Abstract
The skin is the largest organ in the human body and provides the first line of defence against environmental attack and pathogen invasion. It harbor multiple commensal microbial communities at different body sites, which play important roles in sensing the environment, protecting against colonization and infection of pathogens, and guiding the host immune system in response to foreign invasions. The skin microbiome is largely variable between individuals and body sites, with several core commensal members commonly shared among individuals at the healthy state. These microbial commensals are essential to skin health and can potentially lead to disease when their abundances and activities change due to alterations in the environment or in the host. While recent advances in sequencing technologies have enabled a large number of studies to characterize the taxonomic composition of the skin microbiome at various body sites and under different physiological conditions, we have limited understanding of the microbiome composition and dynamics at the strain level, which is highly important to many microbe‐related diseases. Functional studies of the skin microbial communities and the interactions among community members and with the host are currently scant, warranting future investigations. In this review, we summarize the recent findings on the skin microbiome, highlighting the roles of the major commensals, including bacteria, fungi and bacteriophages, in modulating skin functions in health and disease. Functional studies of the skin microbiota at the metatranscriptomic and proteomic levels are also included to illustrate the interactions between the microbiota and the host skin.
Abbreviations
- AMP
antimicrobial peptide
- GAS
Group A Streptococcus
- ITS
internal transcribed spacer
- SCFAs
short chain fatty acids
- TLR
Toll‐like receptor
Introduction
The skin is the largest organ in the human body and plays important roles in human physiology. Organized as an assembly of cells in highly structured layers including the epidermis, dermis and subcutaneous regions, the skin acts as the physical barrier protecting the internal organs from environmental changes and pathogen invasion (Madison, 2003).
The skin is also a host for hundreds of microorganisms, including bacteria, eukaryotes and viruses. Immediately after birth, diverse microbial communities colonize the skin at different sites with unique physiological and immunological niches. The resident microorganisms sequester nutrients from skin secretions and form a dynamic ecological system with the host skin through complex interactions within the microbial communities and with the host. The composition, dynamics and function of the skin microbiota have a significant impact on skin health and function.
Skin microorganisms have been recognized mostly in their role in various skin diseases, and the emphasis in medical implication has been on how to remove the pathogenic organisms. The research in recent years through microbiome studies has revealed that the microorganisms on the skin are an essential part of the host–microbiota symbiotic system, suggesting that skin commensals play important roles in maintaining skin health and proper function (Sanford & Gallo, 2013). This new view calls for paradigm‐shifting recognition of the functions of the skin microorganisms in skin health and new treatment strategies for microorganism‐associated skin diseases.
Taxonomic composition of the human skin microbiome
Studies investigating the composition of the human skin microbiome have revealed the presence of hundreds of microorganisms including bacteria, fungi, parasites and viruses (Grice & Segre, 2011). To date, the majority of culture‐ and sequencing‐based microbiome studies have focused on characterizing the skin bacterial and fungal communities. Based on sequencing analysis of phylogenetic marker genes, such as the bacterial 16S ribosomal RNA (rRNA) and fungal internal transcribed spacer (ITS), the bacterial residents identified mainly belong to four phyla: Actinobacteria, Firmicutes, Proteobacteria and Bacteroidetes (Gao et al. 2007; Grice et al. 2009), while the majority of fungal species identified are from a single genus, Malassezia (Findley et al. 2013).
While the taxonomic composition of the skin microbiome has been well characterized at the genus or sometimes species level, its strain‐level composition and dynamics are still poorly understood. Two studies have shown that two of the most abundant skin bacterial species, Propionibacterium acnes and Staphylococcus epidermidis, exhibit strain‐level diversity between individuals, skin status, and the skin site sampled (Fitz‐Gibbon et al. 2013; Oh et al. 2014). Fitz‐Gibbon et al. identified strain‐level differences in the skin microbiota between acne patients and healthy individuals. Certain P. acnes strains were highly associated with acne while some other strains were enriched in healthy skin (Fitz‐Gibbon et al. 2013). Genome comparison based on the single nucleotide polymorphisms (SNPs) observed in a large number of sequenced P. acnes strains revealed that strains isolated from the same individuals were often more closely related to each other than to the strains isolated from different individuals, suggesting individuality of the skin microbiota at the strain level (Tomida et al. 2013). Consistently, metagenomic shotgun sequencing analysis by Oh et al. revealed that P. acnes strain types and abundances were primarily driven by individuality as opposed to body site. S. epidermidis strains, on the other hand, exhibited less individual specificities. Instead, the strains were correlated with the body sites from where they were collected (Oh et al. 2014).
In contrast to bacterial and fungal compositions, only a few studies have described the remaining skin inhabitants such as the viral and parasitic components. Metagenomic shotgun sequencing analysis and culture‐based studies have started to unfold the composition and function of the skin viral community. Double stranded DNA (dsDNA) eukaryotic viruses, including herpesviruses, papillomaviruses, polyomaviruses, circoviruses, adenoviruses, anelloviruses and paroviruses, were identified in the healthy skin microbiota (Foulongne et al. 2012; Ma et al. 2014; Wylie et al. 2014). In addition, prokaryotic viruses of the major skin bacteria, in particular P. acnes and S. epidermidis phages, were found at multiple skin sites (Oh et al. 2014; Liu et al. 2015). These initial studies suggest the existence of a complex and dynamic virome on the human skin.
Microscopic analysis of skin samples has revealed the presence of parasitic mites on the human skin (Crosti et al. 1983; Kligman & Christensen, 2011). However, further investigation of the skin viral and parasitic communities has thus far been hampered in part due to the low abundances of these organisms. Limited cultivation methods, a lack of genomic reference databases, and few molecular tools to enrich and identify these organisms also pose challenges in studying these communities on the skin. Future developments in molecular methods and sequencing technologies will improve our understanding of the role of the less abundant skin microorganisms and their interactions with others in the community and with the host in shaping the function of the human skin.
Factors influencing the composition of the skin microbiome
The composition of the human skin microbiome is influenced by multiple factors. Similar to the microbial communities at other body sites, individual variation is the major factor differentiating the skin microbiome among the populations (Gao et al. 2007; Costello et al. 2009). Age, sex and hygiene practice have been suggested to contribute to the individual variation of the skin microbial composition (Larson, 2001; Fierer et al. 2008; Song et al. 2013).
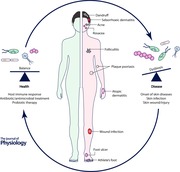
The spatial site is another factor affecting the skin microbiome composition. The skin is composed of a number of compartmentalized regions with distinct physiological properties such as pH, temperature, moisture, sweat level and lipid content. Each site represents an ecological niche that favours the growth of its own unique collection of microorganisms. The microbial communities at dry, moist and lipid‐rich sites are largely different (Fig. 1). The most diverse skin microbial communities are found on the dry and exposed skin sites, such as the forearm and palm (Gao et al. 2007; Costello et al. 2009; Grice et al. 2009). The skin microbiota of the moist and sweat‐rich axilla (underarm) is dominated by aerobic Corynebacterium and Staphylococcus species, which prefer conditions of higher temperature and humidity (Costello et al. 2009; Grice et al. 2009). The lipid‐rich areas of the skin, such as the sebaceous sites of the face and upper trunk, exhibit the lowest microbial diversity, colonized primarily by lipophilic microorganisms including Propionibacterium and Malassezia species, as well as the demodex mite, Demodex folliculorum (Costello et al. 2009; Grice et al. 2009; Kligman & Christensen, 2011; Fitz‐Gibbon et al. 2013). In an attempt to transplant the skin microbial community from different topographical sites, Costello et al. found that over an 8 hour period, forehead and forearm bacterial communities inoculated onto forearm and forehead, respectively, deviated from the original composition and became more similar to the community of the inoculated site. This suggests that the physiological properties of the skin site are a strong driver in defining the composition of the microbial community (Costello et al. 2009).
Figure 1. The composition and function of the human skin microbiota.
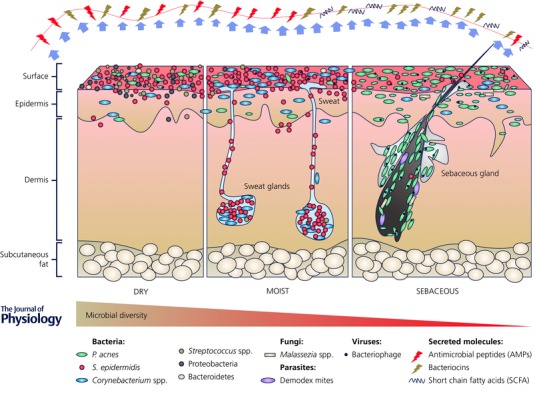
Driven by physiological properties of the skin, the microbial communities at dry, moist and sebaceous skin sites are largely different with various species dominating at each site. Staphylococcus epidermidis, Propionibacterium acnes and Malassezia spp. metabolize skin nutrients, produce physiologically important molecules, such as antimicrobial peptides, bacteriocins and short chain fatty acids, and play a role in defending against pathogen colonization and infection.
Open to continual contact with surroundings, the skin microbiota is influenced by environmental factors. It has been suggested that human‐to‐human (Hamburger, 1947; Pittet et al. 1999; Meadow et al. 2013; Song et al. 2013), human‐to‐pet (Song et al. 2013), and even human‐to‐object (Lax et al. 2014; Wood et al. 2015) contacts shape the composition of the skin microbial community. Song et al. showed that skin bacterial communities can be shared among co‐habiting family members, while pet ownership could also lead to the transfer of skin microorganisms between human and animals (Song et al. 2013). Diversity analysis of P. acnes phages on the skin revealed that the same phage strains were shared among related individuals, suggesting transmission of either phage or the phage‐associated bacterial host can occur between individuals (Liu et al. 2015).
Transfer of microorganisms between human and surfaces has also been shown in a number of different settings including the computer keyboard, mobile phone, home, classroom, restroom and hospital wards (Hambraeus, 1973; Fierer et al. 2010; Flores et al. 2011; Lax et al. 2014; Meadow et al. 2014a,b). These studies indicate common microbial pools existing in the population, which is important to issues regarding pathogen transmission, health care and hygiene practices (Pittet et al. 1999; Flores et al. 2011; Meadow et al. 2014b), and also suggest a potential application of individual microbiome signatures in forensic science (Fierer et al. 2010).
In addition to individual differences, topographical variation, and environmental influences, the host health status and the skin condition can also affect the composition of the microbiota. Shifts in skin bacterial and fungal communities have been linked to a number of skin diseases and conditions including psoriasis (Gao et al. 2008; Alekseyenko et al. 2013; Statnikov et al. 2013), atopic dermatitis (Dekio et al. 2007; Zhang et al. 2011; Kong et al. 2012), acne (Fitz‐Gibbon et al. 2013), dandruff (Clavaud et al. 2013), and damaged or wounded skin (Robson, 1997; Price et al. 2009; Gontcharova et al. 2010; Misic et al. 2014). The parasitic mite Demodex folliculorum, as well as its own associated microbiota, has been implicated in rosacea (Bonnar et al. 1993; Murillo et al. 2014).
Despite variations in the skin microbiome due to multiple contributing factors, skin microbial communities of healthy individuals appear relatively stable over at least several months (Costello et al. 2009; Grice et al. 2009). The stable nature of the human skin microbiome and persistence of core skin microorganisms suggest important functions of the commensal microbiota in skin health.
Key players of the commensal skin microbiota
Taxonomic studies have identified a number of key players in the healthy skin microbiota. Resident skin microorganisms are often considered commensal or mutualistic; however, with changes to the condition of the skin, including injury or the introduction of medical devices such as implants or catheters, some of the resident microorganisms can behave as opportunistic pathogens. To date, the dominant and most extensively studied members of the healthy skin microbiota include Staphylococcus, Propionibacterium, Streptococcus, Corynebacterium and Malassezia. Changes in the abundances of these organisms are often linked to diseased states (Paulino et al. 2006; Gao et al. 2008; Kong et al. 2012; Alekseyenko et al. 2013; Clavaud et al. 2013; Fitz‐Gibbon et al. 2013) (Table 1). Studies have also implicated phages as potential modulators of the skin bacterial community (Soothill, 1994; Vieira et al. 2012; Mendes et al. 2013; Liu et al. 2015; Pincus et al. 2015). Although these skin microorganisms are believed to be beneficial residents of the healthy skin microbiota, their functions in protecting against the action of pathogenic species and in maintaining skin health are not yet fully understood. Below we discuss the roles of the most representative skin commensals, S. epidermidis, P. acnes, and eukaryotic microorganisms Malassezia species, as well as phages, in skin health.
Table 1.
The functions of the major skin commensals in health and disease
| Microorganism | Function in skin health | Disease association | References |
|---|---|---|---|
| Staphylococcus epidermidis | Producing antimicrobial peptides and bacteriocins | Hospital‐acquired, open wound, skin burns and medical device infections | Wisplinghoff et al. 2004; Fontana et al. 2006; Li et al. 2007; Bastos et al. 2009; Rogers et al. 2009; Cogen et al. 2010; Lai et al. 2010; Coates et al. 2014 |
| Promoting host immune responses via TLR signalling | |||
| Propionibacterium acnes | Metabolizing sebum and producing SCFAs | Acne, SAPHO syndrome, sarcoidosis, sciatica, endophthalmitis, prostate cancer | Ushijima et al. 1984; Schaeverbeke et al. 1998; Eishi et al. 2002; Cohen et al. 2005; Schmid‐Wendtner & Korting, 2006; Javey et al. 2010; Fitz‐Gibbon et al. 2013; Rollason et al. 2013; Shu et al. 2013 |
| Maintaining acidic skin pH | |||
| Producing bacteriocins | |||
| Promoting commensal growth | |||
| Corynebacterium spp. | Commensal organisms | Atopic dermatitis | Kong et al. 2012; Oh et al. 2012 |
| Streptococcus spp. | Commensal organisms | Atopic dermatitis | Oh et al. 2012 |
| Malassezia spp. | Producing antimicrobials, such as azelaic acid | Dandruff, atopic dermatitis, folliculitis, psoriasis | Nazzaro‐Porro & Passi, 1978; Leeming et al. 1986; Brasch & Christophers, 1993; Xu et al. 2007; Gaitanis et al. 2012 |
| Bacteriophage | Specific lytic activities against bacterial species and strains | Soothill, 1994; Vieira et al. 2012; Mendes et al. 2013; Liu et al. 2015; Pincus et al. 2015 | |
| Modulating skin bacterial populations |
Staphylococcus epidermidis
The Gram‐positive bacterium S. epidermidis is a dominant skin resident found at multiple body sites. Multi‐locus sequence typing (MLST) of S. epidermidis has revealed a high level of strain diversity, with nearly 600 sequence types currently identified (http://sepidermidis.mlst.net). Unlike its coagulase‐positive relative Staphylococcus aureus, coagulase‐negative S. epidermidis is widely accepted as a beneficial skin microorganism of low pathogenicity. Genomic analysis of S. epidermidis has revealed a reduced virulence potential of this species compared to other staphylococci (Zhang et al. 2003). The roles of commensal S. epidermidis in skin health are twofold. Firstly, S. epidermidis produces and secretes a number of antimicrobial peptides (AMPs), such as phenol soluble modulins (PSMs) and bacteriocins, which can directly prevent the colonization of skin pathogens including Group A Streptococcus (GAS), S. aureus and even other S. epidermidis strains (Fontana et al. 2006; Bastos et al. 2009; Cogen et al. 2010) (Fig. 1). Secondly, S. epidermidis functions as a bacterial primer on the skin, regulating and promoting host inflammatory responses via Toll‐like receptor (TLR) signalling. Wanke et al. showed that when co‐colonized with pathogenic S. aureus, commensal S. epidermidis not only upregulated AMP expression but also abolished the inhibition of NF‐κB signalling asserted by S. aureus, leading to amplified host immunity in response to pathogen invasion (Wanke et al. 2011). S. epidermidis can enhance host immune responses in defence against other bacterial pathogens in addition to S. aureus, such as GAS, as well as against viral infections, such as vaccinia virus and human papillomavirus (HPV), while maintaining its own colonization on the skin (Li et al. 2007; Lai et al. 2010). Despite being typically considered a commensal organism, S. epidermidis can act as an opportunistic pathogen, with biofilm formation as a pathogenic mechanism (Cogen et al. 2010). The ubiquitous nature of S. epidermidis on the human skin and its ability to form biofilms have resulted in a high incidence of S. epidermidis in hospital‐acquired infections, medical device failure and even bacteraemia (Wisplinghoff et al. 2004; Rogers et al. 2009).
Propionibacterium acnes
Gram‐positive lipophilic P. acnes is a dominant skin resident species, particularly at sebaceous sites, such as the face, neck and upper trunk. Other Propionibacterium species, including Propionibacterium granulosum, Propionibacterium avidum and Propionibacterium humerusii, have also been identified on the human skin, but at a much lower prevalence and abundance than P. acnes. Propionibacteria are believed to play a beneficial role in maintaining skin health via their ability to metabolize triglycerides in sebum to short chain fatty acids (SCFAs). SCFAs exhibit antimicrobial properties and contribute to the acidic skin pH, thus preventing the colonization of pathogenic skin species including S. aureus (Ushijima et al. 1984; Shu et al. 2013) (Fig. 1). In addition to the production of SCFAs, some Propionibacterium species are capable of producing bacteriocins (Faye et al. 2011). P. acnes bacteriocins have been shown to inhibit the growth of some P. acnes strains as well as other bacteria (Fujimura & Nakamura, 1978). Consistent with their role in skin health, studies have revealed reduced relative abundance of Propionibacteria in skin diseases including psoriasis (Gao et al. 2008) and atopic dermatitis (Kong et al. 2012). Historically, P. acnes has been implicated in the pathogenesis of the common skin disease, acne, mostly due to a high frequency of isolation of the species from acne lesions (Marples et al. 1973; Gehse et al. 1983). Yet this association remains a topic of much debate due, in part, to the dominance of the species on healthy, non‐acneic skin. Analysis of the first P. acnes genome highlighted the virulence potential of this organism (Brüggemann et al. 2004). Sequencing and comparative genome analysis of large collections of P. acnes strains isolated from acne patients and healthy individuals have since revealed significant phylogenetic diversity within this species (McDowell et al. 2005, 2008; Kilian et al. 2012; Fitz‐Gibbon et al. 2013; Tomida et al. 2013). Certain lineages of strains have been associated with disease while others are associated with health (Lomholt & Kilian, 2010; McDowell et al. 2011; Fitz‐Gibbon et al. 2013; Kasimatis et al. 2013). While a causal relationship is yet to be determined, it has been increasingly recognized that communities of microorganisms colonize the skin. Mere presence or absence of disease‐associated strains may not be sufficient in determining the clinical outcome of disease or health. The presence and activities of other strains and species in the community may also contribute to skin health and disease and need to be considered when defining disease association.
Malassezia species
Recent metagenomic analyses have revealed that bacteria represent the main fraction of the skin microbiota; however, the skin also harbor eukaryotic species. Metagenomic shotgun sequencing and ITS‐based analysis of the fungal community from healthy skin have revealed low fungal diversity at most core body sites, with Malassezia species being the predominant colonizers (Paulino et al. 2006, 2008; Findley et al. 2013; Oh et al. 2014). Malassezia are lipophilic yeasts that colonize sebaceous areas of the skin and degrade sebum. Malassezia, in particular M. restricta and M. globosa, are generally recognized as commensal fungi, due to their prevalence on healthy skin (Ashbee & Evans, 2002). Genome analysis of M. restricta and M. globosa has revealed an abundance of lipases and phospholipases that are believed to aid in fatty acid metabolism (Dawson, 2007; Xu et al. 2007). One of the by‐products from fatty acid metabolism by Malassezia species is azelaic acid (Nazzaro‐Porro & Passi, 1978), which exhibits antimicrobial properties against skin bacteria and fungi (Leeming et al. 1986; Brasch & Christophers, 1993). Similar to other skin commensal microorganisms, Malassezia species have also been linked to a number of skin diseases. M. sympodialis has been implicated in atopic dermatitis, whereby it contributes to skin inflammation via the release of allergens (Selander et al. 2006). M. restricta has been controversially associated with dandruff, an inflammatory scalp disorder (Gaitanis et al. 2012). Despite associations with skin inflammatory conditions, the prevalence of Malassezia species on healthy skin suggests that these species are commensals and may become harmful when unfavorable conditions are presented. Further understanding of the functions of these fungal species will provide important insight in skin health and disease.
Bacteriophages
Phages are prokaryotic viruses that infect bacterial hosts, and are a dominant part of the skin virome. They are commonly found at multiple skin sites, naturally co‐occurring with their preferred bacterial hosts. Metagenomic shotgun sequencing analysis suggested that Propionibacterium and Staphylococcus phages are the most abundant skin phages, while other phages, such as Streptococcus and Corynebacterium phages, are also present but at lower relative abundances (Oh et al. 2014). Using culture‐based approaches and genome analysis of skin samples, Liu et al. revealed an increased frequency of P. acnes phages isolated from healthy individuals compared to acne patients, and suggested that phages may play a role in modulating the skin bacterial populations (Liu et al. 2015). Despite being used for over a century in Eastern European countries to treat bacteria‐associated diseases (Sulakvelidze et al. 2001), the interest in phage therapy to modulate bacterial communities in health and disease has recently generated substantial interest (Nobrega et al. 2015). Skin pathogens, such as S. aureus and Pseudomonas aeruginosa, can colonize the open wound upon skin injury, and subsequently cause skin infections that can be difficult to manage and treat (Church et al. 2006). Phage therapy, demonstrated in vitro and ex vivo, was found to be an efficient and promising treatment strategy to clear skin infections caused by P. aeruginosa (Soothill, 1994; Vieira et al. 2012; Pincus et al. 2015). With the emergence of many drug‐resistant pathogens and the increased failure rate in common skin antibiotics and antimicrobials, phage therapy presents a promising approach to treat bacterial infection and to maintain a healthy state of the skin microbial community.
Beyond the taxonomy and metagenome of the skin microbiota
Despite the existence of many microorganisms on the human skin, the limited microbial biomass available from skin samples has hindered the study of the functional role of the skin microbiota as a whole. To date, only a few published studies have characterized the skin microbiome at the metagenomic level (Human Microbiome Project Consortium et al. 2012; Mathieu et al. 2013; Oh et al. 2014). Metatranscriptomic, metaproteomic, and metabolomic analyses of the skin communities are not quite on a par with the success shared by the microbiome studies at other body sites such as the gut and oral cavity. Continued advances in molecular methods and next‐generation sequencing technologies have allowed ‘omic'‐based analysis of the skin microbiota from limited biological materials, and as a result, researchers have begun to expand their focus from taxonomic characterization to the functional determination of the skin microbiota and how they interact with the host. To understand the role of the skin microbiota in health and disease beyond the metagenomic level, recently Kang et al. performed the first skin metatranscriptomic analysis and revealed significant differences in the transcriptional activities of the skin microbiota between healthy individuals and acne patients (Kang et al. 2015). A host–bacteria interaction mechanism via metabolites was discovered from the study, providing one molecular explanation for acne pathogenesis. In the presence of externally available vitamin B12, P. acnes was shown to repress its own vitamin B12 biosynthesis and shunt the metabolic flow towards the production of porphyrins, a group of bacterial metabolites inducing inflammation in host tissues and leading to acne development (Kang et al. 2015). This suggests that the skin microbiota constantly senses the host metabolite level, reacts to its changes, and in turn plays a role in skin health or disease.
Bek‐Thomsen et al. performed a proteomic analysis of the host and bacterial proteins identified from the skin follicles of acne patients and healthy individuals (Bek‐Thomsen et al. 2014). Surface adhesion proteins, namely dermatan sulfate binding proteins and Christie‐Atkins‐Munch‐Petersen (CAMP) factors (CAMP1 and CAMP2), which have both been previously linked to the virulence property of P. acnes (Valanne et al. 2005; McDowell et al. 2011, 2013; Nakatsuji et al. 2011), were found more frequently in healthy skin than in acne‐affected skin (Bek‐Thomsen et al. 2014). While these data are seemingly contradictory to the association of these factors with diseased states, further investigations of the functions of these bacterial proteins are needed to fully understand the roles of the skin microorganisms in health and disease, and to determine whether these secreted molecules are essential to the microorganisms and/or are virulent to the host.
Proteomic analysis of the Malassezia secretome has also revealed the functional potential of the skin fungal community. Of the 14 lipases and 9 phospholipases encoded in the M. globosa genome, 13 and 6, respectively, are believed to be secreted (Xu et al. 2007). The clustering of the genes on the chromosome and the secretion of multiple gene products are thought to aid in host specificity and imply an efficient mechanism for nutrient biosynthesis in these microorganisms. Their full health benefit to the human skin yet remains to be elucidated.
The role of the skin microbiota in shaping skin functions
The functions of the human skin include insulation, sensation, thermoregulation, absorption and synthesis. Additionally, the skin plays a central role in immune defence, preventing infection and host damage. Keratinocytes, the cells that coat the outer skin layers, constantly monitor the skin surface to recognize foreign or pathogen‐associated molecular patterns (PAMPs), and in their presence, initiate an innate immune response via TLRs and Nod‐like receptors, resulting in the production and secretion of cytokines, chemokines and AMPs (Heath & Carbone, 2013).
The skin microbiota plays an important role in shaping host immunity and aiding in the stimulation of host immune responses to defend against the colonization of pathogenic microorganisms. Naik et al. compared germ‐free mice with mice raised under specific pathogen‐free (SPF) conditions to understand how the skin commensal microorganisms modulate host immunity (Naik et al. 2012). Compared to SPF mice, who exhibited diverse immune signalling, germ‐free mice had weakened skin immune responses, producing significantly lower levels of microbial‐derived signalling molecules, interferon‐γ (IFN‐γ) and interleukin‐17A (IL‐17A). Colonization of germ‐free mice by commensal S. epidermidis restored IL‐17A production on the skin (Naik et al. 2012). When exposed to the protozoan parasite Leishmania major, germ‐free mice had impaired immune responses, which were rescued by colonization with S. epidermidis on the skin. This further supports a role for commensal skin bacteria in promoting host immunity (Naik et al. 2012).
Wound healing is a critical process in skin barrier function. Upon skin injury, skin cells deploy a highly efficient wound healing process involving inflammation, tissue repair and scar formation (Singer & Clark, 1999). In contrast to the many health benefits offered by the skin microbiota, in damaged or broken skin, when the physical barrier function is compromised, the otherwise commensal microorganisms can often behave as opportunistic pathogens, hindering the processes of tissue formation and wound healing. Commensal species, such as P. acnes and S. epidermidis, can infect the open wound, tightly adhering to damaged and exposed tissue via biofilm formation (Fig. 2). Biofilms are complex microbial communities encased in an extracellular polymeric substance (EPS). This encasing facilitates microbe–microbe communications and leads to increased virulence and resistance to many antimicrobial agents. As a result, biofilm formation complicates the wound healing process (Black & Costerton, 2010; Percival et al. 2012; Bertesteanu et al. 2014). Foot ulcers are chronic wounds commonly found in diabetic patients. The observation of biofilm formation from otherwise non‐pathogenic skin microorganisms in diabetic foot ulcers has led to the concept of functional equivalent pathogroups, whereby alone these species are harmless but together they can elicit a virulent potential equivalent to that of a known pathogen (Dowd et al. 2008).
Figure 2. Schematic diagram of biofilm formation in impaired wound healing.
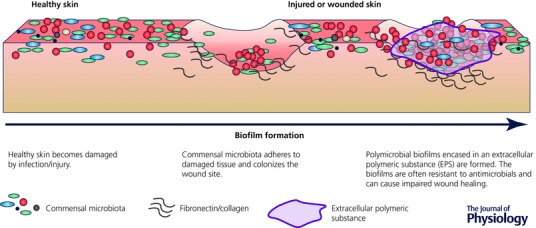
Commensal skin microorganisms colonize the wound site and adhere to exposed substratum tissues of the wound surface. Secretions of extracellular polymeric substances facilitate biofilm maturation, leading to the formation of polymicrobial biofilms.
In addition, microbial colonization of wounded sites can result in the release of microbial molecules that further damage the skin tissue, promoting chronic inflammation and delaying the healing process (Eming et al. 2007). Wound infection with the skin pathogen S. aureus has been shown to cause impaired healing due to the production of extracellular adherence protein (Eap). Eap is an anti‐inflammatory molecule that interferes with normal skin repair by reducing neutrophil and macrophage recruitment, and thus reducing inflammation, an important process in tissue repair (Athanasopoulos et al. 2006).
More recently, studies have revealed that wound healing is accelerated in the absence of the skin microbiota (Canesso et al. 2014). Skin wound healing was scarless in germ‐free mice, with reduced infiltration of neutrophils and inflammation, compared to conventionally raised mice (Canesso et al. 2014).
While evidence points towards the microbiota as a cause of delayed wound healing, the ability of commensal microbes to produce AMPs and bacteriocins to prevent pathogen colonization is central to reducing wound infection by pathogens. Recent advances in understanding the role of the microbiota in wound healing through mouse models (Grice et al. 2010; Canesso et al. 2014; Zhang et al. 2015) has opened up new avenues to further investigate the role of commensals in skin healing, and thus providing insight on host–microbial interactions in this essential skin process.
Exploiting microorganisms to enhance skin function in health
With the many health benefits conferred by commensal microorganisms, research has turned towards exploiting the properties of commensal skin microorganisms, such as those with potential probiotic properties, to manipulate the skin microbiota and enhance skin health. Examples include the topical application of the commensal skin bacterium Janthinobacterium lividum to treat athlete's foot, a common fungal skin infection, via the control of bacterial–fungal interactions (Ramsey et al. 2015). Ramsey et al. revealed that growth of the fungal species Trichophyton rubrum was inhibited by J. lividum. The in vitro and amphibian animal models used in the study warrant additional research to investigate the use of J. lividum as a probiotic treatment in humans (Ramsey et al. 2015).
Commensal skin microorganisms can be exploited to correct dysbiosis in the skin microbiota in diseases. S. epidermidis has been suggested as a probiotic in treating acne (Wang et al. 2014). While S. epidermidis and P. acnes naturally co‐exist on the skin, Wang et al. found that commensal S. epidermidis can inhibit the overgrowth of P. acnes, which has been linked to acne. On the other hand, the health‐association of certain P. acnes strains implies that supplementation with health‐associated strains may help to treat acne and to maintain skin health (Fitz‐Gibbon et al. 2013). While typical acne treatments include antibiotic administration, the extensive use of antibiotics has led to the emergence of antibiotic‐resistant strains and thus increased rate of treatment failure (Ross et al. 2003). Exploiting probiotic and prebiotic therapeutics will ultimately reduce the prevalence of antibiotic resistance in the population and potentially result in better treatment outcomes.
Additionally, non‐pathogenic microorganisms that are not usually part of the normal skin microbiota have been investigated for their potential applications in enhancing immune responses. Vitreoscilla filiformis, a Gram‐negative bacterium recognized by keratinocytes, can stimulate antioxidant and antimicrobial defence mechanisms via TLR‐2 signalling (Mahe et al. 2013; Volz et al. 2014). Application of topical V. filiformis to lesional skin significantly improved the skin condition in atopic dermatitis patients by inducing high levels of the anti‐inflammatory cytokine IL‐10 (Guéniche et al. 2008; Volz et al. 2014).
Conclusion
An increasing number of studies have shown that the human microbiome exhibits a high level of individuality (Schloss et al. 2014), and at the strain level it can be used as ‘individual fingerprints’ (Schloissnig et al. 2013). While we have gathered ample knowledge of the taxonomic composition of the skin microbiome at the phylum, genus and sometimes species level, our current understanding at the strain level is limited. A few studies have highlighted associations of specific strains of skin bacteria with disease pathogenesis, such as the increased prevalence of specific P. acnes lineages on acneic skin compared to healthy skin (Fitz‐Gibbon et al. 2013), and the increased prevalence of multi‐drug‐resistant S. epidermidis strains isolated from prosthetic joint infections (Hellmark et al. 2013). Therefore, strain‐level differentiation is important in defining the role of the resident microorganisms in skin health and disease. Recently, new methods have been developed to infer the strain‐level composition of a microbial community from metagenomic shotgun sequencing data, such as PathoScope and ConStrains (Francis et al. 2013; Luo et al. 2015). Improved methods for strain‐level identification and analysis will enable future studies to reveal the population structure and dynamics of the skin microbiome at the strain level and the complex interactions between strains, species, bacterial prey and viral predators, microbiota and human host. Strain‐level understanding of the microbiome will provide unprecedented insight into the role of the skin microbiota in health and disease.
The core skin microbiota consists of a number of key commensals, including species from Staphylococcus, Propionibacterium, Streptococcus and Corynebacterium, as well as fungi and viruses, which are dominant and prevalent among healthy individuals. Despite the health benefits that these key players confer, a number of studies have implicated a role for these same species in diseases, mainly due to frequent detection and isolation of these species at diseased sites (Marples et al. 1973; Gehse et al. 1983; O'Gara & Humphreys, 2001; Ramage et al. 2003; Jahns et al. 2012). With the dominance and prevalence of these organisms on the healthy skin, one must question if these skin microorganisms are truly representative of a diseased state, or if they are nothing more than normal constituents of the resident skin microbiota, innocent bystanders in skin disease and guilty by association. A key issue in determining a role for the skin microbiota in disease pathogenesis is to establish whether alterations in the healthy skin microbiota are a cause or consequence of the diseased state. Additionally, sample contamination due to the ubiquitous nature of skin microorganisms presents another challenge when defining a pathogenic role for skin commensals found outside their normal environment.
Factors influencing the role of commensal microorganisms in skin health and disease include changes in the environmental niche that they colonize or the host status. While these organisms are typically considered commensal, when they find residence outside of their preferred environmental niche or when opportunistic conditions are presented, they can often pose a pathogenic threat. Such examples include the cases of P. acnes and S. epidermidis found in medical device and implant infections (Tunney et al. 1998; Sampedro et al. 2009), and the high incidence of infection from common skin species, such as Malassezia, in immuno‐compromised patients (Tragiannidis et al. 2010). The microbial properties that allow these commensal microorganisms to benefit the host, for example biofilm formation or host‐adhesion mechanisms, are often the traits linking them with virulence in diseased states (O'Gara & Humphreys, 2001; Ramage et al. 2003; Jahns et al. 2012). Understanding their environmental niche and molecular mechanism in host interactions will provide significant insight in treating commensal‐associated infections and diseases.
Advances in modern technologies have allowed researchers to expand from the studies of individual microorganisms in human health and disease to investigations of the role of the microbial community as a whole in human physiology. Given the multitude and complexity of the microbiota residing on the human skin, investigating the molecular interactions between microbe and microbe and between microbe and host, in addition to taxonomic characterizations, will advance our knowledge of the role of the commensal skin microbiota in health and disease. Future functional studies of the skin microbiota at the metatranscriptomic, metaproteomic and metabolic levels are vital to our understanding of disease mechanisms involved with the microbiota and potential future manipulations of the microbiota in disease therapeutics and skin health maintenance.
Additional information
Competing interests
None declared.
Funding
This work is funded by NIH grant R01GM099530 from the National Institute of General Medical Sciences (NIGMS).
Biographies
Emma Barnard is a postdoctoral fellow at the David Geffen School of Medicine, University of California, Los Angeles. She obtained her PhD in molecular biology from the Queen's University of Belfast, Northern Ireland, UK. She currently conducts research to investigate the human skin microbiome and its interaction with the host in health and disease.

Huiying Li is an assistant professor in the Department of Molecular and Medical Pharmacology at the UCLA David Geffen School of Medicine. She received her PhD in biochemistry and molecular biology from the University of California, Los Angeles. Her main research interest is to understand the human microbiome in health and disease at both molecular and systems levels. Currently her research group studies the human skin microbiome and oral microbiome and their associations with diseases including acne, atopic dermatitis and periodontitis.
This is an Editor's Choice article from the 15 January 2017 issue.
References
- Alekseyenko AV, Perez‐Perez GI, De Souza A, Strober B, Gao Z, Bihan M, Li K, Methé BA & Blaser MJ (2013). Community differentiation of the cutaneous microbiota in psoriasis. Microbiome 1, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbee HR & Evans EG (2002). Immunology of diseases associated with Malassezia species. Clin Microbiol Rev 15, 21–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasopoulos AN, Economopoulou M, Orlova VV, Sobke A, Schneider D, Weber H, Augustin HG, Eming SA, Schubert U, Linn T, Nawroth PP, Hussain M, Hammes HP, Herrmann M, Preissner KT & Chavakis T (2006). The extracellular adherence protein (Eap) of Staphylococcus aureus inhibits wound healing by interfering with host defense and repair mechanisms. Blood 107, 2720–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos MC, Ceotto H, Coelho ML & Nascimento JS (2009). Staphylococcal antimicrobial peptides: relevant properties and potential biotechnological applications. Curr Pharm Biotechnol 10, 38–61. [DOI] [PubMed] [Google Scholar]
- Bek‐Thomsen M, Lomholt HB, Scavenius C, Enghild JJ & Brüggemann H (2014). Proteome analysis of human sebaceous follicle infundibula extracted from healthy and acne‐affected skin. PLoS One 9, e107908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertesteanu S, Triaridis S, Stankovic M, Lazar V, Chifiriuc MC, Vlad M & Grigore R (2014). Polymicrobial wound infections: pathophysiology and current therapeutic approaches. Int J Pharm 463, 119–126. [DOI] [PubMed] [Google Scholar]
- Black CE & Costerton JW (2010). Current concepts regarding the effect of wound microbial ecology and biofilms on wound healing. Surg Clin North Am 90, 1147–1160. [DOI] [PubMed] [Google Scholar]
- Bonnar E, Eustace P & Powell FC (1993). The Demodex mite population in rosacea. J Am Acad Dermatol 28, 443–448. [DOI] [PubMed] [Google Scholar]
- Brasch J & Christophers E (1993). Azelaic acid has antimycotic properties in vitro. Dermatology 186, 55–58. [DOI] [PubMed] [Google Scholar]
- Brüggemann H, Henne A, Hoster F, Liesegang H, Wiezer A, Strittmatter A, Hujer S, Dürre P & Gottschalk G (2004). The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science 305, 671–673. [DOI] [PubMed] [Google Scholar]
- Canesso MC, Vieira AT, Castro TB, Schirmer BG, Cisalpino D, Martins FS, Rachid MA, Nicoli JR, Teixeira MM & Barcelos LS (2014). Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J Immunol 193, 5171–5180. [DOI] [PubMed] [Google Scholar]
- Church D, Elsayed S, Reid O, Winston B & Lindsay R (2006). Burn wound infections. Clin Microbiol Rev 19, 403–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaud C, Jourdain R, Bar‐Hen A, Tichit M, Bouchier C, Pouradier F, El Rawadi C, Guillot J, Ménard‐Szczebara F, Breton L, Latgé JP & Mouyna I (2013). Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS One 8, e58203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates R, Moran J & Horsburgh MJ (2014). Staphylococci: colonizers and pathogens of human skin. Future Microbiol 9, 75–91. [DOI] [PubMed] [Google Scholar]
- Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, Torpey JW, Otto M, Nizet V, Kim JE & Gallo RL (2010). Selective antimicrobial action is provided by phenol‐soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol 130, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RJ, Shannon BA, McNeal JE, Shannon T & Garrett KL (2005). Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J Urol 173, 1969–1974. [DOI] [PubMed] [Google Scholar]
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI & Knight R (2009). Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosti C, Menni S, Sala F & Piccinno R (1983). Demodectic infestation of the pilosebaceous follicle. J Cutan Pathol 10, 257–261. [DOI] [PubMed] [Google Scholar]
- Dawson TL Jr (2007). Malassezia globosa and restricta: breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole‐genome analysis. J Investig Dermatol Symp Proc 12, 15–19. [DOI] [PubMed] [Google Scholar]
- Dekio I, Sakamoto M, Hayashi H, Amagai M, Suematsu M & Benno Y (2007). Characterization of skin microbiota in patients with atopic dermatitis and in normal subjects using 16S rRNA gene‐based comprehensive analysis. J Med Microbiol 56, 1675–1683. [DOI] [PubMed] [Google Scholar]
- Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E & Rhoads D (2008). Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS One 3, e3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eishi Y, Suga M, Ishige I, Kobayashi D, Yamada T, Takemura T, Takizawa T, Koike M, Kudoh S, Costabel U, Guzman J, Rizzato G, Gambacorta M, du Bois R, Nicholson AG, Sharma OP & Ando M (2002). Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol 40, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Krieg T & Davidson JM (2007). Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 127, 514–525. [DOI] [PubMed] [Google Scholar]
- Faye T, Holo H, Langsrud T, Nes IF & Brede DA (2011). The unconventional antimicrobial peptides of the classical propionibacteria. Appl Microbiol Biotechnol 89, 549–554. [DOI] [PubMed] [Google Scholar]
- Fierer N, Hamady M, Lauber CL & Knight R (2008). The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci USA 105, 17994–17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer N, Lauber CL, Zhou N, McDonald D, Costello EK & Knight R (2010). Forensic identification using skin bacterial communities. Proc Natl Acad Sci USA 107, 6477–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, Schoenfeld D, Nomicos E, Park M, NIH Intramural Sequencing Center Comparative Sequencing Program , Kong HH & Segre JA (2013). Topographic diversity of fungal and bacterial communities in human skin. Nature 498, 367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz‐Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, Elashoff D, Erfe MC, Loncaric A, Kim J, Modlin RL, Miller JF, Sodergren E, Craft N, Weinstock GM & Li H (2013). Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol 133, 2152–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores GE, Bates ST, Knights D, Lauber CL, Stombaugh J, Knight R & Fierer N (2011). Microbial biogeography of public restroom surfaces. PLoS One 6, e28132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana MB, de Bastos MOC & Brandelli A (2006). Bacteriocins Pep5 and epidermin inhibit Staphylococcus epidermidis adhesion to catheters. Curr Microbiol 52, 350–353. [DOI] [PubMed] [Google Scholar]
- Foulongne V, Sauvage V, Hebert C, Dereure O, Cheval J, Gouilh MA, Pariente K, Segondy M, Burguière A, Manuguerra JC, Caro V & Eloit M (2012). Human skin microbiota: high diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS One 7, e38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis OE, Bendall M, Manimaran S, Hong C, Clement NL, Castro‐Nallar E, Snell Q, Schaalje GB, Clement MJ, Crandall KA & Johnson WE (2013). Pathoscope: species identification and strain attribution with unassembled sequencing data. Genome Res 23, 1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura S & Nakamura T (1978). Purification and properties of a bacteriocin‐like substance (acnecin) of oral Propionibacterium acnes . Antimicrob Agents Chemother 14, 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitanis G, Magiatis P, Hantschke M, Bassukas ID & Velegraki A (2012). The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev 25, 106–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Tseng CH, Pei Z & Blaser MJ (2007). Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci USA 104, 2927–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Tseng CH, Strober BE, Pei Z & Blaser MJ (2008). Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS One 3, e2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehse M, Höffler U, Gloor M & Pulverer G (1983). Propionibacteria in patients with acne vulgaris and in healthy persons. Arch Dermatol Res 275, 100–104. [DOI] [PubMed] [Google Scholar]
- Gontcharova V, Youn E, Sun Y, Wolcott RD & Dowd SE (2010). A comparison of bacterial composition in diabetic ulcers and contralateral intact skin. Open Microbiol J 4, 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guéniche A, Knaudt B, Schuck E, Volz T, Bastien P, Martin R, Röcken M, Breton L & Biedermann T (2008). Effects of nonpathogenic gram‐negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: a prospective, randomized, double‐blind, placebo‐controlled clinical study. Br J Dermatol 159, 1357–1363. [DOI] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, NISC Comparative SequencingProgram , Bouffard GG, Blakesley RW, Murray PR, Green ED, Turner ML & Segre JA (2009). Topographical and temporal diversity of the human skin microbiome. Science 324, 1190–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA & Segre JA (2011). The skin microbiome. Nat Rev Microbiol 9, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Snitkin ES, Yockey LJ, Bermudez DM, NISC Comparative Sequencing Program , Liechty KW, Segre JA et al (2010). Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc Natl Acad Sci USA 107, 14799–14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambraeus A (1973). Dispersal and transfer of Staphylococcus aureus in an isolation ward for burned patients. J Hyg (Lond) 71, 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger M Jr (1947). Transfer of beta hemolytic streptococci by shaking hands. Am J Med 2, 23–25. [DOI] [PubMed] [Google Scholar]
- Heath WR & Carbone FR (2013). The skin‐resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol 14, 978–985.24048119 [Google Scholar]
- Hellmark B, Söderquist B, Unemo M & Nilsdotter‐Augustinsson Å (2013). Comparison of Staphylococcus epidermidis isolated from prosthetic joint infections and commensal isolates in regard to antibiotic susceptibility, agr type, biofilm production, and epidemiology. Int J Med Microbiol 303, 32–39. [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium et al (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahns AC, Lundskog B, Ganceviciene R, Palmer RH, Golovleva I, Zouboulis CC, McDowell A, Patrick S & Alexeyev OA (2012). An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case–control study. Br J Dermatol 167, 50–58. [DOI] [PubMed] [Google Scholar]
- Javey G, Albini TA & Flynn HW Jr (2010). Resolution of pigmented keratic precipitates following treatment of pseudophakic endophthalmitis caused by Propionibacterium acnes . Ophthalmic Surg Lasers Imaging, 1–3. [DOI] [PubMed] [Google Scholar]
- Kang D, Shi B, Erfe MC, Craft N & Li H (2015). Vitamin B12 modulates the transcriptome of the skin microbiota in acne pathogenesis. Sci Transl Med 7, 293ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasimatis G, Fitz‐Gibbon S, Tomida S, Wong M & Li H (2013). Analysis of complete genomes of Propionibacterium acnes reveals a novel plasmid and increased pseudogenes in an acne associated strain. Biomed Res Int 2013, 918320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M, Scholz CF & Lomholt HB (2012). Multilocus sequence typing and phylogenetic analysis of Propionibacterium acnes . J Clin Microbiol 50, 1158–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman AM & Christensen MS (2011). Demodex folliculorum: requirements for understanding its role in human skin disease. J Invest Dermatol 131, 8–10. [DOI] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, NISC Comparative Sequence Program , Murray PR, Turner ML & Segre JA et al (2012). Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22, 850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, Ryan AF, Di Nardo A & Gallo RL (2010). Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol 130, 2211–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E (2001). Hygiene of the skin: when is clean too clean? Emerg Infect Dis 7, 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax S, Smith DP, Hampton‐Marcell J, Owens SM, Handley KM, Scott NM, Gibbons SM, Larsen P, Shogan BD, Weiss S, Metcalf JL, Ursell LK, Vázquez‐Baeza Y, Van Treuren W, Hasan NA, Gibson MK, Colwell R, Dantas G, Knight R & Gilbert JA (2014). Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 345, 1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeming JP, Holland KT & Bojar RA (1986). The in vitro antimicrobial effect of azelaic acid. Br J Dermatol 115, 551–556. [DOI] [PubMed] [Google Scholar]
- Li M, Lai Y, Villaruz AE, Cha DJ, Sturdevant DE & Otto M (2007). Gram‐positive three‐component antimicrobial peptide‐sensing system. Proc Natl Acad Sci USA 104, 9469–9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yan R, Zhong Q, Ngo S, Bangayan NJ, Nguyen L, Lui T, Liu M, Erfe MC, Craft N, Tomida S & Li H (2015). The diversity and host interactions of Propionibacterium acnes bacteriophages on human skin. ISME J 9, 2078–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomholt HB & Kilian M (2010). Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS One 5, e12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Knight R, Siljander H, Knip M, Xavier RJ & Gevers D (2015). ConStrains identifies microbial strains in metagenomic datasets. Nat Biotechnol 33, 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Madupu R, Karaoz U, Nossa CW, Yang L, Yooseph S, Yachimski PS, Brodie EL, Nelson KE & Pei Z (2014). Human papillomavirus community in healthy persons, defined by metagenomics analysis of human microbiome project shotgun sequencing data sets. J Virol 88, 4786–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell A, Gao A, Barnard E, Fink C, Murray PI, Dowson CG, Nagy I, Lambert PA & Patrick S (2011). A novel multilocus sequence typing scheme for the opportunistic pathogen Propionibacterium acnes and characterization of type I cell surface‐associated antigens. Microbiology 157, 1990–2003. [DOI] [PubMed] [Google Scholar]
- McDowell A, Nagy I, Magyari M, Barnard E & Patrick S (2013). The opportunistic pathogen Propionibacterium acnes: insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS One 8, e70897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell A, Perry AL, Lambert PA & Patrick S (2008). A new phylogenetic group of Propionibacterium acnes . J Med Microbiol 57, 218–224. [DOI] [PubMed] [Google Scholar]
- McDowell A, Valanne S, Ramage G, Tunney MM, Glenn JV, McLorinan GC, Bhatia A, Maisonneuve JF, Lodes M, Persing DH & Patrick S (2005). Propionibacterium acnes types I and II represent phylogenetically distinct groups. J Clin Microbiol 43, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison KC (2003). Barrier function of the skin: ‘la raison d'être’ of the epidermis. J Invest Dermatol 121, 231–241. [DOI] [PubMed] [Google Scholar]
- Mahe YF, Perez MJ, Tacheau C, Fanchon C, Martin R, Rousset F & Seite S (2013). A new Vitreoscilla filiformis extract grown on spa water‐enriched medium activates endogenous cutaneous antioxidant and antimicrobial defenses through a potential Toll‐like receptor 2/protein kinase C, zeta transduction pathway. Clin Cosmet Investig Dermatol 6, 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marples RR, McGinley KJ & Mills OH (1973). Microbiology of comedones in acne vulgaris. J Invest Dermatol 60, 80–83. [DOI] [PubMed] [Google Scholar]
- Mathieu A, Delmont TO, Vogel TM, Robe P, Nalin R & Simonet P (2013). Life on human surfaces: skin metagenomics. PLoS One 8, e65288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow JF, Altrichter AE & Green JL (2014a). Mobile phones carry the personal microbiome of their owners. PeerJ 2, e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow JF, Altrichter AE, Kembel SW, Moriyama M, O'Connor TK, Womack AM, Brown GZ, Green JL & Bohannan BJ (2014b). Bacterial communities on classroom surfaces vary with human contact. Microbiome 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow JF, Bateman AC, Herkert KM, O'Connor TK & Green JL (2013). Significant changes in the skin microbiome mediated by the sport of roller derby. PeerJ 1, e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes JJ, Leandro C, Corte‐Real S, Barbosa R, Cavaco‐Silva P, Melo‐Cristino J, Górski A & Garcia M (2013). Wound healing potential of topical bacteriophage therapy on diabetic cutaneous wounds. Wound Repair Regen 21, 595–603. [DOI] [PubMed] [Google Scholar]
- Misic AM, Gardner SE & Grice EA (2014). The wound microbiome: modern approaches to examining the role of microorganisms in impaired chronic wound healing. Adv Wound Care (New Rochelle) 3, 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo N, Aubert J & Raoult D (2014). Microbiota of Demodex mites from rosacea patients and controls. Microb Pathog 71–72, 37–40. [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA & Belkaid Y (2012). Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Tang DC, Zhang L, Gallo RL & Huang CM (2011). Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: potential targets for inflammatory acne treatment. PLoS One 6, e14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzaro‐Porro M & Passi S (1978). Identification of tyrosinase inhibitors in cultures of Pityrosporum . J Invest Dermatol 71, 205–208. [DOI] [PubMed] [Google Scholar]
- Nobrega FL, Costa AR, Kluskens LD & Azeredo J (2015). Revisiting phage therapy: new applications for old resources. Trends Microbiol 23, 185–191. [DOI] [PubMed] [Google Scholar]
- O'Gara JP & Humphreys H (2001). Staphylococcus epidermidis biofilms: importance and implications. J Med Microbiol 50, 582–587. [DOI] [PubMed] [Google Scholar]
- Oh J, Byrd AL, Deming C, Conlan S, NISC Comparative Sequencing Program , Kong HH & Segre JA et al (2014). Biogeography and individuality shape function in the human skin metagenome. Nature 514, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Conlan S, Polley EC, Segre JA & Kong HH (2012). Shifts in human skin and nares microbiota of healthy children and adults. Genome Med 4, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulino LC, Tseng CH & Blaser MJ (2008). Analysis of Malassezia microbiota in healthy superficial human skin and in psoriatic lesions by multiplex real‐time PCR. FEMS Yeast Res 8, 460–471. [DOI] [PubMed] [Google Scholar]
- Paulino LC, Tseng CH, Strober BE & Blaser MJ (2006). Molecular analysis of fungal microbiota in samples from healthy human skin and psoriatic lesions. J Clin Microbiol 44, 2933–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival SL, Emanuel C, Cutting KF & Williams DW (2012). Microbiology of the skin and the role of biofilms in infection. Int Wound J 9, 14–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus NB, Reckhow JD, Saleem D, Jammeh ML, Datta SK & Myles IA (2015). Strain specific phage treatment for Staphylococcus aureus infection is influenced by host immunity and site of infection. PLoS One 10, e0124280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet D, Dharan S, Touveneau S, Sauvan V & Perneger TV (1999). Bacterial contamination of the hands of hospital staff during routine patient care. Arch Intern Med 159, 821–826. [DOI] [PubMed] [Google Scholar]
- Price LB, Liu CM, Melendez JH, Frankel YM, Engelthaler D, Aziz M, Bowers J, Rattray R, Ravel J, Kingsley C, Keim PS, Lazarus GS & Zenilman JM (2009). Community analysis of chronic wound bacteria using 16S rRNA gene‐based pyrosequencing: impact of diabetes and antibiotics on chronic wound microbiota. PLoS One 4, e6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, Tunney MM, Patrick S, Gorman SP & Nixon JR (2003). Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials 24, 3221–3227. [DOI] [PubMed] [Google Scholar]
- Ramsey JP, Mercurio A, Holland JA, Harris RN & Minbiole KP (2015). The cutaneous bacterium Janthinobacterium lividum inhibits the growth of Trichophyton rubrum in vitro . Int J Dermatol 54, 156–159. [DOI] [PubMed] [Google Scholar]
- Robson MC (1997). Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 77, 637–650. [DOI] [PubMed] [Google Scholar]
- Rogers KL, Fey PD & Rupp ME (2009). Coagulase‐negative staphylococcal infections. Infect Dis Clin North Am 23, 73–98. [DOI] [PubMed] [Google Scholar]
- Rollason J, McDowell A, Albert HB, Barnard E, Worthington T, Hilton AC, Vernallis A, Patrick S, Elliott T & Lambert P (2013). Genotypic and antimicrobial characterisation of Propionibacterium acnes isolates from surgically excised lumbar disc herniations. Biomed Res Int 2013, 530382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JI, Snelling AM, Carnegie E, Coates P, Cunliffe WJ, Bettoli V, Tosti G, Katsambas A, Galvan Peréz Del Pulgar JI, Rollman O, Török L, Eady EA & Cove JH (2003). Antibiotic‐resistant acne: lessons from Europe. Br J Dermatol 148, 467–478. [DOI] [PubMed] [Google Scholar]
- Sampedro MF, Piper KE, McDowell A, Patrick S, Mandrekar JN, Rouse MS, Steckelberg JM & Patel R (2009). Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Microbiol Infect Dis 64, 138–145. [DOI] [PubMed] [Google Scholar]
- Sanford JA & Gallo RL (2013). Functions of the skin microbiota in health and disease. Semin Immunol 25, 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeverbeke T, Lequen L, de Barbeyrac B, Labbé L, Bébéar CM, Morrier Y, Bannwarth B, Bébéar C & Dehais J (1998). Propionibacterium acnes isolated from synovial tissue and fluid in a patient with oligoarthritis associated with acne and pustulosis. Arthritis Rheum 41, 1889–1893. [DOI] [PubMed] [Google Scholar]
- Schloissnig S, Arumugam M, Sunagawa S, Mitreva M, Tap J, Zhu A, Waller A, Mende DR, Kultima JR, Martin J, Kota K, Sunyaev SR, Weinstock GM & Bork P (2013). Genomic variation landscape of the human gut microbiome. Nature 493, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Iverson KD, Petrosino JF & Schloss SJ (2014). The dynamics of a family's gut microbiota reveal variations on a theme. Microbiome 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid‐Wendtner MH & Korting HC (2006). The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol 19, 296–302. [DOI] [PubMed] [Google Scholar]
- Selander C, Zargari A, Möllby R, Rasool O & Scheynius A (2006). Higher pH level, corresponding to that on the skin of patients with atopic eczema, stimulates the release of Malassezia sympodialis allergens. Allergy 61, 1002–1008. [DOI] [PubMed] [Google Scholar]
- Shu M, Wang Y, Yu J, Kuo S, Coda A, Jiang Y, Gallo RL & Huang CM (2013). Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin‐resistant Staphylococcus aureus . PLoS One 8, e55380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ & Clark RA (1999). Cutaneous wound healing. N Engl J Med 341, 738–746. [DOI] [PubMed] [Google Scholar]
- Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg‐Lyons D, Caporaso JG, Knights D, Clemente JC, Nakielny S, Gordon JI, Fierer N & Knight R (2013). Cohabiting family members share microbiota with one another and with their dogs. Elife 2, e00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soothill JS (1994). Bacteriophage prevents destruction of skin grafts by Pseudomonas aeruginosa . Burns 20, 209–211. [DOI] [PubMed] [Google Scholar]
- Statnikov A, Alekseyenko AV, Li Z, Henaff M, Perez‐Perez GI, Blaser MJ & Aliferis CF (2013). Microbiomic signatures of psoriasis: feasibility and methodology comparison. Sci Rep 3, 2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulakvelidze A, Alavidze Z & Morris JG (2001). Bacteriophage therapy. Antimicrob Agents Chemother 45, 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida S, Nguyen L, Chiu BH, Liu J, Sodergren E, Weinstock GM & Li H (2013). Pan‐genome and comparative genome analyses of Propionibacterium acnes reveal its genomic diversity in the healthy and diseased human skin microbiome. MBio 4, e00003–00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tragiannidis A, Bisping G, Koehler G & Groll AH (2010). Minireview: Malassezia infections in immunocompromised patients. Mycoses 53, 187–195. [DOI] [PubMed] [Google Scholar]
- Tunney MM, Patrick S, Gorman SP, Nixon JR, Anderson N, Davis RI, Hanna D & Ramage G (1998). Improved detection of infection in hip replacements. A currently underestimated problem. J Bone Joint Surg Br 80, 568–572. [DOI] [PubMed] [Google Scholar]
- Ushijima T, Takahashi M & Ozaki Y (1984). Acetic, propionic, and oleic acid as the possible factors influencing the predominant residence of some species of Propionibacterium and coagulase‐negative Staphylococcus on normal human skin. Can J Microbiol 30, 647–652. [DOI] [PubMed] [Google Scholar]
- Valanne S, McDowell A, Ramage G, Tunney MM, Einarsson GG, O'Hagan S, Wisdom GB, Fairley D, Bhatia A, Maisonneuve JF, Lodes M, Persing DH & Patrick S (2005). CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiology 151, 1369–1379. [DOI] [PubMed] [Google Scholar]
- Vieira A, Silva YJ, Cunha A, Gomes NC, Ackermann HW & Almeida A (2012). Phage therapy to control multidrug‐resistant Pseudomonas aeruginosa skin infections: in vitro and ex vivo experiments. Eur J Clin Microbiol Infect Dis 31, 3241–3249. [DOI] [PubMed] [Google Scholar]
- Volz T, Skabytska Y, Guenova E, Chen KM, Frick JS, Kirschning CJ, Kaesler S, Röcken M & Biedermann T (2014). Nonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL‐10‐producing dendritic cells and regulatory Tr1 cells. J Invest Dermatol 134, 96–104. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kuo S, Shu M, Yu J, Huang S, Dai A, Two A, Gallo RL & Huang CM (2014). Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol 98, 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke I, Steffen H, Christ C, Krismer B, Götz F, Peschel A, Schaller M & Schittek B (2011). Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J Invest Dermatol 131, 382–390. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP & Edmond MB (2004). Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39, 309–317. [DOI] [PubMed] [Google Scholar]
- Wood M, Gibbons SM, Lax S, Eshoo‐Anton TW, Owens SM, Kennedy S, Gilbert JA & Hampton‐Marcell JT (2015). Athletic equipment microbiota are shaped by interactions with human skin. Microbiome 3, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie KM, Mihindukulasuriya KA, Zhou Y, Sodergren E, Storch GA & Weinstock GM (2014). Metagenomic analysis of double‐stranded DNA viruses in healthy adults. BMC Biol 12, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Saunders CW, Hu P, Grant RA, Boekhout T, Kuramae EE, Kronstad JW, Deangelis YM, Reeder NL, Johnstone KR, Leland M, Fieno AM, Begley WM, Sun Y, Lacey MP, Chaudhary T, Keough T, Chu L, Sears R, Yuan B & Dawson TL (2007). Dandruff‐associated Malassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc Natl Acad Sci USA 104, 18730–18735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang E, Tanaka T, Tajima M, Tsuboi R, Nishikawa A & Sugita T (2011). Characterization of the skin fungal microbiota in patients with atopic dermatitis and in healthy subjects. Microbiol Immunol 55, 625–632. [DOI] [PubMed] [Google Scholar]
- Zhang M, Jiang Z, Li D, Jiang D, Wu Y, Ren H, Peng H & Lai Y (2015). Oral antibiotic treatment induces skin microbiota dysbiosis and influences wound healing. Microb Ecol 69, 415–421. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Ren SX, Li HL, Wang YX, Fu G, Yang J, Qin ZQ, Miao YG, Wang WY, Chen RS, Shen Y, Chen Z, Yuan ZH, Zhao GP, Qu D, Danchin A & Wen YM (2003). Genome‐based analysis of virulence genes in a non‐biofilm‐forming Staphylococcus epidermidis strain (ATCC 12228). Mol Microbiol 49, 1577–1593. [DOI] [PubMed] [Google Scholar]


