Abstract
There is a growing realisation that the gut–brain axis and its regulation by the microbiota may play a key role in the biological and physiological basis of neurodevelopmental, age‐related and neurodegenerative disorders. The routes of communication between the microbiota and brain are being unravelled and include the vagus nerve, gut hormone signalling, the immune system, tryptophan metabolism or by way of microbial metabolites such as short chain fatty acids. The importance of early life gut microbiota in shaping future health outcomes is also emerging. Disturbances of this composition by way of antibiotic exposure, lack of breastfeeding, infection, stress and the environmental influences coupled with the influence of host genetics can result in long‐term effects on physiology and behaviour, at least in animal models. It is also worth noting that mode of delivery at birth influences microbiota composition with those born by Caesarean section having a distinctly different microbiota in early life to those born per vaginum. At the other extreme of life, ageing is associated with a narrowing in microbial diversity and healthy ageing correlates with a diverse microbiome. Recently, the gut microbiota has been implicated in a variety of conditions including depression, autism, schizophrenia and Parkinson's disease. There is still considerable debate as to whether or not the gut microbiota changes are core to the pathophysiology of such conditions or are merely epiphenomenal. It is plausible that such neuropsychiatric disorders might be treated in the future by targeting the microbiota either by microbiota transplantation, antibiotics or psychobiotics.
Keywords: ageing, gut‐brain axis, stress
Introduction
Claude Bernard, William James, Ivan Pavlov and Walter Cannon, the fathers of modern physiology, have all described the bidirectional communication between the gut and the brain and its importance in maintaining homeostasis (Aziz & Thompson, 1998; Mayer, 2011). However, over the past decade increasing emphasis has been placed on the role of intestinal microbiota in regulating the gut–brain axis. Concurrently, a growing body of evidence points to the microbiota playing a significant role in modulating brain function and behaviour and that the microbiota–gut–brain axis is poised as a bidirectional communication pathway enabling gut microbes to communicate with the brain, and the brain with the gut (Rhee et al. 2009; Canfora et al. 2015; Foster et al. 2016). The mechanisms of communication are complex and are slowly being unravelled; they include immune, neural, endocrine and metabolic pathways (Grenham et al. 2011; Mayer et al. 2014a; El Aidy et al. 2015; Koh et al. 2016; Sommer & Backhed, 2016). Preclinical studies have implicated the vagus nerve as a key route of neural communication between gut microbes and centrally mediated behavioural effects, as demonstrated by the prevention of the in vivo effects of specific bacterial strains by selective vagotomy (Bravo et al. 2011; Bercik et al. 2011b). The gut microbiota also regulates key central neurotransmitters by altering levels of precursors; for example Bifidobacterium infantis has been shown to elevate plasma tryptophan levels and thus influence central 5‐HT transmission (Desbonnet et al. 2008; O'Mahony et al. 2015a). Furthermore, synthesis and release of neurotransmitters from bacteria has been reported; the inhibitory neurotransmitter γ‐aminobutyric acid (GABA) can be produced by Lactobacillus and Bifidobacterium species, whereas Escherichia, Bacillus and Saccharomyces spp. can produce noradrenaline (norepinephrine). On the other hand Candida, Streptococcus, Escherichia and Enterococcus spp. have been reported to produce serotonin, and Bacillus can produce dopamine, whereas certain Lactobacillus spp. can produce acetylcholine (Lyte, 2013, 2014; Wall et al. 2014). These microbially synthesised neurotransmitters can cross the mucosal layer of the intestines, and possibly mediate physiological events in the brain.
Short chain fatty acids (SCFAs), which include propionate, butyrate and acetate, are important metabolic products of gut microbial activity and may exert central effects indirectly or directly either through G‐protein coupled receptors or in the case of butyrate as an epigenetic modulator acting through histone deacetylases (HDACs) (Galland, 2014; Stilling et al. 2014, 2016; Paul et al. 2015). Moreover, SCFAs are involved in a plethora of physiological functions ranging from energy balance and metabolism to the modulation of adipose tissue, liver tissue and skeletal muscle function (Canfora et al. 2015). Immune signalling from gut to brain mediated by cytokine molecules is another documented route of communication (El Aidy et al. 2014). The epithelial layer of the gut and its mucous layer have many functions, including the regulation of nutrient and fluid absorption from the gut lumen in addition to serving as a physical barrier from invading pathogens or harmful substances (Farhadi et al. 2003; Scaldaferri et al. 2012; Johansson et al. 2013; Kelly et al. 2015). Interactions between microbes and the immune system of the gut help the latter to identify self and non‐self antigens and potentially harmful pathogens (Fasano, 2012; Sonnenberg & Artis, 2012; Kamada et al. 2013; Sommer & Backhed, 2013).
The hypothalamic–pituitary–adrenal (HPA) axis, which provides the core regulation of the stress response, can significantly impact the microbiota–gut–brain axis (O'Mahony et al. 2009, 2011, 2015b; Scott et al. 2013; Moloney et al. 2014; Tillisch, 2014; Wang & Kasper, 2014). It is increasingly clear and probably of relevance in a number of pathological conditions that psychological or physical stress can significantly dysregulate the microbiota–gut–brain, for example in irritable bowel syndrome (Dinan et al. 2006).
Multiple lines of approach have been used to interrogate the microbiota–gut–brain axis, especially in animal model systems; these include the use of microbiota deficient animals known as germ‐free mice, specific bacterial species often incorrectly referred to as probiotics, antibiotics, animals exposed to pathogens and the use of stress to determine the effects of dysregulating the HPA axis. There is an emerging consensus, at least from animal studies, that the gut microbiota plays a pivotal role in regulating early brain development (Bercik et al. 2012; Collins et al. 2012; Mayer et al. 2014a; Borre et al. 2014; Sampson & Mazmanian, 2015). Determining the mechanisms and pathways underlying microbiota–brain interactions is an exciting and evolving area of research that may yield novel insights into individual variations and perhaps enable the development of new treatments for a range of neurodevelopmental and neurodegenerative disorders, ranging from autism to Parkinson's disease. Moreover, there is increased emphasis on understanding the factors contributing to healthy brain ageing and the microbiome is poised to play a crucial role here. Here we will review important recent findings in the field.
Maturation and decline of bacterial gut microbiota
It is generally estimated that the gut is inhabited by 1013–1014 microorganisms, although the ratio of microbial to human cells has been recently revised downward (Sender et al. 2016). In terms of genes we have over 100 times as many genes in our microbiome as we have in our genome. The total weight of these gut microbes is 1–2 kg, which is similar to the weight of the human brain (Stilling et al. 2014). Mammals have never existed without microbes, except in laboratory situations. The reality is that we have co‐evolved, and we are fundamentally dependent upon our colonisers for survival, as of course are they on us (Bordenstein & Theis, 2015).
With over 1000 species and greater than 7000 strains the microbiota is an ecosystem dominated by bacteria, mainly strict anaerobes, but also includes other microorganisms such as viruses and bacteriophages, protozoa, archaea and fungi (Lankelma et al. 2015). At a phylum level the microbiome is largely defined by two dominant bacterial phylotypes, Bacteroidetes and Firmicutes with Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia phyla present in relatively low abundance (Qin et al. 2010; Lankelma et al. 2015).
Colonisation of the infant gut for the most part begins at birth, when delivery through the birth canal exposes the infant to its mother's microbiota and thus initiates an initial maternal signature to the microbiota (Donnet‐Hughes et al. 2010; Collado et al. 2012; Matamoros et al. 2013; Backhed et al. 2015). The impact of mode of delivery, be it vaginal birth or Caesarean section (C. section) on neonatal microbial composition is now being appreciated. Indeed, C. section results in an altered microbial composition in the neonate resembling that of the mother's skin with Staphylococcus, Corynebacterium and Propionibacterium spp. dominating in comparison with vaginally delivered infants whose microbiome is more akin to that from the mother's vagina, which have a predominance of Lactobacillus, Prevotella or Sneathia spp. (Dominguez‐Bello et al. 2010). Moreover, this signature has been shown to persist into adulthood with a distinctly different faecal microbiota composition being detected in those individuals who have been born by C. section (Goedert et al. 2014), compared with their natural born counterparts (but see Yassour et al. 2016). Significant differences in microbiota composition have been described between preterm and normal‐term neonates (Barrett et al. 2013, 2015). Barrett and colleagues (2013) showed that preterm infants, albeit just two infants, lacked two of the main bacterial genera seen in normal‐term infants: Bifidobacterium and Lactobacillus, with a compensatory dominance of the Proteobacteria observed. It is worth noting that there is a growing appreciation of the connection between microbial composition and the nutritional needs of the infant host (Voreades et al. 2014). Indeed exclusively breastfed infants display an increase in the relative composition of Bifidobacterium species that have evolved specifically to utilise human milk oligosaccharides (Costello et al. 2012; Hinde & Lewis, 2015). Each diet induces a specialised microbiota with the capability of microbial digestion of that specific diet. Indeed, an increase in the relative composition of strict anaerobes as a function of diet and environment occurs early in life, and a complex adult‐like microbiome emerges by the age of 1 year old. Although inter‐individual variations in the composition of gut microbiota is apparent, an internal balance exists that confers a propensity towards health benefits (Gilbert et al. 2016), whereas perturbations in this ecosystem has the potential to negatively impact health, increasing vulnerability to a range of diseases (Sankar et al. 2015; Gilbert et al. 2016). Infection, disease, diet and antibiotics are among the many factors that may change microbial composition (Borre et al. 2014). However, the composition tends to revert to its previous level of diversity once the distorting factor has subsided (Vandenplas, 2015). This is especially true in relation to antibiotic exposure (Blaser, 2014), but far less so in relation to dietary patterns which tend to be relatively permanent. Taken together it is clear that infancy is a critical period for both microbiota colonisation and neurodevelopment. Thus, the time is now ripe for longitudinal studies to assess the impact of altered microbiota composition in early life on neurocognitive function in humans (Yang et al. 2016).
Major shifts in diet in adults likewise show dramatic changes in microbiota composition (David et al. 2014). It is clear from studies of remote hunter‐gatherer tribes that Western guts have undergone a significant reduction in bacterial diversity and globalisation is driving this trend forward (Leach, 2013; Schnorr et al. 2014; Clemente et al. 2015; Martinez et al. 2015; Rampelli et al. 2015; Sonnenburg et al. 2016).
As we age, it has been shown that the core microbiota undergoes a dynamic shift (Claesson et al. 2011). Such age‐related changes in the composition, and in particular the diversity, of the microbiota correlate with health outcomes in the elderly, especially in the context of frailty measures (O'Toole & Jeffery, 2015; Zapata & Quagliarello, 2015). Of interest is the fact that the number of probiotic Bifidobacteria strains decreases with age (Rondanelli et al. 2015). Recent studies in centenarians have reinforced the importance of microbial diversity in maintaining health as we age (Biagi et al. 2016). Thus it is becoming clear that maintaining a healthy microbiome is crucial to having a healthy brain across the lifespan from cradle to grave (see Fig. 1).
Figure 1. We are living in a microbial world throughout our lifespan.
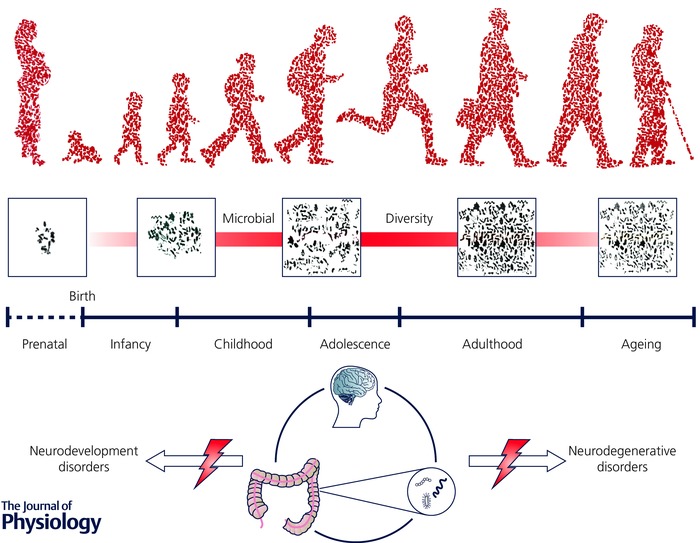
A growing body of evidence suggests that gut microbiota is essential to human health and is a key player in the bidirectional communication across the gut–brain axis. The microbiota dynamically changes across the lifespan, establishing its relationship with the host at critical windows during infancy, adolescence and ageing. At these time windows, there is an increased vulnerability to external insults, which may result in enhanced susceptibility to brain disorders. Early life disturbance of the developing gut microbiota has the potential to significantly impact on neurodevelopment and potentially lead to adverse mental health outcomes later in life. Similarly, the microbiota may contribute to the ageing process and the trajectory of neurodegenerative disorders.
Microbiota and brain development
Germ‐free mice have been instrumental in highlighting a key role of the microbiota in early brain development (Gareau, 2014; Sampson & Mazmanian, 2015; Luczynski et al. 2016a). Key changes in multiple neurotransmitter systems and their receptors have been described in a variety of different brain regions in germ‐free mice. Using a genome‐wide transcriptomic approach Diaz Heijtz and colleagues (2011) demonstrated that germ‐free mice have an upregulation of genes associated with a variety of plasticity and metabolic pathways including synaptic long‐term potentiation, steroid hormone metabolism, and cyclic adenosine 5‐phosphate‐mediated signalling. Of note, the cerebellum and hippocampus were the two most sensitised brain areas to such changes in gene expression, with the hypothalamus being relatively resistant. The serotonergic system is particularly vulnerable to early‐life manipulations of the microbiome. We have shown that germ‐free mice have a marked increase in 5‐HT concentrations in the hippocampus (Clarke et al. 2013), whereas Neufeld and colleagues have shown a decreased 5‐HT1A receptor gene expression in the hippocampal dentate gyrus of female germ‐free animals using in situ hybridisation (Neufeld et al. 2011). Brain‐derived neurotrophic factor (BDNF) is an important plasticity‐related protein that promotes neuronal growth, development and survival and plays a key role in learning, memory and mood regulation. In germ‐free mice, Bdnf expression is lower in the cortex and amygdala compared with controls (Diaz Heijtz et al. 2011). In the hippocampus, the changes in Bdnf levels documented are inconsistent with some studies reporting an increase (Neufeld et al. 2011), whereas most others show a decrease in expression (Sudo et al. 2004; Diaz Heijtz et al. 2011; Gareau et al. 2011; Clarke et al. 2013).
Somewhat intriguingly, many of the CNS alterations, including changes in BDNF levels found in germ‐free mice occur in a sex‐specific manner with only male germ‐free animals exhibiting the serotonergic alterations described above as well as the decrease in Bdnf expression (Clarke et al. 2013). Germ‐free mice have been shown to have modest increases in hippocampal volume and the pyramidal neurons within the ventral hippocampus were shorter and less branched, and had deficits in the number of stubby and mushroom spines. On the other hand decreased branching was observed for dentate granule cells without any overt change at the spine density level in germ‐free mice (Luczynski et al. 2016b). The birth of new neurons in the adult hippocampus, neurogenesis, plays a critical role in modulating learning and memory and in mediating the behavioural responses to stress and antidepressant drugs (O'Leary & Cryan, 2014). Recent data using germ‐free animals have shown that neurogenesis is also regulated by the microbiome. Germ‐free mice exhibit increased adult hippocampal neurogenesis in the dorsal hippocampus (Ogbonnaya et al. 2015). Postweaning microbial colonisation of germ‐free mice failed to reverse the changes in adult hippocampal neurogenesis, suggesting that a critical window exists in the pre‐weaning period during which the microbiota exerts its influences on adult hippocampal neurogenesis (Ogbonnaya et al. 2015). Intriguingly antibiotic administration, which depletes the microbiota, to adult animals actually decreases neurogenesis. Moreover, this effect was reversed by exercise or administration of a probiotic cocktail (Mohle et al. 2016).
The effects of the microbiome on brain development are not specific to the hippocampus with data emerging that there are alterations in amygdala function as well. The amygdala is a critical brain area for social behaviour as well as being a key node for the gating of anxiety and fear‐related behaviour (Ledoux, 2007; Stilling et al. 2015). Structural and functional changes in the amygdala are associated with a variety of neuropsychiatric disorders ranging from autism spectrum (Schumann & Amaral, 2006; Mosconi et al. 2009) to anxiety disorders (Ledoux, 2007; Janak & Tye, 2015). Germ‐free mice have increased amygdala volume and have dendritic hypertrophy in the basolateral amygdala (BLA). Moreover, the pyramidal neurons of the BLA in germ‐free mice have more thin, stubby and mushroom spines compared to mice with normal microbiota (Luczynski et al. 2016b). Using RNA‐sequencing significant differences at the levels of differential gene expression, exon usage and RNA editing were found in the amygdala. The expression of immediate early response genes such as Fos, Fosb, Egr2 or Nr4a1 were increased in the amygdala of germ‐free mice in concert with increased signalling of the transcription factor CREB in germ‐free mice (Stilling et al. 2015). Moreover, differential expression and recoding of several genes involved in fundamental brain processes ranging from neuronal plasticity, metabolism, neurotransmission and morphology were identified. A significant downregulation was noted for immune system‐related genes (Stilling et al. 2015), which is in line with the underdeveloped immune system and immature microglia reported in germ‐free mice (Erny et al. 2015). This adds further evidence to a key role of the immune system in mediating the effects of the microbiota on brain physiology and behaviour. Indeed, the recently discovered lymphatic branches in the central nervous system may be one such mechanism (Louveau et al. 2015).
Another aspect of neurodevelopment shown to be critically regulated by the microbiome is prefrontal cortical myelination (Hoban et al. 2016). Germ‐free mice have hypermyelination and increased expression of genes involved in myelination and myelin plasticity processes in the prefrontal cortex but not other brain areas investigated (Hoban et al. 2016). Similar findings have also emerged in adult animals treated with antibiotics (Gacias et al. 2016).
It should be noted that although germ‐free mice have been instrumental in advancing all aspects of microbiome research including microbiome‐to‐brain signalling (Grover & Kashyap, 2014; Luczynski et al. 2016a), there are many challenges in their use. This is especially true with regards to the marked alterations in the immune system and gastrointestinal tract and in terms of lacking any true clinical translation (Nguyen et al. 2015; Al‐Asmakh & Zadjali, 2015; Arrieta et al. 2016). That said the preclinical use of germ‐free mice is not to directly mimic the human condition but to provide a platform to explore the role of bacteria on host development and function and answer the question whether the microbiome is involved or not (Nguyen et al. 2015; Faith et al. 2014; Al‐Asmakh & Zadjali, 2015; Arrieta et al. 2016). Nonetheless, germ‐free mice have limited utility to address experimental questions regarding the impact of altered microbiota composition that first occurs later in life. Thus studies using antibiotic treatment have proven useful complementary alternatives. Antibiotic treatment has also been shown to affect other brain neurodevelopment processes. Indeed, antibiotic treatment in adult mice alters BDNF protein levels in both the amygdala and hippocampus (Bercik et al. 2011a). On the other hand administration of antibiotics early in life to male rats has been shown to increase visceral hypersensitivity in adulthood without affecting anxiety, cognitive, immune or stress‐related responses (O'Mahony et al. 2014). The increased visceral hypersensitivity was paralleled by specific changes in the spinal expression of pain‐associated genes (transient receptor potential cation channel subfamily V member 1, the α‐2A adrenergic receptor and cholecystokinin B receptor) (O'Mahony et al. 2014). More recently we targeted the adolescent period in mice whereby many of the phenotypic changes in germ‐free mice could be recapitulated by treating with antibiotics. Indeed, at a behavioural level, post‐weaning depletion of the gut microbiota led to reduced anxiety coupled with cognitive deficits (Desbonnet et al. 2015). Moreover, at a neurochemical level in the adult brain altered tryptophan metabolism was observed coupled with significantly reduced BDNF, oxytocin and vasopressin expression in adolescent antibiotic‐treated mice (Desbonnet et al. 2015).
Role for the microbiota in neurological conditions?
A growing body of evidence, originating with studies in irritable bowel syndrome, has implicated the microbiota–gut–brain axis in the pathogenesis of stress‐related disorders (De Palma et al. 2014; Moloney et al. 2015). Recently, however, the gut microbiota has been implicated in other brain disorders including autism, schizophrenia and Parkinson's disease. There is still considerable debate and much research needed to determine whether or not gut microbiota changes are core to the pathophysiology of such conditions or are merely epiphenomenal.
Autism
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with a clear genetic basis whose prevalence is on the increase (Ziats et al. 2015). Autism is characterised by a constellation of symptoms including deficits in social behaviour, communication and interaction across multiple domains and repetitive, narrow repertoire of patterns of behaviour, interests, or activities (Chen et al. 2015). In general, symptoms emerge early in the developmental period and significantly impair social and occupational functioning. Although genetics is key in autism pathogenesis there is a very strong gene–environment interaction at play with over 50% of the neurobiology driven by non‐heritable factors (Chen et al. 2015). Moreover, up to 70% of patients with the syndrome co‐present with gastrointestinal symptoms and hence the view that a disruption of the gut–brain axis is involved (Mayer et al. 2014b).
Another facet contributing to social deficits in this condition is a lack of social recognition, a symptom which can be modelled in animals. Our group examined the behaviour of germ‐free mice in the three‐chamber test, a well‐validated assay to assess social behaviour (Moy et al. 2004), where a germ‐free mouse was placed in the centre chamber with a familiar mouse in one chamber and a novel mouse in the other chamber (Desbonnet et al. 2014). Interestingly, germ‐free mice spent as much time with the familiar as with the novel mouse; this is in contrast to the behaviour of conventionally colonised mice who spend more time with the novel than the familiar mouse. Germ‐free mice are more likely to spend time with an object or an empty chamber than with another mouse, a decidedly abnormal behaviour for a sociable animal. Colonisation of the germ‐free mice does partially normalise their behavioural patterns especially in the context of sociability deficits and increased repetitive behaviours – hallmark traits of autism; however, social cognitive deficits remained despite colonisation (Desbonnet et al. 2014). These behavioural changes are also associated with significant alterations in underlying neurochemistry and gene expression (Stilling et al. 2015; Hoban et al. 2016). These findings of social deficits in germ‐free mice have recently been replicated (Buffington et al. 2016) but opposite findings have also been reported (Arentsen et al. 2015).
Preclinical work demonstrated that the microbiota modulates behavioural and selective gastrointestinal abnormalities associated with autism and other neurodevelopmental disorders (Hsiao et al. 2013). The authors used the maternal immune activation model induced by Polyinosinic‐polycytidylic acid (poly‐IC) injection during pregnancy and found altered gastrointestinal barrier defects and microbiota alterations. Dietary administration of the human commensal Bacteroides fragilis, given three times during adolescence, was sufficient to correct gut permeability as well as stereotyped and other abnormal behaviours. Interestingly, as in our germ‐free studies following recolonisation (Desbonnet et al. 2014) social cognition deficits were insensitive to reversal by the bacteria. Furthermore, metabolomics approaches identified a number of bacteria‐derived metabolites that may be involved in the autism‐related behaviours and that were sensitive to manipulation by Bacteroides fragilis.
In humans, prenatal exposure to the mood stabiliser valproate is a major risk‐factor for autism (Christensen et al. 2013). It is of interest that de Theije and colleagues have shown that the autism‐like behavioural changes that occur in mouse models of valproate exposure are coincident with alterations in microbiota (de Theije et al. 2014). Sex differences were observed in these studies, which is in agreement with the clinical literature in autism (Young & Pfaff, 2014).
Clinically, a number of relatively underpowered studies investigating the faecal microbiota in patients with autism spectrum disorder have been reported (Mayer et al. 2014b; Rosenfeld, 2015). Tomova and colleagues examined the microbiota in Slovakian children and noted a significant decrease of the Bacteroidetes/Firmicutes ratio and increases in the amount of Lactobacillus spp. and a modest elevation in Desulfovibrio spp. in the faecal microbiota (Tomova et al. 2015). Administration of a probiotic diet was shown to normalise the Bacteroidetes/Firmicutes ratio and Desulfovibrio spp. levels in these children. In a recent study no significant difference in microbiota diversity or composition was detected between autistic children with their neurotypical siblings (Son et al. 2015). On analysis of the 16S rRNA sequencing data an increase in the low abundance Chloroplast genus was observed in ASD. However, the authors caution that such changes could reflect a relatively high ingestion of chia seeds by these children reinforcing the strong link between diet and microbiome analysis and the need for dietary information in any cross‐sectional or intervention study of the microbiome in human samples. As recently summarised by Mayer and colleagues there is a paucity of large comprehensive studies of the microbiome in autism (Mayer et al. 2014b). Again the issue of chicken or egg emerges; are these changes induced by stereotyped diets seen in many individuals as a product of obsessional behaviour patterns? Also the heterogeneous nature of the disease needs to be taken into account and much more effort is needed to tease out the precise role of the microbiome in both the aetiology and treatment of the disorder.
Increasing attention is currently being paid to oxytocin, the hypothalamic peptide which has been shown to increase sociability. The oxytocin receptor knockout mouse shows considerable deficits in social behaviour (Chini et al. 2014), and intriguingly, a recent study demonstrated that a probiotic bacterium (Lactobacillus reutri) can influence hypothalamic posterior pituitary activity and increase oxytocin levels raising the possibility of influencing social behaviour by targeting the gut microbiota (Erdman & Poutahidis, 2014). Moreover, a recent study found that in a mouse model of maternal obesity (a risk factor for autism in humans) there were alterations in social behaviour, oxytocin cell numbers, synaptic plasticity and microbiota composition. Remarkably, Lactobacillus reuteri could reverse these changes (Buffington et al. 2016). Some preliminary studies in humans indicate that intra‐nasally administered oxytocin may positively alter social behaviour patterns (Yatawara et al. 2016), and a series of large clinical trials are underway to test oxytocin and related therapies for autism spectrum disorder (Shen, 2015). However, there is still considerable debate as to whether preclinical findings of an oxytocin‐mediated increase in social behaviour will translate to the clinical setting, and if they do, which patients and which aspects of the syndrome are likely to benefit most.
Schizophrenia
Schizophrenia is another neurodevelopmental disorder and one of the most debilitating illnesses affecting relatively young people, usually commencing in the late teens and early twenties (Miyamoto et al. 2012). There is a global prevalence of around 1% and the condition is characterised by altered thought processes (delusions and hallucinations) and frequently a deterioration in cognitive functioning. For most patients the condition is life‐long, often with chronic psychosocial deterioration. Current treatments, which primarily target the dopaminergic system, are either ineffective or only partially effective in many patients. There is thus a major requirement for the emergence of alternative, more effective therapeutic targets.
The microbiota has come under the spotlight in relation to the condition. Certain drugs such as phencyclidine (PCP) are used to model schizophrenia‐like syndromes (Moghaddam & Javitt, 2012). In rodents sub‐chronic PCP treatment induces cognitive deficits and hyperlocomotor activity. Recently it was found that in rodents, sub‐chronic PCP significantly altered the gut microbiota and that such changes correlated highly with memory performance (Pyndt Jorgensen et al. 2015). Interestingly, administration of the antibiotic ampicillin blocked the PCP‐induced memory deficits. The authors speculate that the cognitive deficits seen in some patients with schizophrenia may be induced by changes in the gut microbiota. It is important to note that the atypical antipsychotic olanzapine, which targets the dopamine D4 and 5‐HT2 receptors, and is one of the most widely prescribed antipsychotics, exerts significant impact on the gut microbiota (Davey et al. 2013). Indeed, recent studies from our group (Davey et al. 2012; 2013) and others (Morgan et al. 2014; Bahr et al. 2015) point to a key role of the microbiome in manifesting antipsychotic‐induced weight gain. A growing body of literature is now focusing on the collateral impact of various medications outside of CNS drugs on microbiome composition (Lu et al. 2015; Spanogiannopoulos et al. 2016).
Reviewing the clinical literature we have argued (Dinan et al. 2014) strongly that genomic studies in schizophrenia should include a study of microbial DNA. In support of this a recent investigation has found that antibiotic therapy that alters the gut microbiota can be used to potentiate the action of antipsychotics in patients with schizophrenia (Khodaie‐Ardakani et al. 2014). What we lack at this point is any detailed analysis of the gut microbiota in patients with the disorder.
Microbiota and ageing
Over 100 years ago the Pasteur Institute's Elie Metchnikoff received the Nobel Prize for his discovery of the macrophage (Cryan & Dinan, 2015). Later in his career he focused on the concept of longevity and proposed that people lived longer in parts of Bulgaria and Eastern Europe because of the high amount of fermented foods containing lactic acid bacteria that they eat (Mackowiak, 2013). With the advent of germ‐free mice in the 1940s, which lived longer than their conventionalised controls (Gustafsson, 1946; Glimstedt, 1959), it became clear that there was a direct link between microbiota and senescence. More recently, with the ELDERMET study, the relationship between microbiota and functional outcomes in the elderly, especially in terms of frailty, are being realised (Claesson et al. 2011, 2012). Importantly, the microbiota in the elderly was shown to be strongly influenced by diet, opening up dietary‐based intervention strategies in the elderly. This has given rise to the view that healthy ageing is associated with microbial diversity (Biagi et al. 2010; Lynch et al. 2015).
There has been much focus on the neurobiological mechanisms underlying ageing. It is clear that neuroinflammatory processes play a key role in ageing, with a growing emphasis on the role of the brain's resident immune cells, the microglia (Jyothi et al. 2015). More recently, it has been shown that microglia activation is under constant regulation by the gut microbiome (Erny et al. 2015). These provocative findings suggest that it is possible to manipulate neuroimmune responses by targeting the gut microbiome. In particular, bacterial metabolites, especially short chain fatty acids, are crucial to these effects.
Another consequence of ageing and age‐related disorders such as Alzheimer's disease is the progressive leakiness of the blood–brain barrier (BBB). In a very provocative finding Braniste and colleagues have shown, using a variety of techniques, that the integrity of the BBB is dependent on appropriate microbiota composition in the gut (Braniste et al. 2014). Once again short chain fatty acids are key metabolites in mediating such effects.
It is clear that stress can have marked effects on microbiota (Moloney et al. 2014) and the impact of stress on the ageing brain can be particularly pernicious (Prenderville et al. 2015). Both ageing and stress can weaken gastrointestinal barrier function and drive a proinflammatory phenotype via the microbiota (Kelly et al. 2015). In addition both ageing and stress can also negatively impact BBB permeability (Esposito et al. 2002; Montagne et al. 2015). The consequences of both barriers being compromised has the potential to accelerate ‘inflamm‐aging’ processes in the brain. Understanding the mechanisms underlying how the microbiome can influence such processes is now worthy of attention.
Parkinson's disease, Alzheimer's disease and cognitive impairment
Parkinson's disease is generally a disorder seen in the elderly and characterised by degeneration of the dopaminergic nigro‐striatal pathway, with a characteristic pattern of abnormal movements. Studies indicate that the enteric nervous system is frequently involved due to the effects of α‐synuclein (Miraglia et al. 2015). It is well established that alteration in bowel function, mainly in the form of constipation, can precede the onset of prototypical motor symptoms. Svensson and colleagues reviewed all Danish patients who underwent vagotomy over the period of 1977–1995 and compared them with a matched general population cohort (Svensson et al. 2015). They explored the risk of developing Parkinson's disease following a full truncal vagotomy or a selective vagotomy. The risk of developing Parkinson's disease was significantly decreased in patients who underwent a full truncal vagotomy compared to those who underwent a selective vagotomy. The latter had a risk similar to that of the general population. These data offer the suggestion that the vagus nerve may be critically involved in the pathogenesis of the disorder. This is particularly important given the key role of the vagus nerve in mediating microbiome‐to‐brain signalling (Bravo et al. 2011). However, it is worth noting that vagotomy also affects efferent vagal signalling, which has important anti‐inflammatory effects (Olofsson et al. 2012). Moreover, vagotomy also slows down gastrointestinal motor function that can lead to bacterial overgrowth (Grace et al. 2013), which also may complicate interpretation of vagotomy experiments.
In the first study of its kind the gut microbiota has recently been sequenced in patients with Parkinson's disease (Scheperjans et al. 2015). The microbiota of 72 patients and 72 matched controls were pyrosequenced. There was a major reduction in the levels of Prevotellaceae in the patients. There was a positive association between the levels of Enterobacteriaceae and the severity of postural instability and gait difficulty. The authors point out that their study does not address either the temporal or causal relationship between the gut microbiota and the core features of the disease. Another analysis of microbiota composition in Parkinson's disease (PD) pointed to a reduction in butyrate‐producing bacteria (Blautia, Coprococcus and Roseburia) in faeces and Faecalibacterium in the mucosa. This was coincident with an increase in Ralstonia in mucosal samples more abundant in mucosa of PD than controls (Keshavarzian et al. 2015). While some have argued that microbiota transplantation might benefit patients there is certainly no conclusive evidence as yet. Neither are there any reports of controlled trials of probiotics. It is clear that much more research is needed to determine the relative role of the microbiome in Parkinson's disease (Dobbs et al. 2016; Felice et al. 2016).
Alzheimer's disease and vascular dementias are the most common causes of cognitive decline in ageing populations in Western countries. The hippocampus plays a major role in information processing and memory storage, with long‐term potentiation (LTP) – the physiological process thought to underlie cognitive events in the hippocampus – disrupted in animal models of Alzheimer's disease (Lynch, 2004). Studies in rodents indicate that LTP begins to decline in middle age but most dramatically in ageing animals (Lynch, 2004).
VSL#3 is a widely studied probiotic mixture of eight different gram‐positive bacterial strains. When aged animals were treated with this combination the microbiota showed a significant change, with increases in Actinobacteria and Bacteroidetes, both of which were reduced in vehicle‐treated animals (Distrutti et al. 2014). The age‐related attenuation of LTP was decreased in the VSL#3‐treated animals. Furthermore, microglial activation was decreased, the pivotal trophic factor BDNF was increased, and a gene array found alterations in the expression of inflammation and neuronal plasticity‐related genes. More recently we have shown that a bifidobacterium B. longum 1714 had a positive impact on cognition in the mouse (Savignac et al. 2015). These results are of interest but clearly require translation in humans.
Surprisingly, a detailed analysis of the microbiota in patients with Alzheimer's disease is lacking (Alam et al. 2014). However, in type 2 diabetes (T2D), which is a risk factor for Alzheimer's disease, there is alteration in the gut microbiota (Allin et al. 2015; He et al. 2015). This is seen as an important feature of this condition, but whether it is important in Alzheimer's disease or not is an open question.
More recently, preliminary data published in preprint format has implicated the microbiota in the accumulation of amyloid plaques in a mouse model of Alzheimer's disease (Harach et al. 2015). In this study the authors generated a transgenic Alzheimer's disease mouse model under germ‐free conditions and found a dramatic reduction of cerebral Aβ amyloid pathology when compared to control Alzheimer's disease animals, which had a normal intestinal microbiota, albeit one different to healthy, wild‐type mice. Most intriguingly, colonisation of germ‐free Alzheimer's disease mice with microbiota harvested from conventionally raised Alzheimer's disease mice dramatically increased cerebral Aβ pathology. Also in a similar vein, antibiotic treatment has been recently shown to limit Aβ pathology and neuroinflammation (Minter et al. 2016). Simultaneously, a growing body of research is investigating the microbial basis for triggering Aβ pathology (Kumar et al. 2016). Together, these data offer hope for the future generation of a novel microbiota‐based approach to ameliorate symptoms of Alzheimer's disease.
Conclusions
Understanding the manner in which gut microbes influence gut–brain axis communication has been the subject of considerable research effort in recent times. It is now generally accepted that the gut microbiota influences psychological processes such as the stress response and cognition (Dinan et al. 2015). Whether changes in the microbiota are central to the pathophysiology of at least some psychiatric disorders is unproven, though widely speculated upon. Evidence is accumulating for a role for the gut microbiota in autism and age‐related disorders such as Parkinson's and Alzheimer's diseases. Future work must investigate whether or not the exciting data that have largely emerged from animal work can be translated to humans, especially given the different diets and microbiota composition between species. Moreover, the exact mechanisms underlying the communication between microbiome and brain need to be further elucidated.
Additional information
Competing interests
None declared.
Funding
The authors are supported in part by Science Foundation Ireland in the form of a centre grant (Alimentary Pharmabiotic Centre Grant Number SFI/12/RC/2273); by the Health Research Board of Ireland (Grant Numbers HRA_POR/2011/23 and HRA_POR/2012/32) and received funding from the European Community's Seventh Framework Programme Grant MyNewGut under Grant Agreement No. FP7/2007–2013. The Centre has conducted studies in collaboration with several companies including GSK, Pfizer, Cremo, Suntory, Wyeth 4D Pharma, Danone and Mead Johnson. T.G.D. has until recently been on the Board of Alimentary Health.
Acknowledgements
We would like to thank Dr Roman Stilling for producing the figure and Dr Kieran Rea for editorial assistance and comments on the paper.
Biography
John F. Cryan is Professor and Chair, Department of Anatomy and Neuroscience and a Principal Investigator in the APC Microbiome Institute. University College Cork His research is focused on understanding the interaction between brain, gut and microbiome and how it applies to stress, psychiatric and immune‐related disorders at key time‐windows across the lifespan. Professor Cryan has published over 340 peer‐reviewed articles and is President‐elect of the European Behavioural Pharmacology Society. Timothy G. (Ted) Dinan is Professor of Psychiatry and a Principal Investigator in the APC Microbiome Institute at University College Cork. He has worked in research laboratories on both sides of the Atlantic. His main research interest is in the role of the gut microbiota in stress‐related disorders. He has also worked extensively on the regulation of the hypothalamic‐pituitary‐adrenal axis. He has published over 400 papers and numerous books on pharmacology and neurobiology.

This is an Editor's Choice article from the 15 January 2017 issue.
References
- Alam MZ, Alam Q, Kamal MA, Abuzenadah AM & Haque A (2014). A possible link of gut microbiota alteration in type 2 diabetes and Alzheimer's disease pathogenicity: an update. CNS Neurol Disord Drug Targets 13, 383–390. [DOI] [PubMed] [Google Scholar]
- Al‐Asmakh M & Zadjali F (2015). Use of germ‐free animal models in microbiota‐related research. J Microbiol Biotechnol 25, 1583–1588. [DOI] [PubMed] [Google Scholar]
- Allin KH, Nielsen T & Pedersen O (2015). Mechanisms in endocrinology: Gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol 172, R167–R177. [DOI] [PubMed] [Google Scholar]
- Arrieta MC, Walter J, Finlay BB (2016). Human microbiota‐associated mice: a model with challenges. Cell Host Microbe 19, 575–578. [DOI] [PubMed] [Google Scholar]
- Arentsen T, Raith H, Qian Y, Forssberg H & Diaz Heijtz R (2015). Host microbiota modulates development of social preference in mice. Microb Ecol Health Dis 26, 29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz Q & Thompson DG (1998). Brain‐gut axis in health and disease. Gastroenterology 114, 559–578. [DOI] [PubMed] [Google Scholar]
- Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva‐Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al‐Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J & Wang J (2015). Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 852. [DOI] [PubMed] [Google Scholar]
- Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Teesch LM, Oltman CL, Azcarate‐Peril MA, Kirby JR & Calarge CA (2015). Use of the second‐generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry 5, e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E, Deshpandey AK, Ryan CA, Dempsey EM, Murphy B, O'Sullivan L, Watkins C, Ross RP, O'Toole PW, Fitzgerald GF & Stanton C (2015). The neonatal gut harbours distinct bifidobacterial strains. Arch Dis Child Fetal Neonatal Ed 100, F405–F410. [DOI] [PubMed] [Google Scholar]
- Barrett E, Guinane CM, Ryan CA, Dempsey EM, Murphy BP, O'Toole PW, Fitzgerald GF, Cotter PD, Ross RP & Stanton C (2013). Microbiota diversity and stability of the preterm neonatal ileum and colon of two infants. Microbiologyopen 2, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Collins SM & Verdu EF (2012). Microbes and the gut‐brain axis. Neurogastroenterol Motil 24, 405–413. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF & Collins SM (2011a). The intestinal microbiota affect central levels of brain‐derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609.e3. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM & Verdu EF (2011b). The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut‐brain communication. Neurogastroenterol Motil 23, 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, Capri M, Brigidi P & Candela M (2016). Gut microbiota and extreme longevity. Curr Biol 26, 1480–1485. [DOI] [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C, Brigidi P & De Vos W (2010). Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5, e10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser MJ (2014). The microbiome revolution. J Clin Invest 124, 4162–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein SR & Theis KR (2015). Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol 13, e1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF (2014). Treds Mol Med 20, 509–518. [DOI] [PubMed] [Google Scholar]
- Braniste V, Al‐Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyas B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B & Pettersson S (2014). The gut microbiota influences blood‐brain barrier permeability in mice. Sci Transl Med 6, 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J & Cryan JF (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108, 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF & Costa‐Mattioli M (2016). Microbial reconstitution reverses maternal diet‐induced social and synaptic deficits in offspring. Cell 165, 1762–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfora EE, Jocken JW & Blaak EE (2015). Short‐chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 11, 577–591. [DOI] [PubMed] [Google Scholar]
- Chen JA, Penagarikano O, Belgard TG, Swarup V & Geschwind DH (2015). The emerging picture of autism spectrum disorder: genetics and pathology. Annu Rev Pathol 10, 111–144. [DOI] [PubMed] [Google Scholar]
- Chini B, Leonzino M, Braida D & Sala M (2014). Learning about oxytocin: pharmacologic and behavioral issues. Biol Psychiatry 76, 360–366. [DOI] [PubMed] [Google Scholar]
- Christensen J, Grønborg TK, Sørensen MJ, Schendel D, Parner ET, Pedersen LH & Vestergaard M (2013). Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309, 1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Cusack S, O'Sullivan O, Greene‐Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, van Sinderen D, O'Connor M, Harnedy N, O'Connor K, Henry C, O'Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Ross RP & O'Toole PW (2011). Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 108, Suppl. 1, 4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, Fitzgerald GF, Deane J, O'Connor M, Harnedy N, O'Connor K, O'Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP & O'Toole PW (2012). Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184. [DOI] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG & Cryan JF (2013). The microbiome‐gut‐brain axis during early life regulates the hippocampal serotonergic system in a sex‐dependent manner. Mol Psychiatry 18, 666–673. [DOI] [PubMed] [Google Scholar]
- Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B, Magris M, Hidalgo G, Contreras M, Noya‐Alarcón O, Lander O, McDonald J, Cox M, Walter J, Oh PL, Ruiz JF, Rodriguez S, Shen N, Song SJ, Metcalf J, Knight R, Dantas G & Dominguez‐Bello MG (2015). The microbiome of uncontacted Amerindians. Sci Adv 1, e1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado MC, Cernada M, Baüerl C, Vento M & Pérez‐Martínez G (2012). Microbial ecology and host‐microbiota interactions during early life stages. Gut Microbes 3, 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM, Surette M & Bercik P (2012). The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10, 735–742. [DOI] [PubMed] [Google Scholar]
- Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ & Relman DA (2012). The application of ecological theory toward an understanding of the human microbiome. Science 336, 1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF & Dinan TG (2015). Gut microbiota: Microbiota and neuroimmune signalling – Metchnikoff to microglia. Nat Rev Gastroenterol Hepatol 12, 494–496. [DOI] [PubMed] [Google Scholar]
- Davey KJ, O’Mahony SM, Schellekens H, O’Sullivan O, Bienenstock J, Cotter PD, Dinan TG, Cryan JF (2012). Gender‐dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (Berl) 221, 155–169. [DOI] [PubMed] [Google Scholar]
- Davey KJ, Cotter PD, O'Sullivan O, Crispie F, Dinan TG, Cryan JF & O'Mahony SM (2013). Antipsychotics and the gut microbiome: olanzapine‐induced metabolic dysfunction is attenuated by antibiotic administration in the rat. Transl Psychiatry 3, e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ & Turnbaugh PJ (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma G, Collins SM, Bercik P & Verdu EF (2014). The microbiota–gut–brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J Physiol 592, 2989–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG & Cryan JF (2014). Microbiota is essential for social development in the mouse. Mol Psychiatry 19, 146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O'Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG & Cryan JF (2015). Gut microbiota depletion from early adolescence in mice: Implications for brain and behavior. Brain Behav Immun 48, 165–173. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Bienenstock J & Dinan TG (2008). The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res 43, 164–174. [DOI] [PubMed] [Google Scholar]
- de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, Garssen J, Kraneveld AD & Oozeer R (2014). Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun 37, 197–206. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H & Pettersson S (2011). Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 108, 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Borre YE & Cryan JF (2014). Genomics of schizophrenia: time to consider the gut microbiome? Mol Psychiatry 19, 1252–1257. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Stilling RM, Stanton C, Cryan JF (2015). Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res 63, 1–9. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Quigley EM, Ahmed SM, Scully P, O'Brien S, O'Mahony L, O'Mahony S, Shanahan F & Keeling PW (2006). Hypothalamic‐pituitary‐gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology 130, 304–311. [DOI] [PubMed] [Google Scholar]
- Distrutti E, O'Reilly JA, McDonald C, Cipriani S, Renga B, Lynch MA & Fiorucci S (2014). Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age‐related deficit in LTP. PLoS One 9, e106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs SM, Dobbs RJ, Weller C, Charlett A, Augustin A, Taylor D, Ibrahim MA & Bjarnason I (2016). Peripheral aetiopathogenic drivers and mediators of Parkinson's disease and co‐morbidities: role of gastrointestinal microbiota. J Neurovirol 22, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez‐Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N & Knight R (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 107, 11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnet‐Hughes A, Perez PF, Dore J, Leclerc M, Levenez F, Benyacoub J, Serrant P, Segura‐Roggero I & Schiffrin EJ (2010). Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc Nutr Soc 69, 407–415. [DOI] [PubMed] [Google Scholar]
- El Aidy S, Dinan TG, Cryan JF (2014). Immune modulation of the brain‐gut‐microbiota axis. Front Microbiol 5, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Aidy S, Dinan TG & Cryan JF (2015). Gut microbiota: The conductor in the orchestra of immune‐neuroendocrine communication. Clin Ther 37, 954–967. [DOI] [PubMed] [Google Scholar]
- Erdman SE & Poutahidis T (2014). Probiotic ‘glow of health’: it's more than skin deep. Benef Microbes 5, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren‐Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermohlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I & Prinz M (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18, 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, Connolly R, Tutor D & Theoharides TC (2002). Corticotropin‐releasing hormone and brain mast cells regulate blood‐brain‐barrier permeability induced by acute stress. J Pharmacol Exp Ther 303, 1061–1066. [DOI] [PubMed] [Google Scholar]
- Faith JJ, Ahern PP, Ridaura VK, Cheng J & Gordon JI (2014). Identifying gut microbe‐host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med 6, 220ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi A, Banan A, Fields J & Keshavarzian A (2003). Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol 18, 479–497. [DOI] [PubMed] [Google Scholar]
- Fasano A (2012). Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol 10, 1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice VD, Quigley EM, Sullivan AM, O'Keeffe GW & O'Mahony SM (2016). Microbiota‐gut‐brain signalling in Parkinson's disease: Implications for non‐motor symptoms. Parkinsonism Relat Disord 27, 1–8. [DOI] [PubMed] [Google Scholar]
- Foster JA, Lyte M, Meyer E & Cryan JF (2016). Gut microbiota and brain function: An evolving field in neuroscience. Int J Neuropsychopharmacol 19, pyv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacias M, Gaspari S, Santos PM, Tamburini S, Andrade M, Zhang F, Shen N, Tolstikov V, Kiebish MA, Dupree JL, Zachariou V, Clemente JC & Casaccia P (2016). Microbiota‐driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. Elife 5, e13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland L (2014). The gut microbiome and the brain. J Med Food 17, 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG (2014). Microbiota‐gut‐brain axis and cognitive function. Adv Exp Med Biol 817, 357–371. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G & Sherman PM (2011). Bacterial infection causes stress‐induced memory dysfunction in mice. Gut 60, 307–317. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, Jansson JK, Dorrestein PC & Knight R (2016). Microbiome‐wide association studies link dynamic microbial consortia to disease. Nature 535, 94–103. [DOI] [PubMed] [Google Scholar]
- Glimstedt G (1959). The germfree animal as a research tool. Ann N Y Acad Sci 78, 281–284. [DOI] [PubMed] [Google Scholar]
- Goedert JJ, Hua X, Yu G & Shi J (2014). Diversity and composition of the adult fecal microbiome associated with history of cesarean birth or appendectomy: Analysis of the American Gut Project. EBioMedicine 1, 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace E, Shaw C, Whelan K & Andreyev HJ (2013). Review article: small intestinal bacterial overgrowth – prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment Pharmacol Ther 38, 674–688. [DOI] [PubMed] [Google Scholar]
- Grenham S, Clarke G, Cryan JF & Dinan TG (2011). Brain‐gut‐microbe communication in health and disease. Front Physiol 2, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover M & Kashyap PC (2014). Germ‐free mice as a model to study effect of gut microbiota on host physiology. Neurogastroenterol Motil 26, 745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B (1946). Germ‐free rearing of rats. Acta Anat (Basel) 2, 376–391. [DOI] [PubMed] [Google Scholar]
- Harach T, Marungruang N, Dutilleul N, Cheatham V, Mc Coy KD, Neher JJ, Jucker M, Fåk F, Lasser T, Bolmont T (2015). Reduction of Alzheimer's disease beta‐amyloid pathology in the absence of gut microbiota. Available at: https://arxiv.org/ftp/arxiv/papers/1509/1509.02273.pdf.
- He C, Shan Y & Song W (2015). Targeting gut microbiota as a possible therapy for diabetes. Nutr Res 35, 361–367. [DOI] [PubMed] [Google Scholar]
- Hinde K & Lewis ZT (2015). MICROBIOTA. Mother's littlest helpers. Science 348, 1427–1428. [DOI] [PubMed] [Google Scholar]
- Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G & Cryan JF (2016). Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 6, e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH & Mazmanian SK (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH & Tye KM (2015). From circuits to behaviour in the amygdala. Nature 517, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Sjovall H & Hansson GC (2013). The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 10, 352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyothi HJ, Vidyadhara DJ, Mahadevan A, Philip M, Parmar SK, Manohari SG, Shankar SK, Raju TR & Alladi PA (2015). Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta. Neurobiol Aging 36, 3321–3333. [DOI] [PubMed] [Google Scholar]
- Kamada N, Seo SU, Chen GY & Nunez G (2013). Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13, 321–335. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G & Hyland NP (2015). Breaking down the barriers: the gut microbiome, intestinal permeability and stress‐related psychiatric disorders. Front Cell Neurosci 9, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E & Shannon KM (2015). Colonic bacterial composition in Parkinson's disease. Mov Disord 30, 1351–1360. [DOI] [PubMed] [Google Scholar]
- Khodaie‐Ardakani MR, Mirshafiee O, Farokhnia M, Tajdini M, Hosseini SM, Modabbernia A, Rezaei F, Salehi B, Yekehtaz H, Ashrafi M, Tabrizi M & Akhondzadeh S (2014). Minocycline add‐on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized double‐blind placebo‐controlled study. Psychiatry Res 215, 540–546. [DOI] [PubMed] [Google Scholar]
- Koh A, De Vadder F, Kovatcheva‐Datchary P & Backhed F (2016). From dietary fibre to host physiology: short‐chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345. [DOI] [PubMed] [Google Scholar]
- Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE & Moir RD (2016). Amyloid‐β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med 8, 340ra372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankelma JM, Nieuwdorp M, de Vos WM & Wiersinga WJ (2015). The gut microbiota in internal medicine: implications for health and disease. Neth J Med 73, 61–68. [PubMed] [Google Scholar]
- Leach J (2013). Gut microbiota: Please pass the microbes. Nature 504, 33. [DOI] [PubMed] [Google Scholar]
- LeDoux J (2007). The amygdala. Curr Biol 17, R868–874. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH & Kipnis J (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Mahbub R & Fox JG (2015). Xenobiotics: Interaction with the intestinal microflora. ILAR J 56, 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, McVey Neufeld KA, Oriach CS, Clarke G, Dinan TG & Cryan JF (2016a). Growing up in a bubble: using germ‐free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol 19, pyw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, Whelan SO, O'Sullivan C, Clarke G, Shanahan F, Dinan TG & Cryan JF (2016b). Adult microbiota‐deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci (in press; DOI: 10.1111/ejn.13291). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA (2004). Long‐term potentiation and memory. Physiol Rev 84, 87–136. [DOI] [PubMed] [Google Scholar]
- Lynch DB, Jeffery IB, Cusack S, O'Connor EM & O'Toole PW (2015). Diet‐microbiota‐health interactions in older subjects: implications for healthy aging. Interdiscip Top Gerontol 40, 141–154. [DOI] [PubMed] [Google Scholar]
- Lyte M (2013). Microbial endocrinology in the microbiome‐gut‐brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog 9, e1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M (2014). Microbial endocrinology: Host‐microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 5, 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak PA (2013). Recycling Metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Health 1, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez I, Stegen JC, Maldonado‐Gomez MX, Eren AM, Siba PM, Greenhill AR & Walter J (2015). The gut microbiota of rural papua new guineans: composition, diversity patterns, and ecological processes. Cell Rep 11, 527–538. [DOI] [PubMed] [Google Scholar]
- Matamoros S, Gras‐Leguen C, Le Vacon F, Potel G & de La Cochetiere MF (2013). Development of intestinal microbiota in infants and its impact on health. Trends Microbiol 21, 167–173. [DOI] [PubMed] [Google Scholar]
- Mayer EA (2011). Gut feelings: the emerging biology of gut‐brain communication. Nat Rev Neurosci 12, 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Knight R, Mazmanian SK, Cryan JF & Tillisch K (2014a). Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 34, 15490–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Padua D & Tillisch K (2014b). Altered brain‐gut axis in autism: comorbidity or causative mechanisms? Bioessays 36, 933–939. [DOI] [PubMed] [Google Scholar]
- Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler‐Castrillo P, Musch MW, Liao F, Ward JF, Holtzman DM, Chang EB, Tanzi RE & Sisodia SS (2016). Antibiotic‐induced perturbations in gut microbial diversity influences neuro‐inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep 6, 30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraglia F, Betti L, Palego L & Giannaccini G (2015). Parkinson's disease and alpha‐synucleinopathies: from arising pathways to therapeutic challenge. Cent Nerv Syst Agents Med Chem 15, 109–116. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW & Lieberman JA (2012). Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry 17, 1206–1227. [DOI] [PubMed] [Google Scholar]
- Moghaddam B & Javitt D (2012). From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 37, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR & Wolf SA (2016). Ly6Chi monocytes provide a link between antibiotic‐induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep 15, 1945–1956. [DOI] [PubMed] [Google Scholar]
- Moloney RD, Desbonnet L, Clarke G, Dinan TG & Cryan JF (2014). The microbiome: stress, health and disease. Mamm Genome 25, 49–74. [DOI] [PubMed] [Google Scholar]
- Moloney RD, Dinan TG & Cryan JF (2015). Stress & the microbiota‐gut‐brain axis in visceral pain. Psychoneuroendocrinology 61, 8. [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M & Zlokovic BV (2015). Blood‐brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, Bogue MA, Paredes SH, Yourstone S, Carroll IM, Kawula TH, Bower MA, Sartor RB & Sullivan PF (2014). The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One 9, e115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Cody‐Hazlett H, Poe MD, Gerig G, Gimpel‐Smith R & Piven J (2009). Longitudinal study of amygdala volume and joint attention in 2‐ to 4‐year‐old children with autism. Arch Gen Psychiatry 66, 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J & Crawley JN (2004). Sociability and preference for social novelty in five inbred strains: an approach to assess autistic‐like behavior in mice. Genes Brain Behav 3, 287–302. [DOI] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J & Foster JA (2011). Reduced anxiety‐like behavior and central neurochemical change in germ‐free mice. Neurogastroenterol Motil 23, 255–264, e119. [DOI] [PubMed] [Google Scholar]
- Nguyen TL, Vieira‐Silva S, Liston A & Raes J (2015). How informative is the mouse for human gut microbiota research? Dis Model Mech 8, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF & O'Leary OF (2015). Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry 78, e7–9. [DOI] [PubMed] [Google Scholar]
- O’Leary OF, Cryan JF (2014). A ventral view on antidepressant action: roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends in Pharmacological Sciences 35, 675–687. [DOI] [PubMed] [Google Scholar]
- Olofsson PS, Rosas‐Ballina M, Levine YA & Tracey KJ (2012). Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev 248, 188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony SM, Clarke G, Borre YE, Dinan TG & Cryan JF (2015a). Serotonin, tryptophan metabolism and the brain‐gut‐microbiome axis. Behav Brain Res 277, 32–48. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Clarke G, Dinan TG & Cryan JF (2015b). Early‐life adversity and brain development: Is the microbiome a missing piece of the puzzle? Neuroscience (in press; DOI: 10.1016/j.neuroscience.2015.09.068). [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Felice VD, Nally K, Savignac HM, Claesson MJ, Scully P, Woznicki J, Hyland NP, Shanahan F, Quigley EM, Marchesi JR, O'Toole PW, Dinan TG & Cryan JF (2014). Disturbance of the gut microbiota in early‐life selectively affects visceral pain in adulthood without impacting cognitive or anxiety‐related behaviors in male rats. Neuroscience 277, 885–901. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Hyland NP, Dinan TG & Cryan JF (2011). Maternal separation as a model of brain‐gut axis dysfunction. Psychopharmacology (Berl) 214, 71–88. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF & Dinan TG (2009). Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illesses. Biol Psychiatry 65, 263–267. [DOI] [PubMed] [Google Scholar]
- O'Toole PW & Jeffery IB (2015). Gut microbiota and aging. Science 350, 1214–1215. [DOI] [PubMed] [Google Scholar]
- Paul B, Barnes S, Demark‐Wahnefried W, Morrow C, Salvador C, Skibola C & Tollefsbol TO (2015). Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin Epigenetics 7, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenderville JA, Kennedy PJ, Dinan TG, Cryan JF (2015). Adding fuel to the fire: the impact of stress on the ageing brain. Trends Neurosci 38, 13–25. [DOI] [PubMed] [Google Scholar]
- Pyndt Jorgensen B, Krych L, Pedersen TB, Plath N, Redrobe JP, Hansen AK, Nielsen DS, Pedersen CS, Larsen C & Sorensen DB (2015). Investigating the long‐term effect of subchronic phencyclidine‐treatment on novel object recognition and the association between the gut microbiota and behavior in the animal model of schizophrenia. Physiol Behav 141, 32–39. [DOI] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto J‐M, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz‐Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD & Wang J (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampelli S, Schnorr SL, Consolandi C, Turroni S, Severgnini M, Peano C, Brigidi P, Crittenden AN, Henry AG & Candela M (2015). Metagenome sequencing of the Hadza hunter‐gatherer gut microbiota. Curr Biol 25, 1682–1693. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C & Mayer EA (2009). Principles and clinical implications of the brain‐gut‐enteric microbiota axis. Nat Rev Gastroenterol hepatol 6, 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondanelli M, Giacosa A, Faliva MA, Perna S, Allieri F & Castellazzi AM (2015). Review on microbiota and effectiveness of probiotics use in older. World J Clin Cases 3, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS (2015). Microbiome disturbances and autism spectrum disorders. Drug Metab Dispos 43, 1557–1571. [DOI] [PubMed] [Google Scholar]
- Sampson TR & Mazmanian SK (2015). Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17, 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar SA, Lagier JC, Pontarotti P, Raoult D & Fournier PE (2015). The human gut microbiome, a taxonomic conundrum. Syst Appl Microbiol 38, 276–286. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Tramullas M, Kiely B, Dinan TG & Cryan JF (2015). Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav Brain Res 287, 59–72. [DOI] [PubMed] [Google Scholar]
- Scaldaferri F, Pizzoferrato M, Gerardi V, Lopetuso L & Gasbarrini A (2012). The gut barrier: new acquisitions and therapeutic approaches. J Clin Gastroenterol 46, Suppl., S12–17. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola‐Rautio J, Pohja M, Kinnunen E, Murros K & Auvinen P (2015). Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord 30, 350–358. [DOI] [PubMed] [Google Scholar]
- Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, Turroni S, Biagi E, Peano C, Severgnini M, Fiori J, Gotti R, De Bellis G, Luiselli D, Brigidi P, Mabulla A, Marlowe F, Henry AG & Crittenden AN (2014). Gut microbiome of the Hadza hunter‐gatherers. Nat Commun 5, 3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM & Amaral DG (2006). Stereological analysis of amygdala neuron number in autism. J Neurosci 26, 7674–7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LV, Clarke G & Dinan TG (2013). The brain‐gut axis: a target for treating stress‐related disorders. Mod Trends Pharmacopsychiatri 28, 90–99. [DOI] [PubMed] [Google Scholar]
- Sender R, Fuchs S & Milo R (2016). Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164, 337–340. [DOI] [PubMed] [Google Scholar]
- Shen HH (2015). News feature: Microbes on the mind. Proc Natl Acad Sci USA 112, 9143–9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F & Backhed F (2013). The gut microbiota – masters of host development and physiology. Nat Rev Microbiol 11, 227–238. [DOI] [PubMed] [Google Scholar]
- Sommer F & Backhed F (2016). Know your neighbor: Microbiota and host epithelial cells interact locally to control intestinal function and physiology. Bioessays 38, 455–464. [DOI] [PubMed] [Google Scholar]
- Son JS, Zheng LJ, Rowehl LM, Tian X, Zhang Y, Zhu W, Litcher‐Kelly L, Gadow KD, Gathungu G, Robertson CE, Ir D, Frank DN & Li E (2015). Comparison of fecal microbiota in children with autism spectrum disorders and neurotypical siblings in the Simons Simplex Collection. PLoS One 10, e0137725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF & Artis D (2012). Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity 37, 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS & Sonnenburg JL (2016). Diet‐induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanogiannopoulos P, Bess EN, Carmody RN & Turnbaugh PJ (2016). The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol 14, 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilling RM, Dinan TG & Cryan JF (2014). Microbial genes, brain & behaviour ‐ epigenetic regulation of the gut‐brain axis. Genes Brain Behav 13, 69–86. [DOI] [PubMed] [Google Scholar]
- Stilling RM, Ryan FJ, Hoban AE, Shanahan F, Clarke G, Claesson MJ, Dinan TG & Cryan JF (2015). Microbes & neurodevelopment – Absence of microbiota during early life increases activity‐related transcriptional pathways in the amygdala. Brain Behav Immun 50, 209–220. [DOI] [PubMed] [Google Scholar]
- Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG & Cryan JF (2016). The neuropharmacology of butyrate: The bread and butter of the microbiota‐gut‐brain axis? Neurochem Int 99, 110–132. [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C & Koga Y (2004). Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol 558, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E, Horvath‐Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P & Sorensen HT (2015). Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol 78, 522–529. [DOI] [PubMed] [Google Scholar]
- Tillisch K (2014). The effects of gut microbiota on CNS function in humans. Gut Microbes 5, 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K & Ostatnikova D (2015). Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav 138, 179–187. [DOI] [PubMed] [Google Scholar]
- Vandenplas Y (2015). Healthy gut microbiota and long term health. Benef Microbes 6, 173–179. [DOI] [PubMed] [Google Scholar]
- Voreades N, Kozil A & Weir TL (2014). Diet and the development of the human intestinal microbiome. Front Microbiol 5, 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG & Stanton C (2014). Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol 817, 221–239. [DOI] [PubMed] [Google Scholar]
- Wang Y & Kasper LH (2014). The role of microbiome in central nervous system disorders. Brain Behav Immun 38, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I, Corwin EJ, Brennan PA, Jordan S, Murphy JR & Dunlop A (2016). The infant microbiome: Implications for infant health and neurocognitive development. Nurs Res 65, 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassour M, Vatanen T, Siljander H, Hamalainen AM, Harkonen T, Ryhanen SJ, Franzosa EA, Vlamakis H, Huttenhower C, Gevers D, Lander ES, Knip M, Group DS & Xavier RJ (2016). Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 8, 343ra381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA & Guastella AJ (2016). The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry 21, 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ & Pfaff DW (2014). Sex differences in neurological and psychiatric disorders. Front Neuroendocrinol 35, 253–254. [DOI] [PubMed] [Google Scholar]
- Zapata HJ & Quagliarello VJ (2015). The microbiota and microbiome in aging: potential implications in health and age‐related diseases. J Am Geriatr Soc 63, 776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziats MN, Grosvenor LP & Rennert OM (2015). Functional genomics of human brain development and implications for autism spectrum disorders. Transl Psychiatry 5, e665. [DOI] [PMC free article] [PubMed] [Google Scholar]


