giallauria F, Bossone E, Vigorito C, Lenzi A, Pasquali D, Isidori AM, Cittadini A. MANAGEMENT OF ENDOCRINE DISEASE: Klinefelter syndrome, cardiovascular system and thromboembolic disease. Review of literature and clinical perspectives. Eur J Endocrinol. 2016 doi: 10.1530/EJE-15-1025. [DOI] [PubMed] [Google Scholar]
- Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–23. [PubMed] [Google Scholar]
- Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA United Kingdom Clinical Cytogenetics G. Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab. 2005;90:6516–22. doi: 10.1210/jc.2005-1077. [DOI] [PubMed] [Google Scholar]
- Traish AM. Outcomes of testosterone therapy in men with testosterone deficiency (TD): part II. Steroids. 2014;88:117–26. doi: 10.1016/j.steroids.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Traish AM, Abdallah B, Yu G. Androgen deficiency and mitochondrial dysfunction: implications for fatigue, muscle dysfunction, insulin resistance, diabetes, and cardiovascular disease. Horm Mol Biol Clin Investig. 2011;8:431–44. doi: 10.1515/HMBCI.2011.132. [DOI] [PubMed] [Google Scholar]
- Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68:314–29. doi: 10.1111/ijcp.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom AM, Raivio T, Hadziselimovic F, Wikstrom S, Tuuri T, Dunkel L. Klinefelter syndrome in adolescence: onset of puberty is associated with accelerated germ cell depletion. J Clin Endocrinol Metab. 2004;89:2263–70. doi: 10.1210/jc.2003-031725. [DOI] [PubMed] [Google Scholar]
- Zeger MP, Zinn AR, Lahlou N, Ramos P, Kowal K, Samango-Sprouse C, Ross JL. Effect of ascertainment and genetic features on the phenotype of Klinefelter syndrome. J Pediatr. 2008;152:716–22. doi: 10.1016/j.jpeds.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol. 2009;5:673–81. doi: 10.1038/nrendo.2009.212. [DOI] [PubMed] [Google Scholar]