Abstract
Acute leukemia (AL) is a bone marrow malignancy of hematopoietic progenitors that historically is poorly responsive to treatment. With the widespread adoption of dose-intense chemotherapy, more human patients attain long-term survivals, but whether comparable progress has been made in canine AL is unknown. To investigate this question, medical records from three academic veterinary hospitals were reviewed. Fifty dogs met the criteria for AL, having excess circulating or marrow blasts, a major cytopenia(s), and no substantial lymphadenopathy. Thirty-six dogs received cytotoxic chemotherapy; 23 achieved a complete or partial response for a median of 56 days (range, 9 – 218). With failure or relapse, 14 dogs were rescued. Median survival with treatment was poor at 55 days (range, 1 – 300). Untreated (n=6) and palliatively-treated (n= 8) dogs lived a median of 7.5 days. Most dogs developed chemoresistance within weeks of initiating treatment, and consequently, survival times for AL remain disappointingly short.
Keywords: Acute leukemia, canine, chemotherapy, CHOP, immunophenotype
Introduction
Lymphoid cancers are among the most frequently diagnosed malignancies of dogs. When characterized by their behavior and responsiveness to treatment, a number of distinct forms can be discriminated, ranging from comparatively indolent types, such as chronic lymphocytic leukemia and T-zone lymphomas, to more aggressive forms, such as non-Hodgkin lymphoma (NHL) and acute lymphoblastic leukemia (ALL). Their susceptibility to chemotherapy also varies widely, as seen in highly responsive nodal NHLs, or contrastingly, in those cancers arising in the skin and gastrointestinal tract that usually exhibit only marginal, short-lived responses.1–6 Historically, canine ALL has been perceived as a rapidly progressive and chemotherapy-resistant cancer, and hence, can be an extremely discouraging diagnosis for both clinician and owner.7, 8
Acute lymphoblastic leukemia arises from the malignant transformation of lymphoid progenitors in bone marrow, which results in myelophthisis and subsequent invasion of peripheral tissues. Clinical signs are typically acute in onset, caused by the infiltrative and functional effects of the expanding burden of malignant cells, and are most commonly a consequence of disrupted hematopoiesis. The identification and morphologic classification of leukemic cells is the first preliminary step in the diagnosis of ALL. In humans, flow cytometric demonstration of abnormally high numbers of cells expressing immature surface antigens (“maturation shift”) or the aberrant, marked predominance of a single mature lymphoid population (“distribution shift”) in peripheral blood is confirmatory.9 Additional analyses of leukemic cells by conventional cytogenetic and new molecular genetic assays can help predict outcomes and guide the selection of risk-adapted therapies.10 Despite these advancements in ALL profiling, however, most regimens used for remission induction are similar, consisting of corticosteroids, an anthracycline, vincristine, and often, L-asparaginase.10 While remission rates with such multiagent chemotherapy are high (80–95%), outcomes are heterogeneous, with age being the single most important predictor of failure. Most adults with ALL eventually relapse and succumb to their disease, with 5-year survival rates usually <40%; in contrast, 80% of pediatric patients survive long-term.11 This differential therapeutic outcome is attributed to more intensive chemotherapy regimens and better treatment compliance in children. Indeed, when adults are treated with pediatric-type protocols, 5-year survivals are boosted to >60%, demonstrating the importance of dose-intense chemotherapy for this cancer.10
In dogs, ALL is considered to behave and respond to chemotherapy analogously to the adult form in humans, based on the anecdotal experiences of clinicians and the sparse canine literature. In the sole comprehensive clinical study, Matus et al. reported an objective response attained by only 8 of 21 dogs treated with vincristine and prednisone, with a median survival of 120 days.7 With the use of dose-intense, multi-agent chemotherapy protocols for NHL in dogs now much more commonplace than in 1983, it is tempting to speculate that the outcome of canine ALL has improved since that initial publication. Accordingly, we retrospectively analyzed cases of suspected ALL in dogs to investigate that possibility. Because diagnostic evaluations of these patients were non-standardized and frequently incomplete - a common challenge in veterinary medicine, and particularly so in a study spanning multiple institutions and decades – two populations emerged: dogs with definitive ALL, and dogs with leukemias suspected to be lymphoid in origin, but lacking conclusive lineage assignment. These latter cases were designated as acute undifferentiated leukemias, or AULs. The objectives of our study were to derive a picture of common clinicopathologic abnormalities in cases of ALL and AUL (referred to collectively as acute leukemia [AL]); determine the response and outcome following treatment with multi-agent chemotherapy; and identify any patient factors at diagnosis that could predict risk for therapeutic failure.
Materials and methods
The medical records of dogs diagnosed with AL between 1989 and 2014 were reviewed. Detailed survey questionnaires were sent to participating institutions, with the intent to acquire information on each patient’s signalment, historical and physical examination findings at initial presentation, results of diagnostic testing, treatment regimen(s), response to therapy and eventual outcome. The number of cases from each participating institutions were: North Carolina State University, 34 patients; University of Georgia, 11 patients; and Cornell University, 5 patients. Specific data was collected retrospectively via review of the patient’s medical record. When possible, follow-up conversations with pet owners and/or referring veterinarians were performed to determine outcome.
Data regarding signalment, presenting complaint(s) and historical information, physical examination findings, results of clinicopathologic analyses (complete blood count [CBC]; chemistry panel; urinalysis, blood flow cytometric analysis and immunophenotyping; cytologic findings from blood and aspirates of lymphoid organs and bone marrow), imaging (thoracic radiography and abdominal sonography), treatment protocols (if any), responses to therapy, durations of response and date/reason for death (where available) were collected and tabulated.
The lineage of leukemic cells was assessed by several means. Flow cytometric analysis was performed as previously described using the antibodies listed in Table 1.12 In many cases, the authors were unable to gain access to flow cytometry data files, and thus relied upon notations within the patient’s medical record for phenotypic information. Cases contributed by Cornell University had flow cytometry performed at North Carolina State University. Immunocytochemistry was performed using the following antibodies (clone and suppliers listed parenthetically): anti-CD3 (CA17.2A12; Dako), anti-CD18; (CA16.3C10; Moore), anti-CD20 (rabbit polyclonal; Biocare Medical, Concord, CA), anti-CD21 (clone not specified, Cell Marque, Rocklin, CA), anti-CD34 (clone not specified; P. McSweeny & R. Nash, Seattle, WA), anti-CD45 (CA12.10C12; Moore) and anti-CD79a (HM57; Dako) as previously detailed.13 Cases in which the majority of leukemic cells expressed B5, CD20, CD21, and/or CD79a/b were considered to be B-cell leukemias, and cases in which the majority stained positively for CD3, CD4, CD5 and/or CD8 were considered to be T-cell leukemias. The remaining cases in which cells expressed none of the tested lineage markers (but may have stained positively with non-differentiating CD antigens, such as CD11d, CD18, CD34 or CD45) were designated AULs. Lymphoid lineage determination by PCR for Antigen Receptor Rearrangement (PARR) assay was made by observing a single, heat-resistant band on gel electrophoresis of PCR products following amplification with T-cell receptor gamma locus (T cell) or immunoglobulin heavy chain locus (B cell) primers.12
Table 1.
Antibodies used for flow cytometric analysis of blood from dogs with AL
| Antibody | Antibody Clone | Source (Site) | Specificity |
|---|---|---|---|
| Anti-B5 antigen | B5 | Tompkins‡ (NCSU) | B cells |
| Anti-CD3 | CA17.2A12 | AbD Serotec§ (NCSU) Moore† (UGA) |
T cells |
| Anti-CD4 | YKIX302.9 | AbD Serotec (NCSU) | Helper T cells; neutrophils |
| Anti-CD5 | YKIX322.3 | AbD Serotec (UGA) eBioscience§ (NCSU) |
T cells |
| Anti-CD8 | 1.140 | Tompkins (NCSU) | Cytotoxic T cells |
| Anti-CD11d | CA11.8H2 | Novus Biologicals§ (NCSU) | T cells; Macrophages |
| Anti-CD14 | 61D3 | eBioscience (NCSU) | Monocytes; Macrophages |
| Anti-CD18 | CA16.3C10 | Moore (UGA) | All leukocytes |
| Anti-CD20 | L26 | Dako§ (UGA) | B cells |
| Anti-CD21 | CA21D6 | AbD Serotec (NCSU) Moore (UGA) |
B cells |
| Anti-CD34 | BIRMA-K3 1H6 |
Dako (UGA) Novus Biologicals (NCSU) |
Hematopoietic stem cells |
| Anti-CD45 | YKIX716.13 CA12.10C12 |
AbD Serotec (NCSU) Moore (UGA) |
All leukocytes |
| Anti-CD79b | AT107-2 | AbD Serotec (NCSU) | B cells |
Dr. Mary B. Tompkins, North Carolina State University, Raleigh, NC, USA
Commercial suppliers: AbD Serotec, Raleigh, NC, USA; eBioscience, San Diego, CA, USA; Novus Biologicals, Littleton, CO, USA; Dako, Carpinteria, CA, USA
Dr. Peter F. Moore, University of California, Davis, CA, USA
Study inclusion required the following minimum AL criteria: 1) blasts constituted >20% of circulating leukocytes or >30% of the nucleated cells in the bone marrow; 2) the concurrent presence of neutropenia, anemia or thrombocytopenia; and for those definitively diagnosed as ALL, 3) confirmed lymphoid origin of leukemic cells (by flow cytometry or immunocytochemistry).14 These criteria are similar to those previously described. Adam et al. adopted a uniform cut-off value of 30% blasts in blood or marrow, but 20% has since been advocated.15, 16 Dogs were excluded if the morphologic analysis described exclusively small (mature) lymphocytes in peripheral blood and/or bone marrow, or if lymphadenopathy was moderate-to-marked and deemed more consistent with a diagnosis of stage V NHL by the clinician. Lymph node sizes were not measured, but characterized as normal, or mildly, moderately, or markedly enlarged in the patient’s medical record.
The response to treatment was based on leukemic cell count and status of cytopenia(s) on a CBC, and/or cytologic re-evaluation of a bone marrow aspirate. Based on these parameters, responses were classified according to the following scheme: complete remission (CR), no leukemic cells and normal total lymphocyte count, resolution of cytopenia(s), and <5% lymphocytes on bone marrow evaluation (when performed); partial remission (PR), >50% reduction in leukemic cell count or lymphocyte percentage on bone marrow evaluation, and persistent cytopenia(s); stable disease (SD), <50% reduction to ≤25% increase in leukemic cells or lymphocyte percentage on bone marrow evaluation, and persistent cytopenia(s); and progressive disease (PD),: >25% increase in circulating leukemic cell count or lymphocyte percentage on bone marrow evaluation, and worsening of cytopenia(s).7 Responses typically were determined just prior to the next planned chemotherapy administration, at the time of the expected white blood cell nadir (7–14 days post-treatment, depending on the agent).
From this dataset, the rates of response, remission durations and survivals were calculated. The objective response rate (ORR) was defined as the sum of dogs achieving CR or PR expressed as a percentage of the total number of dogs treated. Given the variations in the frequency of evaluation, the date at which remission was achieved could not always be precisely determined, and therefore, remission duration was defined as the time in days from initial chemotherapy administration until relapse. Overall remission duration was calculated as the summed length in days of the first remission plus any subsequent remission achieved by additional chemotherapy. Survival was measured from the time of diagnosis to death (due to any cause) or last known follow-up; patients remaining in remission or lost to follow-up were censored in survival analyses.
Graphical (pie chart) summaries of treatments and outcomes were prepared with Excel (Microsoft, Bellevue, WA, USA). Survival curves (remission duration; survival) were generated by the product-limit method of Kaplan and Meier, using Prism (GraphPad, San Diego, CA, USA). Analyses of prognostic factors were also performed with Prism. The effects of categorical values (CD34 expression; cranial abdominal organomegaly) were compared using the Mann-Whitney U test. The effects of continuous variables (leukemic cell count; age) were assessed by comparing survival curves of each patient quartile to the lowest reference quartile, using the log-rank test (Mantel-Haenszel method), as described previously.17 In all statistical analyses, the threshold for significance was set at P < 0.05.
Results
Case descriptions
Sixty-two potential cases of ALL were reviewed. Twelve cases were excluded due to the suspicion of acute myeloid leukemia based on the clinical pathologist’s light microscopic assessment, morphological classification of leukemic cells as exclusively small-to-intermediate in size, or moderate-to-marked peripheral lymphadenopathy. Fifty dogs met the inclusion criteria and were considered to have a probable diagnosis of ALL. Mixed-breed dogs constituted the majority of cases (n=14), followed by 7 Labrador retrievers, 6 German shepherds, 5 Golden retrievers, 2 Australian shepherds, 2 Collies, 2 Rottweilers, and 1 each of the following breeds: Corgi, English bulldog, English setter, Giant Schnauzer, Jack Russell terrier, Keeshond, Pug, Samoyed, Silky terrier, Shih Tzu, Standard Poodle and Weimaraner. Golden retrievers have previously been found to be significantly over-represented in cases of ALL.15 The age of patients ranged from 2 – 14 years (median, 7.1), and their weights ranged from 5.4 – 46 kg (median, 29.8), similar to other reports.15, 18
The most commonly reported clinical signs were non-specific, including lethargy (n=30; 60%) and inappetance in (n=32; 64%). Other historical signs of illness included vomiting (n=14), fever (n=11), diarrhea (n=10), neurologic abnormalities (n=7), weight loss (n=6), polyuria and polydipsia (n=6) and epistaxis (n=2). Commonly encountered physical examination findings included mild peripheral lymphadenopathy (n=27; 54%), cranial abdominal organomegaly (n=24; 48%), abnormal neurologic exam (n=8), pale mucous membranes (n=7) and hemorrhage (n=6), consisting of hyphema, ecchymoses and/or petechiae. Not surprisingly, these clinical findings mirror those described in other case series of canine ALL.7, 15
Results of patient evaluations
A variety of clinicopathologic abnormalities were noted. (Table 2). A CBC was available for review for all dogs at the time of diagnosis. Thirty-four (68%) dogs were anemic (hematocrit <34%) and 43 (86%) were thrombocytopenic (platelets <145 × 103 μL−1). Eleven patients (22%) were markedly neutropenic (<1.6 × 103 μL−1). The median leukemic cell count was 73.5 × 103 μL−1 (range, 684 – 886.5 × 103 μL−1), in all cases consisting predominantly of intermediate-to-large-sized cells; a pathology review of leukemic cells on blood smears resulted in an initial designation of acute leukemia. Hemogram abnormalities of the same type and magnitude have been observed by others in canine AL.8, 15, 19 At diagnosis, every patient was also evaluated by serum biochemical profile. The most prevalent finding was moderate elevation in alkaline phosphatase, which was present in 30 (60%) of the 50 dogs. Hypercalcemia was present in 6 patients. Bone marrow cell populations were evaluated by aspiration (n=27); malignant blasts were identified in 26 (96%) of these samples. One specimen was described as “hypercellular”, but the record did not indicate whether or not leukemic cells were present.
Table 2.
Selected clinicopathologic abnormalities in dogs with AL
| Clinical Pathology Findings | Frequency of Occurrence (Range) |
|---|---|
| Leukemia | 100% (684 – 886,580 cells/μL) |
| Thrombocytopenia | 86% (500 – 145,000 cells/μL) |
| Anemia | 68% (packed red cell volume, 9 – 34%) |
| Serum alkaline phosphatase elevation | 60% (136 – 8180 IU/L) |
| Neutropenia | 22% (0 – 1,600 cells/μL) |
| Hypercalcemia | 12% (total calcium, 11.7 – 19.1 mg/dL) |
A majority of patients were additionally staged by routine diagnostic imaging. Of the 37 dogs assessed by thoracic radiography, 12 (32%) exhibited sternal or cranial mediastinal lymphadenopathy. On abdominal ultrasonography (n=33), the most commonly observed abnormalities were splenopathy (n=25; 76%), mild lymphadenopathy (n=19; 58%) and hepatopathy (n=13; 39%). Documented splenopathies and hepatopathies included subjective enlargement of the affected organ, alterations in echogenicity, and the presence of focal or diffuse nodules.
Leukemic cells were immunophenotyped for B- or T-cell lineage in 37 dogs by various means: flow cytometry (n=31); immunocytochemistry (n=4); and PARR assay (n=2). Twenty patients had definitive lineage assignment. Five had a B-cell leukemia (CD79a+) diagnosed via immunocytochemistry (n=3) or flow cytometry (n=2). In four of these dogs, the leukemic cells also expressed the hematopoietic progenitor cell antigen CD34. Fifteen patients had T-cell leukemias, including all 5 hypercalcemic dogs that were evaluated (of 6 total), as determined by PARR (n=2) or flow cytometry (n=13). In the latter group, the following phenotypes were observed: CD3+ (n=5); CD3+CD4+ (n=1); CD4+ (n=2); CD3+CD8+ (n=2); CD8+ (n=2); and unspecified (n=1). Only one of the T-cell patients (CD4+) had blasts expressing CD34. None of these samples exhibited a mixed pattern of T- and B-cell antigens, as has been observed in canine leukemias and lymphomas.15, 20 In the remaining 17 evaluated dogs, a definitive phenotype could not be established for the leukemic cells: six were CD34+ and negative for all other B- and T-cell-lineage markers; six were CD34+ with no other markers reported in the medical record; and five were CD34- and negative for all other B- and T-cell-lineage markers. In one individual, CD14 was detected on the circulating blasts; expression of this myeloid antigen is seen occasionally in human ALL and canine lymphomas20, 21, and was therefore not considered in deciding the origin. At necropsy, this patient’s leukemic cells stained positively with anti-BLA.36, consistent with a B-cell ALL.22 Thirteen of the 50 dogs did not have lineage assessment performed.
Treatment selection and responses to therapy
Given the short survivals historically associated with a diagnosis of AL in dogs, a reluctance to pursue intensive cytotoxic chemotherapy was often encountered. Not surprisingly, for 8 of the 50 dogs, only palliative therapy was elected (Figure 1A). Prednisone was prescribed for at-home administration of indefinite duration; 3 of those patients survived for 14, 24 and 29 days, while the remaining 5 were lost to follow-up. Six dogs received no treatment, and survived 5, 7 and 8 days, or were similarly lost to further evaluation.
Figure 1.
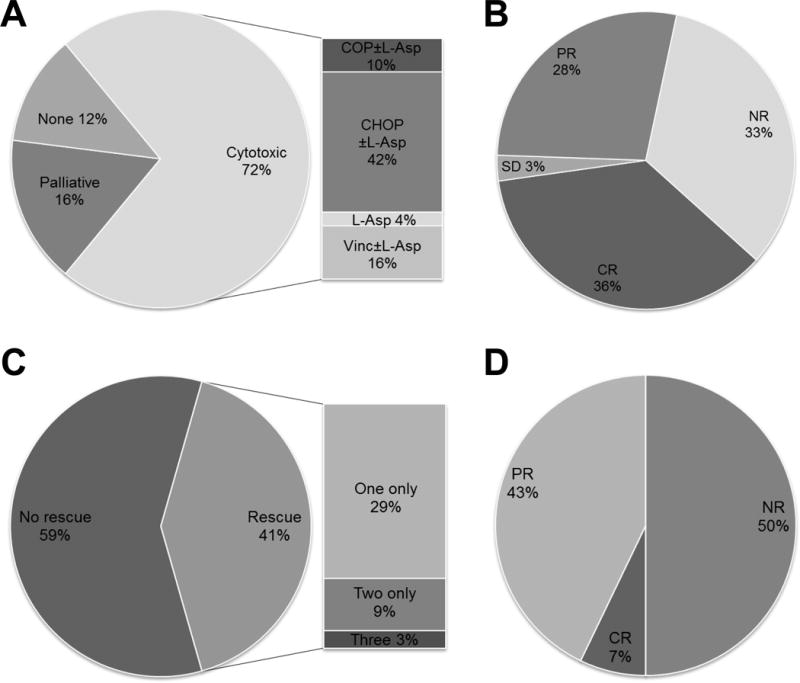
Treatment selection and responses to cytotoxic chemotherapy in dogs with AL. (A) Slightly less than three-quarters of patients were treated with an injectable, cytotoxic chemotherapy protocol. (B) The objective response rate to induction cytotoxic chemotherapy was 64%. (C) Approximately two-fifths of patients that either failed induction chemotherapy or relapsed were administered one or more rescue protocols. (D) Half of 14 dogs responded to their first rescue. All 4 dogs subjected to additional attempts at rescue (two only [n=4] or three [n=1]) had short-lived, partial responses to these chemotherapy regimens (not depicted).
Thirty- six dogs (72%) were treated with cytotoxic chemotherapy that included at least one injectable agent (Figure 1A). On an intent-to-treat basis, the most frequently instituted (21 of 36; 58%) regimen consisted of cyclophosphamide, doxorubicin, vincristine and prednisone, and hereafter is designated simply as CHOP. In almost all patients, CHOP was modified by inclusion of L-asparaginase (n=19). Other modifications included spinal radiation for central nervous system involvement (n=1); incorporation of cytosine arabinoside (n=3); and total body irradiation followed by autologous peripheral stem cell transplantation (n=1). Of dogs treated with CHOP, 12 attained a complete remission (CR) and 6 attained a partial remission (PR), for an overall response rate (ORR) of 85%. The median remission duration for CHOP responders was 41 days (range, 16 – 218 days). One dog maintained stable disease and survived 75 days. A smaller subset of patients was treated with cyclophosphamide, vincristine and prednisone (COP), which yielded one CR of 130 days’ duration, and two PRs of 9 and 31 days’ duration (60% ORR). Four of the five dogs also received L-asparaginase in the protocol. Five dogs received a regimen consisting of vincristine, L-asparaginase and corticosteroids (prednisone or dexamethasone). One of these patients was lost to follow-up evaluation, while the remaining 4 dogs had survival times ranging from 6 to 45 days. Three dogs received vincristine and prednisone, and had survival times of 4, 10 and 16 days. Two patients were treated with L-asparaginase and prednisone and survived 1 and 5 days. The summed overall responses of dogs treated with cytotoxic chemotherapy is shown in Figure 1B; notably, almost one-third of dogs (n=12) failed to respond. The overall response rate of canine AL patients receiving an injectable cytotoxic agent was 64%. Equally disappointing was that fact that responses, when obtained, were generally short-lived. The median duration of first remissions (partial or complete) was only 56 days (Figure 2A).
Figure 2.
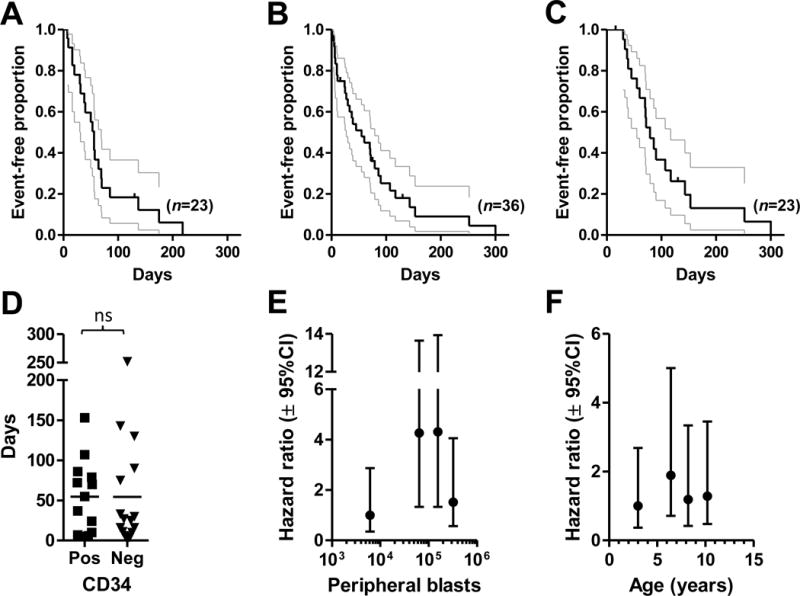
The durability of responses, outcomes and putative prognostic factors for dogs with AL treated with cytotoxic chemotherapy. (A) The first remission durations for patients that had objective responses to induction cytotoxic chemotherapy are shown. Two patients that were lost to follow-up while still in remission at 16 and 130 days are censored. The median duration was 56 days. (B) The survivals of all patients treated with cytotoxic chemotherapy are shown. Five patients were lost to follow-up at 16, 16, 72, 75 and 130 days and are censored. The median survival was 55 days. (C) The survivals of patients that had objective responses to cytotoxic chemotherapy are shown. Four patients were lost to follow-up at 16, 16, 72 and 130 days and are censored. The median survival was 79 days. In (A), (B) and (C), gray lines indicate 95% confidence intervals; ticks represent censored individuals. (D) There was no significant (ns) difference in survival between patients treated with cytotoxic chemotherapy (n=29) whose circulating lymphoblasts expressed surface CD34 at initial presentation versus those negative for the antigen (Mann-Whitney U test, p=0.6769). Peripheral lymphocyte/blast counts (E) in the 2nd and 3rd, but not 4th, quartiles were associated with significantly increased hazard for decreased survival in patients treated with cytotoxic chemotherapy. Increasing age at diagnosis (F) was not associated with a significantly greater hazard for poorer survival in treated dogs. For each variable, the patient population was divided into quartiles, represented by the median value depicted on the x-axis (lymphocyte/blast count [n=36]: 6,100; 63,510; 154,600; 321,948 cells/μL; age [n=35; the age of 1 dog was not recorded]: 3; 6.4; 8.2; 10.2 years). Survival curves for each quartile were then compared to the designated reference (lowest) quartile by log-ranktest, and computed hazard ratios and their 95% confidence intervals (Mantel-Haenszel method) were plotted on the y-axis.
Fourteen dogs that either developed progressive disease (PD) during their initial protocol or relapsed after the end of induction were administered a second cytotoxic chemotherapy regimen (Figure 1C). These rescue therapies included treatment with a multi-agent protocol containing mechlorethamine, vincristine, procarbazine and prednisone (MOPP); re-initiation of CHOP; or treatment with either mitoxantrone, L-asparaginase, lomustine or cytosine arabinoside as single agents (some in combination with corticosteroids). The overall response rate for dogs receiving a first rescue was 7 of 14 (50%; Figure 1D), consisting of 1 CR and 6 PR, with a median remission duration of 19 days (range: 14 – 110 days). Four dogs subsequently were administered a second rescue protocol, which included L-asparaginase, cyclophosphamide, cytosine arabinoside, or doxorubicin. Partial responses of 19, 21, 21 and 36 days’ duration were achieved. One of these patients received a third rescue protocol consisting of L-asparaginase, mechlorethamine, vinblastine, procarbazine and prednisone, which provided a partial remission for 7 days. The median overall remission duration for treated patients was 57 days.
Outcomes for patients treated with cytotoxic chemotherapy
In our study population, dogs with AL treated with modified CHOP, CHOP or various combinations of its constituent agents, had poor survivals, which is not unexpected, given the observed inability of these induction protocols to provide durable responses. The median survival of all dogs treated with cytotoxic chemotherapy, including rescue treatments, was just 55 days (Figure 2B; range 4 1 – 300 days). Even when this analysis was confined to patients who demonstrated measurable responses to their initial protocol (Figure 2C), lifespans were only marginally longer (median, 79 days). Indeed, just two treated dogs survived for longer than six months; none were alive at one year.
In the adult form of human ALL, the outcomes achieved with intensive chemotherapy induction regimens can be correlated with a number of biological and clinical factors at diagnosis, including T- or B-cell immunophenotype, the presence of a mediastinal mass or hepatosplenomegaly, cytogenetic status, total white blood cell and platelet counts, and most importantly, age.23–25 We sought to determine whether some of the same or analogous factors might be helpful in predicting the outcome for dogs with ALL treated with cytotoxic chemotherapy. However, too few treated patients had definitive lineage identification (n=8 T cell; n=5 B cell) or radiographic evidence of a mediastinal mass (n=2) to permit analyses of these parameters. It has been reported that, in dogs with lymphoproliferative diseases, CD34 expression on circulating abnormal lymphocytes is associated with a poor prognosis.26 In our study population, however, there was no difference in survivals between dogs whose leukemic cells expressed the antigen and those which lacked expression (Figure 2D). Similarly, the mean survival times of patients presenting with or without cranial organomegaly at diagnosis was not different (not shown). In childhood ALL, total white blood cell and blast counts are highly correlated and offer equivalent prognostic information.17 Accordingly, we asked whether leukemic cell count had predictive significance for survival in our treated patients, using the method of analyzing continuous variables in ALL described by Donadieu et al.17 For dogs lost to follow-up, survival was considered to end at the last date of evaluation. As seen in Figure 2E, dogs in two of the quartiles with higher median cell counts (63,510; 154,600) had a significantly increased hazard of death when compared to those with the lowest count (6,100 cells/μL). However, survival in the quartile with the highest lymphocyte/blast numbers was not different than reference. The lack of a uniform trend across counts, together with the small sample number per quartile (n=9) and the large magnitude of confidence interval overlap, suggests that this parameter’s significance as a prognostic factor is uncertain, and it may not be clinically important for dogs with AL. Advancing age did not carry a greater risk for shorter survival among our patients (Figure 2F).
Discussion
In this study, we provide clinicopathologic and outcome information on the largest number of dogs with AL since the series of Matus et al.7 Our population comprised cases of AUL of suspected, but unconfirmed lymphoid lineage, and those with a definitive diagnosis of ALL. Data from these AL patients show that injectable cytotoxic chemotherapy only provides modest rates of remission induction (36% CR). Further, the lack of durability of these responses demonstrates that the development of chemoresistance is an early and frequently occurring event, resulting in the short survival rate that continues to characterize this disease.
This bleak outcome with AL might be seen as surprising, when contrasted to the relatively good results that can be obtained with chemotherapy in canine NHL, where malignant lymphocytes appear to have a greater inherent responsiveness to CHOP agents, and to retain their chemosensitivity for longer periods during treatment. In fact, because of this discordant susceptibility, as well as the potentially overlapping clinical presentations of AL and stage V NHL, which has a far greater prevalence in dogs, we were careful to try to exclude cases of the latter, which would have provided a skewed, and in retrospect, overly optimistic portrait of canine AL. Despite this precaution, discriminating between the two can be difficult, particularly as there are no universally accepted criteria for the diagnosis of AL in dogs. We considered cases to represent AL when the presence of excess circulating (or marrow) blasts and cytopenias of one or more major lines were dominant findings, in the absence of substantial lymphadenopathy. Others have defined ALL as marked lymphoblastic leukocytosis characterized by thrombocytopenia and anemia7, 27, and suggested that ALL can be differentiated from lymphoma by clinically significant marrow involvement by immature cells at initial diagnosis.28 The fact that six dogs (five T-cell, one undetermined phenotype) in our study were hypercalcemic could suggest the unintended inclusion of T-cell NHL cases, which are commonly associated with this finding.29 However, hypercalcemia is present in up to 4.8% of cases of human (pre-B) ALL30, 31, and has been observed in a dog with ALL32, so it is also possible that this metabolic derangement occurs more frequently in AL than previously reported. In humans, the distinction between ALL and stage V NHL is considered arbitrary.33 Our results suggest that, for dogs, this differentiation is important, at the least in choosing among therapeutic options. Confronted with a diagnosis of AL, some owners might decide that no treatment is appropriate, when realistically considering the costs and probable outcome; conversely, if cytotoxic chemotherapy is elected, the most condensed, dose-intense schedule of CHOP and L-asparaginase that can be tolerated should be administered to induce a CR. Therapeutic half-measures are unlikely to be rewarding in this disease. In contrast, palliation of NHL in dogs with less-intense chemotherapy regimens can often produce satisfactorily long survivals with minimal disruption of quality of life.
In providing a framework for investigating the clinical features of canine AL, a comparison to the disease in humans naturally arises and is often instructive, since many of the presenting findings, clinicopathologic abnormalities, and responses and outcomes with treatment appear quite similar. Moreover, there is no comparative veterinary oncology literature to serve as a guide, as spontaneous AL has only been sporadically observed in a very small number of other species, such as the cat, rat, gorilla, rhinoceros, and pygmy hippopotamus.34–38 In the dog, the incidence of this cancer is unknown. In one study, cases of ALL represented only 2.1% of bone marrow aspirate samples submitted for cytologic analysis.39 No causative factors have been incriminated in canine AL. Similarly, the pathogenesis in most human cases are undetermined, with less than <5% of cases associated with predisposing genetic conditions (e.g., trisomy 21); exposures to ionizing radiation, chemotherapeutic drugs or toxins; or potentially, infections with viruses, such as Epstein-Barr virus.33
Flow cytometric evaluation (immunophenotyping) of leukemic cells is now the standard means for categorizing ALL in children and adults, and has been increasingly used in dogs.15, 18 With the use of extensive panels of monoclonal antibodies reactive against lineage and maturation markers, precise identification of the transformed progenitor, and consequently, information on prognosis, can be more readily assessed in leukemias in people. For example, 80% of human ALL cases are classified as early (precursor) B-cell lineage40, which conveys a better prognosis than T-cell ALL (15% of cases). In canine NHL, it is well-established that most T-cell-origin cancers are associated with a poorer outcome than their B-cell counterparts41, but it is unknown whether the same holds true in canine ALL.42 We were unable to make this determination, since insufficient study dogs had definitive immunophenotyping. Unlike the disease in humans, cases of T-cell ALL in our study outnumbered B-cell cases (15 vs. 5), but again, this difference may only reflect the small number of dogs for whom lineage was assigned. Moreover, two dogs in our study were considered to have T-cell-origin cancers based on PARR analysis, and these designations may have been erroneous. In humans, T-cell receptor rearrangements are frequently found in B cell tumors43 and acute myeloid leukemia44, and thus, PARR is likely not a useful test in determining lineage in canine ALL. Acknowledging these limitations, our data approximate those of Ruslander et al., who found that in 9 cases of ALL, 3 were T-cell-origin, 2 were B-cell-origin, and 4 were null cell (undetermined lineage).41 A predominance of T-cell ALLs (12 of 13 cases) was also reported by Stokol et al.19 In contrast, in a study of 71 dogs with ALs, when myeloid leukemias were excluded from analysis, B-cell-origin cancers constituted 43% and T-cell-origin cancers constituted 20% of these malignancies (37% were undifferentiated)8. In another publication reporting on flow cytometric assessment of canine leukemias, 15 of 25 ALL cases were B-cell-origin, 6 were T-cell-origin, and the remaining 4 were either of mixed or undetermined lineage.15 Finally, Tasca et al. observed that 47 of 51 ALL cases (92%) were B-cell cancers.18 Currently, based on these conflicting reports, the true prevalence of B- and T-cell leukemias in dogs should be considered uncertain.
The other marker of potential prognostic significance that was assessed in a majority of patients in this study was CD34, a surface antigen found on hematopoietic progenitor cells, but not normally on mature leukocytes. It has been suggested that positive CD34 staining is a defining characteristic of canine ALL45; however, this parameter is not a criterion for ALL in humans. Here, 37% of dogs that had immunophenotyping performed had CD34+ malignant blasts in circulation. Another group reported 76% expression of CD34 in canine ALL patients.15 Expression of CD34 on leukemic cells in human ALL ranges from 44 – 70%, with evidence for correlation between positivity and a poorer prognosis.46 As described above, an analogous association with shorter survival times was reported in dogs with lymphoproliferative diseases26; however, we found no such relationship between expression and outcome in our treated patient population (Figure 2D).
The literature describing the treatment of canine ALL patients is limited. As previously mentioned, Matus et al. found a 4-month survival in a subset of 30 dogs with ALL that were treated with vincristine and prednisone.7 Morris et al. described 11 dogs with ALL (10 treated with injectable chemotherapy) that had a median survival of 62 days (range, 4 – 119).47 Novacco et al. reported median survivals of just 8 (B-cell) and 10 (T-cell) days in treated and untreated dogs; the longest survival was 120 days.8 Other authors reported similarly poor outcomes45, 48, 49, with two isolated exceptions.50, 51 In our study, the two dogs with the longest survivals [252 (T-cell ALL) and 300 days (AUL)] were administered CHOP, in combination with L-asparaginase or cytosine arabinoside, respectively. Intensive protocols using cyclophosphamide, daunorubicin, vincristine, and prednisone, with or without L-asparaginase, are used successfully for remission induction in adults with ALL.24, 52 With such treatment, CR rates are high (85–89% CR), but long-term survivals are stubbornly limited; in Larson et al., the median survival of 197 patients was just 36 months.24 Nonetheless, these outcomes exceed what was attained in our patients treated with similar drugs (albeit given in sequential rather than concurrent fashion). Adults with ALL treated with CHOP agents survive ~4% of a normal lifespan, vs. only ~1.7% in dogs (assuming average US lifespans of 76 and 10 years) – less than half as long. Of course, these varying outcomes could reflect intrinsic differences in the biology of human and canine ALL. More likely, however, the differences are due to the dose intensity achievable in humans, given the much greater acceptance of risk and adverse effects, and the more widespread availability of advanced supportive care and financial resources. Support for this contention is provided by the fact that none of the dogs in this study died from chemotherapy, while in the Larson study, the treatment-related death rate was 9%. The mortality of adults undergoing remission induction for ALL typically ranges from 2 – 8%, primarily due to opportunistic infections.23 Whether such intensive treatments and hazards would be acceptable for dogs, and automatically translate into higher CR rates and longer survivals, remain open questions. In our study, one dog did receive total body irradiation (and autologous peripheral blood stem cell transplant) following a short course of dose-intense chemotherapy. This patient achieved a CR, but was euthanized only 2.5 months after discharge from the hospital.
Not surprisingly, the limited long-term survival conferred by dose-intense chemotherapy in adult ALL has also spurred investigations into other therapeutic (and possibly less toxic) approaches. Towards that end, extensive study has been applied to evaluate the cytogenetic variations within categories of ALL, yielding important implications for treatment. The presence of the Philadelphia chromosome (a reciprocal translocation of chromosomes 9 and 22, which generates the oncogenic bcr-abl fusion product) is a significantly negative prognostic factor associated with very high relapse rates and median survival times of only 9 months.53 More than 50% of B-cell-lineage ALLs in patients over 60 years have this translocation, which partly explains the dismal outcomes with treatment in this age group. With the introduction of the bcr-abl-inhibitor drug, imatinib, during the induction, consolidation and maintenance phases of treatment, survival times have dramatically improved in Philadelphia chromosome-positive patient subgroups.53 It seems likely that analogous cytogenetic discoveries in canine leukemias54 will eventually point to the use of small molecule inhibitors of specific survival pathways in lymphoblasts as the most expedient and least toxic means to achieve better outcomes for ALL in dogs.
The limitations of this study are inherent to any multi-institutional, retrospective analysis and have been well-described. The most salient additional limitation here is the heterogeneity of treatment protocols, which is not unexpected, given the paucity of literature on effective therapy for this cancer. Further, patient responses to chemotherapy were primarily judged only by changes on serial CBCs. Without follow-up bone marrow cytologic analyses during induction to more accurately assess remission status, it seems likely that the CR rate, as well as the efficacy of the chemotherapy being administered, were frequently overestimated. Finally, the marked systemic signs of illness and historically poor prognosis commonly attached to a diagnosis of AL likely dampened owners’ enthusiasm for aggressive treatment, thereby worsening outcomes. Because many patients are euthanized not long after initial presentation, measuring survival time as an endpoint also produces a not entirely accurate picture, but nonetheless, does reflect the reality of this aggressive malignancy.
In conclusion, the use of injectable cytotoxic chemotherapy, such as CHOP, in canine AL yields a reasonable overall response rate (64%), but few durable CRs and, most importantly, no apparent cures, presumably due to the rapid onset of chemoresistance during treatment. Long-term survivals, if possible – and data from human ALL patients suggests such outcomes are achievable – will require substantial advances in dose-intense chemotherapy protocols, supportive care, and potentially, the concurrent use of more targeted therapeutic agents.
Acknowledgments
The authors are grateful to Kenneth M. Rassnick, DVM, DACVIM, Veterinary Medical Center of Central New York, East Syracuse, NY 13057, USA and Mitchell Kaye, DVM, DACVIM, Massachusetts Veterinary Referral Hospital, Woburn, MA 01801, USA for contributing cases to this study. We also thank Julie Nettifee-Osborne (NCSU) for assistance with data compilation. This work was performed at the Veterinary Teaching Hospital, NCSU College of Veterinary Medicine, Raleigh, NC 27606, USA. Paul Hess was supported in part by a National Institutes of Health grant K08 DK082264.
Abbreviations
- AL
acute leukemia
- ALL
acute lymphoblastic leukemia
- AUL
acute undifferentiated leukemia
- CBC
complete blood count
- CHOP
chemotherapy protocol consisting of cyclophosphamide, doxorubicin, vincristine and prednisone
- COP
chemotherapy protocol consisting of cyclophosphamide, vincristine and prednisone
- CR
complete remission
- MOPP
chemotherapy protocol consisting of mechlorethamine, vincristine, procarbazine and prednisone
- ORR
objective response rate
- PARR
polymerase chain reaction for antigen receptor rearrangement
- PD
progressive disease
- PR
partial remission
- SD
stable disease
Footnotes
This research was presented, in part, at the Veterinary Cancer Society Annual Meeting, 2010.
Conflict of Interests
The authors declare no conflict of interests.
References
- 1.Chun R, Garrett LD, Vail DM. Evaluation of a high-dose chemotherapy protocol with no maintenance therapy for dogs with lymphoma. Journal of Veterinary Internal Medicine. 2000;14(2):120–4. doi: 10.1892/0891-6640(2000)014<0120:eoahcp>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Garrett LD, Thamm DH, Chun R, Dudley R, Vail DM. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. Journal of Veterinary Internal Medicine. 2002;16(6):704–9. doi: 10.1892/0891-6640(2002)016<0704:eoacpw>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Keller ET, MacEwen EG, Rosenthal RC, Helfand SC, Fox LE. Evaluation of prognostic factors and sequential combination chemotherapy with doxorubicin for canine lymphoma. Journal of Veterinary Internal Medicine. 1993;7(5):289–95. doi: 10.1111/j.1939-1676.1993.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald VS, Thamm DH, Kurzman ID, Turek MM, Vail DM. Does L-asparaginase influence efficacy or toxicity when added to a standard CHOP protocol for dogs with lymphoma? Journal of Veterinary Internal Medicine. 2005;19(5):732–6. doi: 10.1892/0891-6640(2005)19[732:dlieot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Rassnick KM, Moore AS, Collister KE, Northrup NC, Kristal O, Chretin JD, Bailey DB. Efficacy of combination chemotherapy for treatment of gastrointestinal lymphoma in dogs. Journal of Veterinary Internal Medicine. 2009;23(2):317–22. doi: 10.1111/j.1939-1676.2008.0270.x. [DOI] [PubMed] [Google Scholar]
- 6.Williams LE, Rassnick KM, Power HT, Lana SE, Morrison-Collister KE, Hansen K, Johnson JL. CCNU in the treatment of canine epitheliotropic lymphoma. Journal of Veterinary Internal Medicine. 2006;20(1):136–43. doi: 10.1892/0891-6640(2006)20[136:cittoc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Matus RE, Leifer CE, MacEwen EG. Acute lymphoblastic leukemia in the dog: a review of 30 cases. Journal of the American Veterinary Medical Association. 1983;183(8):859–62. [PubMed] [Google Scholar]
- 8.Novacco M, Comazzi S, Marconato L, Cozzi M, Stefanello D, Aresu L, Martini V. Prognostic factors in canine acute leukaemias: a retrospective study. Vet Comp Oncol. 2015 doi: 10.1111/vco.12136. [DOI] [PubMed] [Google Scholar]
- 9.Riley RS, Massey D, Jackson-Cook C, Idowu M, Romagnoli G. Immunophenotypic analysis of acute lymphocytic leukemia. Hematology/Oncology Clinics of North America. 2002;16(2):245–99, v. doi: 10.1016/s0889-8588(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 10.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. Journal of Clinical Oncology. 2011;29(5):532–43. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- 11.Chiaretti S, Vitale A, Cazzaniga G, Orlando SM, Silvestri D, Fazi P, Valsecchi MG, Elia L, Testi AM, Mancini F, Conter V, te Kronnie G, Ferrara F, Di Raimondo F, Tedeschi A, Fioritoni G, Fabbiano F, Meloni G, Specchia G, Pizzolo G, Mandelli F, Guarini A, Basso G, Biondi A, Foa R. Clinico-biological features of 5202 patients with acute lymphoblastic leukemia enrolled in the Italian AIEOP and GIMEMA protocols and stratified in age cohorts. Haematologica. 2013;98(11):1702–10. doi: 10.3324/haematol.2012.080432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thalheim L, Williams LE, Borst LB, Fogle JE, Suter SE. Lymphoma immunophenotype of dogs determined by immunohistochemistry, flow cytometry, and polymerase chain reaction for antigen receptor rearrangements. Journal of Veterinary Internal Medicine. 2013;27(6):1509–16. doi: 10.1111/jvim.12185. [DOI] [PubMed] [Google Scholar]
- 13.Caniatti M, Roccabianca P, Scanziani E, Paltrinieri S, Moore PF. Canine lymphoma: immunocytochemical analysis of fine-needle aspiration biopsy. Veterinary Pathology. 1996;33(2):204–12. doi: 10.1177/030098589603300210. [DOI] [PubMed] [Google Scholar]
- 14.Weiss DJ. Flow cytometric and immunophenotypic evaluation of acute lymphocytic leukemia in dog bone marrow. Journal of Veterinary Internal Medicine. 2001;15(6):589–94. doi: 10.1892/0891-6640(2001)015<0589:fcaieo>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Adam F, Villiers E, Watson S, Coyne K, Blackwood L. Clinical pathological and epidemiological assessment of morphologically and immunologically confirmed canine leukaemia. Vet Comp Oncol. 2009;7(3):181–95. doi: 10.1111/j.1476-5829.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 16.Vallie VE. Veterinary Comparative Hematopathology. Ames, IA: Blackwell Publishing; 2007. [Google Scholar]
- 17.Donadieu J, Auclerc MF, Baruchel A, Perel Y, Bordigoni P, Landman-Parker J, Leblanc T, Cornu G, Sommelet D, Leverger G, Schaison G, Hill C. Prognostic study of continuous variables (white blood cell count, peripheral blast cell count, haemoglobin level, platelet count and age) in childhood acute lymphoblastic leukaemia. Analysis Of a population of 1545 children treated by the French Acute Lymphoblastic Leukaemia Group (FRALLE) British Journal of Cancer. 2000;83(12):1617–22. doi: 10.1054/bjoc.2000.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tasca S, Carli E, Caldin M, Menegazzo L, Furlanello T, Gallego LS. Hematologic abnormalities and flow cytometric immunophenotyping results in dogs with hematopoietic neoplasia: 210 cases (2002–2006) Vet Clin Pathol. 2009;38(1):2–12. doi: 10.1111/j.1939-165X.2008.00099.x. [DOI] [PubMed] [Google Scholar]
- 19.Stokol T, Schaefer DM, Shuman M, Belcher N, Dong L. Alkaline phosphatase is a useful cytochemical marker for the diagnosis of acute myelomonocytic and monocytic leukemia in the dog. Vet Clin Pathol. 2015;44(1):79–93. doi: 10.1111/vcp.12227. [DOI] [PubMed] [Google Scholar]
- 20.Wilkerson MJ, Dolce K, Koopman T, Shuman W, Chun R, Garrett L, Barber L, Avery A. Lineage differentiation of canine lymphoma/leukemias and aberrant expression of CD molecules. Veterinary Immunology and Immunopathology. 2005;106(3–4):179–96. doi: 10.1016/j.vetimm.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Nakase K, Kita K, Shiku H, Tanaka I, Nasu K, Dohy H, Kyo T, Tsutani H, Kamada N. Myeloid antigen, CD13, CD14, and/or CD33 expression is restricted to certain lymphoid neoplasms. American Journal of Clinical Pathology. 1996;105(6):761–8. doi: 10.1093/ajcp/105.6.761. [DOI] [PubMed] [Google Scholar]
- 22.Imam A, Stathopoulos E, Taylor CR. BLA.36: a glycoprotein specifically expressed on the surface of Hodgkin’s and B cells. Anticancer Research. 1990;10(4):1095–104. [PubMed] [Google Scholar]
- 23.Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, Lazarus HM, Franklin IM, Litzow MR, Ciobanu N, Prentice HG, Durrant J, Tallman MS, Goldstone AH. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106(12):3760–7. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 24.Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P, Duggan D, Davey FR, Sobol RE, Frankel SR, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood. 1995;85(8):2025–37. [PubMed] [Google Scholar]
- 25.Hoelzer D, Thiel E, Loffler H, Buchner T, Ganser A, Heil G, Koch P, Freund M, Diedrich H, Ruhl H, et al. Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults. Blood. 1988;71(1):123–31. [PubMed] [Google Scholar]
- 26.Williams MJ, Avery AC, Lana SE, Hillers KR, Bachand AM, Avery PR. Canine lymphoproliferative disease characterized by lymphocytosis: immunophenotypic markers of prognosis. Journal of Veterinary Internal Medicine. 2008;22(3):596–601. doi: 10.1111/j.1939-1676.2008.0041.x. [DOI] [PubMed] [Google Scholar]
- 27.Couto CG. Clinicopathologic aspects of acute leukemias in the dog. Journal of the American Veterinary Medical Association. 1985;186(7):681–5. [PubMed] [Google Scholar]
- 28.Leifer CE, Matus RE. Lymphoid leukemia in the dog. Acute lymphoblastic leukemia and chronic lymphocytic leukemia. Veterinary Clinics of North America Small Animal Practice. 1985;15(4):723–39. doi: 10.1016/s0195-5616(85)50032-7. [DOI] [PubMed] [Google Scholar]
- 29.Fournel-Fleury C, Ponce F, Felman P, Blavier A, Bonnefont C, Chabanne L, Marchal T, Cadore JL, Goy-Thollot I, Ledieu D, Ghernati I, Magnol JP. Canine T-cell lymphomas: a morphological, immunological, and clinical study of 46 new cases. Veterinary Pathology. 2002;39(1):92–109. doi: 10.1354/vp.39-1-92. [DOI] [PubMed] [Google Scholar]
- 30.Hibi S, Funaki H, Ochiai-Kanai R, Ikushima S, Todo S, Sawada T, Imashuku S. Hypercalcemia in children presenting with acute lymphoblastic leukemia. International Journal of Hematology. 1997;66(3):353–7. doi: 10.1016/s0925-5710(97)00052-2. [DOI] [PubMed] [Google Scholar]
- 31.Trehan A, Cheetham T, Bailey S. Hypercalcemia in acute lymphoblastic leukemia: an overview. Journal of Pediatric Hematology/Oncology. 2009;31(6):424–7. doi: 10.1097/MPH.0b013e3181a1c12b. [DOI] [PubMed] [Google Scholar]
- 32.Henry CJ, Lanevschi A, Marks SL, Beyer JC, Nitschelm SH, Barnes S. Acute lymphoblastic leukemia, hypercalcemia, and pseudohyperkalemia in a dog. Journal of the American Veterinary Medical Association. 1996;208(2):237–9. [PubMed] [Google Scholar]
- 33.Conter V, Rizzari C, Sala A, Chiesa R, Citterio M, Biondi A. Acute lymphoblastic leukemian. Orphanet Encyclopedia. http://www.orpha.net/data/patho/GB/uk-ALL.pdf.
- 34.Cotter SM, Essex M. Animal model: feline acute lymphoblastic leukemia and aplastic anemia. American Journal of Pathology. 1977;87(1):265–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Svoboda T, Jiricka Z, Klir P. Spontaneous acute lymphoblastic leukemia in Sprague-Dawley rats. II. Clinicopathologic observations. Neoplasma. 1989;36(2):149–54. [PubMed] [Google Scholar]
- 36.Barrie MT, Backues KA, Grunow J, Nitschke R. Acute lymphocytic leukemia in a six-month-old western lowland gorilla (Gorilla gorilla gorilla) J Zoo Wildl Med. 1999;30(2):268–72. [PubMed] [Google Scholar]
- 37.Radcliffe RW, Paglia DE, Couto CG. Acute lymphoblastic leukemia in a juvenile southern black rhinoceros (Diceros bicornis minor) J Zoo Wildl Med. 2000;31(1):71–6. doi: 10.1638/1042-7260(2000)031[0071:ALLIAJ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.McCurdy P, Sangster C, Lindsay S, Vogelnest L. Acute lymphoblastic leukemia in a pygmy hippopotamus (Hexaprotodon liberiensis) J Zoo Wildl Med. 2014;45(4):906–10. doi: 10.1638/2014-0005.1. [DOI] [PubMed] [Google Scholar]
- 39.Weiss DJ. A retrospective study of the incidence and the classification of bone marrow disorders in the dog at a veterinary teaching hospital (1996–2004) Journal of Veterinary Internal Medicine. 2006;20:955–61. doi: 10.1892/0891-6640(2006)20[955:arsoti]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.McKenna RW. Multifaceted approach to the diagnosis and classification of acute leukemias. Clinical Chemistry. 2000;46(8 Pt 2):1252–9. [PubMed] [Google Scholar]
- 41.Ruslander DA, Gebhard DH, Tompkins MB, Grindem CB, Page RL. Immunophenotypic characterization of canine lymphoproliferative disorders. In Vivo. 1997;11(2):169–72. [PubMed] [Google Scholar]
- 42.Dobson JM, Blackwood LB, McInnes EF, Bostock DE, Nicholls P, Hoather TM, Tom BD. Prognostic variables in canine multicentric lymphosarcoma. Journal of Small Animal Practice. 2001;42(8):377–84. doi: 10.1111/j.1748-5827.2001.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 43.Szczepanski T, Beishuizen A, Pongers-Willemse MJ, Hahlen K, Van Wering ER, Wijkhuijs AJ, Tibbe GJ, De Bruijn MA, Van Dongen JJ. Cross-lineage T cell receptor gene rearrangements occur in more than ninety percent of childhood precursor-B acute lymphoblastic leukemias: alternative PCR targets for detection of minimal residual disease. Leukemia. 1999;13(2):196–205. doi: 10.1038/sj.leu.2401277. [DOI] [PubMed] [Google Scholar]
- 44.Chapiro E, Delabesse E, Asnafi V, Millien C, Davi F, Nugent E, Beldjord K, Haferlach T, Grimwade D, Macintyre EA. Expression of T-lineage-affiliated transcripts and TCR rearrangements in acute promyelocytic leukemia: implications for the cellular target of t(15;17) Blood. 2006;108(10):3484–93. doi: 10.1182/blood-2005-09-009977. [DOI] [PubMed] [Google Scholar]
- 45.Vernau W, Moore PF. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Veterinary Immunology and Immunopathology. 1999;69(2–4):145–64. doi: 10.1016/s0165-2427(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 46.Thomas X, Archimbaud E, Charrin C, Magaud JP, Fiere D. CD34 expression is associated with major adverse prognostic factors in adult acute lymphoblastic leukemia. Leukemia. 1995;9(2):249–53. [PubMed] [Google Scholar]
- 47.Morris JS, Dunn JK, Dobson JM. Canine lymphoid leukaemia and lymphoma with bone marrow involvement: a review of 24 cases. J Sm Anim Pract. 1993;34:72–9. [Google Scholar]
- 48.Grindem CB, Stevens JB, Perman V. Morphological classification and clinical and pathological characteristics of spontaneous leukemia in 17 dogs. Journal of the American Animal Hospital Association. 1985;21:219–26. [Google Scholar]
- 49.Couto CG. Clinicopathologic aspects of acute leukemias in the dog. Journal of the American Veterinary Medical Association. 1985;186(7):681–5. [PubMed] [Google Scholar]
- 50.Moldovanu G. Continuing long-term remission after cyclophosphamide(NSC-26271) therapy for canine leukemia. Cancer Chemotherapy Reports Part 1. 1969;53(4):223–7. [PubMed] [Google Scholar]
- 51.MacEwen EG, Patnaik AK, Hayes AA, Wilkins RJ, Hardy WD, Jr, Kassel RL, Old LJ. Temporary plasma-induced remission of lymphoblastic leukemia in a dog. American Journal of Veterinary Research. 1981;42(8):1450–2. [PubMed] [Google Scholar]
- 52.Thomas X, Danaila C, Bach QK, Dufour P, Christian B, Corront B, Bosly A, Bastion Y, Gratecos N, Leblay R, et al. Sequential induction chemotherapy with vincristine, daunorubicin, cyclophosphamide, and prednisone in adult acute lymphoblastic leukemia. Annals of Hematology. 1995;70(2):65–9. doi: 10.1007/BF01834381. [DOI] [PubMed] [Google Scholar]
- 53.Rowe JM. Optimal management of adults with ALL. British Journal of Haematology. 2009;144(4):468–83. doi: 10.1111/j.1365-2141.2008.07513.x. [DOI] [PubMed] [Google Scholar]
- 54.Breen M, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans–man and his best friend share more than companionship. Chromosome Research. 2008;16(1):145–54. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]


