Abstract
The aim of this study was to determine whether maternal exposure to persistent perfluoroalkyl substances (PFASs) affect the capability to breastfeed.
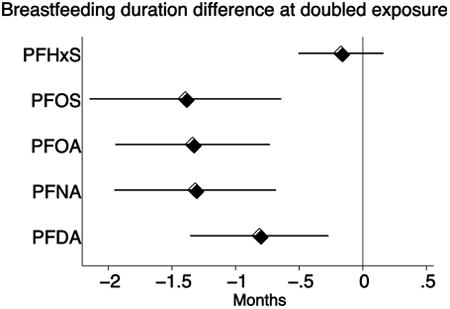
In two Faroese birth cohorts (N=1,130), concentrations of five PFASs were measured in maternal serum during pregnancy or two weeks after term. Duration of breastfeeding was assessed by questionnaire and clinical interview. In adjusted linear regression models, a doubling of maternal serum PFASs was associated with a reduction in duration of both total and exclusive breastfeeding, most pronounced for perfluorooctane sulfonic acid (PFOS) where a doubling was associated with a reduction in total breastfeeding of 1.4 (95% CI: 0.6; 2.1) months and perfluorooctanoic acid (PFOA) where a doubling was associated with a reduction in exclusive breastfeeding of 0.5 (0.3; 0.7) months. The associations were evident among both primiparous and multiparous women, and thus cannot be explained by confounding from previous breastfeeding.
Keywords: Breast milk, Breastfeeding, Endocrine disruption, Lactation, Maternal health, Perfluoroalkyl substances
Graphical abstract
1. Introduction
Breastfeeding has many positive effects on the infant as well as the mother [1]. Thus, the World Health Organization (WHO) recommends that infants are breastfed exclusively for the first six months of life and partially up to two years or beyond [2]. However, globally only 38% of infants are exclusively breastfed as recommended [3].
Initiation and duration of breastfeeding is thought to depend mainly on individual choice and societal factors [4], but recent research shows that nearly half of the mothers in a US cohort study experienced early undesired weaning [5]. Inadequate milk supply is frequently reported to be one of the leading causes for early weaning [6-8]. Although the perception of inadequate milk supply is often attributed to social and psychological factors, insufficient supply may also be real and caused by physiological factors [9].
Still, little is known about the physiological reasons for insufficient milk production, and the focus has been on hormonal abnormalities, maternal disease and contraindications [10]. Persistent environmental chemicals could potentially result in endocrine disruption that could affect the hormonal processes responsible for maternal breast milk production. For example, dichlorodiphenyldichloroethylene (DDE) has been associated with reduced duration of breastfeeding in studies carried out in the United States [11] and in Mexico [12]. In the Mexican study, differences remained after censoring of women who ceased breastfeeding for known external causes. However, the associations were confined to those who had previously breastfed, which suggests that at least some of the effect could be a result of confounding from previous breastfeeding. Thus, women who had previously breastfed for a longer period would both have lowered their current DDE concentrations and be more likely to breastfeed longer again, as compared to women who had previously breastfed only shortly [12].
Also of possible concern are the perfluoroalkyl substances (PFASs), a group of highly persistent chemicals frequently used in consumer products [13], and which are ubiquitously present in humans [14-16]. Duration of breastfeeding in Danish women was found to decrease with increasing serum-concentrations of PFASs, but since the findings were seen only in multiparous women, previous breastfeeding could have confounded the association [17].
Using data from two Faroese cohorts we aimed to examine whether exposure to the five most common PFASs was associated with the duration of breastfeeding among primiparous and multiparous women.
2. Methods
2.1 Study design
Two birth cohorts were formed in 1997–2000 [18-20] and in 2007-2009 [21] in the Faroe Islands. Located in the North Atlantic, the Faroe Islands is a self-governing marine community within the Danish kingdom. This Nordic population is approximately 50,000, rather homogenous, and has free access to health care, including obstetric care. The birth cohorts were formed to examine associations between environmental chemical exposures and adverse health outcomes. The hypothesis being tested in the present study was formulated after data collection, as triggered by a recently published study [17].
A blood sample was obtained from the mother between week 34 and 36 of pregnancy (older cohort) or two weeks after their term date (younger cohort). Background and medical information about the mother, the gestational period and the childbirth was recorded at recruitment and at subsequent follow-up. At age 5, parents of both cohorts filled out a questionnaire followed by an interview by a research nurse. The younger cohort additionally filled out a questionnaire followed by an interview at age 18 months in which information about breastfeeding (yes/no), duration of exclusive breastfeeding, and breastfeeding in addition to formula or other food sources (mixed breastfeeding) was obtained (in terms of number of months). Total duration of breastfeeding was calculated as the sum of exclusive and mixed breastfeeding. Information about total duration of breastfeeding was, however, replaced with information from the questionnaire at age 5 years if the mother stated that the child had been breast-fed for more than 18 months. In the older cohort, information about duration of total and exclusive (no other food) breastfeeding was obtained from the 5-year questionnaire only. The WHO definition of exclusive breastfeeding states that no other liquids (including water) and solids are given [22]. We did, however, not inquire about supplementation with water; only trace amounts of PFASs have been detected in Faroese water samples [23].
Information retrieved from the hospital records included maternal age (maternal birthday – child birthday), parity (zero/at least one previous birth), pregnancy smoking (none/any), pregnancy alcohol intake (none/any), pre-pregnancy body mass index (BMI: weight/height2), education (none/any education above primary school), and employment (employed including maternity leave/unemployed, under education, home maker, early retirement or sick leave). Furthermore, in the younger cohort, more detailed information about education was also obtained in a questionnaire to the mothers two weeks after their term date. If information about education was missing from the hospital records, we used information from the subsequent maternal questionnaire. Twins were excluded from our analyses.
Written informed consent was obtained from all women included in the study. The Faroese cohort study was performed in accordance with the Helsinki declaration and was approved by the Faroese ethical review committee and the institutional review board at Harvard T.H. Chan School of Public Health.
2.2 Exposure assessment
The five most common PFASs, i.e., perfluorohexane sulfonic acid (PFHxS), perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), and perfluorodecanoic acid (PFDA) were measured in maternal serum along with p,p′-DDE and polychlorinated biphenyls (PCBs). Quantitation of PFAS was carried out by isotope dilution and online solid-phase extraction followed by analysis using high-pressure liquid chromatography with tandem mass spectrometry [24]. PFOS was quantified by integration of two adjacent peaks, which represent the branched isomers and the linear isomer. The limit of detection (LOD) was 0.03 ng/mL. Values below LOD were assumed to be 0.015 ng/mL. The accuracy of the method was assessed by regular participation in the German Quality Assessment program (G-EQUAS) organized by the German Society of Occupational Medicine. The between batch-imprecision during the analysis of the samples were <7.7% for the older cohort and <5.0% for the younger cohort.
DDE and PCB serum analyses were conducted as previously described [18, 24]. The accuracy for this analysis was also assessed by participation in the G-EQUAS program. Use of quality control samples verified that the results from the two cohorts were comparable. A simplified ΣPCB concentration was calculated as the sum of congeners CB-138, CB-153, and CB-180 multiplied by 2.
The population distribution of PFASs, DDE and ΣPCB concentrations is skewed to the right, and these measures were therefore log-transformed using log10 in order to reduce the influence of outlying values when they were used as predictors and to avoid violating model assumptions when performing analyses of associations with PFASs as the outcome.
2.3 Statistical analysis
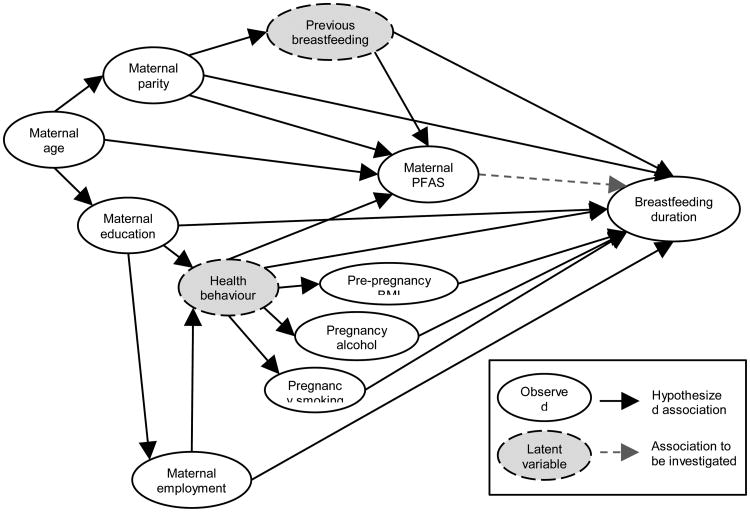
Women who had not provided information about duration of breastfeeding or provided a blood sample for PFAS analysis were compared to those who had provided the information using unadjusted logistic regression models, and the two cohorts were compared with regard to exposures, outcomes and covariates using Wilcoxon's rank-sum test (continuous variables) or Chi squared test (categorical variables). Confounding was identified using a directed acyclic graph (DAG, Figure 1) based on existing literature about predictors for breastfeeding and pregnancy serum-PFAS concentrations. Maternal smoking [6, 7, 9], maternal alcohol intake [10], high maternal BMI [7, 9], low maternal age [6-9], low maternal education [6-9], maternal employment [6], and primiparity [6, 8] have all been associated with reduced duration of breastfeeding. These factors might also be associated with PFAS exposure [25-29], although only the association between higher parity and reduced serum-PFAS concentrations is consistent across studies [25-27, 29]. Confounding was identified as backdoor paths between maternal serum-PFAS concentrations and breastfeeding [30], and these paths were blocked by conditioning on one of the variables on each path if information was available [31].
Figure 1. Association between maternal serum-PFAS and duration of breastfeeding.
Hypothesized causal associations and potential confounding paths are depicted in a Directed Acyclic Graph (DAG).
Since knowledge about sources of PFAS exposure is limited and results from existing studies of predictors are inconsistent [25-29], we also tested associations between potential confounding variables and the log-transformed serum-PFAS concentrations in linear regression models adjusted for cohort, and the estimates of association were converted to express the percent differences in serum-PFAS concentrations with a one-unit increase in the covariate.
Associations between maternal serum concentrations of each PFAS (log-transformed) and duration of total and exclusive breastfeeding were determined in linear regression models, and estimates from these models were converted to express the change in breastfeeding duration with a doubling of the serum-PFAS concentration. The assumption about a linear association between log-transformed serum-PFAS concentrations and breastfeeding was tested by tentatively including non-log-transformed serum-PFAS concentration measures. Furthermore, model residuals were inspected in graphs.
All analyses were adjusted for cohort. In addition, we performed adjusted association analyses, in which we included potential confounding variables as identified in the DAG. We also examined correlations between serum concentrations of DDE, ΣPCB and PFASs and tentatively adjusted the association models for serum concentrations of DEE and ΣPCB to determine if these exposures explained any association between serum-PFAS concentrations and breastfeeding duration. To further examine whether an association could be ascribed to confounding from previous breastfeeding, we tested interactions between parity and serum-PFAS concentrations. Furthermore, we performed analyses testing for cohort differences to ascertain whether any results were confined to one of the cohorts. Finally, we performed sensitivity analyses in which we stratified the associations by parity or cohort rather than testing interactions to examine if this would change the estimates.
Although serum concentrations of different PFASs are correlated, we attempted to include all five PFASs in a single model, while removing insignificant PFASs using the backwards elimination approach.
To adjust for dependence between women being included twice, all regression analyses were performed with cluster-robust standard errors. All associations were tested in complete-case-analyses, and all analyses were performed in Stata version 14.1 (StataCorp, College Station, TX, USA). A significance level of 0.05 was applied for detecting associations and interactions.
3. Results
A total of 1,130 mother-child dyads were included in the study. Twenty-five mothers were included once in both the older and the younger cohort, nine were included twice in the older cohort, and two were included twice in the younger cohort. The study thus encompassed 640 mother-child dyads in the older and 490 in the younger cohort. Among these, 1,092 (97%) maternal blood samples were drawn in which PFASs were measured, and information about duration of total and exclusive breastfeeding was provided in 1,030 and 1,029 (91%) dyads respectively. Women who provided information about breastfeeding were older, more likely to have an education beyond primary school and to have given birth previously, and they had lower serum concentrations of PFOA but higher serum concentrations of PFNA and PFDA. Those who provided a blood sample for PFAS analysis were more likely to have an education and a job and less likely to have consumed alcohol during pregnancy and deliver preterm (results not shown).
The distribution of maternal serum concentrations of all five PFASs differed between the two cohorts, with the median PFHxS, PFOS, PFOA, and PFDA being lower in the younger cohort, whereas the median PFNA was higher (Table 1). In the younger cohort, 14 mothers had serum PFHxS concentrations below LOD. PFOS, PFOA, PFNA and PFDA were detectable in all serum samples. The median duration of total and exclusive breastfeeding was 9 and 5 months, respectively, with no significant difference between the cohorts (Table 1). In the older cohort, 1.5% did not breastfeed at all and 4.2% introduced other sources of nutrition from the beginning. In the younger cohort the corresponding figures were 3.3% and 5.1%. Smoking and alcohol consumption were significantly less frequent in the younger cohort, and the mothers of the younger cohort were on average 5 months older when their child was born, as compared to the mothers of the older cohort. Mothers of the younger cohort were also more likely to have an education beyond primary school and more likely to have a job (Table 1).
Table 1. Cohort characteristics.
| Overall | Older cohort | Younger cohort | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Continuous variables | N | Median (IQR a) | N | Median (IQR a) | N | Median (IQR a) | p w |
| PFHxS (ng/mL) | 1092 | 1.45 (0.22; 5.15) | 605 | 4.49 (2.24; 8.43) | 487 | 0.20 (0.13; 0.31) | <0.001 |
| PFOS (ng/mL) | 1092 | 19.47 (8.67; 28.22) | 605 | 27.13 (23.18; 33.13) | 487 | 8.26 (6.22; 10.71) | <0.001 |
| PFOA (ng/mL) | 1092 | 2.40 (1.45; 3.59) | 605 | 3.34 (2.56; 4.01) | 487 | 1.40 (0.95; 1.95) | <0.001 |
| PFNA (ng/mL) | 1092 | 0.62 (0.48; 0.83) | 605 | 0.59 (0.46; 0.79) | 487 | 0.66 (0.52; 0.86) | <0.001 |
| PFDA (ng/mL) | 1092 | 0.28 (0.21; 0.37) | 605 | 0.28 (0.22; 0.38) | 487 | 0.26 (0.19; 0.35) | 0.006 |
| Total breastfeeding (months) | 1030 | 9 (6; 12) | 577 | 9 (6; 12) | 453 | 9.5 (6; 12) | 0.91 |
| Exclusive breastfeeding (months) | 1029 | 5 (4; 6) | 576 | 5 (4; 6) | 453 | 5 (4; 6) | 0.49 |
| Maternal age (years) | 1130 | 30.3 (26.2; 33.9) | 640 | 30.1 (25.9; 33.2) | 490 | 30.6 (26.7; 34.5) | 0.02 |
| Pre-pregnancy BMI (kg/m2) | 1129 | 23.2 (21.2; 25.9) | 640 | 23.0 (21.2; 25.6) | 489 | 23.7 (21.2; 26.0) | 0.06 |
| Birth weight (g) | 1130 | 3700 (3400; 4030) | 640 | 3725 (3400; 4000) | 490 | 3700 (3400; 4050) | 0.94 |
|
| |||||||
| Binary variables | N | n (%) | N | n (%) | N | n (%) | p c |
|
| |||||||
| Previous births | 1128 | 804 (71.3) | 640 | 464 (72.5) | 488 | 340 (69.7) | 0.30 |
| Pregnancy alcohol intake | 1127 | 288 (25.6) | 639 | 264 (41.3) | 488 | 24 (4.9) | <0.001 |
| Pregnancy smoking | 1128 | 254 (22.2) | 640 | 177 (27.7) | 488 | 77 (15.8) | <0.001 |
| Maternal education beyond primary school | 1124 | 872 (77.6) | 640 | 454 (70.9) | 484 | 418 (86.4) | <0.001 |
| Maternal employment | 1129 | 843 (74.7) | 640 | 451 (70.5) | 489 | 392 (80.2) | <0.001 |
| Caesarean section | 1127 | 128 (11.4) | 640 | 65 (10.2) | 487 | 63 (12.9) | 0.15 |
| Preterm delivery (GA < 37 weeks) | 1127 | 28 (2.5) | 640 | 13 (2.0) | 487 | 15 (3.1) | 0.26 |
IQR: Inter Quartile Range: 25; 75 percentile
p-value for difference between cohorts (Wilcoxon's rank-sum test)
p-value for difference between cohorts (Chi squared test)
GA Gestational Age
When adjusting for cohort only, older women, and those employed, had higher serum-PFNA concentrations, and older age was also associated with higher serum-PFDA but lower serum-PFOA concentrations (Table 2). Higher maternal BMI, pregnancy smoking, and pregnancy alcohol intake were associated with increased serum concentrations of PFOS, PFOA, and PFHxS respectively, and having given birth previously was associated with decreased serum concentrations of PFHxS and PFOA but increased serum-PFDA concentrations (Table 2). Finally, maternal education was associated with lower concentrations of PFOS, PFOA, PFNA, and PFDA (Table 2). As determined by identification of backdoor paths in the DAG, these factors were all taken into account when examining the association between maternal serum-PFAS concentrations and duration of breastfeeding.
Table 2. Percent difference in serum-PFAS concentrations associated with a one-unit increase in covariates.
| PFHxS | PFOS | PFOA | PFNA | PFDA | ||
|---|---|---|---|---|---|---|
|
| ||||||
| N | %-difference (95 % CI) | %-difference (95 % CI) | %-difference (95 % CI) | %-difference (95 % CI) | %-difference (95 % CI) | |
| Maternal age (years) | 1092 | 0.21 (-0.65; 1.09) | 0.28 (-0.11; 0.67) | -1.61 (-2.07; -1.15) | 1.23 (0.80; 1.67) | 1.63 (1.14; 2.12) |
| Pre-pregnancy BMI (kg/m2) | 1091 | -0.02 (-1.07; 1.03) | 0.67 (0.12; 1.21) | -0.29 (-0.91; 0.34) | 0.47 (-0.12; 1.07) | -0.06 (-0.68; 0.56) |
| Previous births (yes/no) | 1090 | -16.87 (-25.00; -7.86) | -4.08 (-8.37; 0.42) | -31.40 (-34.83; -27.80) | 0.98 (-4.15; 6.38) | 7.84 (1.76; 14.29) |
| Pregnancy alcohol intake (yes/no) | 1089 | 19.06 (5.50; 34.36) | -3.02 (-7.40; 1.52) | 4.31 (-1.68; 10.67) | -4.05 (-9.66; 1.90) | -2.26 (-8.46; 4.37) |
| Pregnancy smoking (yes/no) | 1090 | -5.20 (-15.12; 5.88) | -0.53 (-5.17; 4.33) | 6.20 (0.43; 12.30) | -4.92 (-10.19; 0.65) | -3.85 (-9.65; 2.32) |
| Maternal education (yes/no) | 1086 | 1.09 (-9.84; 13.34) | -5.75 (-10.62; -0.61) | -7.94 (-13.41; -2.13) | -7.95 (-13.54; -2.01) | -7.78 (-13.97; -1.15) |
| Maternal employment (yes/no) | 1091 | 7.06 (-4.07; 19.49) | 2.54 (-2.34; 7.67) | -3.34 (-9.17; 2.87) | 6.06 (0.08; 12.41) | 6.50 (-0.03; 13.45) |
All analyses were adjusted for cohort
We found a strong negative association between maternal serum-PFAS concentrations and duration of both total and exclusive breastfeeding (Table 3). No deviation from log-linearity was found in the associations between PFAS exposure and breastfeeding. Residual plots indicated a slightly right-skewed distribution of the residuals for total duration of breastfeeding, and we therefore repeated the analyses after a square root transformation of total duration of breastfeeding. This change produced normally distributed residuals and revealed similar p-values for the associations.
Table 3. Differences in duration of breastfeeding associated with a doubling of the maternal serum-PFAS concentrations.
| Model 1 a | Model 2 a b c | Model 3 a b d | Model 4 b c e | |||||
|---|---|---|---|---|---|---|---|---|
| Primiparous | Multiparous | Older cohort | Younger cohort | |||||
|
| ||||||||
| Total breastfeeding (months) | ||||||||
|
| ||||||||
| Difference (95 % CI) | Difference (95 % CI) | Difference (95 % CI) | Difference (95 % CI) | p i | Difference (95 % CI) | Difference (95 % CI) | p i | |
| N=998 | N=987 | N=987 | N=987 | |||||
| PFHx S | -0.1 (-0.5; 0.2) | -0.2 (-0.5; 0.2) | -0.1 (-0.5; 0.3) | -0.2 (-0.5; 0.2) | 0.66 | -0.2 (-0.7; 0.2) | -0.1 (-0.6; 0.4) | 0.75 |
| PFOS | -1.4 (-2.1; -0.6) | -1.4 (-2.1; -0.6) | -1.3 (-2.3; -0.3) | -1.4 (-2.2; -0.7) | 0.75 | -2.2 (-3.5; -0.8) | -1.0 (-1.9; -0.1) | 0.14 |
| PFOA | -1.6 (-2.1; -1.0) | -1.3 (-1.9; -0.7) | -1.1 (-2.0; -0.1) | -1.4 (-2.0; -0.8) | 0.45 | -1.8 (-3.0; -0.7) | -1.1 (-1.7; -0.4) | 0.21 |
| PFNA | -1.0 (-1.6; -0.3) | -1.3 (-2.0; -0.7) | -1.6 (-2.6; -0.6) | -1.2 (-2.0; -0.5) | 0.60 | -1.8 (-2.8; -0.9) | -0.7 (-1.5; -0.0) | 0.07 |
| PFDA | -0.4 (-1.0; 0.2) | -0.8 (-1.4; -0.3) | -1.6 (-2.6; -0.7) | -0.5 (-1.2; 0.1) | 0.06 | -1.2 (-2.0; -0.5) | -0.3 (-1.1; 0.5) | 0.10 |
|
| ||||||||
| Exclusivebreastfeeding (months) | ||||||||
|
| ||||||||
| Difference (95 % CI) | Difference (95 % CI) | Difference (95 % CI) | Difference (95 % CI) | p i | Difference (95 % CI) | Difference (95 % CI) | p i | |
|
| ||||||||
| N=997 | N=986 | N=986 | N=986 | |||||
| PFHx S | -0.0 (-0.2; 0.1) | -0.1 (-0.2; 0.1) | -0.0 (-0.1; 0.1) | -0.1 (-0.2; 0.0) | 0.28 | -0.1 (-0.2; 0.1) | -0.0 (-0.2; 0.1) | 0.91 |
| PFOS | -0.4 (-0.7; -0.1) | -0.3 (-0.6; -0.1) | -0.2 (-0.6; 0.1) | -0.3 (-0.6; -0.1) | 0.52 | -0.4 (-0.8; 0.1) | -0.3 (-0.6; -0.0) | 0.88 |
| PFOA | -0.6 (-0.8; -0.4) | -0.5 (-0.7; -0.3) | -0.4 (-0.8; 0.0) | -0.5 (-0.7; -0.3) | 0.55 | -0.6 (-0.9; -0.2) | -0.4 (-0.7; -0.2) | 0.52 |
| PFNA | -0.2 (-0.4; -0.0) | -0.2 (-0.5; -0.0) | -0.3 (-0.8; 0.1) | -0.2 (-0.4; 0.0) | 0.64 | -0.2 (-0.5; 0.1) | -0.3 (-0.6; 0.0) | 0.76 |
| PFDA | -0.1 (-0.3; 0.1) | -0.2 (-0.4; 0.0) | -0.5 (-0.9; -0.1) | -0.1 (-0.3; 0.1) | 0.06 | -0.1 (-0.3; 0.2) | -0.3 (-0.6; -0.0) | 0.27 |
Adjusted for cohort
Adjusted for maternal age, pre-pregnancy BMI, pregnancy alcohol intake, pregnancy smoking, education, and employment
Adjusted for parity
Interaction between parity and PFAS
Interaction between cohort and PFAS
p-value for interaction
Adjusting for maternal parity, age, pre-pregnancy BMI, pregnancy alcohol intake, pregnancy smoking, education, employment, and cohort weakened the association between PFOA and total breastfeeding but strengthened the association between PFNA and PFDA and total breastfeeding. Other associations were only affected to a very limited degree or not affected at all. Thus, a doubling of maternal serum PFOS, PFOA, and PFNA concentrations remained significantly associated with a reduction in duration of total and exclusive breastfeeding, and a doubling of maternal serum PFDA concentrations was now additionally associated with a reduction in total duration of breastfeeding (Table 3, Model 2). Serum concentrations of DDE and ΣPCB were weakly to moderately correlated to the PFASs, and adjusting for maternal serum concentrations of DDE or ΣPCB slightly weakened some of the associations but did not substantially change them (results not shown).
The results were not confined to multiparous women, since we found equally strong associations in each group when including an interaction-term between parity and serum-PFAS concentrations in the analyses (Table 3, Model 3). Stratification by parity in most analyses resulted in estimates very similar to those obtained when performing interaction analyses, although the association between PFOA and total duration of breastfeeding was slightly attenuated among primiparous women, where a doubling of PFOA was associated with a reduction of 0.6 (95% CI: -1.7; 0.5) months.
Finally, differences between the two cohorts were not statistically significant at the 5% level, but a tendency was seen towards the association between serum-PFAS concentrations and total duration of breastfeeding being weaker in the less-exposed younger cohort, as compared to the older cohort (Table 3, Model 4). Stratifying the analyses by cohort rather than performing interactions did not change the associations.
When including all five PFASs in a single model, only PFOA was significantly associated with the duration of exclusive breastfeeding, while both PFOA and PFNA were significantly associated with the total duration of breastfeeding after backwards elimination of the other PFASs.
4. Discussion
In this study, we found that increased maternal serum-PFAS concentrations were associated with a significantly shorter duration of both exclusive and total breastfeeding. This in accordance with a recent U.S. study, which found significant associations between high serum-PFOA concentrations and increased risk of early termination of breastfeeding [32], and it agrees well with the overall findings in 1,400 women from the Danish National Birth Cohort, although the Danish findings were confined to multiparous women [17]. Questions have, however, been raised about the validity of the serum-PFAS measurements in the Danish National Birth Cohort [33], thus possibly explaining the inability of the study to detect associations among primiparous women. In order to eliminate any confounding from the duration of previous breastfeeding, we would have had to include this factor in our analyses. However, this information was not obtained in our study, but similar strong associations among primiparous and multiparous women would argue against confounding from previous breastfeeding having played a major role in our study.
The PFAS exposure among the Faroese women is similar to that of women elsewhere in Western countries examined in the same years. Thus, the mothers in the younger cohort had median serum-PFAS concentrations very similar to those of Danish pregnant women examined in 2010-2012 [34] but lower than those of American pregnant women examined in 2003-2006 [35]. The median serum-PFAS concentrations among the mothers in the older cohort were somewhat higher, but their median PFOS and PFOA serum concentrations were close to those of American pregnant women examined in 1999-2002 [29] and slightly lower than those of pregnant women in the Danish National Birth Cohort in 1996-2002 [17]. The differences in serum-PFAS concentrations and temporal trends between the two cohorts could be part of the reason why some of the associations between PFAS exposure and total duration of breastfeeding appeared weaker in the younger cohort, as compared to the older cohort.
In addition to differences in serum-PFAS concentrations, mothers in the younger cohort were also older, better educated, more likely to work, and less likely to consume alcohol and smoke during pregnancy all of which is line with the time trends in the Faroe Islands.
In mouse studies, PFAS exposure at serum concentration levels similar to elevated human exposures has been shown to delay the differentiation of the lactating gland in the exposed dam [36, 37], and in addition to direct effects on the dam, this animal model has also revealed that both in utero [36-38] and peripubertal [39] PFAS exposure might stunt the subsequent development of the mammary gland tissue in females, although results could depend on mouse strain [39]. Furthermore, PFOS exposure has been associated with significantly reduced serum concentrations of prolactin-family hormone in pregnant mice [40]. It therefore seems plausible that cumulated PFAS exposures affect the ability to lactate.
We adjusted our analyses for several potential confounders, all of which had limited impact on the associations. However, residual confounding may still be present. In addition to physiological factors, termination of breastfeeding is also thought to be dependent on individual choice and societal factors [4], as well as social and psychological factors [9]. Societal factors such as paid maternity leave are uniform within the Faroese community and are thus unlikely to have confounded the associations in our study. We did not have information on psychological factors or individual choice, such as wanting to terminate breastfeeding because of work, but these factors are unlikely to be associated with serum-PFAS concentrations, and thus would seem unlikely to have confounded the observed associations between PFAS exposure and early termination of breastfeeding. As for social factors, some were included in our analyses, but additional factors might also be important. Although maternal ethnicity has been associated with initiation and duration of breastfeeding in other settings [6, 7, 9], the Faroese population is highly homogenous, and ethnicity was therefore not considered in our analyses. Of greater interest, the Faroese population is highly exposed to other environmental chemicals, which may also pass into breast milk to an extent that they might affect the children's health [41]. Adjustment for maternal serum concentrations of DDE and ΣPCB, however, did not remove the associations. Knowledge about harmful substances being transferred in breast milk could, however, conceivably have made some women choose to terminate breastfeeding earlier than otherwise planned, but since the women in our study were unaware of their own exposure to all of the environmental chemicals, this factor is also an unlikely confounder.
Some sources of imprecision may play a role. Thus, blood samples, in which PFASs were measured, were obtained at different time points in the two cohorts. Maternal serum-PFAS concentrations have been found to decline during pregnancy [27, 42], but serum-PFAS concentrations measured in the third trimester were highly correlated with serum-PFAS concentrations measured three weeks after delivery [42]. Timing of blood sampling is therefore unlikely to have affected our results, especially as all analyses were adjusted for cohort to account for any systematic differences in serum-PFAS concentrations between the cohorts. Furthermore, all serum samples were measured at the same laboratory with a documented record of excellent quality.
Some women may choose to terminate breastfeeding for other reasons than diminished milk production, which makes duration of breastfeeding less appropriate as a valid proxy measure for the capability to breastfeed, and any misclassification could possibly have resulted in an underestimation of the true association between PFAS exposure and the capability to breastfeed. Recall of breastfeeding duration may also involve some degree of imprecision and thus a loss of power, but since the maternal serum-PFAS concentrations were unknown to the women, differential misclassification is highly unlikely.
Women who did not provide information about breastfeeding had higher PFOS and lower PFNA and PFDA concentrations. We cannot exclude the possibility of selection bias, but any bias would likely weaken some associations between PFASs and breastfeeding while strengthening others, and since data was only missing for a minority of women, the associations found in our study are unlikely to have been caused by selection bias.
PFAS exposure is considered a public health concern for other reasons as well. Thus, maternal PFAS burdens can be transferred to the infant through breast milk [43], and PFAS exposure during infancy may lead to adverse health effects [16]. Studies on the benefits of long durations of breastfeeding therefore ought to consider the balance between nutritional and psychological benefits to the child and the possible toxicity from increased transfer of toxicants via human milk. Even if long-term breastfeeding is recommended, the adverse effects on lactation may make this difficult or impossible for some women. Our findings, in concert with other evidence, suggest that exposure to environmental chemicals, such as the PFASs, might interfere with women's ability to lactate in accordance with health recommendations. Although additional studies would be desirable, current knowledge on associations between environmental chemicals and the capability to lactate should be considered when assessing the causes of short breastfeeding duration.
4. Conclusions
Increased maternal serum-PFAS concentrations are associated with a decreased duration of breastfeeding. The results are not confined to multiparous women and are unlikely to be explained by confounding from maternal age, education, employment, and factors related to health behavior. This association may be due to deficient lactation caused by endocrine disruption effects and suggests that exposure to environmental chemicals should be taken into account when assessing the causes for early weaning.
Highlights.
Elevated maternal PFAS exposure is associated with reduced breastfeeding duration
A doubled serum-PFOS reduced total duration of breastfeeding by 1.4 months
A doubled serum-PFOA reduced exclusive breastfeeding duration by 0.5 months
Associations were seen among both primiparous and multiparous women
Acknowledgments
We thank the Faroese mothers and their children for participating in the CHEF cohort studies, and we thank the research nurses, Mari Ann Ellendersen and Nanna Kallsberg, for their contribution. Funding: This work was supported by the National Institute of Environmental Health Sciences, NIH (ES012199); the U.S. Environmental Protection Agency (R830758); the Danish Council for Strategic Research (09-063094); and the Danish Environmental Protection Agency as part of the environmental support program DANCEA (Danish Cooperation for Environment in the Arctic). The funding agencies were not involved in the study or writing the manuscript. The authors are solely responsible for all results and conclusions, which do not necessarily reflect the position of any funding agencies.
The study was designed by CAGT and PG. CAGT performed the data analysis and drafted the manuscript. EBJ provided assistance with the statistical analyses. MSP provided assistance with data management. PW was responsible for the cohort studies and the clinical examinations. US carried out the clinical examinations. FN was in charge of the PFAS analyses. TKJ contributed to the data analysis. PG participated in drafting of the manuscript. All authors contributed to the data interpretation and manuscript development and approved the final version.
Abbreviations
- BMI
Body mass index
- DDE
Dichlorodiphenyldichloroethylene
- DAG
Directed acyclic graph
- LOD
Limit of detection
- PFAS
Perfluoroalkyl substance
- PFDA
Perfluorodecanoic acid
- PFHxS
Perfluorohexane sulfonic acid
- PFNA
Perfluorononanoic acid
- PFOS
Perfluorooctane sulfonic acid
- PFOA
Perfluorooctanoic acid
- PCB
Polychlorinated biphenyl
- WHO
World Health Organization
Footnotes
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, Rollins NC G Lancet Breastfeeding Series. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–90. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 2.WHO. The optimal duration of exclusive breastfeeding. World Health Organization (WHO); Geneva: 2001. [Google Scholar]
- 3.UNICEF. The State of the World's Children 2015: Executive Summary. United Nations Children's Fund (UNICEF); New York: 2014. [Google Scholar]
- 4.Wolf JH. Low breastfeeding rates and public health in the United States. Am J Public Health. 2003;93(12):2000–10. doi: 10.2105/ajph.93.12.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuebe AM, Horton BJ, Chetwynd E, Watkins S, Grewen K, Meltzer-Brody S. Prevalence and risk factors for early, undesired weaning attributed to lactation dysfunction. J Womens Health (Larchmt) 2014;23(5):404–12. doi: 10.1089/jwh.2013.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wambach K, Campbell SH, Gill SL, Dodgson JE, Abiona TC, Heinig MJ. Clinical lactation practice: 20 years of evidence. J Hum Lact. 2005;21(3):245–58. doi: 10.1177/0890334405279001. [DOI] [PubMed] [Google Scholar]
- 7.Ahluwalia IB, Morrow B, Hsia J. Why do women stop breastfeeding? Findings from the Pregnancy Risk Assessment and Monitoring System. Pediatrics. 2005;116(6):1408–12. doi: 10.1542/peds.2005-0013. [DOI] [PubMed] [Google Scholar]
- 8.Li R, Fein SB, Chen J, Grummer-Strawn LM. Why mothers stop breastfeeding: mothers' self-reported reasons for stopping during the first year. Pediatrics. 2008;122(Suppl 2):S69–76. doi: 10.1542/peds.2008-1315i. [DOI] [PubMed] [Google Scholar]
- 9.Thulier D, Mercer J. Variables associated with breastfeeding duration. J Obstet Gynecol Neonatal Nurs. 2009;38(3):259–68. doi: 10.1111/j.1552-6909.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann RL, Bergmann KE, von Weizsacker K, Berns M, Henrich W, Dudenhausen JW. Breastfeeding is natural but not always easy: intervention for common medical problems of breastfeeding mothers - a review of the scientific evidence. J Perinat Med. 2014;42(1):9–18. doi: 10.1515/jpm-2013-0095. [DOI] [PubMed] [Google Scholar]
- 11.Rogan WJ, Gladen BC, McKinney JD, Carreras N, Hardy P, Thullen J, Tingelstad J, Tully M. Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: effects on growth, morbidity, and duration of lactation. American journal of public health. 1987;77(10):1294–7. doi: 10.2105/ajph.77.10.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gladen BC, Rogan WJ. DDE and shortened duration of lactation in a northern Mexican town. American journal of public health. 1995;85(4):504–8. doi: 10.2105/ajph.85.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds--exposure assessment for the general population in Western countries. International journal of hygiene and environmental health. 2009;212(3):239–70. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. Biological monitoring of polyfluoroalkyl substances: A review. Environmental science & technology. 2006;40(11):3463–73. doi: 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- 15.Perez F, Nadal M, Navarro-Ortega A, Fabrega F, Domingo JL, Barcelo D, Farre M. Accumulation of perfluoroalkyl substances in human tissues. Environment international. 2013;59:354–62. doi: 10.1016/j.envint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Grandjean P, Clapp R. Perfluorinated Alkyl Substances: Emerging Insights Into Health Risks. New Solut. 2015;25(2):147–63. doi: 10.1177/1048291115590506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal concentrations of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) and duration of breastfeeding. Scand J Work Environ Health. 2010;36(5):413–21. doi: 10.5271/sjweh.2908. [DOI] [PubMed] [Google Scholar]
- 18.Heilmann C, Budtz-Jorgensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environmental health perspectives. 2010;118(10):1434–8. doi: 10.1289/ehp.1001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grandjean P, Poulsen LK, Heilmann C, Steuerwald U, Weihe P. Allergy and sensitization during childhood associated with prenatal and lactational exposure to marine pollutants. Environmental health perspectives. 2010;118(10):1429–33. doi: 10.1289/ehp.1002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmermann CA, Osuna CE, Steuerwald U, Weihe P, Poulsen LK, Grandjean P. Asthma and allergy in children with and without prior measles, mumps, and rubella vaccination. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2015;26(8):742–9. doi: 10.1111/pai.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim BM, Choi AL, Ha EH, Pedersen L, Nielsen F, Weihe P, Hong YC, Budtz-Jorgensen E, Grandjean P. Effect of hemoglobin adjustment on the precision of mercury concentrations in maternal and cord blood. Environmental research. 2014;132:407–12. doi: 10.1016/j.envres.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. e-Library of Evidence for Nutrition Actions (eLENA) Exclusive Breastfeeding. World Health Organization (WHO); Geneva: 2016. [Google Scholar]
- 23.Eriksson U, Karrman A, Rotander A, Mikkelsen B, Dam M. Perfluoroalkyl substances (PFASs) in food and water from Faroe Islands. Environmental science and pollution research international. 2013;20(11):7940–8. doi: 10.1007/s11356-013-1700-3. [DOI] [PubMed] [Google Scholar]
- 24.Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA : the journal of the American Medical Association. 2012;307(4):391–7. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brantsaeter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, Thomsen C, Meltzer HM, Becher G, Sabaredzovic A, Hoppin JA, Eggesbo M, Longnecker MP. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environment international. 2013;54:74–84. doi: 10.1016/j.envint.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg V, Nost TH, Huber S, Rylander C, Hansen S, Veyhe AS, Fuskevag OM, Odland JO, Sandanger TM. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environment international. 2014;69:58–66. doi: 10.1016/j.envint.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Kato K, Wong LY, Chen A, Dunbar C, Webster GM, Lanphear BP, Calafat AM. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003-2006. Environmental science & technology. 2014;48(16):9600–8. doi: 10.1021/es501811k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain RB. Contribution of diet and other factors to the levels of selected polyfluorinated compounds: data from NHANES 2003-2008. International journal of hygiene and environmental health. 2014;217(1):52–61. doi: 10.1016/j.ijheh.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Sagiv SK, Rifas-Shiman SL, Webster TF, Mora AM, Harris MH, Calafat AM, Ye X, Gillman MW, Oken E. Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environmental science & technology. 2015;49(19):11849–58. doi: 10.1021/acs.est.5b02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearl J. Causal diagrams for empirical research. Biometrika. 1995;82(4):669–688. [Google Scholar]
- 31.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. American journal of epidemiology. 2002;155(2):176–84. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 32.Romano ME, Xu Y, Calafat AM, Yolton K, Chen A, Webster GM, Eliot MN, Howard CR, Lanphear BP, M BJ. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environmental research. 2016 doi: 10.1016/j.envres.2016.04.034. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bach CC, Henriksen TB, Bossi R, Bech BH, Fuglsang J, Olsen J, Nohr EA. Perfluoroalkyl Acid Concentrations in Blood Samples Subjected to Transportation and Processing Delay. PLoS One. 2015;10(9):e0137768. doi: 10.1371/journal.pone.0137768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen TK, Andersen LB, Kyhl HB, Nielsen F, Christesen HT, Grandjean P. Association between perfluorinated compound exposure and miscarriage in Danish pregnant women. PLoS One. 2015;10(4):e0123496. doi: 10.1371/journal.pone.0123496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, Lanphear BP. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity (Silver Spring) 2016;24(1):231–7. doi: 10.1002/oby.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, Strynar MJ, Lindstrom AB, Thibodeaux JR, Wood C, Fenton SE. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicological sciences : an official journal of the Society of Toxicology. 2007;96(1):133–44. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 37.White SS, Stanko JP, Kato K, Calafat AM, Hines EP, Fenton SE. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environmental health perspectives. 2011;119(8):1070–6. doi: 10.1289/ehp.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White SS, Kato K, Jia LT, Basden BJ, Calafat AM, Hines EP, Stanko JP, Wolf CJ, Abbott BD, Fenton SE. Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross-foster and restricted gestational exposures. Toxicol. 2009;27(3-4):289–98. doi: 10.1016/j.reprotox.2008.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang C, Tan YS, Harkema JR, Haslam SZ. Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reprod Toxicol. 2009;27(3-4):299–306. doi: 10.1016/j.reprotox.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CK, Kang SG, Lee JT, Lee SW, Kim JH, Kim DH, Son BC, Kim KH, Suh CH, Kim SY, Park YB. Effects of perfluorooctane sulfuric acid on placental PRL-family hormone production and fetal growth retardation in mice. Mol Cell Endocrinol. 2015;401:165–72. doi: 10.1016/j.mce.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 41.Grandjean P, Budtz-Jorgensen E, Steuerwald U, Heinzow B, Needham LL, Jorgensen PJ, Weihe P. Attenuated growth of breast-fed children exposed to increased concentrations of methylmercury and polychlorinated biphenyls. FASEB J. 2003;17(6):699–701. doi: 10.1096/fj.02-0661fje. [DOI] [PubMed] [Google Scholar]
- 42.Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, Darnerud PO. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996-2010. Environmental science & technology. 2012;46(16):9071–9. doi: 10.1021/es301168c. [DOI] [PubMed] [Google Scholar]
- 43.Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jorgensen E. Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environmental science & technology. 2015;49(17):10466–73. doi: 10.1021/acs.est.5b02237. [DOI] [PMC free article] [PubMed] [Google Scholar]