Abstract
Glia (from Greek γλοία meaning ‘glue’) pertains to non‐neuronal cells in the central (CNS) and peripheral nervous system (PNS) that nourish neurons and maintain homeostasis. In addition, glia are now increasingly appreciated as active regulators of numerous physiological processes initially considered exclusively under neuronal regulation. For instance, enteric glia, a collection of glial cells residing within the walls of the intestinal tract, regulate intestinal motility, a well‐characterized reflex controlled by enteric neurons. Enteric glia also interact with various non‐neuronal cell types in the gut wall such as enterocytes, enteroendocrine and immune cells and are therefore emerging as important local regulators of diverse gut functions. The intricate molecular mechanisms that govern glia‐mediated regulation are beginning to be discovered, but much remains unknown about the functions of enteric glia in health and disease. Here we present a current view of the enteric glia and their regulatory roles in gastrointestinal (GI) (patho)physiology; from GI motility and epithelial barrier function to enteric neuroinflammation.
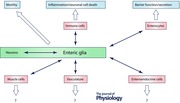
Introduction
At its heart, the discipline of physiology aims to understand processes that govern homeostasis (Michael et al. 2009). In this regard, glia can be considered the seat of nervous system physiology. Indeed, we are now well aware of many of the essential roles glia play in the maintenance of homeostasis within the central nervous system (CNS) and some of the potentially catastrophic effects if these functions are perturbed. Astroglia, in particular, are essential for the regulation of neuronal microenvironments and neural network functions (reviewed in Parpura et al. 2012). Similar types of glial cells are associated with neurons in peripheral neural networks, but the roles of these glial cells in the regulation of homeostasis outside the brain are much less defined.
The largest collection of glia outside the brain and spinal cord is housed within the walls of the intestines in the enteric nervous system (ENS). The ENS is the largest division of the autonomic nervous system (ANS) and consists of approximately 100 million enteric neurons that are surrounded by 1–7 times as many glial cells depending on the species (reviewed in Gulbransen, 2014). Enteric neurons and glia are housed in two major ganglionated plexuses in the gut wall (Fig. 1) and the integrative circuitry within these networks is sufficient to control reflex behaviours of the intestine such as peristalsis (Bayliss & Starling, 1900), fluid exchange across the mucosal surface and the regulation of local blood flow.
Figure 1. Schematic depiction of the intestine showing the general arrangement of the enteric nervous system in the gut wall.
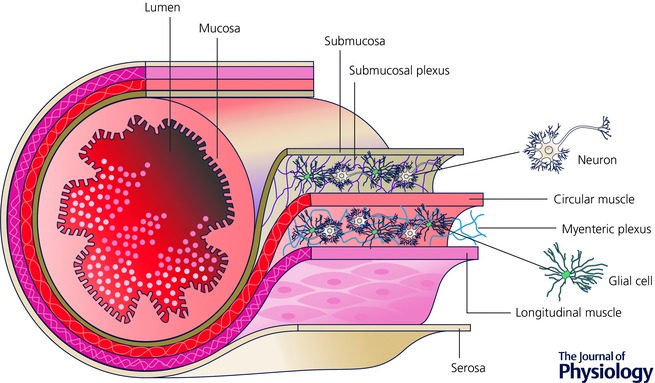
Enteric neurons and glia are housed within the submucosal and myenteric plexuses. Neural programmes in the submucosal plexus regulate fluid exchange across the intestinal mucosa and neural programmes in the myenteric plexus coordinate the contractile activity of the intestine. Image courtesy of David E. Fried.
The basic neural circuitry underlying intestinal reflexes is now relatively well understood and many of the key neurotransmitters are known (Furness, 2012). Yet, new observations suggest that this level of understanding of ENS function is grossly inadequate to understand the control of intestinal reflexes. Indeed, a growing body of work is currently modifying old neurocentric models to include an unexpected layer of complexity provided by glial cells. The picture that is emerging is one where glial cells have a strong influence on the physiological control of gut functions at multiple levels (see recent reviews by Neunlist et al. 2014; Coelho‐Aguiar Jde et al. 2015; Sharkey, 2015). This is an intriguing concept but many important questions remain unanswered about basic glial functions in the gut. In this review, we focus on our current understanding of how enteric glia participate in the regulation of intestinal homeostasis and discuss some of the important unanswered questions in the field. We examine recent studies that probe enteric glial cell identity and discuss novel findings that demonstrate active roles of enteric glia in the regulation of gut motility reflexes, barrier function and inflammation.
What are enteric glia?
Enteric glia are a large population of non‐myelinating peripheral glial cells that derive from neural crest precursors that colonize the intestinal tract between embryonic (E) days 9 and 13.5 in mice (Rothman et al. 1986; Kapur et al. 1992). Precursor cells begin to commit to a glial fate near E11.5 and cells expressing markers of terminally differentiated glia such as the calcium‐binding protein S100β and the intermediate filament glial fibrillary acidic protein (GFAP) are present by E14.5–16 (Fig. 2). Mature enteric glial cells display a strong morphological resemblance to astrocytes (Reichenbach et al. 1992) and express similar molecular markers such as the astrocyte‐associated determinant of GFAP (Jessen et al. 1983), vimentin (Jessen & Mirsky, 1983), connexin‐43 (McClain et al. 2014) and S100β (Ferri et al. 1982). No unique enteric glial marker has been identified, but the unique compilation of characteristics displayed by enteric glia specifically set this class of glia apart from other classes of glia (Fig. 3). Indeed, not all astrocytic properties can be generalized to enteric glia because the two cell types are fundamentally different. For example, enteric glia require neuregulin signalling through the ErbB3 receptor for development while astrocytes do not (Riethmacher et al. 1997). Likewise, enteric glia lack expression of some key astrocytic proteins such as aldehyde dehydrogenase 1 family member L1 (Aldh1L1) (Boesmans et al. 2014) and express non‐astrocytic molecules like Sox10 (Young et al. 2002), a transcription factor more common in oligodendrocytes. Indeed, the transcriptional profile of enteric glia shows significant overlap with oligodendrocytes, astrocytes and even neurons of the CNS (Fig. 3 A; Rao et al. 2015). This finding may partly illuminate the remarkable plasticity of enteric glial cells. For example, enteric glia are capable of forming enteric neurons in vitro (Joseph et al. 2011) or performing the functions of oligodendrocytes and astrocytes when transplanted into the CNS (Jiang et al. 2003, 2005). However, enteric glia are mainly restricted to a glial fate in their native intestinal environment (Joseph et al. 2011) and only form neurons under very rare circumstances (Laranjeira et al. 2011). These studies clearly show that the fate of the enteric glia is heavily influenced by external signals. Yet the specific conditions and factors that drive the heterogeneity of enteric glia are still poorly understood. A deeper understanding of these transformative factors holds great promise for the development of novel therapies for many diseases by harnessing the plastic capabilities of enteric glia.
Figure 2. Enteric glial cells derive from neural crest precursors and mature into neuroglia in the enteric nervous system.
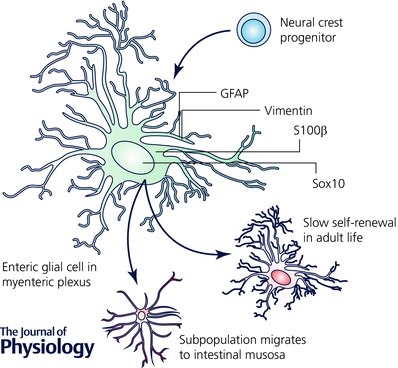
Enteric glia within the myenteric plexus are slowly replaced under physiological conditions (Joseph et al. 2011; Laranjeira et al. 2011) and are responsible for generating glia that migrate to the intestinal mucosa (Kabouridis et al. 2015).
Figure 3. Gene expression in enteric glia.
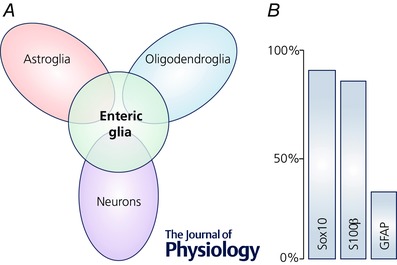
A, transcriptional profile of enteric glia compared with the profiles of neurons and glia from the CNS (Rao et al. 2015). Not drawn to scale. B, expression of common markers for enteric glia estimated from Boesmans et al. (2015); co‐localization among the glial markers omitted for clarity.
Beyond inter‐glial expression differences, new data indicate significant intra‐glial variability in the expression levels of key markers such as GFAP, S100β and Sox10 (Fig. 3 B; Boesmans et al. 2015). The significance of this variability within enteric glia is not currently understood and at this point, there is no consensus on what the ‘best’ enteric glial cell marker might be. In light of these discoveries, future studies should consider the following points. (i) Current markers may not be pan‐enteric glial at any given time. Sox10 and proteolipid protein 1 (PLP1) are the closest pan‐enteric glia markers and even these are not entirely reliable (Boesmans et al. 2015; Rao et al. 2015). (ii) Current ‘enteric glial‐specific’ markers are not confined to enteric glia. Sox10 and GFAP are widely expressed by other glia, as mentioned above, and other non‐glial cells such as melanocytes (Potterf et al. 2001) and hepatic stellate cells (Gard et al. 1985), respectively. Likewise, S100β is expressed in subpopulations of neurons in the CNS (Vives et al. 2003). This is a significant problem because it confounds the interpretation of experiments that aim to understand the integrative functions of enteric glia by selectively modulating their functions in vivo. This problem is made even more challenging by the recent discovery of intramucosal neuroglial cells that express both neuronal and glial markers (Badizadegan et al. 2014). (iii) The expression of various markers by enteric glia is a dynamic process that reflects many changes in glial maturity and phenotype. For example, expression of GFAP appears to reflect both enteric glial cell maturity and glial cell reactivity as a response to inflammatory stimuli (von Boyen et al. 2004). Clearly, understanding these processes will be an important aspect when both planning and interpreting future experiments.
Enteric glial cell numbers and their morpho‐functional characteristics also vary widely depending on their location in the GI tract, age, sex and species (Table 1). This is an important consideration while comparing findings from multiple studies and interpolating results from animal models to human physiology. For instance, the glia index (glia‐to‐neuron ratio) in the human intestine is approximately sevenfold greater than in the mouse intestine (Gabella & Trigg, 1984; Hoff et al. 2008). This potentially indicates a more prominent role of glia in the human intestine than in rodents, but this concept is still theoretical and direct experimental confirmation is still lacking. Additionally, enteric glia may exhibit differences in their expression of particular receptor subtypes (Table 2) and signal transduction cascades in different species and regions of the gut. Some signal pathways seem well conserved, but it is unknown how similar human and murine enteric glia actually are. Future efforts, therefore, should be directed towards closing the gap in knowledge between human enteric glia and those in experimental animals.
Table 1.
Variability in numbers and morpho‐functional characteristics of enteric glial cells
| Variables | Description | References | ||
|---|---|---|---|---|
| Species | ↑ Glia index in larger species (1 in mouse and 7 in human MP) | (Gabella & Trigg, 1984; Hoff et al. 2008) | ||
| Sex | ↑ Glia index in males (human ileum SMP) | (Hoff et al. 2008) | ||
| ↑ GFAP expression in females (mouse MP) | Unpublished* | |||
| Age | ↓ Number of Sox10 expressing cells with age (mouse MP) | (Stenkamp‐Strahm et al. 2013) | ||
| ↑ Glial density with age (human ileum/ sigmoid colon MP) | (Hoff et al. 2008) | |||
| ↑ transcription of Cx43 | (McClain et al. 2014) | |||
| Location along the gut length | ↑ Glial density in ileum (guinea pig MP, interganglionc area) | (Hoff et al. 2008) | ||
| Location within the gut wall | ↑ Glia index in MP than in SMP (mouse, guinea pig, rabbit, sheep, | (Gabella & Trigg, 1984; | ||
| human) | Hoff et al. 2008) | |||
| Four types | Within SMP/MP | I – protoplasmic (intraganglionic) | (Gulbransen & Sharkey, 2012; Boesmans et al. 2015) | |
| II – fibrous (interganglionic) | ||||
| Extraganglionic | IIIMUCOSA – mucosal | |||
| IIISMP/MP – at the level of ganglia | ||||
| IV – intramuscular | ||||
Glia index, number of glial cells per neuron. MP, myenteric plexus. SMP, submucosal plexus. *Courtesy of Ninotchska Del Valle Dorta (Gulbransen lab).
Table 2.
Neurotransmitters that (could) activate enteric glia
| Neurotransmitter | Receptor (subunit or substrate) | Method | Source | References |
|---|---|---|---|---|
| Acetylcholine | Muscarinic* | fluo4 | CRL‐2690 cell line and human duodenal SMP in vitro | (Boesmans et al. 2013) |
| Nicotinic* | fluo4 | Mouse colon MP in situ | (Broadhead et al. 2012) | |
| α3‐Nicotinic | IHC | Mouse colon MP in situ | (MacEachern et al. 2011) | |
| Catecholamines | α2a‐Adrenergic receptor | IHC | Rat ileum/colon MP in situ | (Nasser et al. 2006b) |
| Glutamate | mGluR5 | IHC | Guinea pig ileum/colon SMP/MP in situ | (Nasser et al. 2007) |
| Mouse ileum/colon SMP/MP in situ | ||||
| Rat ileum/colon SMP/MP in situ | ||||
| AMPA (GluR1 and GluR3) | ICC | Rat small intestine MP in vitro | (von Boyen et al. 2006) | |
| KA (GluR5) | ||||
| NMDA (NR2A/B) | ||||
| NMDA (NR1) | IHC | Human colon MP in situ | (Giaroni et al. 2003) | |
| Purinergic | A2B (adenosine) | IHC | Rat ileum MP in situ | (Vieira et al. 2011) |
| Human jejunum/colon in situ | (Christofi et al. 2001) | |||
| P2Y1 (ADP) | fluo4 | Mouse colon MP in situ | (McClain et al. 2014) | |
| fluo4 | Guinea pig colon MP in situ | (Gulbransen & Sharkey, 2009) | ||
| fluo4 | Mouse embryonic cultures in vitro | (Gulbransen et al. 2012) | ||
| P2Y4 (ATP, UTP) | IHC/fluo4 | Mouse/guinea pig colon MP in situ | (Gulbransen & Sharkey, 2009) | |
| IHC | Guinea pig ileum/colon SMP in situ | (Van Nassauw et al. 2006) | ||
| ICC/fura2 | Guinea pig colon MP in vitro | (Kimball & Mulholland, 1996) | ||
| Serotonin | Not tested | fura2 | Guinea pig MP in vitro | (Kimball & Mulholland, 1996) |
| 5‐HT2 * | fluo4 | CRL‐2690 cell line and human duodenal SMP in vitro | (Boesmans et al. 2013) |
AMPA, α‐amino‐3‐hydroxy‐5‐methylisoxazole‐4‐propionate; ATP, adenosine triphosphate; ICC, immunocytochemistry; IHC, immunohistochemistry; KA, kainate; metabotropic glutamate receptor; MP, myenteric plexus; NMDA, N‐methyl‐d‐aspartate; SMP, submucosal plexus. *Receptor determined only by pharmacological inhibition and not confirmed by ICC/IHC.
Neuron–glia crosstalk and the regulation of gut motility
Until recently, neurons were considered the only active cells in the ENS. Consequently, the major part of our understanding of the enteric reflexes that underlie gut motor activity is extremely neurocentric. However, mounting evidence over the past decade shows that enteric glia play an active role in enteric neural circuits that control motility (Gulbransen & Sharkey, 2009; Broadhead et al. 2012) and that manipulating enteric glia can have profound effects on gut functions (Aube et al. 2006; Nasser et al. 2006a). We are now well aware of the fact that glia are also excitable cells. Like astrocytes, enteric glial excitability is mainly encoded by transient elevations of intracellular calcium concentration ([Ca2+]i) and a number of studies have shown that glial activity is recruited by neurotransmitters/neuromodulators released during synaptic communication (Table 2). Importantly, Broadhead et al. (2012) demonstrated that these glial [Ca2+]i transients are entrained with endogenous neuronal reflexes that underlie peristalsis (Broadhead et al. 2012). Yet the significance of neural recruitment of enteric glial activity has remained enigmatic. Enteric glia are clearly capable of ‘listening’ to enteric neurons (Table 2), but if and how they ‘talk back’ is only beginning to come to light. Two studies from our own laboratory provide the first hints that the activation of enteric glia is an important modulator of enteric reflexes. First, we found that agonist‐evoked [Ca2+]i responses in enteric glia lead to the opening of glial connexin‐43 (Cx43) hemichannels (Fig. 4) and that the selective ablation of Cx43 in GFAP‐expressing enteric glia limits the propagation of [Ca2+]i responses through the glial network (McClain et al. 2014). Importantly, we found that impairing the activity of glial cells in vivo disrupts the neural control of gut motility and produces constipation in mice (Fig. 5 A–C). Based on these data, it is tempting to hypothesize that the mechanisms enacted by [Ca2+]i responses in enteric glia function to regulate the activity of enteric neural networks. We recently tested this hypothesis using GFAP::hM3Dq transgenic ‘DREADD’ (designer receptors exclusively activated by designer drugs) mice to selectively trigger Gq‐G protein‐coupled receptor (GPCR)‐dependent [Ca2+]i responses in GFAP‐expressing enteric glia (McClain et al. 2015). Our results show that enteric glia exert a surprisingly robust and selective influence on neuronal circuits in the gut. Perhaps the most surprising finding in this study was that the activation of glial [Ca2+]i responses alone was sufficient to drive intestinal contractility (Fig 5 D–F). Glial‐driven contractions were entirely tetrodotoxin‐sensitive so presumably the effects of glial activation were mediated through direct actions on neurons. Importantly, glial cell activation had no effect on neurogenic relaxations in the intestine. This is important because it suggests that gliotransmission in the intestine is highly specific.
Figure 4. Enteric glia actively participate in purinergic neuron–glia signalling.
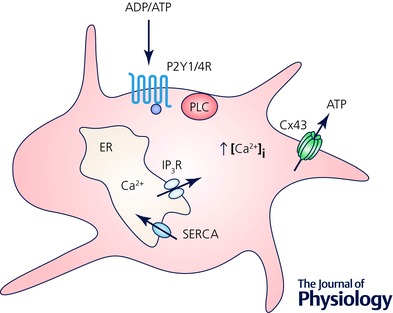
ADP and ATP bind to G‐protein coupled purinergic receptors P2Y1R and P2Y4R, respectively, and activate phospholipase C (PLC) and subsequent production of inositol 1,4,5‐trisphosphate (IP3). This consequently activates IP3 receptors (IP3R) inducing the release of Ca2+ from endoplasmic reticulum (ER). Increase in the intracellular Ca2+ concentration [Ca2+]i induces ATP release through Cx43 hemichannels. Sarco/endoplasmic reticulum Ca2+‐ATPase (SERCA) pumps Ca2+ ions back into the ER. Based from original research on enteric glial cells (Kimball & Mulholland 1996; Zhang et al. 2003; McClain et al. 2014). Not drawn to scale.
Figure 5. Enteric glia actively regulate gut motility.
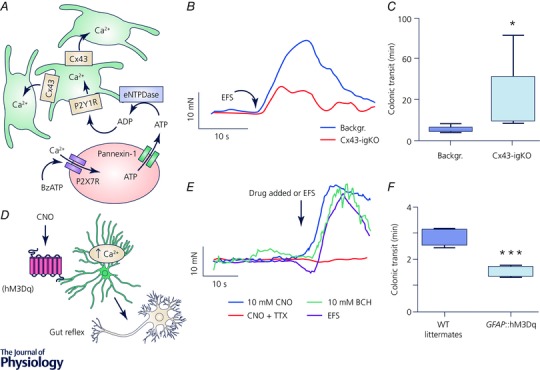
Inhibition (A–C) or activation (D–F) of glial calcium (Ca2+) signalling (A and D) results in reduction or stimulation of the gut motor reflex assessed by smooth muscle tension recordings (B and E), respectively, and corresponds to changes in the distal colon motility tested in vivo (C and F). A–C, experiments from tamoxifen‐induced glia‐specific knock out (igKO) of connexin 43 (Cx43) mice (Cx43‐igKO) and the tamoxifen‐treated background strains (Backgr.); figures obtained from McClain et al. (2014). A, neuron‐specific stimulation activates Ca2+ responses in enteric glia and Cx43 is required for the propagation of the glial Ca2+ response (see original work for details). B, electrical field stimulation (EFS) elicits muscle contractions and the contraction force is reduced in the Cx43‐igKO mice. C, selective reduction of the Ca2+ response in the enteric glia reduces distal colon motility in vivo. D–F, experiments from GFAP::hM3Dq transgenic mice, where glial fibrillary acidic protein (GFAP) promoter drives expression of an engineered Gq‐coupled human M3 muscarinic receptor (hM3Dq) and their wild‐type (WT) littermates; figures obtained from McClain et al. (2015). D, enteric glia expressing hM3Dq respond to clozapine N‐oxide (CNO) with an increase in cytosolic Ca2+ and subsequently affect neurally controlled gut reflexes. E, glia‐specific stimulation with CNO evoked response in GFAP::hM3Dq mice similar to stimuli with bethanechol (BCH) and EFS that directly activate smooth muscle and enteric neurons, respectively. Note that CNO effect was blocked by tetrodotoxin (TTX) indicating that glia‐specific effects are mediated via enteric neurons. Also, CNO stimulation evoked no response in WT littermates (see original work). F, selective activation of glial Ca2+ signalling enhances in vivo motility of the distal colon.
The studies described above provide strong support for the notion that enteric glia actively participate in regulation of gut motility (Fig. 5). However, a great deal of work is still needed to dissect the exact mechanisms involved. One obvious question at this point is how glia excite neurons. Is this via gliotransmitter release? If so, what is the identity of the gliotransmitter(s), what are the release mechanisms and how does the transmitter exert a selective effect on excitatory circuits? Some data from our lab (Brown et al. 2015) and the work of others (Zhang et al. 2003) suggest that ATP fits the criteria for a candidate gliotransmitter in the ENS. Enteric glial cells release ATP through Cx43 hemichannels when stimulated (McClain et al. 2014; Brown et al. 2015), but whether glial ATP release is responsible for the observed excitatory effects in vivo is unclear. Likewise, it is not clear how Cx43‐dependent ATP release from glia could exert such specific effects on enteric circuits. Furthermore, it is still unknown whether enteric glia exhibit other modes of gliotransmission, such as Ca2+‐dependent exocytosis, a well‐studied process in astoglia of the CNS (Zorec et al. 2012). If they do, it will be important to determine how certain conditions favour and/or modulate any certain mode of gliotransmission.
Beyond understanding transmitters and release mechanisms, it is also important to understand how enteric glia process information. Glial information processing could occur at multiple levels including within single cells or within networks of glia. At the single cell level, the soma appears to be a centre of integration for [Ca2+]i transients generated in fine processes (Broadhead et al. 2012). However, significant integration also seems to occur directly in the processes prior to summation and propagation to the cell soma (Broadhead et al. 2012). [Ca2+]i responses in the soma recruit activity in the surrounding glia in the form of Ca2+ waves. This network level integration could be an extremely important aspect of GI physiology, but its significance remains relatively unclear. In our experiments, we find that reducing the propagation of Ca2+ waves through the glial network by ablating Cx43 blunts GI motility (McClain et al. 2014). However, it is unknown if this outcome reflects abnormalities in glia‐to‐neuron communication mediated by Cx43 hemichannels or altered glial network integration mediated by Cx43 hemichannels and gap junctions. In any case, changes that affect glial integration such as changes in glia numbers or their cellular Ca2+ handling could play major roles in GI dismotility disorders such as chronic constipation (Bassotti et al. 2013) and functional dyspepsia (Cirillo et al. 2015), and this will be an important point to address in future work.
One major deficiency in our current understanding of glial activity is in regard to the activation of intracellular signal transduction that does not involve Ca2+. The change in [Ca2+]i is currently the most studied mode of the glial activation simply due to the availability of Ca2+‐sensitive dyes and genetically encoded proteins. Glial Ca2+ dynamics are clearly important for normal gut physiology (Broadhead et al. 2012; McClain et al. 2014) and also play an important role in pathological processes (see below). Yet Ca2+ is only one second‐messenger and there are many signalling pathways in glial cells. For example, Christofi et al. (1993) showed that glia are the major contributors to cAMP formation in the myenteric plexus, but the significance of cAMP elevations in glia is not understood at all. Could glial cAMP signalling be of equal or greater importance than glial Ca2+ signalling? Questions such as this clearly need more attention and addressing other second messengers such as cGMP, an effector molecule of NO signalling (Denninger & Marletta, 1999), or cAMP is becoming more feasible with the use of readily available sensors (Nikolaev et al. 2006; Borner et al. 2011). As a final note, the glial influence on gut motility may extend well beyond their interactions with neurons. Indeed, enteric glia interact with many non‐neuronal cells that are important for peristalsis. For instance, glia‐derived ATP could also signal directly to interstitial cells of Cajal or to smooth muscle (Sanders, 2000). Furthermore, Bohorquez et al. (2014) recently described a novel relationship between enteric glia and enteroendocrine cells (Bohorquez et al. 2014). Such interactions with non‐neuronal cells have the potential for major effects on gut function. But to what extent these interactions influence intestinal reflexes is still unknown (Fig. 6).
Figure 6. Enteric glia as active players in the peristaltic reflex.
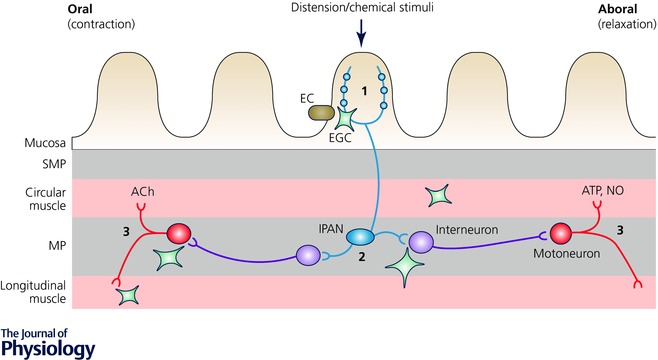
The accepted circuitry of the peristaltic reflex involves the following chain of events: (1) mechanical or chemical stimuli in the gut lumen activate intrinsic primary afferent neurons (IPANs) residing in both plexi; (2) IPANs activate interneurons that project in both oral (ascending) and aboral (descending) directions; (3) ascending interneurons activate excitatory motorneurons that cause smooth muscle contraction by releasing acetylcholine (ACh) and neuropeptides while descending interneurons produce relaxation below the point of stimulation by activating inhibitory motorneurons that release nitric oxide (NO), purines and other inhibitory molecules (Kunze & Furness, 1999). Enteric glia cells (EGC) could interact with the circuit at multiple levels (see text for details), from the release of serotonin from enterochromafine cells (EC) to the direct interaction with the smooth muscle cells. Other abbreviations: MP, myenteric plexus; SMP, submucosal plexus. This schematic representation is not drawn to scale.
Enteric glia at the mucosal interface
A growing number of studies strongly support the notion that enteric glia are important regulators of physiological processes in the gut mucosa. For example, mice with a targeted ablation of enteric glia exhibit a dramatic loss of epithelial barrier function (Bush et al. 1998; Cornet et al. 2001; Aube et al. 2006). Subsequent in vitro work has identified several enteric glial‐derived molecules that impact gut barrier function through direct actions on epithelial cells (Table 3). Based on these findings, Neunlist et al. (2013) recently coined the term ‘neuronal–glial–epithelial unit’ to describe the anatomical proximity and functional interaction between enteric glia and the intestinal epithelium. However, emerging data draw some aspects of the neuronal–glial–epithelial unit into question. For example, inhibition of glial functions with fluoroacetate, a glial cell metabolic toxin, had no effect on electrogenic ion transport under physiological conditions (MacEachern et al. 2015). A regulatory role of glial cells did emerge under pathological conditions during inflammation, but these findings suggest that glia do not play a major role in the regulation of secretomotor functions under normal circumstances. Likewise, mucosal glial cells are absent in both germ‐free mice and mice treated with antibiotics (Kabouridis et al. 2015). Yet secretomotor function and transepithelial resistance are preserved in germ‐free mice (Lomasney et al. 2014). In light of these new findings, it would seem that mucosal glia do not play an essential role in the regulation of epithelial barrier function in the short term.
Table 3.
Enteric glia cell (EGC)‐derived molecules that regulate intestinal epithelial function
| Molecule | Effect on GI epithelium | Source | References |
|---|---|---|---|
| TGF‐β1 | ↓ Proliferation | In vitro (Caco‐2 and primary EGCs, MP derived) | (Neunlist et al. 2007) |
| GSNO | ↓ Permeability | In vitro, in vivo, human tissue | (Savidge et al. 2007) |
| 15dPGJ2 | ↓ Proliferation | In vitro (Caco‐2 and primary EGCs, MP derived) | (Bach‐Ngohou et al. 2010) |
| GDNF* | ↓ Permeability | In vivo | (Zhang et al. 2010) |
| proEGF | ↑ Wound healing | In vitro (Caco‐2 and EGC lines) | (Van Landeghem et al. 2011) |
Abbreviations: 15dPGJ2, 15‐deoxy‐Δ12,14‐prostaglandin J2; Caco‐2, human epithelial colorectal adenocarcinoma cell line; GDNF, glial‐derived neurotrophic factor; GSNO, S‐nitrosoglutathione; proEGF, pro‐epidermal growth factor precursor; TGF‐β1, transforming growth factor β1. *Release of GDNF was not directly associated to EGCs.
Many of these discrepancies may be reconciled by considering the mechanisms and kinetics of the release of the proposed glial‐derived factors. For example, the tonic release of glial factors may play an important role in the maturation of the epithelium but not in the neurogenic regulation of secretomotor functions. In support, germ‐free mice do have less mucosal thickness in the absence of mucosal glia (Lomasney et al. 2014). Many of the studies that have identified glial mediators have used in vitro systems that study the interaction between Caco‐2 cells and cultured enteric glia or the supernatants from glial cultures. These conditions lack neuronal input and thus, any factors released by glia under these conditions do not require glial excitation or activity‐dependent processes. Some glia‐derived substances such as nitric oxide (NO) can freely diffuse across membranes but many others require regulated transport. For example, prostaglandin E2 (PGE2) and small peptides (≤10mer) could be released through Cx43 hemichannels (Jiang & Cherian, 2003; Neijssen et al. 2005) while larger proteins like fibroblast growth factor (FGF) and transforming growth factor β1 (TGF‐β1) may require Ca2+‐dependent exocytosis. One possibility is that glial mediators that act as trophic factors to support the growth and differentiation of enterocytes are constitutively released while the release of those that affect secretomotor functions are more tightly regulated and activity dependent. In support, cholinergic signalling in the ENS induces NO production in enteric glia that modulates the epithelial secretion (MacEachern et al. 2011). However, this is an indirect effect that is mediated by neuron–glia interactions within myenteric ganglia (MacEachern et al. 2011). Furthermore, recent evidence indicates that enteric glial‐derived NO contributes to epithelial barrier dysfunction in animal models of colitis (MacEachern et al. 2015). These studies highlight the need for a more comprehensive understating of glial inter‐ and intracellular signalling mechanisms to understand their roles in health and disease.
One very exciting aspect of glia in the intestinal mucosa is their potential for bi‐directional interactions with the gut microbiome (Liu et al. 2013). Whether glial cells directly influence the microbiome is not currently clear but new data suggest that the presence of gut bacteria has a major effect on the development of mucosal glia. These studies, performed by Kabouridis et al. (2015), show that mucosal glia are continuously replenished by precursor cells in the enteric plexuses and that the replenishment did not occur in antibiotic‐treated animals or germ‐free mice. These results indicate that cues from the gut microbiota are essential to promote the migration of glia from the plexuses into mucosa. Interactions between the microbiota and the immune system are implicated in this process (reviewed in Kabouridis & Pachnis, 2015) but the exact mechanisms are currently unknown. Theoretically, it is possible that bacterial and viral components directly influence enteric glia through actions on glial Toll‐like receptors (TLR‐3, ‐4 and ‐7; Barajon et al. 2009; see below). However, this would imply that glia in the myenteric plexus are exposed to bacteria or bacterial components on a regular basis, and to what extent this occurs under physiological conditions is unclear. Perhaps a more likely explanation is that the microbiota indirectly influence glia through interactions with the gut epithelium (reviewed in Abreu, 2010) and mucosal immune cells (Round & Mazmanian, 2009). Indeed, microbiota‐driven neuroimmune interactions have already been documented (Muller et al. 2014). In any case, these are very exciting findings that raise many questions about the role of microbiota–glial interactions in gut physiology and pathophysiology.
Enteric glia and intestinal inflammation
Enteric glia actively participate in immune responses in the intestine. It is now clear that enteric glia have the potential to modulate immune response by both responding to and secreting inflammatory mediators that include interleukin (IL)‐1 and IL‐6 (Ruhl et al. 2001) and purines (Gulbransen et al. 2012; Brown et al. 2015). However, the mechanisms underlying the glial responses to gut inflammation and their contribution to the development of functional gastrointestinal disorders are still poorly understood. One emerging theme is that pro‐inflammatory stimuli enact glial signalling pathways that involve nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB) and NO. This pathway has emerged as the main effector pathway for pro‐inflammatory stimuli in enteric glia and it seems to be a critical mediator of the detrimental effects of reactive glia. For example, NF‐κB signalling is involved with the elevation of S100β content and release by glia and elevated glial NO production during GI inflammation induced by pathogenic bacteria (Turco et al. 2014), DSS‐colitis in mice (Esposito et al. 2014; MacEachern et al. 2015) and ulcerative colitis in humans (Cirillo et al. 2011; Esposito et al. 2014). Likewise, our group has discovered that the activation of glia by purinergic danger cues released by neurons in the context of neuroinflammation drives glial NO production (Brown et al. 2015). Importantly, glial activation and NO production is a critical mediator of neurodegeneration during intestinal inflammation and neurodegeneration is a major contributor to functional bowel disorders (Gulbransen & Sharkey, 2012; Brown et al. 2015). In this case, the mechanisms involve the pathological opening of glial Cx43 hemichannels, glial ATP release and the activation of neuronal P2X7 receptors (Gulbransen et al. 2012; Brown et al. 2015). Precisely how purines drive an up‐regulation of inducible nitric oxide synthase (iNOS) activity in glia is currently unclear but it may also involve NF‐κB signalling downstream of the βγ subunits of GPCRs or Ca2+/calmodulin‐dependent protein kinase II (CaMKII) (Jones et al. 2007).
Whether similar signalling pathways are involved in other aspects of reactive gliosis in inflamed gut such as the upregulation of major histocompatibility complex class II (MHC‐II) (Koretz et al. 1987; Turco et al. 2014) is unknown. This molecule is typically expressed by antigen presenting cells and the expression by enteric glia suggests that glia may instruct immune cells in unique ways (Geboes et al. 1992). Thus, understanding if shared signalling pathways drive diverse glial contributions to immune responses that include the secretion of inflammatory mediators and a gained antigen presenting capability would be important in the quest for new therapeutics. Turco et al. (2014) observed some support for a common signalling pathway because an upregulation of MHC‐II by glia accompanied the glial response to either S100β or TLR signalling (Turco et al. 2014).
Of course, an important task for future research will be to determine how to mitigate the detrimental effects of reactive glia in the intestine without interfering with their physiological functions. This may be challenging given that glial mediators such as ATP and NO play important roles in both GI physiology (MacEachern et al. 2011; McClain et al. 2014) and pathophysiology (Fig. 7; Brown et al. 2015; MacEachern et al. 2015). However, the distinct signal transduction mechanisms involved with either physiological or pathophysiological glial functions may allow for dampening of pathophysiological functions without interfering with GI physiology. In support, palmitoylethanolammide (PEA) was found to improve colonic inflammation by inhibiting NF‐κB and NO release (Esposito et al. 2014).
Figure 7. The role of enteric glia in inflammation – feed‐forward loop leading to increased cell death.
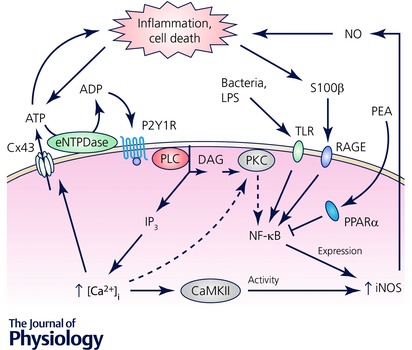
Both ATP and nitric oxide (NO) released from enteric glia regulate normal gut physiology (see text for details). Infection‐induced TLR signalling increases iNOS expression via NF‐κB and results in increased release of NO, a molecule with an antimicrobial effect. Excessive NO release, either by the infection or by other inflammatory signals (omitted for clarity) can also damage the cells leading to a surge of purines and S100β. While S100β enhances NO release via the increased iNOS expression, purine signalling increases intracellular calcium concentration ([Ca2+]i) and increased ATP release via Cx43 hemichannels. Increased [Ca2+] can also lead to increased iNOS activity and expression via CaMKII and PKC, respectively. Both PKC and CaMKII were not directly investigated in enteric glia (light grey); our findings indirectly show that PKC does not play a role in enteric glia (dashed arrows). The main findings are summarized from Esposito et al. (2014), Turco et al. (2014) and Brown et al. (2015); see text for details. Abbreviations: CaMKII, Ca2+/calmodulin‐dependent protein kinase II; eNTPDase, ecto‐nucleoside triphosphate diphosphohydrolase; iNOS, inducible nitric oxide synthase; NF‐κB, nuclear factor kappa‐light‐chain‐enhancer of activated B cells; PKC, protein kinase C; PLC, phospholipase C; PPARα, peroxisome‐proliferator‐activated receptor‐α; S100β, S100 calcium‐binding protein β; TLR, toll‐like receptor.
Conclusions
Enteric glia are clearly necessary for the maintenance of gastrointestinal functions and have the potential to profoundly influence gut physiology (Fig. 6) and pathophysiology (Fig. 7). The field is now poised to begin asking more pointed questions about why enteric glial cells are so important and what mechanisms they contribute to. Specifically, understanding the intricacies of the glial cell interface with multiple cell types is a relatively poorly understood area that holds great promise to further our understanding of the pathogenesis of many gastrointestinal diseases. New experimental tools such as glial‐specific mutant mice (McClain et al. 2014, 2015) and glial‐specific viral vectors (Benskey et al. 2015; Gombash et al. 2015) are now readily available and provide ample opportunities to selectively alter defined mechanisms in enteric glial cells at will. The incorporation of these types of technologies into future work will be extremely important to gain a more in‐depth understanding of these fascinating glial cells and, in turn, the gastrointestinal tract itself.
Additional information
Competing interests
The authors have no financial, professional, or personal conflicts that are relevant to the manuscript.
Author contributions
Both authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
B.D.G.’s research is currently supported by grants from the Crohn's and Colitis Foundation of America (CCFA; Senior Research Award) and the National Institutes of Health (NIH; RO1DK103723).
Acknowledgements
The authors thank David E. Fried and Ninotchska Del Valle Dorta for allowing them to use their unpublished work.
Biographies
Vladimir Grubišić received a medical degree from the University of Zagreb in Croatia in 2007 and a doctorate in Neurobiology from the University of Alabama at Birmingham in 2014. He briefly continued his postdoctoral training with his graduate adviser Dr Vladimir Parpura as a Civitan Emerging Scholar and is currently a Postdoctoral Fellow in the Neuroscience Program and the Department of Physiology at the Michigan State University.

Brian D. Gulbransen received his Bachelor of Science in Zoology and Physiology (with Honors) from the University of Wyoming in 2003 and his Doctor of Philosophy in Neuroscience from the University of Colorado (Anschutz Medical Campus) in 2007. He trained as a Postdoctoral Fellow under Dr Keith Sharkey at the University of Calgary and is currently an Assistant Professor in the Neuroscience Program and the Department of Physiology at the Michigan State University (https://www.msu.edu/~gulbrans/).
References
- Abreu MT (2010). Toll‐like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10, 131–144. [DOI] [PubMed] [Google Scholar]
- Aube AC, Cabarrocas J, Bauer J, Philippe D, Aubert P, Doulay F, Liblau R, Galmiche JP & Neunlist M (2006). Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut 55, 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach‐Ngohou K, Mahe MM, Aubert P, Abdo H, Boni S, Bourreille A, Denis MG, Lardeux B, Neunlist M & Masson D (2010). Enteric glia modulate epithelial cell proliferation and differentiation through 15‐deoxy‐12,14‐prostaglandin J2. J Physiol 588, 2533–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badizadegan K, Thomas AR, Nagy N, Ndishabandi D, Miller SA, Alessandrini A, Belkind‐Gerson J & Goldstein AM (2014). Presence of intramucosal neuroglial cells in normal and aganglionic human colon. Am J Physiol Gastrointest Liver Physiol 307, G1002–G1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A & Rumio C (2009). Toll‐like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem 57, 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassotti G, Villanacci V, Cretoiu D, Cretoiu SM & Becheanu G (2013). Cellular and molecular basis of chronic constipation: taking the functional/idiopathic label out. World J Gastroenterol 19, 4099–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM & Starling EH (1900). The movements and the innervation of the large intestine. J Physiol 26, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskey MJ, Kuhn NC, Galligan JJ, Garcia J, Boye SE, Hauswirth WW, Mueller C, Boye SL & Manfredsson FP (2015). Targeted gene delivery to the enteric nervous system using AAV: a comparison across serotypes and capsid mutants. Mol Ther 23, 488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesmans W, Cirillo C, Van den Abbeel V, Van den Haute C, Depoortere I, Tack J & Vanden Berghe P (2013). Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol Motil 25, e151–160. [DOI] [PubMed] [Google Scholar]
- Boesmans W, Lasrado R, Vanden Berghe P & Pachnis V (2015). Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 63, 229–241. [DOI] [PubMed] [Google Scholar]
- Boesmans W, Rocha NP, Reis HJ, Holt M & Vanden Berghe P (2014). The astrocyte marker Aldh1L1 does not reliably label enteric glial cells. Neurosci Lett 566, 102–105. [DOI] [PubMed] [Google Scholar]
- Bohorquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R & Liddle RA (2014). An enteroendocrine cell‐enteric glia connection revealed by 3D electron microscopy. PLoS One 9, e89881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner S, Schwede F, Schlipp A, Berisha F, Calebiro D, Lohse MJ & Nikolaev VO (2011). FRET measurements of intracellular cAMP concentrations and cAMP analog permeability in intact cells. Nat Protoc 6, 427–438. [DOI] [PubMed] [Google Scholar]
- Broadhead MJ, Bayguinov PO, Okamoto T, Heredia DJ & Smith TK (2012). Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J Physiol 590, 335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IAM, McClain JL, Watson RE, Patel BA & Gulbransen BD (2015). Enteric glia mediate neuron death in colitis through purinergic pathways that require Connexin‐43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2, 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH & Sofroniew MV (1998). Fulminant jejuno‐ileitis following ablation of enteric glia in adult transgenic mice. Cell 93, 189–201. [DOI] [PubMed] [Google Scholar]
- Christofi FL, Hanani M, Maudlej N & Wood JD (1993). Enteric glial cells are major contributors to formation of cyclic AMP in myenteric plexus cultures from adult guinea‐pig small intestine. Neurosci Lett 159, 107–110. [DOI] [PubMed] [Google Scholar]
- Christofi FL, Zhang H, Yu JG, Guzman J, Xue J, Kim M, Wang YZ & Cooke HJ (2001). Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system. J Comp Neurol 439, 46–64. [DOI] [PubMed] [Google Scholar]
- Cirillo C, Bessissow T, Desmet AS, Vanheel H, Tack J & Vanden Berghe P (2015). Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Am J Gastroenterol 110, 1205–1215. [DOI] [PubMed] [Google Scholar]
- Cirillo C, Sarnelli G, Esposito G, Turco F, Steardo L & Cuomo R (2011). S100B protein in the gut: the evidence for enteroglial‐sustained intestinal inflammation. World J Gastroenterol 17, 1261–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho‐Aguiar Jde M, Bon‐Frauches AC, Gomes AL, Verissimo CP, Aguiar DP, Matias D, Thomasi BB, Gomes AS, Brito GA & Moura‐Neto V (2015). The enteric glia: identity and functions. Glia 63, 921–935. [DOI] [PubMed] [Google Scholar]
- Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P & Liblau RS (2001). Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc Natl Acad Sci USA 98, 13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denninger JW & Marletta MA (1999). Guanylate cyclase and the ·NO/cGMP signaling pathway. Biochim Biophys Acta 1411, 334–350. [DOI] [PubMed] [Google Scholar]
- Esposito G, Capoccia E, Turco F, Palumbo I, Lu J, Steardo A, Cuomo R, Sarnelli G & Steardo L (2014). Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4‐dependent PPAR‐α activation. Gut 63, 1300–1312. [DOI] [PubMed] [Google Scholar]
- Ferri GL, Probert L, Cocchia D, Michetti F, Marangos PJ & Polak JM (1982). Evidence for the presence of S‐100 protein in the glial component of the human enteric nervous system. Nature 297, 409–410. [DOI] [PubMed] [Google Scholar]
- Furness JB (2012). The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9, 286–294. [DOI] [PubMed] [Google Scholar]
- Gabella G & Trigg P (1984). Size of neurons and glial cells in the enteric ganglia of mice, guinea‐pigs, rabbits and sheep. J Neurocytol 13, 49–71. [DOI] [PubMed] [Google Scholar]
- Gard AL, White FP & Dutton GR (1985). Extra‐neural glial fibrillary acidic protein (GFAP) immunoreactivity in perisinusoidal stellate cells of rat liver. J Neuroimmunol 8, 359–375. [DOI] [PubMed] [Google Scholar]
- Geboes K, Rutgeerts P, Ectors N, Mebis J, Penninckx F, Vantrappen G & Desmet VJ (1992). Major histocompatibility class II expression on the small intestinal nervous system in Crohn's disease. Gastroenterology 103, 439–447. [DOI] [PubMed] [Google Scholar]
- Giaroni C, Zanetti E, Chiaravalli AM, Albarello L, Dominioni L, Capella C, Lecchini S & Frigo G (2003). Evidence for a glutamatergic modulation of the cholinergic function in the human enteric nervous system via NMDA receptors. Eur J Pharmacol 476, 63–69. [DOI] [PubMed] [Google Scholar]
- Gombash SE, Cowley CJ, Fitzgerald JA, Iyer CC, Fried D, McGovern VL, Williams KC, Burghes AH, Christofi FL, Gulbransen BD & Foust KD (2015). SMN deficiency disrupts gastrointestinal and enteric nervous system function in mice. Hum Mol Genet 24, 3847–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD (2014). Enteric Glia (doi: 10.4199/C00113ED1V01Y201407NGL002). Morgan & Claypool Publishers, San Rafael. [Google Scholar]
- Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ & Sharkey KA (2012). Activation of neuronal P2X7 receptor‐pannexin‐1 mediates death of enteric neurons during colitis. Nat Med 18, 600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD & Sharkey KA (2009). Purinergic neuron‐to‐glia signaling in the enteric nervous system. Gastroenterology 136, 1349–1358. [DOI] [PubMed] [Google Scholar]
- Gulbransen BD & Sharkey KA (2012). Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9, 625–632. [DOI] [PubMed] [Google Scholar]
- Hoff S, Zeller F, von Weyhern CW, Wegner M, Schemann M, Michel K & Ruhl A (2008). Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti‐Sox8/9/10 antibody. J Comp Neurol 509, 356–371. [DOI] [PubMed] [Google Scholar]
- Jessen KR & Mirsky R (1983). Astrocyte‐like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J Neurosci 3, 2206–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Saffrey MJ & Burnstock G (1983). The enteric nervous system in tissue culture. I. Cell types and their interactions in explants of the myenteric and submucous plexuses from guinea pig, rabbit and rat. Brain Res 262, 17–35. [DOI] [PubMed] [Google Scholar]
- Jiang JX & Cherian PP (2003). Hemichannels formed by connexin 43 play an important role in the release of prostaglandin E2 by osteocytes in response to mechanical strain. Cell Commun Adhes 10, 259–264. [DOI] [PubMed] [Google Scholar]
- Jiang S, Khan MI, Lu Y, Werstiuk ES & Rathbone MP (2005). Acceleration of blood‐brain barrier formation after transplantation of enteric glia into spinal cords of rats. Exp Brain Res 162, 56–62. [DOI] [PubMed] [Google Scholar]
- Jiang S, Wang J, Khan MI, Middlemiss PJ, Salgado‐Ceballos H, Werstiuk ES, Wickson R & Rathbone MP (2003). Enteric glia promote regeneration of transected dorsal root axons into spinal cord of adult rats. Exp Neurol 181, 79–83. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Jourd'heuil D, Salerno JC, Smith SM & Singer HA (2007). iNOS regulation by calcium/calmodulin‐dependent protein kinase II in vascular smooth muscle. Am J Physiol Heart Circ Physiol 292, H2634–H2642. [DOI] [PubMed] [Google Scholar]
- Joseph NM, He S, Quintana E, Kim YG, Nunez G & Morrison SJ (2011). Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest 121, 3398–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, Pettersson S & Pachnis V (2015). Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 85, 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabouridis PS & Pachnis V (2015). Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. J Clin Invest 125, 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur RP, Yost C & Palmiter RD (1992). A transgenic model for studying development of the enteric nervous system in normal and aganglionic mice. Development 116, 167–175. [DOI] [PubMed] [Google Scholar]
- Kimball BC & Mulholland MW (1996). Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C‐dependent mechanism. J Neurochem 66, 604–612. [DOI] [PubMed] [Google Scholar]
- Koretz K, Momburg F, Otto HF & Moller P (1987). Sequential induction of MHC antigens on autochthonous cells of ileum affected by Crohn's disease. Am J Pathol 129, 493–502. [PMC free article] [PubMed] [Google Scholar]
- Kunze WA & Furness JB (1999). The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol 61, 117–142. [DOI] [PubMed] [Google Scholar]
- Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden Berghe P & Pachnis V (2011). Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest 121, 3412–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YA, Chung YC, Pan ST, Shen MY, Hou YC, Peng SJ, Pasricha PJ & Tang SC (2013). 3‐D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterol Motil 25, e324–338. [DOI] [PubMed] [Google Scholar]
- Lomasney KW, Houston A, Shanahan F, Dinan TG, Cryan JF & Hyland NP (2014). Selective influence of host microbiota on cAMP‐mediated ion transport in mouse colon. Neurogastroenterol Motil 26, 887–890. [DOI] [PubMed] [Google Scholar]
- McClain JL, Fried DE & Gulbransen BD (2015). Agonist‐evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol 1, 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JL, Grubišić V, Fried D, Gomez‐Suarez RA, Leinninger GM, Sevigny J, Parpura V & Gulbransen BD (2014). Ca2+ responses in enteric glia are mediated by connexin‐43 hemichannels and modulate colonic transit in mice. Gastroenterology 146, 497–507.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEachern SJ, Patel BA, Keenan CM, Dicay M, Chapman K, McCafferty DM, Savidge TC, Beck PL, MacNaughton WK & Sharkey KA (2015). Inhibiting inducible nitric oxide synthase in enteric glia restores electrogenic ion transport in mice with colitis. Gastroenterology 149, 445–455.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEachern SJ, Patel BA, McKay DM & Sharkey KA (2011). Nitric oxide regulation of colonic epithelial ion transport: a novel role for enteric glia in the myenteric plexus. J Physiol 589, 3333–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael J, Modell H, McFarland J & Cliff W (2009). The “core principles” of physiology: what should students understand? Adv Physiol Educ 33, 10–16. [DOI] [PubMed] [Google Scholar]
- Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M & Bogunovic M (2014). Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser Y, Fernandez E, Keenan CM, Ho W, Oland LD, Tibbles LA, Schemann M, MacNaughton WK, Ruhl A & Sharkey KA (2006a). Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am J Physiol Gastrointest Liver Physiol 291, G912–G927. [DOI] [PubMed] [Google Scholar]
- Nasser Y, Ho W & Sharkey KA (2006b). Distribution of adrenergic receptors in the enteric nervous system of the guinea pig, mouse, and rat. J Comp Neurol 495, 529–553. [DOI] [PubMed] [Google Scholar]
- Nasser Y, Keenan CM, Ma AC, McCafferty DM & Sharkey KA (2007). Expression of a functional metabotropic glutamate receptor 5 on enteric glia is altered in states of inflammation. Glia 55, 859–872. [DOI] [PubMed] [Google Scholar]
- Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L & Neefjes J (2005). Cross‐presentation by intercellular peptide transfer through gap junctions. Nature 434, 83–88. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Aubert P, Bonnaud S, Van Landeghem L, Coron E, Wedel T, Naveilhan P, Ruhl A, Lardeux B, Savidge T, Paris F & Galmiche JP (2007). Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF‐β1‐dependent pathway. Am J Physiol Gastrointest Liver Physiol 292, G231–G241. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Rolli‐Derkinderen M, Latorre R, Van Landeghem L, Coron E, Derkinderen P & De Giorgio R (2014). Enteric glial cells: recent developments and future directions. Gastroenterology 147, 1230–1237. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Van Landeghem L, Mahe MM, Derkinderen P, des Varannes SB & Rolli‐Derkinderen M (2013). The digestive neuronal‐glial‐epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol 10, 90–100. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Gambaryan S & Lohse MJ (2006). Fluorescent sensors for rapid monitoring of intracellular cGMP. Nat Methods 3, 23–25. [DOI] [PubMed] [Google Scholar]
- Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R & Verkhratsky A (2012). Glial cells in (patho)physiology. J Neurochem 121, 4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potterf SB, Mollaaghababa R, Hou L, Southard‐Smith EM, Hornyak TJ, Arnheiter H & Pavan WJ (2001). Analysis of SOX10 function in neural crest‐derived melanocyte development: SOX10‐dependent transcriptional control of dopachrome tautomerase. Dev Biol 237, 245–257. [DOI] [PubMed] [Google Scholar]
- Rao M, Nelms BD, Dong L, Salinas‐Rios V, Rutlin M, Gershon MD & Corfas G (2015). Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia 63, 2040–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Siegel A, Senitz D & Smith TG Jr (1992). A comparative fractal analysis of various mammalian astroglial cell types. Neuroimage 1, 69–77. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg‐Riethmacher E, Brinkmann V, Yamaai T, Lewin GR & Birchmeier C (1997). Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature 389, 725–730. [DOI] [PubMed] [Google Scholar]
- Rothman TP, Tennyson VM & Gershon MD (1986). Colonization of the bowel by the precursors of enteric glia: studies of normal and congenitally aganglionic mutant mice. J Comp Neurol 252, 493–506. [DOI] [PubMed] [Google Scholar]
- Round JL & Mazmanian SK (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl A, Franzke S, Collins SM & Stremmel W (2001). Interleukin‐6 expression and regulation in rat enteric glial cells. Am J Physiol Gastrointest Liver Physiol 280, G1163–G1171. [DOI] [PubMed] [Google Scholar]
- Sanders KM (2000). Postjunctional electrical mechanisms of enteric neurotransmission. Gut 47(Suppl 4), iv23–iv25; discussion iv26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R & Sofroniew MV (2007). Enteric glia regulate intestinal barrier function and inflammation via release of S‐nitrosoglutathione. Gastroenterology 132, 1344–1358. [DOI] [PubMed] [Google Scholar]
- Sharkey KA (2015). Emerging roles for enteric glia in gastrointestinal disorders. J Clin Invest 125, 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp‐Strahm C, Patterson S, Boren J, Gericke M & Balemba O (2013). High‐fat diet and age‐dependent effects on enteric glial cell populations of mouse small intestine. Auton Neurosci 177, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco F, Sarnelli G, Cirillo C, Palumbo I, De Giorgi F, D'Alessandro A, Cammarota M, Giuliano M & Cuomo R (2014). Enteroglial‐derived S100B protein integrates bacteria‐induced Toll‐like receptor signalling in human enteric glial cells. Gut 63, 105–115. [DOI] [PubMed] [Google Scholar]
- Van Landeghem L, Chevalier J, Mahe MM, Wedel T, Urvil P, Derkinderen P, Savidge T & Neunlist M (2011). Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am J Physiol Gastrointest Liver Physiol 300, G976–G987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nassauw L, Costagliola A, Van Op den Bosch J, Cecio A, Vanderwinden JM, Burnstock G & Timmermans JP (2006). Region‐specific distribution of the P2Y4 receptor in enteric glial cells and interstitial cells of Cajal within the guinea‐pig gastrointestinal tract. Auton Neurosci 126–127, 299–306. [DOI] [PubMed] [Google Scholar]
- Vieira C, Ferreirinha F, Silva I, Duarte‐Araujo M & Correia‐de‐Sa P (2011). Localization and function of adenosine receptor subtypes at the longitudinal muscle–myenteric plexus of the rat ileum. Neurochem Int 59, 1043–1055. [DOI] [PubMed] [Google Scholar]
- Vives V, Alonso G, Solal AC, Joubert D & Legraverend C (2003). Visualization of S100B‐positive neurons and glia in the central nervous system of EGFP transgenic mice. J Comp Neurol 457, 404–419. [DOI] [PubMed] [Google Scholar]
- von Boyen GB, Steinkamp M, Adler G & Kirsch J (2006). Glutamate receptor subunit expression in primary enteric glia cultures. J Recept Signal Transduct Res 26, 329–336. [DOI] [PubMed] [Google Scholar]
- von Boyen GB, Steinkamp M, Reinshagen M, Schafer KH, Adler G & Kirsch J (2004). Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 53, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HM, Jones BR & McKeown SJ (2002). The projections of early enteric neurons are influenced by the direction of neural crest cell migration. J Neurosci 22, 6005–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DK, He FQ, Li TK, Pang XH, Cui J de, Xie Q, Huang XL & Gan HT (2010). Glial‐derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J Pathol 222, 213–222. [DOI] [PubMed] [Google Scholar]
- Zhang W, Segura BJ, Lin TR, Hu Y & Mulholland MW (2003). Intercellular calcium waves in cultured enteric glia from neonatal guinea pig. Glia 42, 252–262. [DOI] [PubMed] [Google Scholar]
- Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A & Parpura V (2012). Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro 4, e00080. [DOI] [PMC free article] [PubMed] [Google Scholar]


