Abstract
Filamentous cyanobacteria are the main primary producers in biological desert sand crusts. The cells are exposed to extreme environmental conditions including temperature, light, and diurnal desiccation/rehydration cycles. We have studied the kinetics of activation of photosynthesis during rehydration of the cyanobacteria, primarily Microcoleus sp., within crust samples collected in the Negev desert, Israel. We also investigated their susceptibility to photoinhibition. Activation of the photosynthetic apparatus, measured by fluorescence kinetics, thermoluminescence, and low temperature fluorescence emission spectra, did not require de novo protein synthesis. Over 50% of the photosystem II (PSII) activity, assembled phycobilisomes, and photosystem I (PSI) antennae were detected within less than 5 min of rehydration. Energy transfer to PSII and PSI by the respective antennae was fully established within 10 to 20 min of rehydration. The activation of a fraction of PSII population (about 20%–30%) was light and temperature-dependent but did not require electron flow to plastoquinone [was not inhibited by 3-(3,4-dichlorophenyl)-1,1-dimethylurea]. The cyanobacteria within the crusts are remarkably resistant to photoinhibition even in the absence of protein synthesis. The rate of PSII repair increased with light intensity and with time of exposure. Consequently, the extent of photoinhibition in high-light-exposed crusts reached a constant, relatively low, level. This is in contrast to model organisms such as Synechocystis sp. strain PCC 6803 where PSII activity declined continuously over the entire exposure to high illumination. Ability of the crust's organisms to rapidly activate photosynthesis upon rehydration and withstand photoinhibition under high light intensity may partly explain their ability to survive in this ecosystem.
Biological sand crusts are found in many deserts around the world. They play an important role in stabilizing sandy areas and affect the vegetation composition (Prasse and Bornkamm, 2000; Hagen, 2001; Abed et al., 2002; Eldridge and Leys, 2003; Rajot et al., 2003). Their destruction by man-made activities such as overgrazing is considered an important promoter of desertification in arid and semiarid regions. The crusts are formed by adhesion of the sand to extracellular polysaccharides excreted mainly by filamentous cyanobacteria. These cyanobacteria are the main primary producers in arid desert crusts; other microorganisms including fungi, microalgae, and bacteria are also abundant, particularly in humid areas often covered by a thick crust (Lange et al., 1994). The microorganisms inhabiting the crusts are exposed to extreme environmental conditions including high daytime temperatures during the summer, low temperatures during the night in the winter, high radiation including visible and UV light, and frequent hydration/dehydration cycles.
To cope with the harsh conditions in the biological crusts the organisms must have developed survival mechanisms, the nature of which remains largely unknown. Crucial for survival is the ability to reversibly activate metabolism and grow in the short periods when water is available and to retard metabolic activity during dehydration. The seminal studies by Potts and colleagues (Potts, 1994, 2001; Billi and Potts, 2002) shed some light on the acclimation of the desiccation tolerant, Nostoc commune, to hydration/dehydration cycles. It was concluded that stabilization of existing proteins during dehydration must be involved to ensure rapid reappearance of growth of Nostoc under favorable conditions. Very little is known about the mechanisms whereby the cyanobacteria in the crusts activate the photosynthetic system upon rehydration (Lange et al., 1994; Tarnawski et al., 1994; Dodds et al., 1995; Garcia-Pichel and Belnap, 1996; Lüttge et al., 1996; Qiu and Gao, 2001; Veste et al., 2001) and protect themselves against photoinhibition under the high light intensities particularly during periods of declining water content. Coordination of light energy flux transfer with the rate of electron transport and CO2 fixation is therefore of particular importance during periods of hydration and dehydration. Otherwise photodynamic damage of the photosynthetic machinery may occur. In lichens where the phycobiont partners are green algae, deepoxidation of violaxanthin to zeaxanthin is involved in dissipation of excess light energy (Heber et al., 2001; Bukhov et al., 2004), but this process does not occur in cyanobacteria.
Theoretically, two distinct mechanisms could operate to reduce photodamage under the dehydration/rehydration conditions prevailing in the crusts. The photosynthetic apparatus may undergo disassembly processes during desiccation, which would require repair of the photosynthetic apparatus upon rehydration. Alternatively, during the hydrated state, the cells may activate an efficient repair of the photosynthetic apparatus, as photodamage occurs, and induce a state of dormancy while entering the dehydration process. This would not require de novo synthesis of proteins and pigment components upon rehydration. It is well established that PSII is highly susceptible to photoinhibition, accompanied by rapid degradation (turnover) of its core proteins (Prasil et al., 1992; Kanervo et al., 1995; Niyogi, 1999). While de novo synthesis of the PSII subunits degraded during photoinhibition may occur at high light intensity, it is likely that reassembly of active PSII units and recovery from photoinhibition is achieved only at low light intensities where the rate of repair matches that of the photodamage (Prasil et al., 1992; Keren and Ohad, 1998; Adir et al., 2003; Allakhverdiev et al., 2003). In addition, although PSI is considered to be more stable than PSII, it also undergoes photodamage under chilling and high light conditions (Hihara and Sonoike, 2001).
Taken together, on the basis of earlier studies with various model systems, it is plausible that repair from photodamage during desiccation in the light requires de novo synthesis/reassembly upon rehydration. However, fast recovery of photosynthetic activity upon rehydration (Satoh et al., 2002) and data presented here suggested that the second path is actually operating in the biological crusts. A large part of the PSII and phycobilisomes population and most of the PSI complex population are reactivated within minutes from the onset of rehydration, in the absence of de novo protein synthesis and sustained electron flow. Despite the high radiation condition, photoinhibition of PSII occurs only to a limited extent even at exceedingly high light intensities, and is largely balanced by a fast rate of PSII repair. These properties of the cyanobacteria inhabiting the crust may explain their survival in this harsh biotope.
RESULTS AND DISCUSSION
Activation of PSII during Rehydration
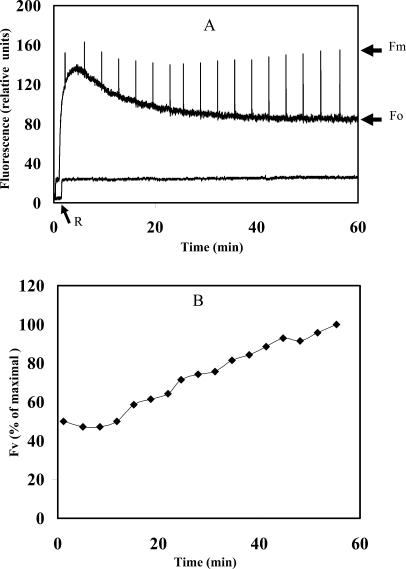
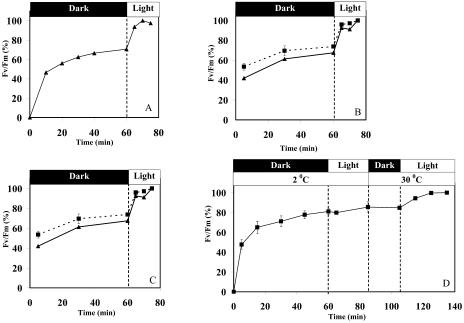
As expected, the dry crusts did not show variable fluorescence and exhibited a very low Fo signal, the fluorescence induced by the modulated weak beam (Fig. 1). Upon rehydration in the light, Fo increased rapidly and reached a maximal value after about 3 min, followed by a gradual decline to a constant value after about 50 min of illumination (Fig. 1A). In cyanobacteria, the Fo signal is ascribed to fluorescence emission due to excitation of closed PSII centers. The fluorescence measurements were carried out with the PAM-101 apparatus (Walz, Effertlich, Germany) where the emission of the modulated measuring beam is at 650 nm and the fluorescence emitted is detected at 710 to 720 nm. Therefore, the emitted fluorescence can be ascribed to excitation of chlorophyll and of allophycocyanin at 650 nm (Glazer, 1988), energy transfer from the phycobilisomes to PSII, and fluorescence emission from the latter. The rapid rise in Fo value after rehydration could be due to the fact that increasing amount of energy reaching closed PSII centers as the phycobilisomes are energetically connected (see Fig. 3). The gradual decline of the Fo signal thereafter occurred while the variable fluorescence of PSII increased (Fig. 1B), both reaching a constant level after about 50 min. Activation of PSII, measured as fluorescence rise induced by saturating light pulses of 1 s, is evident from the rise in variable fluorescence (Fv). The ratio of variable fluorescence to the maximal fluorescence (expressed as maximum fluorescence [Fm] − Fo/Fm = Fv/Fm) increased from a low value of 0.05 after about 3 min of rehydration to 0.5 at the plateau reached after about 50 min, a value common to PSII activity in cyanobacteria (El Bissati et al., 2000). The Fv/Fm value does not provide quantitative assessment of PSII activity per chlorophyll or protein, but the extent of the detected PSII signals. On the other hand, the large changes in the Fo value during the rehydration process (Fig. 1A) could lead to misinterpretation of changes in the PSII activity after rehydration. The extent of PSII activation could be deduced from the rise in Fv with time (Fig. 1B). A Fv3min/Fv55min ratio of approximately 0.5 indicated that one-half of the PSII centers were activated within about 2 to 3 min from the onset of rehydration. The activation of the remaining PSII population progressed gradually and was completed in about 50 min. However, this interpretation of the data is valid only if the total population of PSII centers that can be activated by rehydration of the crust remained constant during the entire duration of the experiment. This is likely to be the case since addition of chloramphenicol did not inhibit the rise of PSII activity during the rehydration process (see below), suggesting that new PSII centers were not formed.
Figure 1.
Kinetics of PSII activation in desert crusts following rehydration in the light. Rehydration of a dried crust disc of 2-cm diameter was carried out at room temperature and 20 μmol photon m−2 s−1. A, Kinetics of the fluorescence parameter Fo elicited by the measuring modulated beam and of Fm induced by 1-s saturating light pulses as a function of the rehydration time (upper trace) and desiccated dried crust (lower trace). Note the low Fo level prior to the initiation of rehydration (oblique arrow, R). B, The variable fluorescence (Fv = Fm − Fo) as a function of rehydration time. The 100% corresponded to the value obtained after 55 min in A. The data presented are the average of four independent experiments; the sds were smaller than 5% of the average.
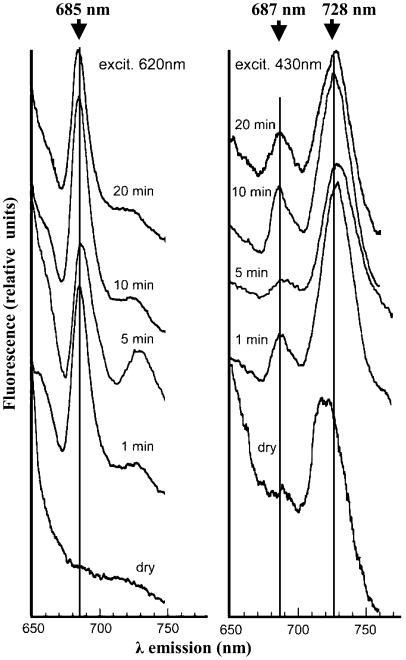
Figure 3.
The 77°K fluorescence emission spectra of crusts as a function of time of rehydration. Samples of crusts were frozen and excited at 620 nm (left) or 430 nm (right), respectively, before (dry) and after rehydration at room temperature and ambient light for 1, 5, 10, and 20 min, respectively. Vertical lines indicate wavelengths of maximal emission. These analyses were repeated four times with very little difference between the results obtained.
Notably, there are large differences in the reported rates of activation of photosynthesis after rehydration (Scherer et al., 1984; Green et al., 1994; Lange et al., 1994; Dodds et al., 1995; Garcia-Pichel and Belnap, 1996; Mazor et al., 1996; Veste et al., 2001; Satoh et al., 2002). Studies by Potts (1994) and our own (data not shown) clearly indicated that the duration of desiccation was an important factor; the shorter the dry phase the faster was the activation of photosynthetic capability. As an example, measurable respiratory activity in Nostoc started about 0.5 h after wetting, photosynthesis after 6 h, and nitrogen fixation after 50 h (Scherer et al., 1984). The experiments performed here were carried out on crusts in which Microcoleus sp. consisted of over 90% of the cyanobacterial population. This crust underwent several consecutive cycles of desiccation/rehydration, thus partially mimicking the conditions in its natural habitat where approximately 200 nights with dew annually were recorded. However, in samples that were maintained in the dry state for longer periods, such as 6 months, PSII activation could not be detected during the first 60 min after rehydration. Nevertheless, following one rehydration/desiccation cycle, the ability to activate PSII within a few minutes of rehydration was reestablished (data not shown). In addition to the duration of the dried period, differences in growth conditions and species-specific may affect the rate of recovery after rehydration, but the exact nature of these differences are poorly understood.
Charge Recombination Activity of PSII during the Rehydration Process
Measurements of thermoluminescence (TL) emissions provided an important tool to follow PSII activation in crust samples (Vass, 2003). This method is based on the fact that excitation of PSII by a single-turnover flash results in primary charge separation and reduction of the QB quinone in PSII to semiquinone radical (QB˙−). The cation radical of the primary donor, P680˙+, is reduced by an electron from the manganese cluster in the PSII donor side that can release up to four electrons following four consecutive excitations, resulting in the oxidized S states. Release of the semiplastoquinone radical from the QB site to the plastoquinone pool can only occur after receiving an additional electron following another charge separation event and protonation to form PQH2. However, upon heating, which supplies the energy required for back electron flow against a potential gradient, the resulting S2,3/QB˙− can recombine, leading to regeneration of the primary charge separated state P680˙+/Pheo˙−. The recombination of this pair is accompanied by photon emission. Thus, in a sample capable of charge separation and recombination, a quantitative relation exists between the photon count and the active PSII population. To form a stable charge separated state, the excitation is given at subzero temperature. Recombination of charges is then initiated by heating the sample at a constant rate (Vass, 2003). The temperature at which maximal rate of charge recombination occurs is proportional to the activation energy required for the process.
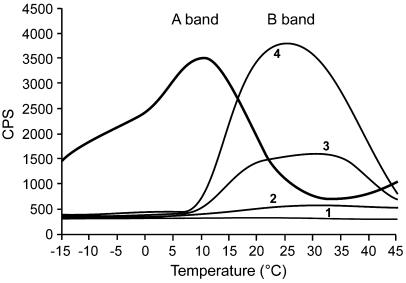
TL measurements were performed using 2-cm diameter discs cut from a crust containing a relatively homogenous density of cyanobacteria, corresponding to about 3 μg chlorophyll. Since the samples are heated up to 50°C during the experiment, separate samples were used in each experiment. Traces of the recorded photon emission as a function of temperature showed the appearance of the B band emission, originating from charge recombination of the QB˙−/S2,3 state with a maximum at about 25°C to 30°C (Vass, 2003), already after 3 min of rehydration in the dark (Fig. 2). At this time, the signal was rather low, presumably since only a fraction of the PSII centers were activated. Notably, the curve obtained showed appearance of the B band as well as residual QA− signal. This may reflect a situation where electron transfer from QA is blocked in some of the PSII centers shortly after rehydration. As expected, the TL curve obtained after 17 min of rehydration showed large rise in the B band as additional PSII centers were activated. Addition of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) to the sample prior to the light excitation prevents reduction of the QB quinone and thus the formation of the stable charge separated QA˙−/S2 state. In the presence of DCMU recombination occurred at about 10°C to 12°C (Fig. 2, A band), in agreement with earlier reports for cyanobacteria and higher plants thylakoids (Vass, 2003). These results are in agreement with those presented in Figure 1 showing the appearance of variable fluorescence at room temperature and the 77°K fluorescence emission elicited by excitation at 430 nm (Fig. 3, below) attributed to presence of assembled PSII.
Figure 2.
TL emission of crust samples prior to and following rehydration. Discs of dried samples (2-cm diameter, chlorophyll content about 2 μg) were rehydrated at room temperature and ambient light. The samples were then placed on the TL apparatus stage, dark-adapted for 3 min, and rapidly cooled by liquid nitrogen to −20°C. Light excitation was followed by heating of the samples at 0.5°C/s and photon emission counted (counts per second, CPS). Trace 1, A disc of hydrated filter paper serving as base line for the apparatus; trace 2, a sample of dry crust prior to rehydration; traces 3 and 4, samples after rehydration for 3 and 17 min, respectively (B band); sample rehydrated for 17 min in the presence of 10 μm DCMU (A band). Similar results were obtained in three other experiments.
Fluorescence Emission Measured at 77°K during the Rehydration Process
Variable fluorescence (Fig. 1) and TL (Fig. 2) provided information about the charge separation and electron flow in PSII but not on the assembly of the phycobilisomes and energy transfer to the reaction centers. To test the latter, we measured the low-temperature (77°K) fluorescence emission spectra as a function of duration of rehydration and of the wavelength of excitation (Fig. 3). In dry crust excited at 620 nm, absorbed by phycocyanin in the phycobilisomes, we could not detected fluorescence at 685 nm (the emission maxima from assembled phycobilisomes (Glazer, 1988; Fig. 3, left). However, a distinct fluorescence emission peak at 685 nm appeared already after 1 min of rehydration, and its intensity did not increase in samples hydrated for a longer duration. These data suggested fast rise in the ability to transfer energy from phycocyanin shortly after rehydration. Excitation of the frozen samples at 380 nm, absorbed by phycocyanin and allophycocyanin (Glazer, 1988), did not result in emission at 645 to 650 nm, i.e. from free phycobiliproteins (data not shown). In addition, fluorescence emission with a maximum at about 730 nm was observed already after 1 to 5 min of rehydration suggesting energy transfer to PSI from either chlorophyll in the antenna of PSI or the phycobilisomes as both absorb at 620 nm. Taken together, the results of the low-temperature fluorescence (Fig. 3) are consistent with our interpretation of the data shown in Figure 1. We propose that the weak modulated beam provided by the PAM apparatus at 650 nm was absorbed by the phycobilisomes cores and contributed to the Fo emission from PSII measured at 710 nm by the PAM-101 (Fig. 1). The fast rise in the fluorescence emission at 685 nm (induced by excitation at 620 nm; Fig. 3), at 77°K, is in agreement with the rise in Fo during that time.
Excitation of the dry crust by 430 nm light, absorbed primarily by chlorophyll a, induced a significant emission at about 715 nm that may be ascribed to PSI antennae, and a small shoulder at 687 nm (Fig. 3, right). However, after 1 min of rehydration, a high fluorescence emission peak at 687 nm, emitted by PSII, was apparent, consistent with data presented in Figure 1. These data provided additional evidence for renewal ability of energy transfer to the activated PSII centers shortly after rewetting. Furthermore, an emission maximum at 728 nm appeared concomitant with the disappearance of the 715-nm peak, indicating that short period of rehydration suffices for the establishment of energy transfer from the antennae to the PSI complex as well. Measurements of absorption changes at 826 nm at room temperature, using the PSI attachment of the PAM-101 apparatus, showed oxidation of P700 in the crusts induced by excitation of the samples with far red light. These signals appeared after about 5 to 10 min of rehydration, but the signal/noise ratio in these experiments did not permit an accurate assessment of the kinetics and extent of PSI oxidation (data not shown).
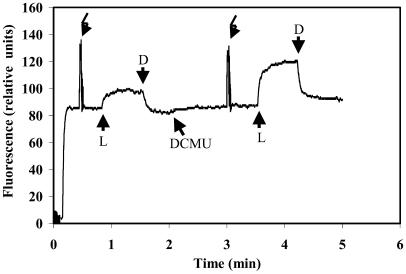
Experiments where the steady state fluorescence was induced by light of 650 nm (37 μmol photon m−2 s−1) in the absence or presence of DCMU showed activation of the entire electron transfer chain within 5 min after rehydration (Fig. 4). As expected, the steady state fluorescence emitted by the DCMU-treated crusts reached values close to the Fm obtained by the saturating pulse. In contrast, the steady state fluorescence emitted by samples that were not treated with DCMU was considerably lower, indicating that QA was not fully reduced and that electron flow to the plastoquinone pool and PSI, via and beyond cytochrome b6f, was functioning already 5 min after rehydration.
Figure 4.
Steady-state photosynthetic activity following rehydration of desiccated desert crusts. The crust was rehydrated for 5 min in the light and the level of the steady-state fluorescence, induced by 40 μmol photon m−2 s−1 of 650 nm light (L), was compared with that obtained from the same sample after addition of DCMU. Saturating white light pulses of 3,000 μmol photons m−2 s−1 were provided to reach Fm; D, dark.
Activation of PSII during Rehydration Does Not Require Photosynthetic Electron Transfer
The data presented in Figures 1 to 3 demonstrated rapid activation of photosynthesis following rehydration of dried crusts. To test whether illumination plays a role in this process, we followed the development of PSII activity in crust samples that were rehydrated in darkness. A rapid rise in variable fluorescence, expressed as Fv/Fm, was observed within about 10 min, followed by a slower increase in the activity that reached a plateau after about 50 to 60 min. Illumination of the crust at this time for 10 min resulted in an additional increase in activity reaching that obtained in samples that were rehydrated under continuous illumination (Fig. 5A). Interestingly, the rise in PSII activity was observed even in the presence of DCMU (Fig. 5B), suggesting that electron transfer to the PQ pool and the S-states cycle were not essential for activation of the light-requiring PSII population. Furthermore, activation of PSII after rehydration did not require de novo synthesis of proteins since addition of chloramphenicol to the rehydration medium did not inhibit this process (Fig. 5C). Clearly, both DCMU and chloramphenicol penetrated the cells and were functional since they affected the variable fluorescence (Fig. 4) and repair from photoinhibition (see Fig. 6), respectively.
Figure 5.
Effect of light, temperature, DCMU, and chloramphenicol on the development of Fv/Fm in crusts after wetting. Dried crusts were rehydrated in the dark and the Fv/Fm values measured as a function of time. At the indicated time points, the samples were transferred to the light (30 μmol photon m−2 s−1) and incubated until a plateau Fv/Fm value was reached. The data are presented as percent of the maximal PSII activity obtained. A, Samples rehydrated with distilled water at 25°C. B, Solid line, samples rehydrated as above; dashed lines, samples rehydrated in presence of 50 μm DCMU. C, Samples rehydrated as in A in the absence (solid line) or presence of 100 μg mL−1 chloramphenicol (dashed lines). D, Sample rehydrated at 2°C and then transferred to the light for 20 min. This was followed by warming the crust to 30°C and illuminated for an additional 30 min. The data are presented as percent of the final values to demonstrate the similarity of the effect of rehydration in the dark or light, in samples that contained different amounts of cyanobacteria and vary to some extent in the initial Fv/Fm values. In these experiments (n = 4), the maximal Fv/Fm values (100%) were in the range of 0.45 to 0.55. The sds are provided only in cases where it was larger than 5% of the average.
Figure 6.
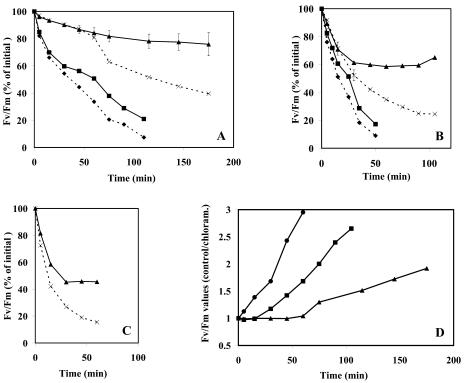
Susceptibility of the crust cyanobacteria to photoinhibition at different light intensities. Crust discs of 2-cm diameter were rehydrated in the light for 1 h at 20°C and 30 μmol photon m−2 s−1 and subsequently exposed to different light intensities at 25°C without (solid lines) or with 100 μg mL−1 chloramphenicol (dashed lines). The Fv/Fm values were measured at the indicated times and plotted as percent of the initial value. Light was provided by tungsten halogen lamps (500 W) filtered through doubled walled glass-Perspex filters cooled by circulating cold water. For comparison, similar experiments were carried out using a suspension of Synechocystis PCC 6803 cells added to a similar amount of sand as that contained in the crusts. The chlorophyll content was about 5 μg/sample. The light intensities in A, B, and C were 500, 1,000, and 1,500 μmol photon m−2 s−1, respectively. Triangles, crust samples; squares, Synechocystis cells. D, Increase in the capacity of the crust cyanobacterial cells to maintain PSII activity as a function of time and light intensity. The data were calculated from those presented in Figures 6, A to C and represent the ratio of Fv/Fm levels of the control samples to those containing chloramphenicol. Triangles, squares, and circles are samples exposed to 500, 1,000 or 1,500 μmol photon m−2 s−1, respectively. The sds are provided only in cases where it was larger than 5% of the average (n = 2).
In the desert area of Nizzana, where the crust samples were collected, moisture in the morning is often accompanied by low, close to freezing temperatures, particularly in the winter. To examine whether the activation of PSII is a temperature-dependent process, we measured the changes in variable fluorescence after rehydration of crust samples in the dark, at 2°C, followed by exposure to the light. We then raised the temperature to 30°C for additional period. The results (Fig. 5D) indicated that activation of PSII was little affected by lowering the temperature to 2°C. About 80% of the maximal signal was obtained in crust samples exposed to this temperature. However, activation of the remaining 20% of PSII population that required light was also temperature-dependent (Fig. 5D).
The finding that application of DCMU did not prevent the light-induced activation of PSII (Fig. 5B) raised the possibility that the effect of light was due to changes within the PSII complex. These could include reduction of QA, back electron flow and charge recombination of P680˙+/Pheo˙−, and possibly involvement of back electron flow within PSII via cytochrome b559 (Barber and Rivas, 1993; Poulson et al., 1995). It is also possible that the manganese cluster of part of the PSII population is partially disassembled during the desiccation, and reassembly might require photoactivation via electron flow-dependent oxidation of the cluster (Nishiyama et al., 2001). To examine these possibilities, we tested whether series of single turnover flashes, delivered at 300-ms intervals in the absence or presence of DCMU, could induce activation of the light-requiring PSII population. We found that 50 single turnover flashes were sufficient to raise the activity of PSII after rehydration in darkness; exposure of the crusts to 300 light flashes did not raise the PSII activity any further (data not shown). The lack of sensitivity to DCMU and the achievement of full activation by 50 single turnover flashes suggested that light-induced conformational changes of the chlorophyll-proteins interactions, rather than excitation, might be responsible for this phenomenon (Cseh et al., 2000). Structural changes in PSII during desiccation or its photoinactivation may lead to photooxidation of chlorophyll, and thus PSII reassembly/reactivation might require de novo synthesis of chlorophyll. However, unlike higher plants, chlorophyll biosynthesis in cyanobacteria is light-independent (Kada et al., 2003), and thus it is unlikely that light-activation of a fraction of the PSII centers was due to de novo chlorophyll synthesis. As indicated, the crusts used here were almost entirely populated by Microcoleus sp. (Supplemental Fig. 1, available at www.plantphysiol.org). Nevertheless, we can't discard the possibility that activation of PSII in another cyanobacterial species, such as Nostoc sp. also present in the crusts, required higher temperatures. Use of artificial crusts composed of single cyanobacterial species, recently produced in our laboratory, might shed light on possible species-specific differences in the temperature dependence of the process of recovery after rehydration.
Low Sensitivity of the Cyanobacteria in the Crust to Photoinhibition
As indicated, the cyanobacteria in the crust are exposed to high light intensity, particularly during the dehydration and the dry period. These conditions are expected to result in severe damage to PSII on one hand and reduced repair capability on the other (Dilley et al., 2001; Adir et al., 2003). To examine photoinhibition and repair of PSII, rehydrated crusts that have reached maximal activity in the light were exposed to 500, 1,000, and 1,500 μmol photon m−2 s−1 for various durations in the absence or presence of chloramphenicol. The latter was used to prevent de novo synthesis of the D1 protein of PSII center that is rapidly degraded under photoinhibitory conditions, and its synthesis is essential for repair of PSII from photodamage. Differences in the rate and extent of PSII inactivation between the control and chloramphenicol-treated samples allowed us to estimate the sensitivity of the samples to light and their ability to repair the photosynthetic activity. For comparison, we examined photoinhibition and repair in an aquatic model cyanobacterium, Synechocystis sp. strain PCC 6803, under the same conditions.
Exposure of the crusts to 500 μmol photon m−2 s−1 for 1 h resulted in a negligible decline of Fv/Fm that was not significantly affected by the presence of chloramphenicol (Fig. 6A). Longer illumination led to continuous decline in PSII activity to about 80% from the initial one after 3 h of illumination. Presence of chloramphenicol enhanced the loss of PSII activity which, at this time point, declined by about 40% (Fig. 6A). Exposure of Synechocystis sp. strain PCC 6803 cells to similar conditions resulted in a considerably faster loss of PSII activity even in the absence of chloramphenicol (Fig. 6A). These results demonstrated that PSII of the cyanobacteria inhabiting the crust was far less sensitive to photoinhibition than Synechocystis, an organism often used as a model system in experiments with cyanobacteria. This conclusion was further supported by experiments where the cells were exposed to 1,000 μmol photon m−2 s−1 (Fig. 6B). The activity of the cyanobacterial crust declined to 60% of the initial Fv/Fm value after 30 min of illumination and remained stable for the rest of the time in the light. Loss of activity was more pronounced in samples where protein synthesis was inhibited, declining to about 25% of the initial within 90 min of illumination. Under these conditions, the PSII activity of Synechocystis sp. PCC 6803 cells decreased by over 80% within 50 min of illumination regardless of whether protein synthesis was inhibited (Fig. 6B). PSII activity of the crust cyanobacteria was not diminished even when exposed to 1,500 μmol photon m−2 s−1 and remained constant at 45% of its initial value from 30 min of illumination onward (Fig. 6C).
The rate and extent of photoinhibition reflects the balance between the photodamage and repair processes. The former can be assessed from the curve obtained in the presence of chloramphenicol where the repair is inhibited, whereas the difference between the curves obtained in the presence and absence of chloramphenicol is indicative of the ability of the cells to repair the damage. Thus, the data presented in Figure 6, A and B clearly indicated that photodamage was considerably smaller and repair was larger in the cyanobacteria within the crust than in Synechocystis sp. PCC 6803. Further, inhibition of protein synthesis in the crust's cyanobacteria did not completely abolish PSII activity even when exposed to high light intensities.
The shape of the curves relating extent of photoinactivation to time of illumination of the crust samples (Fig. 6) suggested that initially the rate of repair did not match that of photodamage but increased thereafter, leading to the plateaus obtained at a lower than initial activity. The ratio of the PSII activity of the control to that observed in the chloramphenicol-treated crusts increased with time of illumination and with light intensity (Fig. 6D). This could be due to a rise in the repair capability with light intensity and duration of exposure. Alternatively, photodamage declined with time of exposure to high light. At this time we cannot distinguish between these possibilities. However, since PSII activity (and thus photosynthesis) and energy trapping in chemical form decreases during photoinhibition, one would expect a decline in the capacity to produce the proteins essential for the repair of photodamage. This is in agreement with the results obtained with Synechocystis PCC 6803 but in contrast to those obtained with the crust's cyanobacteria. To the best of our knowledge an increased resistance to photodamage with time of exposure, due to either of the alternatives raised above, was not observed in other cyanobacteria examined so far; it may reflect a unique capability possessed by the organisms in the biological crusts to withstand photoinhibitory conditions.
A striking property of the crust's cyanobacteria is their ability to repair and assemble functional PSII under high illumination, a process that requires de novo synthesis of protein D1 (Prasil et al., 1992; Aro et al., 1993; Allakhverdiev et al., 2000; Spetea et al., 2000; Adir et al., 2003). The D1 protein is synthesized as a precursor (pD1) with a 9 to 11 amino acid residue extension in its carboxyl end, which is cleaved by the protease CtpA (Anbudurai et al., 1994). The pD1 can be assembled within PSII before or after processing (Adir et al., 2003), but only PSII complexes containing processed D1 can assemble the manganese cluster and thus activate electron flow (Prasil et al., 1992; Gong and Ohad, 1995). Consequently, upon illumination, PSII centers bearing pD1 can only generate the highly oxidizing cation radicals Yz˙− and P680˙− leading to fast degradation of D1 even under a relatively low light intensity (Gong and Ohad, 1995; Adir et al., 2003). Under low light conditions, the probability of survival of PSII centers bearing pD1 protein, prior to stabilization by processing of pD1 and assembly of the manganese cluster, is higher than under high illumination and thus optimal for repair of photoinactivated PSII. The resistance of the crust's cyanobacteria to high illumination during the repair of PSII could be due to rapid processing of pD1 or synthesis of D1 in its mature form disposing of the need for processing activity. Synthesis of D1 as a mature protein lacking the C-terminal extension has been reported in Euglena gracillis (Swensson et al., 1991), but the psbA genes encoding D1 in Nostoc punctiforme (http://www.jgi.doe.gov/JGI_microbial/html/) appear to possess the C terminus. Clearly, better understanding of the ability of cyanobacteria to withstand the harsh conditions in the crusts would have to rest on clarification of the mechanisms whereby a severe photodamage to the photosynthetic machinery during dehydration is prevented.
MATERIALS AND METHODS
Collection of Biological Crusts
Samples were collected from a north-facing dune at the experimental station of the Minerva Arid Ecosystems Research Center (AERC), Nizzana, NW Negev, Israel. The station is located at 34° 23′ E; 30° 56′ N. The average annual rainfall is about 100 mm, occurring mainly between November and March. The mean annual minimum and maximum temperatures (in the shade) are 12.5°C and 25.9°C, respectively. The coldest and warmest months are January and August with mean minimum and maximum temperatures of 5.5°C and 33.5°C, respectively. Temperatures as low as −2°C in the winter and 50 to 55°C in full sunlight (1,500–2,000 μmol photon m−2 s−1) in the summer were frequently encountered. Apart from the rare events of rain during the winter, dew (approximately 200 nights per year; Zangvill, 1996) and fog (about 20 nights per year; S. Berkowicz, Minerva Arid Ecosystem Centre, Hebrew University of Jerusalem, personal communication) are the main sources of moisture for the crusts in Nizzana. The crust samples were placed in petri dishes after removing of excess sand from the bottom layer. Unless otherwise specified, the crust samples were kept in the dry state for at least 1 month, at 30°C, under fluorescent room light.
All the crusts used here were collected in the same place to minimize possible variability due to different composition of the biological consortia (strongly affected by the humidity). Microscopic inspection (Supplemental Fig. 1) and analysis of the 16 s RNA gene sequence, using oligonucleotides specifically designed for cyanobacteria (Rudi et al., 1997) and DNA extracted from the crust, indicated that Microcoleus sp. was the main organism inhabiting this crust, consisting of at least 90% of the cyanobacterial population in the crust.
Rehydration of Dried Biological Crusts
Discs (2-cm diameter) excised from dried crusts were placed in glass dishes mounted in a temperature controlled aluminum block containing six sample holders adequate to the PAM-101 fiber glass light guide. Rehydration of the crust samples (average weight 0.45 ± 0.1 g) was carried out by addition of 0.3 to 0.4 mL/dish distilled water (to maintain the solutes concentration and composition prior to the desiccation), and the samples were kept at the temperatures and light regimes as described for the particular experiments. Unless otherwise mentioned, pieces of crusts dried for various times were rehydrated and exposed to 30 μmol photon m−2 s−1 at room temperature, then allowed to dry again at room temperature for 24 to 48 h before used for rehydration experiments.
Measurements of Photosynthetic Activities
Electron transfer activity of PSII was measured by pulse modulated fluorescence kinetics using the PAM-101 apparatus. The light intensity (measured at the surface of the crust) of the modulated measuring beam (1.6 kHz) was 100 nmol photons m−2 s−1. Actinic light delivered by the PAM-101 system (650 nm; 37 μmol photons m−2 s−1) was used to assess steady state fluorescence. Fm was measured with saturating white light pulses of 3,000 μmol photons m−2 s−1 for 1 s.
Charge separation and recombination in PSII were measured by TL as described in Zer et al. (1994). The samples were placed on the apparatus stage, dark adapted for 3 min, and then rapidly cooled by a stream of liquid nitrogen to −20°C. Light excitation was delivered by a xenon discharge lamp (EG&G, Salem, VA) using a capacitor of 0.05 μF charged at 900V. The samples were then heated at a constant rate (0.5°C/s) and photon emission as a function of the rising temperature was counted (counts per second). Single turnover flashes used for activation of PSII electron flow during rehydration were delivered using a Xenon Micropulser (model 457, Xenon, Woburn, MA); the capacitor used was 0.5 μF charged at 9,000V.
The 77°K fluorescence emission spectra were recorded using a Perkin-Elmer (Foster City, CA) 401spectrofluorimeter according to (Kirilovsky and Ohad, 1986). Crusts fragments of about 3-mm wide and 1-cm long were glued to a glass rod of 5-mm diameter, using transparent tape, and rehydrated in the light. At the indicated times, the rod was rapidly immersed in a dewer containing liquid nitrogen fitted to the sample holder of the spectrophotometer. This mounting allowed us to rotate the glass rod holding the crust to orient the flat piece of crust to the exciting beam and photomultiplier, respectively, giving the maximal emission. Due to the low chlorophyll content of the samples, about 1 μg, the spectra were recorded at maximal instrument sensitivity using a widely open excitation and emission slits, thus somewhat limiting the resolution of the fluorescence emission peaks.
Chlorophyll Measurements
Crusts were homogenized in methanol using a vortex mixer. The samples were centrifuged to sediment the sand and the supernatants used for spectroscopic chlorophyll measurements.
Supplementary Material
Acknowledgments
We thank Mr. Simon Berkowicz for valuable meteorological information and his help in the collection of the crust samples.
This work was supported by grants from the Israel Science Foundation, The Minerva Arid Ecosystem Research Center, and The German Ministerium for Bildung, Wissenshaft, Forschung, und Technologie, and the Bogan Foundation.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.047712.
References
- Abed RMM, Schonhuber W, Amann R, Garcia-Pichel F (2002) Picobenthic cyanobacterial populations revealed by 16S rRNA-targeted in situ hybridization. Environ Microbiol 4: 375–382 [DOI] [PubMed] [Google Scholar]
- Adir N, Zer H, Shochat S, Ohad I (2003) Photoinhibition: a historical perspective. Photosynth Res 76: 343–370 [DOI] [PubMed] [Google Scholar]
- Allakhverdiev SI, Hayashi H, Nishiyama Y, Ivanov AG, Aliev JA, Klimov VV, Murata N, Carpentier R (2003) Glycinebetaine protects the D1/D2/Cytb559 complex of photosystem II against photo-induced and heat-induced inactivation. J Plant Physiol 160: 41–49 [DOI] [PubMed] [Google Scholar]
- Allakhverdiev SI, Sakamoto A, Nishiyama Y, Inaba M, Murata N (2000) Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol 123: 1047–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbudurai PR, Mor TS, Ohad I, Shestakov SV, Pakrasi HB (1994) The ctpa gene encodes the C-terminal processing protease for the D1 protein of the photosystem-I reaction-center complex. Proc Natl Acad Sci USA 91: 8082–8086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro E-M, Virgin I, Andersson B (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113–134 [DOI] [PubMed] [Google Scholar]
- Barber J, Rivas JD (1993) A functional-model for the role of cytochrome-B(559) in the protection against donor and acceptor side photoinhibition. Proc Natl Acad Sci USA 90: 10942–10946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billi D, Potts M (2002) Life and death of dried prokaryotes. Res Microbiol 153: 7–12 [DOI] [PubMed] [Google Scholar]
- Bukhov NG, Govindachary S, Egorova EA, Carpentier R (2004) Recovery of photosystem I and II activities during re-hydration of lichen Hypogymnia physodes thalli. Planta 219: 110–120 [DOI] [PubMed] [Google Scholar]
- Cseh Z, Rajagopal S, Tsonev T, Busheva M, Papp E, Garab G (2000) Thermooptic effect in chloroplast thylakoid membranes. Thermal and light stability of pigment arrays with different levels of structural complexity. Biochemistry 39: 15250–15257 [DOI] [PubMed] [Google Scholar]
- Dilley RA, Nishiyama Y, Gombos Z, Murata N (2001) Bioenergetic responses of Synechocystis 6803 fatty acid desaturase mutants at low temperatures. J Bioenerg Biomembr 33: 135–141 [DOI] [PubMed] [Google Scholar]
- Dodds WK, Gudder DA, Mollenbauer D (1995) The ecology of Nostoc. J Exp Bot 46: 309–319 [Google Scholar]
- El Bissati K, Delphin E, Murata N, Etienne AL, Kirilovsky D (2000) Photosystem II fluorescence quenching in the cyanobacterium Synechocystis PCC 6803: involvement of two different mechanisms. Biochim Biophys Acta 1457: 229–242 [DOI] [PubMed] [Google Scholar]
- Eldridge DJ, Leys JF (2003) Exploring some relationships between biological soil crusts, soil aggregation and wind erosion. J Arid Environ 53: 457–466 [Google Scholar]
- Garcia-Pichel F, Belnap J (1996) Microenvironments and microscale productivity of cyanobacterial desert crusts. J Phycol 32: 774–782 [Google Scholar]
- Glazer AN (1988) Phycobilisomes. In L Packer, AN Glazer, eds, Methods in Enzymology, Vol 167, Cyanobacteria. Academic Press, New York, pp 304–312
- Gong HS, Ohad I (1995) Rapid turnover of the RCII-D1 protein in the dark induced by photoinactivation of photosystem-I in Scenedesmus wild-type and the PSI-donor defective Lf-1 mutant-cells. Biochim Biophys Acta 1228: 181–188 [Google Scholar]
- Green TGA, Lange OL, Cowan IR (1994) Ecophysiology of lichen photosynthesis: the role of water status and thallus diffusion resistances. Cryptogam Bot 4: 166–178 [Google Scholar]
- Hagen LJ (2001) Processes of soil erosion by wind. Ann of Arid Zone 40: 233–250 [Google Scholar]
- Heber U, Bukhov NG, Shuvalov VA, Kobayashi Y, Lange OL (2001) Protection of the photosynthetic apparatus against damage by excessive illumination in homoiohydric leaves and poikilohydric mosses and lichens. J Exp Bot 52: 1999–2006 [DOI] [PubMed] [Google Scholar]
- Hihara Y, Sonoike K (2001) Regulation, inhibition and protection of photosystem I. In E-M Aro, B Andersson, eds, Advances in Photosynthesis and Respiration, Vol 11, Regulation of Photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 507–531
- Kada S, Koike H, Satoh K, Hase T, Fujita Y (2003) Arrest of chlorophyll synthesis and differential decrease of photosystems I and II in a cyanobacterial mutant lacking light-independent protochlorophyllide reductase. Plant Mol Biol 51: 225–235 [DOI] [PubMed] [Google Scholar]
- Kanervo E, Aro EM, Murata N (1995) Low unsaturation level of thylakoid membrane lipids limits turnover of the D1 protein of photosystem II at high irradiance. FEBS Lett 364: 239–242 [DOI] [PubMed] [Google Scholar]
- Keren N, Ohad I (1998) State transition and photoinhibition. In JD Rochaix, M Goldschmidt-Clermont, S Merchant, eds, The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 569–596
- Kirilovsky D, Ohad I (1986) Functional assembly in vitro of phycobilisomes with isolated Photosystem II particles of eukayotic chloroplasts. J Biol Chem 261: 12317–12323 [PubMed] [Google Scholar]
- Lange OL, Meyer A, Budel B (1994) Net photosynthesis activation of a desiccated cyanobacterium without liquid water in high air humidity alone: experiments with Microcoleus sociatus isolated from a desert soil crust. Funct Ecol 8: 52–57 [Google Scholar]
- Lange OL, Meyer A, Zellner H, Heber U (1994) Photosynthesis and water relations of lichen soil crusts: field measurements in the coastal fog zone of the Namib desert. Funct Ecol 8: 253–264 [Google Scholar]
- Lüttge U, Buedel B, Ball E, Strube F, Weber P (1996) Photosynthesis of terrestrial cyanobacteria under light and desiccation stress as expressed by chlorophyll fluorescence and gas exchange. Bot Acta 109: 43–50 [Google Scholar]
- Mazor G, Kidron GJ, Vonshak A, Abeliovich A (1996) The role of cyanobacterial exopolysaccharides in structuring desert microbial crusts. FEMS Microbiol Ecol 21: 121–130 [Google Scholar]
- Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N (2001) Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20: 5587–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333–359 [DOI] [PubMed] [Google Scholar]
- Potts M (1994) Desiccation tolerance of prokaryotes. Microbiol Rev 58: 755–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts M (2001) Desiccation tolerance: a simple process? Trends Microbiol 9: 553–559 [DOI] [PubMed] [Google Scholar]
- Poulson M, Samson G, Whitmarsh J (1995) Evidence that cyt. b559 protects Photosystem II against photoinhibition. Biochemistry 34: 10932–10938 [DOI] [PubMed] [Google Scholar]
- Prasil O, Adir N, Ohad I (1992) Dynamics of photosystem II: mechanism of photoinhibition and recovery in vivo. In J Barber, ed, Topics in Photosynthesis, Vol 11. Elsevier Biomedical Press, Amsterdam, pp 295–348
- Prasse R, Bornkamm R (2000) Effect of microbiotic soil surface crusts on emergence of vascular plants. Plant Ecol 150: 65–75 [Google Scholar]
- Qiu BS, Gao KS (2001) Photosynthetic characteristics of the terrestrial blue-green alga, Nostoc flagelliforme. Eur J Phycol 36: 147–156 [Google Scholar]
- Rajot JL, Alfaro SC, Gomes L, Gaudichet A (2003) Soil crusting on sandy soils and its influence on wind erosion. Catena 53: 1–16 [Google Scholar]
- Rudi K, Skulberg OM, Larsen F, Jakobsen KS (1997) Strain characterization and classification of oxyphotobacteria in clone cultures on the basis of 16S rRNA sequences from the variable regions V6, V7, and V8. Appl Environ Microbiol 63: 2593–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Hirai M, Nishio J, Yamaji T, Kashino Y, Koike H (2002) Recovery of photosynthetic systems during rewetting is quite rapid in a terrestrial cyanobacterium, Nostoc commune. Plant Cell Physiol 43: 170–176 [DOI] [PubMed] [Google Scholar]
- Scherer S, Ernst A, Chen T-W, Böger P (1984) Rewetting of drought-resistant blue-green algae: time course of water uptake and reappearance of respiration, photosynthesis, and nitrogen fixation. Oecologia 62: 418–423 [DOI] [PubMed] [Google Scholar]
- Spetea C, Keren N, Hundal T, Doan JM, Ohad I, Andersson B (2000) GTP enhances the degradation of the photosystem II D1 protein irrespective of its conformational heterogeneity at the Q(B) site. J Biol Chem 275: 7205–7211 [DOI] [PubMed] [Google Scholar]
- Swensson B, Vass I, Styring S (1991) Sequence analysis of the D1 and D2 reaction center proteins of photosystem II. Z Naturforsch 46: 765–776 [DOI] [PubMed] [Google Scholar]
- Tarnawski MG, Green TGA, Buedel B, Meyer A, Zellner H, Lange OL (1994) Diel channels of atmospheric CO2 concentration within, and above, cryptogam stands in a New Zealand temperate rainforest. NZ J Bot 32: 329–336 [Google Scholar]
- Vass I (2003) The history of photosynthetic thermoluminescence. Photosynth Res 76: 303–308 [DOI] [PubMed] [Google Scholar]
- Veste M, Littmann T, Friedrich H, Breckle SW (2001) Microclimatic boundary conditions for activity of soil lichen crusts in sand dunes of the north-western Negev desert, Israel. Flora 196: 465–474 [Google Scholar]
- Zangvill A (1996) Six years of dew observations in the Negev Desert, Israel. J Arid Environ 32: 361–371 [Google Scholar]
- Zer H, Prasil O, Ohad I (1994) Role of plastoquinol oxidoreduction in regulation of photochemical reaction center II-D1 protein turnover in vivo. J Biol Chem 269: 17670–17676 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.