Abstract
Objective
The report of the “Mississippi baby” who was initiated on antiretroviral therapy (ART) within 30 hours of birth and maintained viral suppression off ART for 27 months has increased interest in the timing of ART initiation early in life. We examined associations between age at ART initiation and virologic outcomes in five cohorts of HIV-infected infants and young children who initiated ART before 2 years of age in Johannesburg, South Africa.
Methods
We compared those who initiated ART early (<6 months of age) and those who started ART late (6–24 months of age). Two primary outcomes were examined: (1) initial response to ART in three cohorts and (2) later sustained virologic control after achieving suppression on ART in two cohorts.
Results
We did not observe consistent differences in initial viral suppression rates by age at ART initiation. Overall, initial viral suppression rates were low. Only 31, 40.1, and 26.5% of early-treated infants (<6 months of age) in the three cohorts, respectively, were suppressed <50 copies/mL of HIV-RNA 6 months after starting ART. We did observe better sustained virologic control after achieving suppression on ART among infants starting ART early compared to late. Children who started ART early were less likely to experience viral rebound (>50 copies/mL or >1000 copies/mL) than children who started late in both cohorts.
Conclusions
These findings provide additional support for early initiation of ART in HIV-infected infants.
Keywords: antiretroviral therapy, early treatment, viral suppression, virologic control, infant
Introduction
Evidence from the Children with HIV Early Antiretroviral Therapy (CHER) randomized clinical trial in South Africa and other observational studies indicates that initiation of antiretroviral therapy (ART) early in life reduces morbidity and mortality in HIV-infected infants [1–5]. These findings have informed treatment guidelines which now advise initiation of ART irrespective of clinical and immunological status in all HIV-infected infants and children [6–8]. The report of the “Mississippi baby” who was initiated on antiretroviral therapy (ART) within 30 hours of birth and who maintained viral suppression off therapy for 27 months [9] has further stimulated interest in the possible benefits of initiating ART soon after birth.
Data on the effects of the timing of early ART initiation on virologic outcomes, including initial virologic response and sustained virologic control after suppression are limited. Some studies have reported better initial virologic response in children started on ART <3–6 months of age compared to children started after 6 months of age [4, 10] while others have reported no differences in short-term suppression rates [11, 12]. Reports of infants started on ART in the first months of life with no comparator group of children started at older ages have shown varied rates of initial suppression [13–16]. Few studies have compared later virologic control, i.e. maintenance of suppression without rebound after achieving initial suppression, between children initiating ART at earlier versus later ages. One study of children who initiated ART younger or older than one year of age and who achieved virologic suppression found no association between age at ART start and rebound of HIV-RNA >400 copies/mL at later ages [17].
With increasing attention to the possible benefits of ART initiation in newborns, it is important to investigate the effects of the timing of ART initiation on virologic response in larger cohorts in sub-Saharan Africa where the vast majority of new pediatric infections occur [18]. Here, using data from five study cohorts of HIV-infected children initiating ART before 2 years of age in Johannesburg, South Africa, we examine the association between age at starting ART and (1) initial viral response in three cohorts and (2) later virologic control after achieving suppression in two cohorts.
Methods
Study populations
Five cohorts of HIV-infected infants and young children in Johannesburg, South Africa were included in this analysis (Table 1). Three cohorts of children initiating ART (Cohorts A, B, and C.1) were suitable to examine the association between age at ART initiation and initial response to treatment. Two cohorts of children who had achieved virologic suppression after ART initiation (Cohorts C.2 and D) were suitable to assess the association between age at ART initiation and later virologic control.
Table 1.
Description of five cohorts of HIV-infected infants and young children initiating antiretroviral therapy (ART) before 24 months of age
| Cohort | Relevant citations | N | Years | Brief description | Purpose – to examine: |
|---|---|---|---|---|---|
| Cohort A | [19–21] | 219 | 2011 | Newly-diagnosed, treatment-naïve infants and young children identified as part of a surveillance study | Initial viral response |
| Cohort B | NA | 718 | 2004–2012 | Newly-diagnosed, treatment-naïve infants and young children initiating ART at a routine HIV clinic | Initial viral response |
| Cohort C.1 | [23] | 323 | 2005–2010 | Newly-diagnosed, treatment-naïve infants enrolled into pre- randomization phase of ART strategies trial and initiated on LPV/r-based ART (ClinicalTrials.gov: NCT00117728) | Initial viral response |
| Cohort C.2 | [24, 25] | 195 | 2005–2010 | Children from Cohort C.1 who met criteria for randomization (plasma HIV-RNA <400 copies/mL for at least 3 months within the first 12 months of treatment) and were assigned to remain on LPV/r or switch to nevirapine as part of an ART strategies trial (ClinicalTrials.gov: NCT00117728) | Later virologic control |
| Cohort D | [26] | 293 | 2010–2013 | Children 3–5 years of age who were suppressed on LPV/r (plasma HIV- RNA <50 copies/mL at the time of enrollment into the study) and randomized to remain on LPV/r or switch to efavirenz as part of an ART strategies trial (ClinicalTrials.gov: NCT01146873) | Later virologic control |
Cohort A included newly-diagnosed, treatment-naïve HIV-infected infants and young children identified at hospital admissions, prevention of mother-to-child transmission (PMTCT) follow-up visits, and immunization services as part of an active surveillance study intended to improve engagement in care and ART initiation at three hospitals and two affiliated clinics conducted between January and December 2011 [19–21]. We restricted the analysis to 219 children known to have been initiated on ART before 2 years of age. At enrollment into the surveillance study (pre-treatment), blood samples were collected from each child for plasma HIV-RNA tests (Roche Taqman HIV Test v2.0) and CD4 percentage. Sociodemographic and clinical information was also collected. Subsequent information on survival, HIV-RNA, and CD4 percentage, as close as possible to 6 months post-ART initiation, was abstracted from medical records.
Cohort B included 718 newly-diagnosed, treatment-naïve infants and young children who initiated ART before 2 years of age as part of public HIV services at the Empilweni Services and Research Unit at Rahima Moosa Mother and Child Hospital (RMMCH) between January 2004 and July 2012 and who had at least one follow up visit after ART initiation or died after ART initiation. At this time ritonavir-boosted lopinavir (LPV/r)-based was given to children in this age group and viral load testing was recommended every 6 months after ART initiation [22]. Demographic information, age at ART initiation, HIV-RNA, and CD4 percentage were extracted from a routine database maintained by the site for clinical care purposes.
Cohort C.1 consisted of 323 newly-diagnosed, treatment-naïve infants and young children enrolled into the pre-randomization phase of an ART strategies trial that took place at RMMCH from April 2005 to June 2010 [23]. All children had been exposed to nevirapine used for PMTCT given to the mother, to the child, or both and were initiated on LPV/r-based ART before 2 years of age. Blood samples for plasma HIV-RNA quantification (Roche version 1.5 Amplicor assay, Roche, Branchburg, NJ) and CD4 percentages were measured before treatment began and at 4, 12, 24, 36, and 52 weeks after treatment initiation as part of the pre-randomization phase.
Cohort C.2 includes all 195 of the children from Cohort C.1 who met criteria for randomization (HIV-RNA <400 copies/mL for at least 3 months within the first 12 months of treatment) and were assigned to remain on LPV/r (N=99) or switch to nevirapine (N=96) (ClinicalTrials.gov: NCT00117728) [24, 25]. Children undergoing randomization after viral suppression had been achieved (a median of 9 months after ART initiation) had additional blood samples collected at scheduled post-randomization visits (4, 16, 24, 36, and 52 weeks).
Cohort D included children 3–5 years of age with a history of nevirapine exposure for PMTCT who were suppressed on LPV/r (HIV-RNA <50 copies/mL at the time of enrollment into the study) and randomized to either remain on LPV/r or switch to efavirenz as part of an ART strategies trial that took place at RMMCH from June 2010 to December 2013 (ClinicalTrials.gov: NCT01146873) [26]. This analysis was limited to 293 children who initiated ART before 2 years of age, including 145 children randomized to remain on LPV/r and 148 randomized to switch to efavirenz. Plasma HIV-RNA (AmpliPrep/COBAS® TaqMan® HIV Test, version 2.0, Roche, Branchburg, NJ) measurements were obtained at randomization and scheduled post-randomization visits (4, 8, 16, 24, and 48 weeks). CD4 percentage was measured at study entry and 24 and 48 weeks post-randomization.
Studies were approved by the Institutional Review Boards of Columbia University (New York, New York) and the University of the Witwatersrand (Johannesburg, South Africa). Signed informed consent for all procedures and data was provided by each child’s guardian for Cohorts A, C.1, C.2, and D and for data sharing for Cohort B.
Statistical analysis
To examine effects of the timing of ART initiation, we compared children who initiated ART <6 months of age to those who started ART 6–24 months of age. The <6 months age category was refined further in some analyses. Two primary virologic outcomes were examined: (1) initial response to ART and (2) later sustained virologic control after achieving suppression on ART.
Initial response to ART was examined in Cohorts A, B, and C.1. HIV-RNA (categorized as <50, 50–1000, ≥1000 copies/mL) measured around 6 months post-ART initiation was compared between age at ART initiation groups. We defined the window for “6 months” to be between 3 and 12 months. If more than one result was available, the result nearest to 6 months was chosen. Secondary outcomes for Cohorts A, B, and C.1 included CD4 percentage as a continuous and as a categorical variable (<10, 10–15, and ≥15) at 6 months post-ART initiation. Vital status by 6 months post-ART initiation was also determined.
Later virologic control after suppression on ART was assessed in Cohorts C.2 and D. The proportion of children in three HIV-RNA categories (<50, 50–1000,≥1000 copies/mL) at scheduled visits post-randomization was compared between age at ART initiation groups. A generalized estimating equation (GEE) model with an unstructured correlation structure was used to examine the overall effect of age at ART initiation on HIV-RNA outcomes at repeated visits. Other correlation structures produced similar results. We adjusted for treatment randomization group (LPV/r vs. nevirapine in Cohort C.2; LPV/r vs. efavirenz in Cohort D).
Additionally, we compared area under the plasma viral load curve (AUVLC), a measure of “cumulative viral load,” between age at ART initiation groups. The AUVLC in log10 copy-weeks/mL at scheduled visits in the two cohorts was calculated using the trapezoidal rule, whereby larger areas indicate higher cumulative viremia [27]. The data were normalized so that a child with an AUCVL of 0 was considered fully suppressed and a child with a detectable AUCVL was not fully suppressed at follow-up visits.
Statistical comparisons were conducted using chi-squared or Fisher’s exact tests for categorical variables, ANOVA and t-tests for normally-distributed continuous variables, and Kruskal-Wallis tests for non-normally distributed continuous variables. All p-values are 2-tailed and p-values <0.05 were considered statistically significant. Analyses were performed using SAS version 9.4 (Cary, North Carolina, USA).
Results
Characteristics
Characteristics of the children included in this study are shown in Table 2 stratified by cohort. Cohort A included infants who were actively identified to improve engagement in care and ART initiation and Cohort B included infants accessing routine clinical services. Cohorts C.1, C.2, and D were comprised of infants enrolled in clinical trials. Sex distribution and pretreatment characteristics, including HIV-RNA, CD4 count and percentage, and WAZ were similar across the cohorts. Mean age at ART initiation ranged from 8.7 months in Cohort A to 10.5 months in Cohort B. A higher proportion of children in Cohort A (47.5%) and Cohort D (46.4%) initiated ART early (i.e. <6 months of age) than Cohort B (35.1%), Cohort C.1 (31.6%), and Cohort C.2 (27.7%).
Table 2.
Characteristics of five cohorts of HIV-infected infants and young children initiating antiretroviral therapy (ART) before 24 months of age
| Characteristic | Cohort A | Cohort B | Cohort C.1 | Cohort C.2 | Cohort D |
|---|---|---|---|---|---|
|
| |||||
| N | 219 | 718 | 323 | 195 | 293 |
|
| |||||
| Sex, N (%) | |||||
| Male | 104 (47.5) | 351 (49.0) | 168 (52.0) | 104 (53.3) | 137 (46.8) |
| Female | 115 (52.5) | 366 (51.1) | 155 (48.0) | 91 (46.7) | 156 (53.2) |
|
| |||||
| PMTCT exposure, % | 69.0% | Unknown | 100% | 100% | 100% |
|
| |||||
| Age at ART initiation (months), Mean ± SD | 8.7 ± 6.5 | 10.5 ± 6.7 | 10.1 ± 5.8 | 10.0 ± 10.7 | 8.9 ± 6.4 |
| <6 months | 104 (47.5) | 252 (35.1) | 102 (31.6) | 54 (27.7) | 136 (46.4) |
| 6–12 months | 56 (25.6) | 202 (28.1) | 115 (35.6) | 69 (35.4) | 79 (27.0) |
| 12–24 months | 59 (26.9) | 264 (36.8) | 106 (32.8) | 72 (36.9) | 78 (26.6) |
|
| |||||
| Pretreatment HIV-RNA quantity (copies/mL), N (%) | |||||
| <100000 | 22 (10.8) | 83 (15.8) | 25 (8.8) | 19 (11.0) | 33 (13.7) |
| 100000–750000 | 64 (31.5) | 157 (29.8) | 78 (27.6) | 58 (33.5) | 75 (31.1) |
| ≥750000 | 117 (57.6) | 287 (54.5) | 180 (63.6) | 96 (55.5) | 133 (55.2) |
| Missing | 16 | 191 | 40 | 22 | 52 |
|
| |||||
| Pretreatment CD4 count (cells/mm3), Mean ± SD | 1260 ± 1193 | 985 ± 802 | 1006 ± 754 | 1063 ± 704 | 1213 ± 793 |
|
| |||||
| Pretreatment CD4 percentage, N (%) | |||||
| <10 | 32 (15.0) | 91 (16.6) | 55 (18.1) | 26 (14.0) | 32 (11.7) |
| 10–15 | 33 (15.5) | 106 (19.3) | 61 (20.1) | 37 (19.9) | 51 (18.6) |
| ≥15 | 148 (69.5) | 353 (64.2) | 188 (61.8) | 123 (66.1) | 191 (69.7) |
| Missing | 6 | 168 | 19 | 9 | 19 |
| Mean ± SD | 22.5 ± 12.6 | 19.2 ± 10.1 | 18.5 ± 9.1 | 19.1 ± 8.5 | 21.7 ± 10.6 |
|
| |||||
| Pretreatment weight-for-age z-score (WAZ), Mean ± SD | −2.20 ± 1.9 | −2.53 ± 2.4 | −2.49 ± 1.7 | −2.18 ± 1.7 | −2.16 ± 1.6 |
| WAZ <−2, N (%) | 118 (55.4) | 345 (62.8) | 169 (58.3) | 91 (51.7) | 106 (52.7) |
| N Missing | 6 | 169 | 33 | 19 | 92 |
|
| |||||
| Treatment Initiated On, N (%) | |||||
| LPV/r + lamivudine + stavudine | 0 (0.0) | 559 (77.9) | 163 (50.5) | 108 (55.4) | 234 (79.9) |
| LPV/r + lamivudine + abacavir | 211 (96.4) | 159 (22.1) | 0 (0.0) | 0 (0.0) | 11 (3.8) |
| LPV/r + lamivudine + other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (3.1) |
| Ritonavir + lamivudine + stavudine | 0 (0.0) | 0 (0.0) | 160 (49.5) | 87 (44.6) | 39 (13.3) |
| ATZ/r + lamivudine + abacavir | 3 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 5 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Abbreviations: LPV/r: ritonavir-boosted lopinavir, ATZ/r: ritonavir-boosted atazanavir; PMTCT: prevention of mother-to-child transmission of HIV; SD: standard deviation
Initial response to treatment
Pre-treatment, a higher proportion of children starting ART early (<6 months) had higher HIV-RNA levels (≥750,000 copies/mL) than children starting ART late (6–24 months of age) in all three cohorts (Table 3). Pre-treatment CD4 percentage was consistently higher in the early initiators and the proportion of children underweight (WAZ <−2) was lower in children starting ART early compared to late in Cohort A only (Table 3).
Table 3.
Characteristics, plasma HIV-1 RNA quantity, CD4 percentage, and weight-for-age Z-score pre-antiretroviral therapy (ART) initiation, and plasma HIV-1 RNA quantity, CD4 percentage, and vital status 6 months post-ART initiation in Cohorts A, B, and C stratified by age at ART initiation (<6 months vs. 6–24 months)
| Cohort A | Cohort B | Cohort C.1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age at ART initiation (months) | Age at ART initiation (months) | Age at ART initiation (months) | |||||||
| <6 | 6–24 | P | <6 | 6–24 | P | <6 | 6–24 | P | |
| N | 104 | 115 | 252 | 466 | 102 | 221 | |||
|
| |||||||||
| Characteristics | |||||||||
|
| |||||||||
| Sex, N (%) | |||||||||
| Male | 42 (40.4) | 62 (53.9) | 0.045 | 114 (45.4) | 237 (50.9) | 0.165 | 41 (40.2) | 127 (57.5) | 0.004 |
| Female | 62 (59.6) | 53 (46.1) | 137 (54.6) | 229 (49.1) | 61 (59.8) | 94 (42.5) | |||
|
| |||||||||
| Age at ART initiation (mo), Mean ± SD | 3.3 ± 1.3 | 13.6 ± 5.4 | <0.001 | 3.8 ± 1.3 | 14.1 ± 5.6 | <0.001 | 4.1 ± 1.1 | 12.8 ± 5.0 | <0.001 |
|
| |||||||||
| Pre-treatment | |||||||||
|
| |||||||||
| HIV-RNA quantity (copies/mL), N (%) | |||||||||
| <100000 | 14 (14.3) | 8 (7.6) | 25 (13.5) | 58 (17.0) | 4 (4.7) | 21 (10.7) | |||
| 100000–750000 | 23 (23.5) | 41 (39.1) | 0.036 | 43 (23.2) | 114 (33.3) | 0.011 | 17 (19.8) | 61 (31.0) | 0.019 |
| ≥750000 | 61 (62.2) | 56 (53.3) | 117 (63.2) | 170 (49.7) | 65 (75.6) | 115 (58.4) | |||
| Missing | 6 | 10 | 67 | 124 | 16 | 24 | |||
|
| |||||||||
| HIV-RNA quantity (log copies/mL)1, Median (IQR) | 6.2 (5.4, 6.8) | 5.9 (5.6, 6.4) | 0.196 | 6.1 (5.6, 6.5) | 5.9 (5.2, 6.3) | <0.001 | 5.9 (5.9, 5.9) | 5.9 (5.5, 5.9) | 0.001 |
|
| |||||||||
| CD4 percentage, Mean ± SD | 26.6 ± 13.4 | 18.8 ± 10.4 | <0.001 | 22.9 ± 11.8 | 17.4 ± 8.7 | <0.001 | 23.0 ± 9.6 | 16.4 ± 8.1 | <0.001 |
|
| |||||||||
| CD4 percentage, N (%) | |||||||||
| <10 | 11 (10.8) | 21 (18.9) | 24 (13.0) | 67 (18.3) | 8 (8.3) | 47 (22.6) | |||
| 10–15 | 14 (13.7) | 19 (17.1) | 0.153 | 17 (9.2) | 89 (24.3) | <0.0001 | 13 (13.5) | 48 (23.1) | <0.001 |
| ≥15 | 77 (75.5) | 71 (64.0) | 143 (77.7) | 210 (57.4) | 75 (78.1) | 113 (54.3) | |||
| Missing | 2 | 4 | 68 | 100 | 6 | 13 | |||
|
| |||||||||
| Weight-for-age z-score (WAZ), Mean ± SD | −1.99 ± 2.0 | −2.39 ± 1.8 | 0.127 | −2.55 ± 1.8 | −2.63 ± 1.7 | 0.638 | −2.54 ± 1.8 | −2.47 ± 1.7 | 0.753 |
| WAZ <−2, N (%) | 48 (47.1) | 70 (63.1) | 0.019 | 107 (60.1) | 238 (64.2) | 0.402 | 52 (58.4) | 117 (58.2) | 0.972 |
| Missing | 2 | 4 | 75 | 95 | 13 | 20 | |||
|
| |||||||||
| 6 months post-ART initiation | |||||||||
|
| |||||||||
| Time since treatment start of 6 month HIV-RNA quantity (mo), Mean ± SD | 5.7 ± 1.3 | 5.6 ± 1.3 | 0.702 | 6.3 ± 1.8 | 6.2 ± 1.8 | 0.872 | 6.0 ± 1.3 | 5.8 ± 0.9 | 0.073 |
|
| |||||||||
| HIV-RNA quantity (copies/mL), N (%) | |||||||||
| <50 | 18 (31.0) | 5 (9.8) | 77 (40.1) | 152 (44.2) | 22 (26.5) | 76 (39.2) | |||
| 50–1000 | 16 (27.6) | 12 (23.5) | 0.05 | 57 (29.7) | 103 (29.9) | 0.78 | 39 (47.0) | 70 (36.1) | 0.20 |
| 1000–100000 | 16 (27.6) | 22 (43.1) | 23 (12.0) | 38 (11.1) | 14 (16.9) | 27 (13.9) | |||
| 100000–750000 | 2 (3.5) | 4 (7.8) | 12 (6.3) | 18 (5.2) | 6 (7.2) | 19 (9.8) | |||
| ≥750000 | 6 (10.3) | 8 (15.7) | 10 (5.2) | 12 (3.5) | 2 (2.4) | 2 (1.0) | |||
| Missing | 46 | 64 | 60 | 122 | 19 | 27 | |||
|
| |||||||||
| HIV-RNA quantity (log copies/mL), Median (IQR) | 2.8 (1.59, 4.18) | 3.5 (2.33, 4.93) | 0.025 | 2.3 (1.6, 3.6) | 2.2 (1.6, 3.1) | 0.176 | 2.6 (1.7, 3.2) | 2.2 (1.7, 3.0) | 0.176 |
|
| |||||||||
| CD4 percentage, Mean ± SD | 31.1 ± 10.0 | 24.6 ± 9.1 | <0.001 | 28.3 ± 9.4 | 25.3 ± 8.7 | <0.001 | 28.3 ± 10.2 | 26.1 ± 9.3 | 0.086 |
|
| |||||||||
| CD4 percentage, N (%) | |||||||||
| <10 | 1 (1.7) | 0 (0.0) | 4 (2.2) | 14 (4.2) | 3 (3.8) | 5 (2.6) | |||
| 10–15 | 2 (3.4) | 5 (10.9) | 0.218 | 11 (5.9) | 26 (7.9) | 0.313 | 6 (7.5) | 17 (8.9) | 0.829 |
| ≥15 | 56 (94.9) | 41 (89.1) | 171 (91.9) | 291 (87.9) | 71 (88.8) | 170 (88.5) | |||
| Missing | 45 | 69 | 66 | 135 | 22 | 29 | |||
|
| |||||||||
| Vital status, N (%) | |||||||||
| Alive | 88 (88.9) | 83 (79.8) | 0.076 | 204 (95.8) | 375 (97.2) | 0.370 | 81 (89.0) | 189 (90.9) | 0.618 |
| Dead | 11 (11.1) | 21 (20.2) | 9 (4.2) | 11 (2.9) | 10 (11.0) | 19 (9.1) | |||
| Missing | 5 | 11 | 39 | 80 | 11 | 13 | |||
Note: Assay to assess HIV-RNA quantity in Cohort C.1 used an upper limit of detection of 750,000 copies/mL, which led to truncation of more than half of the measurements.
Abbreviations: SD: standard deviation
Post-treatment, it was only in Cohort A that children who initiated ART early were more likely to be suppressed (<50 copies/mL) at 6 months than children who initiated ART late. This was not observed in Cohorts B and C.1 (Table 3). In a finer stratification of age at ART initiation in Cohort A, the proportions of children suppressed <50 copies/mL 6 months post-ART initiation were 35.5, 25.9, and 9.8% in in those who started ART <3 months, 3–6 months, and 6–24 months, respectively. This trend was not seen in Cohorts B and C.1. Adjustment for pre-treatment viral load or other pre-treatment characteristics including sex, CD4 percentage, and WAZ did not change these results (data not shown). When the three cohorts were combined, there was no difference in suppression to <50 copies by 6 months for children who initiated ART early (<6 months) vs. late (6–24 months), even after adjustment for pretreatment HIV-RNA and CD4 percentage (OR=0.99, 95% CI: 0.71, 1.39).
In Cohorts A, B, and C.1 the mean CD4 percentage at 6 months post-ART initiation was higher in children who initiated ART early (Table 3) but after adjustment for pretreatment CD4 percentage in all three cohorts, the difference was no longer statistically significant (p=0.087, 0.403, 0.201). There was a trend towards lower mortality among children treated <6 months vs treated 6–24 months in Cohort A (p=0.076) (Table 3). In a further stratification of age at ART initiation categories into <3 months, 3–6 months, and 6–24 months, the proportions of children who died by 6 months post-ART initiation were 4.6, 16.4, and 20.2% respectively in Cohort A (p=0.006). No differences between age at ART initiation groups in the proportion of deaths was observed in Cohorts B and C.1.
We combined the data from the three cohorts to investigate effects of the timing of ART initiation in monthly increments in the first 6 months of life. No clear trends in the proportion of children suppressed by 6 months post-ART initiation were observed by age at ART initiation in months. The percentage of children suppressed <50 copies/mL ranged from 25% to 49.3% across all age intervals in months of age at ART initiation (Table S1).
Later virologic control
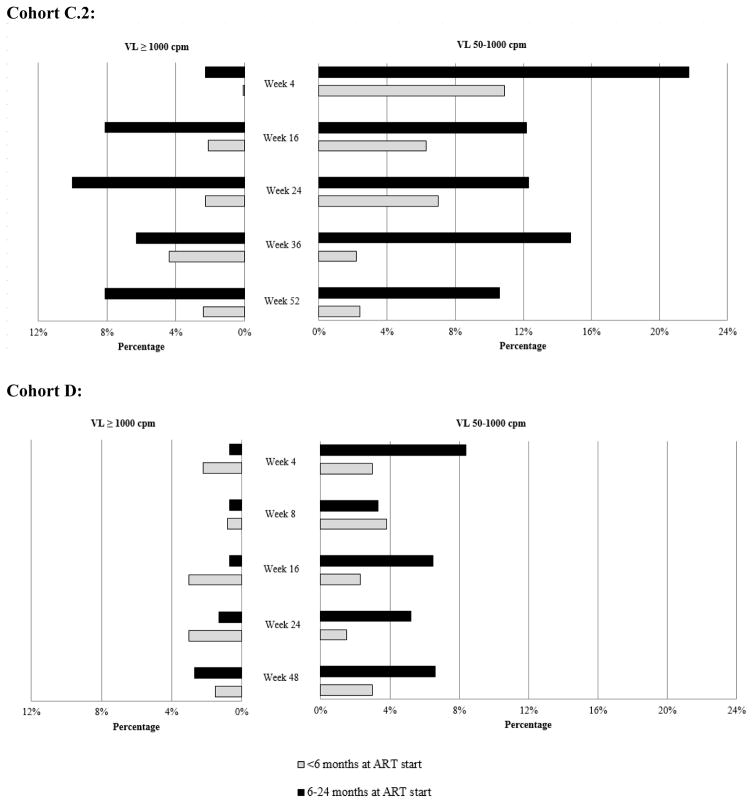
Age at ART start influenced later virologic control after viral suppression in both Cohorts C.2 and D. Of 195 children in Cohort C.2, children <6 months at ART start were less likely to have a plasma HIV-RNA 50–1000 copies/mL and plasma HIV-RNA >1000 copies at all scheduled post-randomization visits than children 6–24 months at ART start (Figure 1). In a GEE model accounting for repeated visits, the odds of having an HIV-RNA 50–1000 vs. <50 copies/mL was lower in children who initiated ART early compared to children who initiated ART late, adjusted for randomization group (OR=0.34, 95% CI: 0.19, 0.61). A similar trend was seen in the GEE model for having an HIV-RNA >1000 vs. <1000 copies/mL (OR=0.29, 95% CI: 0.08, 1.11).
Figure 1.
Percentage of children with plasma HIV-RNA 50–1000 copies/mL (cpm) and VL ≥1000 cpm at scheduled trial visits in Cohort C.2 and Cohort D by age at antiretroviral therapy (ART) initiation (<6 months vs. 6–24 months)
Of 293 children in Cohort D, children <6 months at ART start were less likely to have a plasma HIV-RNA 50–1000 copies/mL at all but one scheduled post-randomization visit than children 6–24 months at ART start. There were no consistent differences in the proportion of children with HIV-RNA >1000 copies/mL by age at starting ART but HIV-RNA >1000 copies/ml was rare in both groups (Figure 1). In a GEE model, the odds of having an HIV-RNA 50–1000 copies/mL vs. <50 copies/mL for children who initiated ART early was 0.46 times that of children who initiated ART late, adjusted for randomization group (OR=0.46, 95% CI: 0.24, 0.87).
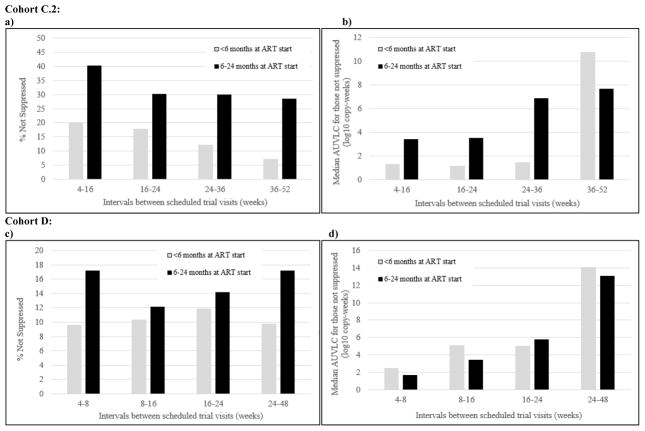
In Cohort C.2, a smaller proportion of the children who started treatment early had a detectable AUVLC compared to children who started treatment late (30.0 vs. 50.7%, p=0.012) across all scheduled trial visits. Among those with a detectable AUVLC, children starting treatment early had a lower median log10 copy-weeks/mL than children starting treatment late (1.7 vs. 9.5, p=0.03). Similarly, in Cohort D, a smaller proportion of the children who started treatment early had a detectable AUVLC compared to children who started treatment late (19.1 vs. 31.9%, p=0.013) across all scheduled trial visits. Among those with a detectable AUVLC, there was no difference in median log10 copy-years/mL between children starting ART early and late (Figure 2).
Figure 2.
Percentage not suppressed (a,c) and median area under the viral load curve (AUVLC) (b,d) at intervals between scheduled trial visits in Cohort C.2 and Cohort D, respectively, by age at antiretroviral therapy (ART) initiation (<6 months vs. 6–24 months)
Discussion
We did not observe a consistent benefit of early ART initiation on rates of initial viral suppression in the first 6 months of treatment in three non-overlapping cohorts from Johannesburg, South Africa. In one of the three cohorts we observed better virological suppression by 6 months in children who initiated ART compared to those who initiated 6–24 months but no differences were observed in the other two. Pre-treatment viral loads and CD4 percentages in all three cohorts were higher in children younger at ART initiation, consistent with the natural history of HIV infection in infancy [28–31]. However, even after accounting for these differences, in a combined analysis of the three cohorts, there were no significant differences in initial viral suppression between children starting treatment early and late. The higher pre-treatment viral loads suggest that the rate of decline was more rapid in the early-treated groups but truncation in the upper range of the assays in use at the time limited our capacity to measure this directly.
While the better virologic response in children initiating ART early observed in Cohort A is encouraging, the lack of confirmation in the other two cohorts leads us to suspect that other factors may be involved. Cohort A was a surveillance study which was intended to actively identify mothers and infants not already engaged in care. These children were predominantly identified through hospital admissions and, in particular, more children starting ART early (<6 months) were identified through routine PMTCT services. Hence these children may have had less advanced disease and benefit most from early treatment than children in the other cohorts starting treatment early. There was some evidence to suggest this speculation may be valid, as pre-treatment weight-for-age Z-scores, a sensitive marker of disease progression, were slightly better in children starting treatment early in Cohort A compared to the other two cohorts. Better surveillance systems in place to identify HIV-infected infants in order to initiate ART at an early age may improve initial outcomes. A prior study has also reported higher rates of viral suppression <500 copies/mL by 6 months in those starting ART ≤3 months of age compared to those starting treatment 3–12 months of age (63 vs. 19%, p<0.001) [10]. Another study reported more early treated infants (<6 months of age) reaching undetectable viral load than later treated infants (6–52 months of age) over a median follow up time of 4.1 years (73.3 vs. 30.1%, p<0.0001) [4]. Two other studies reported no differences in initial virologic suppression at 6 months between children starting early and late [11, 12].
Of note, overall rates of initial viral suppression at 6 months were low. Only 31, 40.1, and 26.5% of early-treated infants in Cohorts A, B, and C.1, respectively, were suppressed <50 copies/mL at 6 months after starting ART. The large proportion of infants at risk for viral failure is concerning, particularly since options for second-line regimens are limited for children.
In children who had initially achieved suppression, we observed a consistent benefit of early ART initiation on rates of sustained viral suppression in the two cohorts we examined. Compared to children who started treatment later in infancy and early childhood (6–24 months of age), children who started treatment early (<6 months) were less likely to experience viral rebound and had a lower cumulative viral load in follow up than children who started treatment later. This benefit was observed in both cohorts and across the different treatment regimens (nevirapine, efavirenz and LPV/r) that were evaluated in these two trials. Although age at ART initiation (<1 year vs. >1 year) in older children who achieved virologic suppression was not associated with rebound of HIV-RNA >400 copies/mL in one study [17], other studies have reported better long-term virologic control in infants starting ART at an early age. In a group of 20 children from the CHER trial maintained on suppressive ART for 7–8 years, 9/12 (75%) of children starting ART before 2 months of age had undetectable plasma HIV-RNA compared to 3/8 (37.5%) of infants who started after 2 months of age (p=0.17) [32]. A study of HIV-infected children maintained on ART for up to 18 years and virally suppressed on conventional assays found plasma viremia by ultrasensitive assays to be below the limit of detection (<2 copies/mL) in all four children who initiated ART early (before 3 months of age) but detectable (median 8 copies/mL) in all four children who did not initiate ART until later childhood (6–15 years) [33].
HIV infection requires life-long ART because of its persistence in latent viral reservoirs located in long-lived resting memory CD4+ T cells and other anatomic sanctuaries that harbor dormant replication-competent proviruses [34, 35]. ART targets replicating virus and has not been shown to eradicate these replication-competent HIV reservoirs. The establishment of the latent viral reservoir occurs early during primary infection [36], and evidence in adults supports that early initiation of ART close to the time of HIV acquisition may limit HIV reservoir size [37–39] and lead to HIV remission in certain adults, termed post-treatment controllers [40, 41]. In infants, several reports have indicated that early ART initiation limits the size of the viral reservoir [13, 32, 42, 43]. A study of 17 HIV-infected infants starting treatment in the first 6 months of life found a significantly smaller viral reservoir in infants treated before 6 weeks of age compared to children initiating ART between 6 weeks and 6 months of age [13]. Although eradication of HIV with early ART has not yet been shown to be feasible, treatment early in life with sustained adherence may restrict the size of the viral reservoir, thereby reducing the likelihood of viral rebound, as demonstrated in this study, and increasing the possibility for sustained suppression with potent ART.
Our study had limitations. Our analysis used a 6 month cut off for early ART initiation, but even earlier age intervals are important to investigate. In all of our cohorts, there were only small numbers of children initiating ART before 6 weeks of age, too few for a subgroup analysis. In addition, although we combined data from three cohorts in our evaluation of initial response to treatment, we were unable to account for differences in viral load assays as well as changes in drug regimens, dosing, and clinical practice taking place in the time period during which children were enrolled.
In conclusion, our study demonstrates improved virologic control after initial viral suppression in children starting ART before 6 months of age compared to those starting at 6–24 months in two large cohorts. We did not find consistent benefits of early treatment on initial viral suppression. Our results suggest that there are virological benefits of early treatment in addition to those shown for reduction of mortality and morbidity [1, 2], growth [44], and neurological outcomes [45]. These results provide additional impetus for early initiation of ART in HIV-infected infants.
Supplementary Material
Acknowledgments
Funding
This study is support by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD 47177, HD 61255), the US President’s Emergency Plan for AIDS Relief (supplement to HD 61255), and by the Secure the Future Foundation (grant RES 219).
We thank Dr. Batya Elul and Dr. Shuang Wang for their advice on the analytical approaches used in the manuscript.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report.
Role of Authors: Cohort data collection - RS, KGT, FP, AC; Data analysis - SS, LK; First draft written by SS and substantive revisions and input from all authors (RS, KGT, FP, SMA, AC, EJA, LK).
References
- 1.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382:1555–1563. doi: 10.1016/S0140-6736(13)61409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faye A, Le Chenadec J, Dollfus C, Thuret I, Douard D, Firtion G, et al. Early versus deferred antiretroviral multidrug therapy in infants infected with HIV type 1. Clin Infect Dis. 2004;39:1692–1698. doi: 10.1086/425739. [DOI] [PubMed] [Google Scholar]
- 4.Chiappini E, Galli L, Tovo PA, Gabiano C, Gattinara GC, Guarino A, et al. Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. AIDS. 2006;20:207–215. doi: 10.1097/01.aids.0000200529.64113.3e. [DOI] [PubMed] [Google Scholar]
- 5.Goetghebuer T, Haelterman E, Le Chenadec J, Dollfus C, Gibb D, Judd A, et al. Effect of early antiretroviral therapy on the risk of AIDS/death in HIV-infected infants. AIDS. 2009;23:597–604. doi: 10.1097/QAD.0b013e328326ca37. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Antiretroviral therapy for HIV infection in infants and children: towards universal access - recommendations for a public health approach. 2010. [PubMed] [Google Scholar]
- 7.World Health Organization. Recommendations for a public health approach. 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [PubMed] [Google Scholar]
- 8.World Health Organization. Recommendations for a public health approach. 2. 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [PubMed] [Google Scholar]
- 9.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M, Jr, Chun TW, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369:1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetghebuer T, Le Chenadec J, Haelterman E, Galli L, Dollfus C, Thorne C, et al. Short- and long-term immunological and virological outcome in HIV-infected infants according to the age at antiretroviral treatment initiation. Clin Infect Dis. 2012;54:878–881. doi: 10.1093/cid/cir950. [DOI] [PubMed] [Google Scholar]
- 11.Luzuriaga K, McManus M, Mofenson L, Britto P, Graham B, Sullivan JL. A trial of three antiretroviral regimens in HIV-1-infected children. N Engl J Med. 2004;350:2471–2480. doi: 10.1056/NEJMoa032706. [DOI] [PubMed] [Google Scholar]
- 12.Tukei VJ, Murungi M, Asiimwe AR, Migisha D, Maganda A, Bakeera-Kitaka S, et al. Virologic, immunologic and clinical response of infants to antiretroviral therapy in Kampala, Uganda. BMC Pediatr. 2013;13:42. doi: 10.1186/1471-2431-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persaud D, Palumbo PE, Ziemniak C, Hughes MD, Alvero CG, Luzuriaga K, et al. Dynamics of the resting CD4(+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS. 2012;26:1483–1490. doi: 10.1097/QAD.0b013e3283553638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Linden D, Hainaut M, Goetghebuer T, Haelterman E, Schmitz V, Maes P, et al. Effectiveness of early initiation of protease inhibitor-sparing antiretroviral regimen in human immunodeficiency virus-1 vertically infected infants. Pediatr Infect Dis J. 2007;26:359–361. doi: 10.1097/01.inf.0000258626.34984.eb. [DOI] [PubMed] [Google Scholar]
- 15.Aboulker JP, Babiker A, Chaix ML, Compagnucci A, Darbyshire J, Debre M, et al. Highly active antiretroviral therapy started in infants under 3 months of age: 72-week follow-up for CD4 cell count, viral load and drug resistance outcome. Aids. 2004;18:237–245. doi: 10.1097/00002030-200401230-00013. [DOI] [PubMed] [Google Scholar]
- 16.Chadwick EG, Yogev R, Alvero CG, Hughes MD, Hazra R, Pinto JA, et al. Long-term outcomes for HIV-infected infants less than 6 months of age at initiation of lopinavir/ritonavir combination antiretroviral therapy. AIDS. 2011;25:643–649. doi: 10.1097/QAD.0b013e32834403f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estripeaut D, Mosser J, Doherty M, Acosta W, Shah H, Castano E, et al. Mortality and long-term virologic outcomes in children and infants treated with lopinavir/ritonavir. Pediatr Infect Dis J. 2013;32:e466–472. doi: 10.1097/INF.0b013e3182a09276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic. 2013. [Google Scholar]
- 19.Kuhn L, Hunt G, Technau KG, Coovadia A, Ledwaba J, Pickerill S, et al. Drug resistance among newly diagnosed HIV-infected children in the era of more efficacious antiretroviral prophylaxis. AIDS. 2014 doi: 10.1097/QAD.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Technau KG, Kalk E, Coovadia A, Black V, Pickerill S, Mellins CA, et al. Timing of Maternal HIV Testing and Uptake of Prevention of Mother-to-Child Transmission Interventions Among Women and Their Infected Infants in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2014;65:e170–178. doi: 10.1097/QAI.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrams EJ, Silva JS, Woldesenbet S, Coovadia A, Black V, Technau KG, et al. Despite access to antiretrovirals for prevention and treatment high rates of mortality persist among HIV-infected infants and young children. doi: 10.1097/INF.0000000000001507. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Department of Health South Africa. Guidelines for the Management of HIV in Children. 2. 2010. [Google Scholar]
- 23.Reitz C, Coovadia A, Ko S, Meyers T, Strehlau R, Sherman G, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis. J Infect Dis. 2010;201:1121–1131. doi: 10.1086/651454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coovadia A, Abrams EJ, Stehlau R, Meyers T, Martens L, Sherman G, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010;304:1082–1090. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn L, Coovadia A, Strehlau R, Martens L, Hu CC, Meyers T, et al. Switching children previously exposed to nevirapine to nevirapine-based treatment after initial suppression with a protease-inhibitor-based regimen: long-term follow-up of a randomised, open-label trial. Lancet Infect Dis. 2012;12:521–530. doi: 10.1016/S1473-3099(12)70051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coovadia A, Abrams EJ, Strehlau R, Shiau S, Pinillos F, Martens L, et al. Efavirenz-Based Antiretroviral Therapy Among Nevirapine-Exposed HIV-Infected Children in South Africa: A Randomized Clinical Trial. Jama. 2015;314:1808–1817. doi: 10.1001/jama.2015.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lima VD, Sierra-Madero J, Wu Z, Singer J, Wood E, Hull MW, et al. Comparing the efficacy of efavirenz and boosted lopinavir using viremia copy-years. J Int AIDS Soc. 2014;17:18617. doi: 10.7448/IAS.17.1.18617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McIntosh K, Shevitz A, Zaknun D, Kornegay J, Chatis P, Karthas N, et al. Age- and time-related changes in extracellular viral load in children vertically infected by human immunodeficiency virus. Pediatr Infect Dis J. 1996;15:1087–1091. doi: 10.1097/00006454-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Tobin NH, Aldrovandi GM. Immunology of pediatric HIV infection. Immunol Rev. 2013;254:143–169. doi: 10.1111/imr.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Schatorje EJ, Gemen EF, Driessen GJ, Leuvenink J, van Hout RW, de Vries E. Paediatric reference values for the peripheral T cell compartment. Scand J Immunol. 2012;75:436–444. doi: 10.1111/j.1365-3083.2012.02671.x. [DOI] [PubMed] [Google Scholar]
- 32.van Zyl GU, Bedison MA, van Rensburg AJ, Laughton B, Cotton MF, Mellors JW. Early Antiretroviral Therapy in South African Children Reduces HIV-1-Infected Cells and Cell-Associated HIV-1 RNA in Blood Mononuclear Cells. J Infect Dis. 2014 doi: 10.1093/infdis/jiu827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luzuriaga K, Tabak B, Garber M, Chen YH, Ziemniak C, McManus MM, et al. Reduced HIV Reservoirs After Early Treatment HIV-1 Proviral Reservoirs Decay Continously Under Sustained Virologic Control in Early-Treated HIV-1- Infected Children. J Infect Dis. 2014 doi: 10.1093/infdis/jiu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- 35.Bruner KM, Hosmane NN, Siliciano RF. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol. 2015 doi: 10.1016/j.tim.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis. 2013;208:1202–1211. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Archin NM, Vaidya NK, Kuruc JD, Liberty AL, Wiegand A, Kearney MF, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci U S A. 2012;109:9523–9528. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191:1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 40.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hocqueloux L, Prazuck T, Avettand-Fenoel V, Lafeuillade A, Cardon B, Viard JP, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS. 2010;24:1598–1601. doi: 10.1097/qad.0b013e32833b61ba. [DOI] [PubMed] [Google Scholar]
- 42.Bitnun A, Samson L, Chun TW, Kakkar F, Brophy J, Murray D, et al. Early initiation of combination antiretroviral therapy in HIV-1-infected newborns can achieve sustained virologic suppression with low frequency of CD4+ T cells carrying HIV in peripheral blood. Clin Infect Dis. 2014;59:1012–1019. doi: 10.1093/cid/ciu432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luzuriaga K, Tabak B, Garber M, Chen YH, Ziemniak C, McManus MM, et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis. 2014;210:1529–1538. doi: 10.1093/infdis/jiu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiau S, Arpadi S, Strehlau R, Martens L, Patel F, Coovadia A, et al. Initiation of antiretroviral therapy before 6 months of age is associated with faster growth recovery in South African children perinatally infected with human immunodeficiency virus. J Pediatr. 2013;162:1138–1145. 1145.e1131–1132. doi: 10.1016/j.jpeds.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laughton B, Cornell M, Grove D, Kidd M, Springer PE, Dobbels E, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 2012;26:1685–1690. doi: 10.1097/QAD.0b013e328355d0ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.