Abstract
The interaction between the human host and the vaginal microbiota is highly dynamic. Major changes in the vaginal physiology and microbiota over a woman's lifetime are largely shaped by transitional periods such as puberty, menopause and pregnancy, while daily fluctuations in microbial composition observed through culture‐independent studies are more likely to be the results of daily life activities and behaviours. The vaginal microbiota of reproductive‐aged women is largely made up of at least five different community state types. Four of these community state types are dominated by lactic‐acid producing Lactobacillus spp. while the fifth is commonly composed of anaerobes and strict anaerobes and is sometimes associated with vaginal symptoms. The production of lactic acid has been associated with contributing to the overall health of the vagina due to its direct and indirect effects on pathogens and host defence. Some species associated with non‐Lactobacillus vaginal microbiota may trigger immune responses as well as degrade the host mucosa, processes that ultimately increase susceptibility to infections and contribute to negative reproductive outcomes such as infertility and preterm birth. Further studies are needed to better understand the functional underpinnings of how the vaginal microbiota affect host physiology but also how host physiology affects the vaginal microbiota. Understanding this fine‐tuned interaction is key to maintaining women's reproductive health.
Abbreviations
- AMP
antimicrobial peptide
- AV
aerobic vaginitis
- BV
bacterial vaginosis
- CCL
chemokine (C‐C motif) ligand
- CDC
cholesterol‐dependent cytolysin
- CST
community state type
- HBD
human β‐defensin
- HIV
human immunodeficiency virus
- HNP
human neutrophil peptide
- HSV
herpes simplex virus
- HPV
human papillomavirus
- IgA
immunoglobulin A
- IFN
interferon
- IgG
immunoglobulin G
- IL
interleukin
- PRR
pattern recognition receptor
- TLR
toll‐like receptor
- SCFA
short chain fatty acid
- SLPI
secretory leukocyte peptidase inhibitor
- SP
surfactant protein
- NF‐κB
nuclear factor‐κB
- TNF
tumor necrosis factor
Introduction
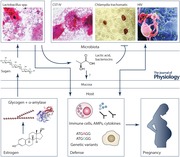
The assemblages of microbes (microbiota) associated with the human body have been shown to affect human physiology, immunity and nutrition (Kau et al. 2011; Chen et al. 2015; Conlon & Bird, 2015). In the vagina, microbes exist in a finely tuned mutualistic relationship with the host and provide the first line of defence against the colonization by opportunistic pathogens. Throughout a woman's lifespan, the vaginal microbiota undergoes major changes associated with transitional reproductive periods such as puberty and menopause, as summarized in Farage & Maibach (2005). During these periods, the vaginal microbiota can affect host reproductive physiology but can also be affected by host physiology.
Recent high‐throughput 16S rRNA gene sequencing studies examining vaginal bacterial species composition and abundance in reproductive‐aged women have shown that there are at least five major types of vaginal microbiota called community state types (CSTs) (Zhou et al. 2007; Ravel et al. 2011; Gajer et al. 2012). Four of these CSTs are dominated by Lactobacillus crispatus (CST‐I), L. iners (CST‐III), L. gasseri (CST‐II) or L. jensenii (CST‐V) and one, CST‐IV, does not contain a significant number of Lactobacillus but is composed of a polymicrobial mixture of strict and facultative anaerobes including species of the genera Gardnerella, Atopobium, Mobiluncus, Prevotella and other taxa in the order Clostridiales (Fig. 1; Fredricks et al. 2005; Campos et al. 2008; Ravel et al. 2011; Gajer et al. 2012). The frequency of these CSTs has been shown to differ in different ethnic backgrounds, with CST‐IV more common (∼40%) in black and Hispanic women (Ravel et al. 2011). The polymicrobial condition known as bacterial vaginosis (BV) is compositionally similar to CST‐IV since it is defined by a loss of Lactobacillus spp., the presence of anaerobes and strict anaerobes, and sometimes associated clinical symptoms including discharge, odour and irritation. In research settings, a Gram‐staining scoring procedure that relies on the identification of bacterial morphotypes known as the Nugent test (appropriately renamed Nugent‐BV by Martin (2012)) is used to establish a BV diagnosis (Nugent et al. 1991). Clinically, the diagnosis of BV is accompanied by an evaluation of the following signs and symptoms: discharge, malodour, the presence of clue cells and vaginal pH > 4.5 as defined by the Amsel criteria (Amsel et al. 1983).
Figure 1. Heat map of vaginal microbiota community state types.
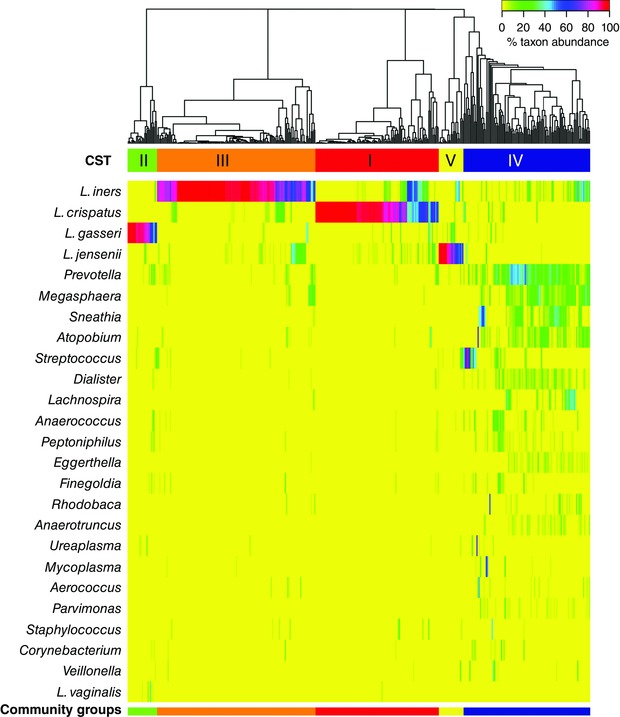
Hierarchical clustering shows that the vaginal microbiota of reproductive‐aged women clusters into five distinct community state types, four of which are dominated by Lactobacillus spp. (Lactobacillus crispatus (CST‐I), L. iners (CST‐III), L. gasseri (CST‐II) or L. jensenii (CST‐V)) and the fifth (CST‐IV) is composed of a polymicrobial mixture of strict and facultative anaerobes including species of the genera Atopobium, Megasphera, Mobiluncus, Prevotella and other taxa in the order Clostridiales. Figure reproduced from Ravel et al. (2011).
Daily fluctuations in the composition of the vaginal microbiota have been previously documented by microscopy and cultivation studies (Hay et al. 1997; Keane et al. 1997; Schwebke et al. 1999). These findings were confirmed and extended in longitudinal culture‐independent analyses such as those of women who self‐collected vaginal swabs twice weekly for 16 weeks (Brotman et al. 2008, 2010; Gajer et al. 2012), or daily for 10 weeks (Ravel et al. 2013) or 4 weeks (Srinivasan et al. 2010). It was observed that some vaginal microbial communities transitioned in and out of CST‐IV. The amount of time spent in a particular CST could vary individually as some women experienced consistent and stable CST longitudinal patterns (defined as community class), while others frequently transitioned between CSTs and most frequently to CST‐IV (Gajer et al. 2012; Ravel et al. 2013). In some cases, CST transitions were triggered by menstruation or sexual behaviours, but in other cases they seem to be driven by uncharacterized factors (Gajer et al. 2012). These longitudinal studies highlight the highly dynamic nature of vaginal microbial communities and emphasize the need to better understand the underlying biological factors modulating fluctuations in composition and functions that affect host physiology.
Historically, Lactobacillus‐dominated vaginal microbial communities have been associated with healthy reproductive‐aged women and are characterized by the production of copious amounts of lactic acid and thus a pH < 4.5 (reviewed in Linhares et al. 2011; Ma et al. 2012; Petrova et al. 2015). This acidic environment is thought to be highly protective against infections or colonization of the vagina by pathogens and non‐indigenous microbes. An additional benefit of Lactobacillus spp. (i.e. L. crispatus and L. gasseri) is the supply of bacteriocins or bacteriocin genes (i.e. gassericin T, acidocin lF221A, type‐A lantibiotic, and Bacteriocins IIa, IIc and J46) to inhibit growth of undesirable species (i.e. Klebsiella spp., Staphylococcus aureus, Escherichia coli or Enterococus faecalis) (Stoyancheva et al. 2014; Zheng et al. 2015). However, the notion that a Lactobacillus‐dominated vaginal microbiota is necessarily the norm has been called into question since mounting evidence suggests that about 25% of asymptomatic women do not possess a Lactobacillus‐dominated microbiota at any given time, a staggering proportion that does not support a diseased state (Zhou et al. 2004; Ravel et al. 2011; Anahtar et al. 2015). These differences between women appear to be driven by a combination of cultural, behavioural, genetic and other unknown underlying factors (Ravel et al. 2011; Gajer et al. 2012; Anahtar et al. 2015). However, a strong association between CST‐IV (as established by Nugent‐BV) and increased risk to sexually transmitted infections (Martin et al. 1999; Peters et al. 2000; Cherpes et al. 2003), including human immunodeficiency virus (HIV) (Cohen et al. 1995; Taha et al. 1998; Cu‐Uvin et al. 2001; Coleman et al. 2007), and reproductive tract and obstetric sequelae has been established through thorough epidemiological studies (Gravett et al. 1986; McDonald et al. 1992; Hillier et al. 1995; Meis et al. 1995; Goldenberg et al. 1997). Hence, while CST‐IV might be normal (asymptomatic) in some women, it is still associated with significantly increased risk of adverse outcomes. An illustration of how CST‐IV can help further foster infections is the case of chlamydial infection, where interferon (IFN)‐γ production is thought to be critical for chlamydia clearance. IFN‐γ activates the human enzyme indoleamine‐2,3‐dioxygenase, which catabolizes tryptophan, eventually leading to tryptophan starvation and chlamydia clearance since genital chlamydia cannot synthesize tryptophan. However, production of indole compounds by anaerobes and strict anaerobes contained in CST‐IV enables chlamydia to shunt its deficiency and produce tryptophan, thus bypassing this host defence mechanism and establishing a long‐term infection (discussed in Aiyar et al. 2014). Similarity, relative to other Lactobacillus‐dominated community states, CST‐IV‐like communities increase the risk of HIV infection (Pyles et al. 2014; Anahtar et al. 2015). However, not all Lactobacillus spp. are necessarily beneficial and protective since, for example, some strains of L. iners might carry pathogenicity factors, such as inerolysin, a cholesterol‐dependent cytolysin (CDC) and a host epithelial cell pore‐forming enzyme, which was found to be up‐regulated at least sixfold in women with BV (Macklaim et al. 2011; 2013). Therefore, when considering the impact of the microbiota on host defence and reproductive physiology, it is important to place it in the context of these dynamic and individualized relationships.
The effect of the microbiota on host defence
The vagina contains a number of immune‐related cells and receptors to help sense the microbial environment (Wira et al. 2005). Surveillance for microbes within the female genital tract of both commensal and pathogenic microbes is generally achieved by microbial motif pattern recognition by pattern recognition receptors (PRRs) such as toll‐like receptors (TLRs) or the dectin‐1 receptor (which helps recognize the fungal pathogen Candida albicans; Carvalho et al. 2012; Usluogullari et al. 2014), and nucleotide‐binding oligomerization domain (NOD) receptors present in and on both squamous epithelial cells lining the vagina and the columnar cells lining the upper female genital tract (as reviewed in Wira et al. 2005; Witkin et al. 2007a; Horne et al. 2008; Mitchell & Marrazzo, 2014). Microbial stimulation of PRRs initiates cytokine/chemokine signalling cascades, for example secretion of interleukin (IL)‐1β, IL‐6, IL‐8 and tumor necrosis factor‐α (TNF‐α), in order to recruit or activate specialized cells, such as NK cells, macrophages, CD4+ helper T‐cells, and CD8+ cytotoxic T‐cell lymphocytes and B lymphocytes (as reviewed in Wira et al. 2005; Brotman et al. 2013a; Nguyen et al. 2014). Genetic variants of PRRs such as the IL‐1R antagonist gene, TLR4, TLR9, IL‐1R2 and TNF‐α may play a role in how a woman responds to a particular microbial challenge or pregnancy outcome, as evidenced by several genetic‐disease association studies (Genç et al. 2004a,b, 2007; Giraldo et al. 2007; Ryckman et al. 2011; Royse et al. 2012; Mackelprang et al. 2015). Women with CST‐IV‐like states show significant increases in IL‐1α, IL‐1β, TNF‐α, IFN‐γ, IL‐4, IL‐8, IL‐10, IL‐12p70, and fms‐like tyrosine kinase 3 ligand relative to CST‐I as well as significantly higher IFN‐γ in CST‐III relative to CST‐I. Specifically, in one study, Prevotella amnii, Mobiluncus mulieris, Sneathia amnii and Sneathia sanguinegens (all commonly found in CST‐IV) were found to induce higher levels of IL‐1α, IL‐1β, and IL‐8 relative to L. crispatus dominated communities (CST‐I), whereas L. iners dominated communities (CST‐III) induced moderate IL‐8 levels relative to CST‐I. The authors also showed how there were significant increases in IL‐1α, IL‐1β and TNF‐α longitudinally in subjects that transition from a CST‐I, to CST‐III and to a CST‐IV (Anahtar et al. 2015). Conversely, mock communities dominated by L. crispatus (CST‐I) and L. jensenii (CST‐V) on reconstructed three‐dimensional vaginal epithelial models do not strongly elicit cytokine IL‐1β or IL‐8 secretion relative to medium control, and also inhibit some pro‐inflammatory responses after TLR 2/6 and 3 agonist induction (Rose et al. 2012). These studies continue to support the notion that the innate immune response is largely driven by vaginal bacterial community states, with CST‐IV potentially having a larger pro‐inflammatory response than CST‐I or CST‐II, and with CST‐III triggering an intermediate response.
Additional factors contributing to vaginal defence include mannose binding lectin (MBL), vaginal antimicrobial peptides (AMPs) and immunoglobulin A and G (IgA, IgG). As its name suggests, MBL binds mannose, N‐acetylglucosamine and fucose carbohydrate moieties present on microbial cell surfaces. Eventually, this interaction leads to cell lysis or targeting for the immune system (Neth et al. 2000; Turner, 2003). IgA and IgG may help to prevent vaginal epithelial cell adherence and uptake, as well as contribute to the neutralization and clearance of infectious microbes from the vagina (Tramont, 1977; Wang et al. 2014). Vaginal AMPs exist in various classes and may recruit immune cells via chemotaxis or possess anti‐endotoxin activity. Mechanisms for each AMP have been thoroughly reviewed elsewhere (Ding et al. 2009; Wilson et al. 2013; Yarbrough et al. 2015), and while the specific association between AMPs and vaginal microbiota has not been extensively investigated, key findings are emphasized here. Defensins are a class of cationic and amphipathic AMPs with diverse mechanisms of action against common vaginal bacteria, pathogens and viruses including HIV, herpes simplex virus (HSV) and human papillomavirus (HPV). In organotypic models of the vaginal epithelium, human β‐defensin (HBD)‐2 expression, but not that of HBD‐1, was associated with colonization by L. iners, Atopobium vaginae and Prevotella bivia (Doerflinger et al. 2014), while in another study using similar experimental in vitro conditions, L. jensenii but not Gardnerella vaginalis was shown to induce HBD‐2 transcription (Valore et al. 2006). As expected, many human defensins bind to viral‐specific proteins to prevent viral attachment to a human cell surface, as for example, with retrocyclin‐1, retrocyclin‐2, human neutrophil peptide (HNP)‐1, HNP‐2, HNP‐3 and to a much lesser degree HNP‐4 (Maddon et al. 1986; Münk et al. 2003; Wang et al. 2003, 2004; Wu et al. 2005; Furci et al. 2007); however, the topic is outside the scope of this review. In addition to defensins, other AMPs are found in the human vagina and include the secretory leukocyte protease inhibitor (SLPI), human epididymis protein 4 (HE4), LL‐37, and surfactant protein (SP)‐A and SP‐D. SLPI expression is associated with BV organisms (Nasioudis et al. 2015) but not with L. crispatus, L. iners, A. vaginae or P. bivia (Doerflinger et al. 2014; Orfanelli et al. 2014). HE4 is associated with G. vaginalis (Orfanelli et al. 2014) and LL‐37 inactivates the sexually transmitted pathogen Neisseria gonorrhoeae while having no effect on L. crispatus and L. jensenii and comparatively little effect on L. iners (Moncla et al. 2012). The lack of AMP stimulation in response to some Lactobacillus spp. is associated with their needed maintenance in the vagina (Valore et al. 2002). Similar to defensins, SP‐A and SP‐D contribute to viral inhibition, including HIV where they act via binding to the viral protein gp120 and human CD4, but with SP‐A simultaneously enhancing gp120 binding to dendritic cells and therefore also facilitating HIV uptake. (Gaiha et al. 2008; Pandit et al. 2014). Thus, overall, microbes, environments, immune regulatory actions and genes tightly interact to govern homeostasis of the vaginal environment.
Bacterial vaginosis and aerobic vaginitis
As mentioned, the vaginal microbiota can be characterized by one of five CSTs, with CST‐IV lacking a relatively high abundance of Lactobacillus spp. Generally, CST‐IV can clinically manifest as aerobic vaginitis (AV) or BV, so the immune response to CST‐IV outlined above overlaps considerably with BV or AV. AV is mainly differentiated from BV by the presence of an inflammatory response predominately associated with aerobes, such as group B Streptococcus, Staphylococcus aureus, Escherichia coli, and Enterococcus (Donders et al. 2002; Donders, 2007). The AV inflammatory response manifests symptomatically as itching or burning, molecularly as increased IL‐6 and IL‐1β, and cellularly as the presence of leukocytes or primary blood cells in a microscopic wet mount (Han et al. 2014). In contrast, the clinical definition of BV does not involve any overt inflammatory responses such as recruitment of neutrophils, redness, itching or burning (reviewed in Cauci, 2004). A number of immune factors including IL‐1β, IL‐2, IL‐4, IL‐6, IL‐8, IL‐10, IL‐12, TNF‐α, IFN‐γ, chemokine C‐C motif ligand 5 (CCL5) and SLPI have been variably and inconsistently associated with BV (summarized in Mitchell & Marrazzo, 2014). These conflicting findings may be due to different study designs (longitudinal versus cross‐sectional, or in vitro versus in vivo), different definitions of BV (symptomatic versus asymptomatic BV or Nugent‐BV versus BV diagnosed according to the Amsel criteria) or that additional features actively suppress the inflammatory response in BV, such as IgA degradation, TLR expression inhibition, or immune‐related genetic variants (Cauci et al. 2003; Witkin et al. 2007b). As an example of BV's effect on host defence, cytokine analysis from a vaginal epithelial cell model co‐colonized with mock communities representing CST‐I to ‐IV as well as Nugent scores corresponding to respective BV diagnosis showed significant increases in IL‐1β, IL‐8, TNF‐α, CCL5 and IL‐1RA in CST‐III or CST‐IV, but not CST‐I or CST‐II (Pyles et al. 2014). The ability of individual BV‐associated bacterial species to elicit an in vitro immune response has also been studied, as in the cases of A. vaginae, which induces expression of chemokine C‐C motif ligand 20 (CCL20), HBD‐2, IL‐1β, IL‐6, IL‐8 and TNF‐α via nuclear factor‐κB (NF‐κB), TLR2 and MyD88 signalling pathways; G. vaginalis, which induces IL‐6 and IL‐8 transcripts; and L. iners, which stimulates PRR signalling but not downstream inflammatory response cytokines IL‐6, IL‐8 or mucins (Libby et al. 2008; Doerflinger et al. 2014). Bacteria‐derived short chain fatty acids (SCFAs), namely acetate, butyrate, propionate and succinate, some of which exist at relatively higher proportions during BV, can induce a pro‐inflammatory response under the hypothesis that SCFAs may act to ultimately inhibit chemotaxis and inflammation in BV (Al‐Mushrif et al. 2000; Chaudry et al. 2004; Mirmonsef et al. 2011; Gajer et al. 2012; O'Hanlon et al. 2013). Relatively high concentrations (2–20 mm) of acetate and butyrate, but not propionate, induce cytokine IL‐6, IL‐8 and IL‐1β secretions and also induce IL‐8 and TNF‐α with TLR2 and TLR7 ligand stimulation in a dose‐ and time‐dependent manner in vitro (Mirmonsef et al. 2011). However, whether or not the host is actively downplaying the sensing of BV‐associated microbes or a specific attribute of BV is evading inflammation remains to be demonstrated since the aetiology of BV is still unknown and the necessary longitudinal studies are lacking (Schwebke, 2009).
Lactic acid and host defence
Lactic acid is produced mainly by vaginal microbes (Boskey et al. 2001) and helps maintain healthy host physiological functions since it has been shown to directly inhibit Chlamydia trachomatis infection (Gong et al. 2014), and potentially both HSV‐2 and HIV in vitro and in vivo if there is sufficient lactic acid to acidify the vagina to pH < 4 (Conti et al. 2009; Aldunate et al. 2013; Isaacs & Xu, 2013, 2014). Lactic acid also inactivates a broad range of BV‐associated microbes at pH < 4.5 (O'Hanlon et al. 2011). When Lactobacillus spp. dominate the vaginal microbiota, they acidify the vagina to a strongly acidic mean pH of 3.5 ± 0.2 that is likely to help protect against a broad range of infections (O'Hanlon et al. 2013). Recent studies aimed to uncover the mechanism by which lactic acid can directly affect host immune functions, as for example by directly inhibiting pro‐inflammatory responses IL‐6, IL‐8 and IL‐1RA, (Hearps et al. 2014), inducing the Th17 lymphocyte pathway via IL‐23 in a dose‐dependent manner upon lipopolysaccharide co‐stimulation (Witkin et al. 2011), and helping to release mediators from vaginal epithelial cells and stimulating antiviral response by release of transforming growth factor‐β (Mossop et al. 2011). In the gut, lactate and acetate from L. casei and Bifidobacterium breve inhibit cell proliferation, but whether these molecules play a similar role in the vagina has not been studied (Matsuki et al. 2013). Interestingly, lactic acid isomers may also play a role in determining host response and the subsequent host–microbiota relationship. Lactic acid exists in the vagina in both d‐(–)‐ and l‐(+)‐isomers, with the host contributing only about 4–30% of the total lactate (Boskey et al. 2001), suggesting a large reliance on microbes to supply the majority of lactic acid for protection. In one study, only d‐(–)‐lactic acid was correlated with α‐amylase, SLPI, hyaluronidase‐1, neutrophil gelatinase‐associated lipocalin and matrix metalloproteinase 8 (MMP‐8) expression in vitro (Nasioudis et al. 2015). The authors suggest that epithelial cell exfoliation and subsequent breakdown of glycogen helps favour Lactobacillus spp. growth, and thus helps sustain d‐(–)‐lactic acid production (see discussion on α‐amylase and glycogen below). Moreover, women with BV were found to be deficient in both isomers, while those with vulvovaginal candidiasis have elevated l‐(+)‐lactic acid as well as CD147 and MMP‐8 genes (Beghini et al. 2014). Lactobacillus iners does not produce d‐(–)‐lactic acid and fails to produce the l‐(+)‐lactic acid in abundance as high as L. crispatus or L. gasseri while L. jensenii produces only d‐(–)‐lactic acid (Witkin et al. 2013), suggesting potential Lactobacillus species‐specific effects on the host. Consequently, the composition of the vaginal microbiota, and specifically the ability of vaginal microbes to produce d‐(–)‐lactic acid, may help to inhibit pathogens and inflammatory responses while also favouring Lactobacillus spp. survival by using host cells resources for carbon sources.
The effect of the microbiota on the vaginal mucosa
The vaginal mucosa plays an important role as a physical barrier to separate host epithelial environment from harmful pathogens, including HIV (Nunn et al. 2014, 2015), whereas vaginal microbes can affect the integrity of the mucosa (Arnold et al. 2014). The BV‐associated species G. vaginalis secretes sialidases, which have been shown to deglycosylate secretory immunoglobulin A (sIgA) and other sialoglycan substrates via the cleavage of sialic acid from gylocoprotein at the α2–3 and α2–6 linkage Neu5Ac present on both N‐ and O‐glycans, thereby hydrolysing protective mucosal sialoglycoproteins (Lewis et al. 2012). Gardnerella vaginalis can consume and neutralize liberated sialic acid residues to further evade the host response (Lewis et al. 2013). In addition, G. vaginalis produces vaginolysin, a cholesterol‐dependent cytolysin that could contribute to BV symptomology by forming pores in the vaginal epithelium with or without CD59 (Gelber et al. 2008; Zilnyte et al. 2015). BV‐associated bacteria quantified by Nugent score also significantly associate with mucins‐1, ‐4, ‐5AC and ‐7 (Moncla et al. 2014). Conversely, certain types of vaginal communities could enhance the integrity of the mucosal barrier. A recent study showed that L. crispatus‐dominated vaginal microbiota were able to reinforce the diffusional barrier properties of cervicovaginal mucus against HIV, hence hindering HIV penetration, while communities dominated by L. iners facilitated the penetration of HIV through the cervicovaginal mucus barrier (Nunn et al. 2015). Thus, a change in vaginal community composition and function is strongly associated with the integrity of the protective mucus layer. Therefore, vaginal bacteria, including species of Lactobacillus, can reduce or increase susceptibility to infectious agents such as HIV.
The effect of the microbiota on reproductive functions
The vaginal microbiota in combination with other factors is associated with adverse reproductive and obstetric outcomes. For example, a meta‐analysis revealed that BV‐like vaginal microbiota are significantly more prevalent in women with tubal infertility when compared with women with other causes of infertility, but is not associated with decreased conception rates (van Oostrum et al. 2013). Preterm labour and delivery has been thought to be in part associated with changes in the vaginal microbiota composition, namely bacteria found in CST‐IV (i.e. G. vaginalis and Ureaplasma), AV or BV (preterm labour and delivery defined as occurring before 37 weeks in Caucasian women (Donders et al. 2009), mostly African American women (Nelson et al. 2014), and mostly Caucasian women (DiGiulio et al. 2015)). Jakovljević et al. assessed differences of gestational time to delivery in BV versus non‐BV women, with a statistically significant difference of 37.72 ± 3.9 versus 39.59 ± 1.1 weeks (Jakovljević et al. 2014). However, another study did not find any association between CST‐IV and preterm births (defined as 28–33.1 weeks versus term births of 38.8–40.7 weeks in mostly African American women) even though the study was well‐powered and preterm births were phenotypically well controlled (Romero et al. 2014). It should be noted that differences in ethnicity, definition of preterm birth and analytical methods of microbiota data could explain these different observations. The possibility remains that functional differences exist and that hypotheses should be further explored using metagenomics‐ or metatranscriptomics‐based approaches.
The interplay between host polymorphism and the vaginal microbiota could play an important role in the mechanisms by which microbes affect reproductive health. Polymorphisms in genes that control inflammatory response (protein kinase Cα, fms‐like tyrosine kinase 1, IL‐6 and TNF‐α) are associated with preterm delivery in combination with CST‐IV vaginal microbiota, although the direct functional impact of these polymorphisms is unknown (Gómez et al. 2010; Jones et al. 2010). These studies and others have suggested that the inflammatory and antimicrobial peptide response, associated with certain vaginal microbiota, exhibit a role in rupturing and invading cervical plug or amniotic membranes, eventually triggering pro‐inflammatory cascades that could lead to premature labour and delivery (reviewed in Goldenberg et al. 2000; Witkin, 2014; Yarbrough et al. 2015). In a rhesus monkey model infected with group B Streptococcus, there was an observed increase in amniotic fluid cytokines TNF‐α, IL‐1β and IL‐6 occurring before uterine contractility or any clinical signs of infection, suggesting a direct role of infection in preterm labour (Gravett et al. 1994). Specific AMPs are expressed upon exposure in vitro and in vivo to the BV‐associated bacteria A. vaginae (CCL20, HBD‐2) or pathogens, such as C. trachomatis (elafin) and N. gonorrhoeae (SLPI), but not L. crispatus or L. jensenii (Libby et al. 2008; King et al. 2009; Cooper et al. 2012; Eade et al. 2012; Doerflinger et al. 2014). The immune response to pathogens can trigger signalling cascades, which could ultimately lead to miscarriage, intrauterine infection, preterm labour, and tubal and ectopic pregnancy. For example, if C. trachomatis ascends past the cervix, such an infection in the upper genital track could lead to tubal scarring and potentially tubal infertility, ectopic pregnancy and chronic pelvic pain (Hafner, 2015, and discussed in Barlow et al. 2001). C. albicans triggers IL‐6 secretion in the placenta ultimately leading to an increase in NF‐κB and an inflammatory response (Tyutyunnik et al. 2014). A study measuring the effects of immunomodulation in pregnant women with BV found that C. albicans and Trichomonas vaginalis, but not C. trachomatis or N. gonorrhoeae, had an effect on vaginal cytokines and none of these pathogens had any effect on anti‐G. vaginalis haemolysin IgA, sialidase or prolidase activity (Cauci & Culhane, 2007). Clearly there is still much to learn about the dynamics, function and mechanisms driving the role of the vaginal microbiome in reproduction health.
Physiology affects vaginal microbiota composition
The composition of the vaginal microbiota changes throughout a woman's lifetime from birth, through puberty, reproductive age and menopause. The early childhood vaginal microbiota comprise a variety of anaerobes, diphtheroids, coagulase‐negative staphylococci, and E. coli, whereas postmenopausal women often experience a loss of Lactobacillus spp. associated with the decrease in oestrogen controlling vaginal epithelial proliferation, maturation, and accumulation of glycogen, which is directly or indirectly nutritionally necessary for the maintenance of Lactobacillus spp. (Hammerschlag et al. 1978a,b; Hillier & Lau, 1997; Galhardo et al. 2006; Brotman et al. 2013b). Indeed, oestrogen levels peak during reproductive age and contribute to shaping the composition of the vaginal microbiota. In menopause, vaginal application of oestrogen cream is associated with vaginal epithelial maturation, the accumulation of glycogen and acidic pH (<4.0), the latter indicative of the presence of high number of Lactobacillus spp. (Nilsson et al. 1995). Interestingly, Lactobacillus spp. was originally thought to directly ferment glycogen in the vagina. However, this idea was gradually refuted and recent evidence suggests that human α‐amylase catabolizes glycogen into smaller polymers, namely maltose and maltotriose, which can then be used by Lactobacillus spp. for metabolism, even in newborns who have residual circulating maternal oestrogen (Cruickshank, 1934; Cruickshank & Sharman, 1934; Stewart‐Tull, 1964; Bernbaum et al. 2007; Spear et al. 2014). This model puts forward that the influence of oestrogen, glycogen and especially α‐amylase provides a positive selection pressure for a Lactobacillus‐dominated microbiota (Spear et al. 2014). These findings highlight the tight interplay between host physiology and the vaginal microbiota.
Conclusion
The human vaginal ecosystem is a dynamic environment in which microbes can affect host physiology but also where host physiology can affect the composition and function of the vaginal microbiota. Species of Lactobacillus have been historically associated with vaginal health in reproductive‐age women due to the direct and indirect protective nature afforded by Lactobacillus products, such as lactic acid and bacteriocin among others, against mucus degradation and inhibition of pathogens. The reported inconsistent innate immune response observed with non‐Lactobacillus‐ or L. iners‐dominated microbiota (CST‐IV, BV, AV and CST‐III, respectively), coupled with recent findings that question the definitions of normality, highlight the need for more in‐depth functional understandings of the interaction between the vaginal microbiota and host physiology, reproduction and defence.
Additional information
Competing interests
None declared.
Author contributions
Both authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported in part by the National Institute of Allergy and Infectious Diseases and the National Institute for General Medical Sciences of the National Institutes of Health under awards number R01GM103604, R01AI089878, R21AI107224, R01AI116799 and U19AI084044.
Biographies
Steven Smith is a PhD candidate at the University of Maryland with a focus on bioinformatics and computational biology. His past and current experiences include integration of experimental and high‐throughput sequencing datasets for use in systems biology approaches. He is currently studying how changes in vaginal bacterial community composition and abundance and associated BV status affect human vaginal transcriptomic responses.

Jacques Ravel is a Professor of Microbiology and Immunology and the Associate Director for Genomics at the Institute for Genome Sciences (IGS) at the University of Maryland School of Medicine in Baltimore, Maryland. His research focuses on applying combinations of modern genomics technologies, systems biology approaches and ecological principles to understand the relationship between the host and the genital microbiota as it relates to women's health.
This is an Editor's Choice article from the 15 January 2017 issue.
References
- Aiyar A, Quayle AJ, Buckner LR, Sherchand SP, Chang TL, Zea AH, Martin DH & Belland RJ (2014). Influence of the tryptophan‐indole‐IFNγ axis on human genital Chlamydia trachomatis infection: role of vaginal co‐infections. Front Cell Infect Microbiol 4, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldunate M, Tyssen D, Johnson A, Zakir T, Sonza S, Moench T, Cone R & Tachedjian G (2013). Vaginal concentrations of lactic acid potently inactivate HIV. J Antimicrob Chemother 68, 2015–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldunate M, Tyssen D, Latham C, Ramsland P, Perlmutter P, Moench T, Cone R & Tachedjian G (2014). Vaginal concentrations of lactic acid potently inactivate HIV‐1 compared to short chain fatty acids present during bacterial vaginosis. AIDS Res Hum Retroviruses 30 Suppl 1, A228. [Google Scholar]
- Al‐Mushrif S, Eley A & Jones BM (2000). Inhibition of chemotaxis by organic acids from anaerobes may prevent a purulent response in bacterial vaginosis. J Med Microbiol 49, 1023–1030. [DOI] [PubMed] [Google Scholar]
- Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D & Holmes KK (1983). Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74, 14–22. [DOI] [PubMed] [Google Scholar]
- Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, Padavattan N, Ismail N, Moodley A, Sabatini ME, Ghebremichael MS, Nusbaum C, Huttenhower C, Virgin HW, Ndung'u T, Dong KL, Walker BD, Fichorova RN & Kwon DS (2015). Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42, 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Birse K, Mckinnon L, Lingappa J, Novak R, Westmacott G, Ball TB, Lauffenburger D & Burgener A (2014). Mucosal integrity factors are perturbed during bacterial vaginosis: a proteomic analysis. AIDS Res Hum Retroviruses 30 Suppl 1, A30. [Google Scholar]
- Barlow RE, Cooke ID, Odukoya O, Heatley MK, Jenkins J, Narayansingh G, Ramsewak SS & Eley A (2001). The prevalence of Chlamydia trachomatis in fresh tissue specimens from patients with ectopic pregnancy or tubal factor infertility as determined by PCR and in‐situ hybridisation. J Med Microbiol 50, 902–908. [DOI] [PubMed] [Google Scholar]
- Beghini J, Linhares I, Giraldo P, Ledger W & Witkin S (2014). Differential expression of lactic acid isomers, extracellular matrix metalloproteinase inducer, and matrix metalloproteinase‐8 in vaginal fluid from women with vaginal disorders. BJOG 122, 1580–1585. [DOI] [PubMed] [Google Scholar]
- Bernbaum JC, Umbach DM, Ragan NB, Ballard JL, Archer JI, Schmidt‐Davis H & Rogan WJ (2007). Pilot studies of estrogen‐related physical findings in infants. Environ Health Perspect 116, 416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey ER, Cone RA, Whaley KJ & Moench TR (2001). Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod 16, 1809–1813. [DOI] [PubMed] [Google Scholar]
- Brotman RM, Ghanem KG, Klebanoff MA, Taha TE, Scharfstein DO & Zenilman JM (2008). The effect of vaginal douching cessation on bacterial vaginosis: a pilot study. Am J Obstet Gynecol 198, 628.e1–628.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman RM, Ravel J, Bavoil PM, Gravitt PE & Ghanem KG (2013a). Microbiome, sex hormones, and immune responses in the reproductive tract: Challenges for vaccine development against sexually transmitted infections. Vaccine 32, 1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman RM, Ravel J, Cone RA & Zenilman JM (2010). Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect 86, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver MI, Viscidi RP, Burke AE, Ravel J & Gravitt PE (2013b). Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 21, 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos ACC, Freitas‐Junior R, Ribeiro LFJ, Paulinelli RR & Reis C (2008). Prevalence of vulvovaginitis and bacterial vaginosis in patients with koilocytosis. Sao Paulo Med J 126, 333–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Giovannini G, De Luca A, D'Angelo C, Casagrande A, Iannitti RG, Ricci G, Cunha C & Romani L (2012). Dectin‐1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell Mol Immunol 9, 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauci S (2004). Vaginal immunity in bacterial vaginosis. Curr Infect Dis Rep 6, 450–456. [DOI] [PubMed] [Google Scholar]
- Cauci S & Culhane JF (2007). Modulation of vaginal immune response among pregnant women with bacterial vaginosis by Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, and yeast. Am J Obstet Gynecol 196, 133.e1–133.e7. [DOI] [PubMed] [Google Scholar]
- Cauci S, Guaschino S, de Aloysio D, Driussi S, De Santo D, Penacchioni P & Quadrifoglio F (2003). Interrelationships of interleukin‐8 with interleukin‐1β and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod 9, 53–58. [DOI] [PubMed] [Google Scholar]
- Chaudry AN, Travers PJ, Yuenger J, Colletta L, Evans P, Zenilman JM & Tummon A (2004). Analysis of vaginal acetic acid in patients undergoing treatment for bacterial vaginosis. J Clin Microbiol 42, 5170–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Tian Y, Huang C, Li D, Zhong Q & Ma X (2015). Interaction between microbes and host intestinal health: modulation by dietary nutrients and gut‐brain‐endocrine‐immune axis. Curr Protein Pept Sci 16, 592–603. [DOI] [PubMed] [Google Scholar]
- Cherpes TL, Meyn LA, Krohn MA, Lurie JG & Hillier SL (2003). Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis 37, 319–325. [DOI] [PubMed] [Google Scholar]
- Cohen CR, Duerr A, Pruithithada N, Rugpao S, Hillier S, Garcia P & Nelson K (1995). Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS 9, 1093–1097. [DOI] [PubMed] [Google Scholar]
- Coleman JS, Hitti J, Bukusi EA, Mwachari C, Muliro A, Nguti R, Gausman R, Jensen S, Patton D, Lockhart D, Coombs R & Cohen CR (2007). Infectious correlates of HIV‐1 shedding in the female upper and lower genital tracts. AIDS 21, 755–759. [DOI] [PubMed] [Google Scholar]
- Conlon M & Bird A (2015). The impact of diet and lifestyle on gut microbiota and human health. Nutrients 7, 17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti C, Malacrino C & Mastromarino P (2009). Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J Physiol Pharmacol 60 Suppl 6, 19–26. [PubMed] [Google Scholar]
- Cooper MD, Roberts MH, Barauskas OL & Jarvis GA (2012). Secretory leukocyte protease inhibitor binds to Neisseria gonorrhoeae outer membrane opacity protein and is bactericidal. Am J Reprod Immunol 68, 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank R (1934). The conversion of the glycogen of the vagina into lactic acid. J Pathol Bacteriol 39, 213–219. [Google Scholar]
- Cruickshank R & Sharman A (1934). The biology of the vagina in the human subject. BJOG 41, 208–226. [Google Scholar]
- Cu‐Uvin S, Hogan JW, Caliendo AM, Harwell J, Mayer KH, Carpenter CC; HIV Epidemiology Research Study (2001). Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clin Infect Dis 33, 894–896. [DOI] [PubMed] [Google Scholar]
- DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, Sun CL, Goltsman DSA, Wong RJ, Shaw G, Stevenson DK, Holmes SP & Relman DA (2015). Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci USA 112, 11060–11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Chou Y‐Y & Chang TL (2009). Defensins in viral infections. J Innate Immun 1, 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger SY, Throop AL & Herbst‐Kralovetz MM (2014). Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species‐specific manner. J Infect Dis 209, 1989–1999. [DOI] [PubMed] [Google Scholar]
- Donders GGG (2007). Definition and classification of abnormal vaginal flora. Best Pract Res Clin Obstet Gynaecol 21, 355–373. [DOI] [PubMed] [Google Scholar]
- Donders GG, Van Calsteren K, Bellen G, Reybrouck R, Van den Bosch T, Riphagen I & Van Lierde S (2009). Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG 116, 1315–1324. [DOI] [PubMed] [Google Scholar]
- Donders GGG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G & Spitz B (2002). Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG 109, 34–43. [DOI] [PubMed] [Google Scholar]
- Eade CR, Diaz C, Wood MP, Anastos K, Patterson BK, Gupta P, Cole AL & Cole AM (2012). Identification and characterization of bacterial vaginosis‐associated pathogens using a comprehensive cervical‐vaginal epithelial coculture assay. PLoS One 7, e50106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage M & Maibach H (2005). Lifetime changes in the vulva and vagina. Arch Gynecol Obstet 273, 195–202. [DOI] [PubMed] [Google Scholar]
- Fredricks DN, Fiedler TL & Marrazzo JM (2005). Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353, 1899–1911. [DOI] [PubMed] [Google Scholar]
- Furci L, Sironi F, Tolazzi M, Vassena L & Lusso P (2007). Alpha‐defensins block the early steps of HIV‐1 infection: interference with the binding of gp120 to CD4. Blood 109, 2928–2935. [DOI] [PubMed] [Google Scholar]
- Gaiha GD, Dong T, Palaniyar N, Mitchell DA, Reid KBM & Clark HW (2008). Surfactant protein A binds to HIV and inhibits direct infection of CD4+ cells, but enhances dendritic cell‐mediated viral transfer. J Immunol 181, 601–609. [DOI] [PubMed] [Google Scholar]
- Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UME, Zhong X, Koenig SSK, Fu L, Ma ZS, Zhou X, Abdo Z, Forney LJ & Ravel J (2012). Temporal dynamics of the human vaginal microbiota. Sci Transl Med 4, 132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galhardo CL, Soares JM, Simões RS, Haidar MA, Rodrigues de Lima G & Baracat EC (2006). Estrogen effects on the vaginal pH, flora and cytology in late postmenopause after a long period without hormone therapy. Clin Exp Obstet Gynecol 33, 85–89. [PubMed] [Google Scholar]
- Gelber SE, Aguilar JL, Lewis KLT & Ratner AJ (2008). Functional and phylogenetic characterization of Vaginolysin, the human‐specific cytolysin from Gardnerella vaginalis . J Bacteriol 190, 3896–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genç MR, Onderdonk AB, Vardhana S, Delaney ML, Norwitz ER, Tuomala RE, Paraskevas L‐R, Witkin SSMAP Study Group (2004a). Polymorphism in intron 2 of the interleukin‐1 receptor antagonist gene, local midtrimester cytokine response to vaginal flora, and subsequent preterm birth. Am J Obstet Gynecol 191, 1324–1330. [DOI] [PubMed] [Google Scholar]
- Genç MR, Vardhana S, Delaney ML, Onderdonk A, Tuomala R, Norwitz E, Witkin SSMAP Study Group (2004b). Relationship between a toll‐like receptor‐4 gene polymorphism, bacterial vaginosis‐related flora and vaginal cytokine responses in pregnant women. Eur J Obstet Gynecol 116, 152–156. [DOI] [PubMed] [Google Scholar]
- Genç MR, Vardhana S, Delaney ML, Witkin SS, Onderdonk ABMAP Study Group (2007). TNFA‐308G>A polymorphism influences the TNF‐alpha response to altered vaginal flora. Eur J Obstet Gynecol 134, 188–191. [DOI] [PubMed] [Google Scholar]
- Giraldo PC, Babula O, Gonçalves AKS, Linhares IM, Amaral RL, Ledger WJ & Witkin SS (2007). Mannose‐binding lectin gene polymorphism, vulvovaginal candidiasis, and bacterial vaginosis. Obstet Gynecol 109, 1123–1128. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Andrews WW, Yuan AC, MacKay HT & St Louis ME (1997). Sexually transmitted diseases and adverse outcomes of pregnancy. Clin Perinatol 24, 23–41. [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC & Andrews WW (2000). Intrauterine infection and preterm delivery. N Engl J Med 342, 1500–1507. [DOI] [PubMed] [Google Scholar]
- Gong Z, Luna Y, Yu P & Fan H (2014). Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2 . PLoS One 9, e107758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez LM, Sammel MD, Appleby DH, Elovitz MA, Baldwin DA, Jeffcoat MK, Macones GA & Parry S (2010). Evidence of a gene‐environment interaction that predisposes to spontaneous preterm birth: a role for asymptomatic bacterial vaginosis and DNA variants in genes that control the inflammatory response. Am J Obstet Gynecol 202, 386.e1–386.e6. [DOI] [PubMed] [Google Scholar]
- Gravett MG, Nelson HP, DeRouen T, Critchlow C, Eschenbach DA & Holmes KK (1986). Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcome. JAMA 256, 1899–1903. [PubMed] [Google Scholar]
- Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ & Novy MJ (1994). An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 171, 1660–1667. [DOI] [PubMed] [Google Scholar]
- Hafner LM (2015). Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections. Contraception 92, 108–115. [DOI] [PubMed] [Google Scholar]
- Hammerschlag MR, Alpert S, Onderdonk AB, Thurston P, Drude E, McCormack WM & Bartlett JG (1978a). Anaerobic microflora of the vagina in children. Am J Obstet Gynecol 131, 853–856. [DOI] [PubMed] [Google Scholar]
- Hammerschlag MR, Alpert S, Rosner I, Thurston P, Semine D, McComb D & McCormack WM (1978b). Microbiology of the vagina in children: normal and potentially pathogenic organisms. Pediatrics 62, 57–62. [PubMed] [Google Scholar]
- Han C, Wu W, Fan A, Wang Y, Zhang H, Chu Z, Wang C & Xue F (2014). Diagnostic and therapeutic advancements for aerobic vaginitis. Arch Gynecol Obstet 291, 251–257. [DOI] [PubMed] [Google Scholar]
- Hay PE, Ugwumadu A & Chowns J (1997). Sex, thrush and bacterial vaginosis. Int J STD AIDS 8, 603–608. [DOI] [PubMed] [Google Scholar]
- Hearps A, Gugasyan R, Srbinovski D, Tyssen D, Aldunate M, Anderson DJ, Cone R & Tachedjian G (2014). Lactic acid, a vaginal microbiota metabolite, elicits an anti‐inflammatory response from vaginal and cervical epithelial cells. AIDS Res Hum Retroviruses 30 Suppl 1, A238–A239. [Google Scholar]
- Hillier SL & Lau RJ (1997). Vaginal microflora in postmenopausal women who have not received estrogen replacement therapy. Clin Infect Dis 25, S123–S126. [DOI] [PubMed] [Google Scholar]
- Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG & Rao AV; the Vaginal Infections and Prematurity Study Group (1995). Association between bacterial vaginosis and preterm delivery of a low‐birth‐weight infant. N Engl J Med 333, 1737–1742. [DOI] [PubMed] [Google Scholar]
- Horne AW, Stock SJ & King AE (2008). Innate immunity and disorders of the female reproductive tract. Reproduction 135, 739–749. [DOI] [PubMed] [Google Scholar]
- Isaacs CE & Xu W (2013). Theaflavin‐3,3'‐digallate and lactic acid combinations reduce herpes simplex virus infectivity. Antimicrob Agents Chemother 57, 3806–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovljević A, Bogavac M, Nikolić A, Tošić MM, Novaković Z & Stajić Z (2014). The influence of bacterial vaginosis on gestational week of the completion of delivery and biochemical markers of inflammation in the serum. Vojnosanit Pregl 71, 931–935. [PubMed] [Google Scholar]
- Jones NM, Holzman C, Friderici KH, Jernigan K, Chung H, Wirth J & Fisher R (2010). Interplay of cytokine polymorphisms and bacterial vaginosis in the etiology of preterm delivery. J Reprod Immunol 87, 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau AL, Ahern PP, Griffin NW, Goodman AL & Gordon JI (2011). Human nutrition, the gut microbiome and the immune system. Nature 474, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane FE, Ison CA & Taylor‐Robinson D (1997). A longitudinal study of the vaginal flora over a menstrual cycle. Int J STD AIDS 8, 489–494. [DOI] [PubMed] [Google Scholar]
- King AE, Wheelhouse N, Cameron S, McDonald SE, Lee K‐F, Entrican G, Critchley HOD & Horne AW (2009). Expression of secretory leukocyte protease inhibitor and elafin in human fallopian tube and in an in‐vitro model of Chlamydia trachomatis infection. Hum Reprod 24, 679–686. [DOI] [PubMed] [Google Scholar]
- Lewis WG, Robinson LS, Gilbert NM, Perry JC & Lewis AL (2013). Degradation, foraging, and depletion of mucus sialoglycans by the vagina‐adapted Actinobacterium Gardnerella vaginalis . J Biol Chem 288, 12067–12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis WG, Robinson LS, Perry J, Bick JL, Peipert JF, Allsworth JE & Lewis AL (2012). Hydrolysis of secreted sialoglycoprotein immunoglobulin A (IgA) in ex vivo and biochemical models of bacterial vaginosis. J Biol Chem 287, 2079–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby EK, Pascal KE, Mordechai E, Adelson ME & Trama JP (2008). Atopobium vaginae triggers an innate immune response in an in vitro model of bacterial vaginosis. Microbes Infect 10, 439–446. [DOI] [PubMed] [Google Scholar]
- Linhares IM, Summers PR, Larsen B, Giraldo PC & Witkin SS (2011). Contemporary perspectives on vaginal pH and lactobacilli. Am J Obstet Gynecol 204, 120.e1–120.e5. [DOI] [PubMed] [Google Scholar]
- Ma B, Forney LJ & Ravel J (2012). Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol 66, 371–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackelprang RD, Scoville CW, Cohen CR, Ondondo RO, Bigham AW, Celum C, Campbell MS, Essex M, Wald A, Kiarie J, Ronald A, Gray G & Lingappa JR (2015). Toll‐like receptor gene variants and bacterial vaginosis among HIV‐1 infected and uninfected African women. Genes Immun 16, 362–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklaim JM, Fernandes AD, Di Bella JM, Hammond J‐A, Reid G & Gloor GB (2013). Comparative meta‐RNA‐seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome 1, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklaim JM, Gloor GB, Anukam KC, Cribby S & Reid G (2011). At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB‐1. Proc Natl Acad Sci USA 108 Suppl 1, 4688–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon PJ, Dalgleish AG, McDougal JS, Clapham PR, Weiss RA & Axel R (1986). The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 47, 333–348. [DOI] [PubMed] [Google Scholar]
- Martin DH (2012). The microbiota of the vagina and its influence on women's health and disease. Am J Med Sci 343, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya‐Achola JO, Bwayo J & Kreiss J (1999). Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 180, 1863–1868. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Pédron T, Regnault B, Mulet C, Hara T & Sansonetti PJ (2013). Epithelial cell proliferation arrest induced by lactate and acetate from lactobacillus casei and bifidobacterium breve. PLoS One 8, e63053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald HM, O'Loughlin JA, Jolley P, Vigneswaran R & McDonald PJ (1992). Prenatal microbiological risk factors associated with preterm birth. Br J Obstet Gynaecol 99, 190–196. [DOI] [PubMed] [Google Scholar]
- Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, Johnson F, Iams JD, Thom E & Andrews WW; National Institute of Child Health and Human Development Maternal‐Fetal Medicine Units Network (1995). The preterm prediction study: significance of vaginal infections. Am J Obstet Gynecol 173, 1231–1235. [DOI] [PubMed] [Google Scholar]
- Mirmonsef P, Zariffard MR, Gilbert D, Makinde H, Landay AL & Spear GT (2011). Short‐chain fatty acids induce pro‐inflammatory cytokine production alone and in combination with toll‐like receptor ligands. Am J Reprod Immunol 67, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C & Marrazzo J (2014). Bacterial vaginosis and the cervicovaginal immune response. Am J Reprod Immunol 71, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncla BJ, Chappell C, Debo BM, Macio IS, Bunge KE & Hillier SL (2014). The effects of hormones and vaginal microflora on the content of MUC1, MUC4, MUC5AC and MUC7 in the cervicovaginal fluid (CVF). AIDS Res Hum Retroviruses 30 Suppl 1, A29. [Google Scholar]
- Moncla BJ, Mietzner TA & Hillier SL (2012). In vitro activity of cationic peptides against Neisseria gonorrhoeae and vaginal Lactobacillus species: The effect of divalent cations. Advances in Bioscience and Biotechnology 3, 249–255. [Google Scholar]
- Mossop H, Linhares IM, Bongiovanni AM, Ledger WJ & Witkin SS (2011). Influence of lactic acid on endogenous and viral RNA‐induced immune mediator production by vaginal epithelial cells. Obstet Gynecol 118, 840–846. [DOI] [PubMed] [Google Scholar]
- Münk C, Wei G, Yang OO, Waring AJ, Wang W, Hong T, Lehrer RI, Landau NR & Cole AM (2003). The theta‐defensin, retrocyclin, inhibits HIV‐1 entry. AIDS Res Hum Retroviruses 19, 875–881. [DOI] [PubMed] [Google Scholar]
- Nasioudis D, Beghini J, Bongiovanni AM, Giraldo PC, Linhares IM & Witkin SS (2015). α‐Amylase in vaginal fluid: association with conditions favorable to dominance of Lactobacillus. Reprod Sci 22, 1393–1398. [DOI] [PubMed] [Google Scholar]
- Nelson DB, Hanlon A, Nachamkin I, Haggerty C, Mastrogiannis DS, Liu C & Fredricks DN (2014). Early pregnancy changes in bacterial vaginosis‐associated bacteria and preterm delivery. Paediatr Perinat Epidemiol 28, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ & Turner MW (2000). Mannose‐binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun 68, 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Kafka JK, Ferreira VH, Roth K & Kaushic C (2014). Innate and adaptive immune responses in male and female reproductive tracts in homeostasis and following HIV infection. Cell Mol Immunol 11, 410–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K, Risberg B & Heimer G (1995). The vaginal epithelium in the postmenopause–cytology, histology and pH as methods of assessment. Maturitas 21, 51–56. [DOI] [PubMed] [Google Scholar]
- Nugent RP, Krohn MA & Hillier SL (1991). Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn K, Wang Y‐Y, Harit D, Cone R & Lai S (2014). Influence of vaginal microbiota on the diffusional barrier properties of cervicovaginal mucus. AIDS Res Hum Retroviruses 30 Suppl 1, A234. [Google Scholar]
- Nunn KL, Wang Y‐Y, Harit D, Humphrys MS, Ma B, Cone R, Ravel J & Lai SK (2015). Enhanced trapping of HIV‐1 by human cervicovaginal mucus is associated with Lactobacillus crispatus‐dominant microbiota. MBio 6, e01084–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanlon DE, Moench TR & Cone RA (2011). In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis 11, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanlon DE, Moench TR & Cone RA (2013). Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One 8, e80074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfanelli T, Jayaram A, Doulaveris G, Forney LJ, Ledger WJ & Witkin SS (2014). Human epididymis protein 4 and secretory leukocyte protease inhibitor in vaginal fluid: relation to vaginal components and bacterial composition. Reprod Sci 21, 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit H, Gopal S, Sonawani A, Yadav AK, Qaseem AS, Warke H, Patil A, Gajbhiye R, Kulkarni V, Al‐Mozaini MA, Idicula‐Thomas S, Kishore U & Madan T (2014). Surfactant protein D inhibits HIV‐1 infection of target cells via interference with gp120‐CD4 interaction and modulates pro‐inflammatory cytokine production. PLoS One 9, e102395‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SE, CM Beck‐Sagué, Farshy CE, Gibson I, Kubota KA, Solomon F, Morse SA, Sievert AJ & Black CM (2000). Behaviors associated with Neisseria gonorrhoeae and Chlamydia trachomatis: cervical infection among young women attending adolescent clinics. Clin Pediatr (Phila) 39, 173–177. [DOI] [PubMed] [Google Scholar]
- Petrova MI, Lievens E, Malik S, Imholz N & Lebeer S (2015). Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol 6, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyles RB, Vincent KL, Baum MM, Elsom B, Miller AL, Maxwell C, Eaves‐Pyles TD, Li G, Popov VL, Nusbaum RJ & Ferguson MR (2014). Cultivated vaginal microbiomes alter HIV‐1 infection and antiretroviral efficacy in colonized epithelial multilayer cultures. PLoS One 9, e93419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J, Brotman RM, Gajer P, Ma B, Nandy M, Fadrosh DW, Sakamoto J, Koenig SS, Fu L, Zhou X, Hickey RJ, Schwebke JR & Forney LJ (2013). Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome 1, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L & Forney LJ (2011). Vaginal microbiome of reproductive‐age women. Proc Natl Acad Sci USA 108 Suppl 1, 4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Bieda J, Chaemsaithong P, Miranda J, Chaiworapongsa T & Ravel J (2014). The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose WA, McGowin CL, Spagnuolo RA, Eaves‐Pyles TD, Popov VL & Pyles RB (2012). Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PLoS One 7, e32728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royse KE, Kempf M‐C, McGwin G, Wilson CM, Tang J & Shrestha S (2012). Toll‐like receptor gene variants associated with bacterial vaginosis among HIV‐1 infected adolescents. J Reprod Immunol 96, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckman KK, Williams SM, Krohn MA & Simhan HN (2011). Interaction between interleukin‐1 receptor 2 and Toll‐like receptor 4, and cervical cytokines. J Reprod Immunol 90, 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwebke JR (2009). New concepts in the etiology of bacterial vaginosis. Curr Infect Dis Rep 11, 143–147. [DOI] [PubMed] [Google Scholar]
- Schwebke JR, Richey CM & Weiss2 HL (1999). Correlation of behaviors with microbiological changes in vaginal flora. J Infect Dis 180, 1632–1636. [DOI] [PubMed] [Google Scholar]
- Spear GT, French AL, Gilbert D, Zariffard MR, Mirmonsef P, Sullivan TH, Spear WW, Landay A, Micci S, Lee B‐H & Hamaker BR (2014). Human α‐amylase present in lower‐genital‐tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis 210, 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, Marrazzo JM & Fredricks DN (2010). Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One 5, e10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart‐Tull DE (1964). Evidence that vaginal lactobacilli do not ferment glycogen. Am J Obstet Gynecol 88, 676–679. [DOI] [PubMed] [Google Scholar]
- Stoyancheva G, Marzotto M, Dellaglio F & Torriani S (2014). Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch Microbiol 196, 645–653. [DOI] [PubMed] [Google Scholar]
- Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, Liomba GN, Broadhead RL, Chiphangwi JD & Miotti PG (1998). Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 12, 1699–1706. [DOI] [PubMed] [Google Scholar]
- Tramont EC (1977). Inhibition of adherence of Neisseria gonorrhoeae by human genital secretions. J Clin Invest 59, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MW (2003). The role of mannose‐binding lectin in health and disease. Mol Immunol 40, 423–429. [DOI] [PubMed] [Google Scholar]
- Tyutyunnik VL, Kan NE, Lomova NA, Karapetyan TE, Kogan EA & Shchyogolev AI (2014). Role of innate immunity in pregnant patients with vulvovaginal infections in the development of intrauterine infection in the newborn. Bull Exp Biol Med 158, 74–76. [DOI] [PubMed] [Google Scholar]
- Usluogullari B, Gumus I, Gunduz E, Kaygusuz I, Simavli S, Acar M, Oznur M, Gunduz M & Kafali H (2014). The role of human dectin‐1 Y238X gene polymorphism in recurrent vulvovaginal candidiasis infections. Mol Biol Rep 41, 6763–6768. [DOI] [PubMed] [Google Scholar]
- Valore EV, Park CH, Igreti SL & Ganz T (2002). Antimicrobial components of vaginal fluid. Am J Obstet Gynecol 187, 561–568. [DOI] [PubMed] [Google Scholar]
- Valore EV, Wiley DJ & Ganz T (2006). Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun 74, 5693–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oostrum N, De Sutter P, Meys J & Verstraelen H (2013). Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta‐analysis. Hum Reprod 28, 1809–1815. [DOI] [PubMed] [Google Scholar]
- Wang W, Cole AM, Hong T & Waring AJ (2003). Retrocyclin, an antiretroviral θ‐defensin, is a lectin. J Immunol 170, 4708–4716. [DOI] [PubMed] [Google Scholar]
- Wang W, Owen SM, Rudolph DL, Cole AM, Hong T, Waring AJ, Lal RB & Lehrer RI (2004). Activity of alpha‐ and theta‐defensins against primary isolates of HIV‐1. J Immunol 173, 515–520. [DOI] [PubMed] [Google Scholar]
- Wang Y‐Y, Kannan A, Nunn KL, Murphy MA, Subramani DB, Moench T, Cone R & Lai SK (2014). IgG in cervicovaginal mucus traps HSV and prevents vaginal Herpes infections. Mucosal Immunol 7, 1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SS, Wiens ME & Smith JG (2013). Antiviral mechanisms of human defensins. J Mol Biol 425, 4965–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Fahey JV, Sentman CL, Pioli PA & Shen L (2005). Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev 206, 306–335. [DOI] [PubMed] [Google Scholar]
- Witkin SS (2014). The vaginal microbiome, vaginal anti‐microbial defence mechanisms and the clinical challenge of reducing infection‐related preterm birth. BJOG 122, 213–218. [DOI] [PubMed] [Google Scholar]
- Witkin SS, Alvi S, Bongiovanni AM, Linhares IM & Ledger WJ (2011). Lactic acid stimulates interleukin‐23 production by peripheral blood mononuclear cells exposed to bacterial lipopolysaccharide. FEMS Immunol Med Microbiol 61, 153–158. [DOI] [PubMed] [Google Scholar]
- Witkin SS, Linhares IM & Giraldo P (2007a). Bacterial flora of the female genital tract: function and immune regulation. Best Pract Res Clin Obstet Gynaecol 21, 347–354. [DOI] [PubMed] [Google Scholar]
- Witkin SS, Linhares IM, Giraldo P & Ledger WJ (2007b). An altered immunity hypothesis for the development of symptomatic bacterial vaginosis. Clin Infect Dis 44, 554–557. [DOI] [PubMed] [Google Scholar]
- Witkin SS, Mendes‐Soares H, Linhares IM, Jayaram A, Ledger WJ & Forney LJ (2013). Influence of vaginal bacteria and D‐and L‐lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. MBio 4, e00460‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Cocchi F, Gentles D, Ericksen B, Lubkowski J, Devico A, Lehrer RI & Lu W (2005). Human neutrophil alpha‐defensin 4 inhibits HIV‐1 infection in vitro. FEBS Lett 579, 162–166. [DOI] [PubMed] [Google Scholar]
- Yarbrough VL, Winkle S & Herbst‐Kralovetz MM (2015). Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update 21, 353–377. [DOI] [PubMed] [Google Scholar]
- Zheng J, Gänzle MG, Lin XB, Ruan L & Sun M (2015). Diversity and dynamics of bacteriocins from human microbiome. Environ Microbiol 17, 2133–2143. [DOI] [PubMed] [Google Scholar]
- Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR & Forney LJ (2004). Characterization of vaginal microbial communities in adult healthy women using cultivation‐independent methods. Microbiology 150, 2565–2573. [DOI] [PubMed] [Google Scholar]
- Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, Foster JA & Forney LJ (2007). Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J 1, 121–133. [DOI] [PubMed] [Google Scholar]
- Zilnyte M, Venclovas Č, Zvirbliene A & Pleckaityte M (2015). The cytolytic activity of vaginolysin strictly depends on cholesterol and is potentiated by human CD59. Toxins 7, 110–128. [DOI] [PMC free article] [PubMed] [Google Scholar]


