Abstract
OBJECTIVES
Early resuscitation may improve outcomes in pediatric traumatic brain injury (TBI). We examined the association between timely treatment of hypotension and hypoxia during early care (prehospital or emergency department locations) and discharge outcomes in children with severe TBI.
METHODS
Hypotension was defined as systolic blood pressure less than 70 + 2 (age in years), and hypoxia was defined as PaO2 < 60 mmHg or oxygen saturation < 90% in accordance with the 2003 Brain Trauma Foundation guidelines. Timely treatment of hypotension and hypoxia during early care was defined as the treatment within 30 minutes of a documented respective episode. Two hundred and thirty-six medical records of children under 18 years with severe TBI from five regional pediatric trauma centers were examined. Main outcomes were in-hospital mortality and discharge Glasgow Outcome Scale (GOS) score.
RESULTS
Hypotension occurred in 26% (60/234) during early care and was associated with in-hospital mortality (23.3% vs 8.6%; p = 0.01). Timely treatment of hypotension during early care occurred in 92% (55/60) by use of intravenous fluids, blood products or vasopressors and was associated with reduced in-hospital mortality (aRR 0.46; 95% CI 0.24, 0.90) and less likelihood of poor discharge GOS (aRR 0.54; 95% CI 0.39, 0.76) when compared to children with hypotension who were not treated in a timely manner. Early hypoxia occurred in 17% (41/236) and all patients received timely oxygen treatment.
CONCLUSIONS
Timely resuscitation during early care was common and associated with lower in-hospital mortality and favorable discharge GOS in severe pediatric TBI.
Keywords: Timely, resuscitation, pediatric, traumatic brain injury, hypotension
INTRODUCTION
Traumatic brain injury (TBI) is a leading cause of death and disability and is responsible for more than 630,000 emergency department visits, 60,000 hospitalizations, and 6,000 deaths in the United States annually among children less than 18 years of age.1,2 Some studies have examined the effect of early hypotension and hypoxia on outcomes in children with TBI3–8 and while these studies suggest that early hypotension and hypoxia post-TBI are associated with poor outcomes, the effect of timely intervention has not been sufficiently examined.
In 1993, Pigula et al reported that hypotension with or without hypoxia in the emergency department (ED) is associated with increased mortality in children with severe TBI.3 Examining Glasgow Outcome Scale (GOS) score at 3-month follow-up and hospital length of stay after initial injury, Kokoska and colleagues later suggested that hypotension occurring during the first 24 hours of hospitalization post injury was associated with worse neurological outcomes and prolonged hospitalization in 72 children with severe TBI.4 In 2005, Coates et al showed that early (pre-hospital [PH] period and/or ED) hypotension was a better predictor of poor outcome than hypotension occurring later.8 In 2008, Samant and colleagues suggested that the first 6 hours after severe pediatric TBI was the period when hypotension was most strongly associated with poor outcomes9 but like previous studies, did not examine the impact of timely resuscitation on outcomes.
In 2003, the Brain Trauma Foundation (BTF) published guidelines recommending that hypotension and hypoxia should be identified and treated “early” to optimize outcomes in severe pediatric TBI,10 but what constituted early was not delineated and these recommendations were removed from the 2012 BTF guidelines due to lack of sufficient supportive evidence.11 In the only study to examine resuscitation during early care (PH or ED), Zebrack and colleagues reported that, after adjusting for injury severity, children with moderate to severe TBI who did not receive an attempt to treat hypotension during early care had a 3.4 fold higher odds of death, and were 3.7 times more likely to suffer disability compared with children in whom treatment was attempted with intraosseous or intravenous catheter placement and fluid administration above hourly maintenance rates.12 Although this study included a mixed moderate and severe TBI severity population, the work was important because it moved past the problem statement (inadequate resuscitation) into addressing treatment (attempted resuscitation) during the early period after TBI. We wanted to extend this work and focus on the association between timely resuscitation during early care and discharge outcomes in severe pediatric TBI, where the prevalence of secondary insults after TBI is known to be particularly detrimental and high. We hypothesized that timely resuscitation during early care would be associated with better discharge outcomes.
MATERIALS AND METHODS
Study Center Selection and Data Sources
As previously reported, in the parent Pediatric Guideline Adherence and Outcomes (PEGASUS) study, data were collected from 2007 to 2011 that included documented resuscitation data and treatment during early care.13 Five geographically dispersed Pediatric Trauma Centers (PTC) affiliated with academic medical centers were recruited based on willingness to participate, a priori ability to contribute data from 30–50 children with severe TBI annually, electronic medical records, representation in the Pediatric Neurocritical Care Research Group (http://www.pncrg.org/), PTC publications, regional PTC status, and recognized expertise severe TBI care. Study centers were the University of Washington’s Harborview Medical Center, Seattle, WA (lead and data coordinating center); Children’s Hospital of Pittsburgh, Pittsburgh, PA; Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL; Harbor-University of California, Los Angeles Medical Center, Torrance, CA; and Nationwide Children’s Hospital, Columbus, OH. Human subjects’ committee approval was obtained at each site.
Study Population
Eligible participants for PEGASUS study13 were subjects 0 to 17 year old with severe TBI, defined as having ≥ one ICD-9 discharge diagnosis code: 800.0–801.9, 803.0–804.9, 850.0–854.1, 959.01, 950.1–950.3, and 995.55, consistent with the Centers for Disease Control and Prevention definition.14 Criteria for severe TBI required: a minimum head Abbreviated Injury Severity (AIS) score ≥ 3, post-resuscitation Glasgow Coma Scale (GCS) score < 9, alive with tracheal intubation in the intensive care unit (ICU) ≥ 48 hours from the time of ICU admission, trauma history, and abnormal admission head computed tomography (CT). We included subjects with extracranial injuries and those who were transferred from the scene to an outside hospital before admission to the study center.
Center Training and Reliability Testing
Data abstraction training modules were created and each data abstractor underwent training followed by data abstraction. At each site, data were abstracted from electronic medical records and included data elements from the early period (PH and ED). Data were remotely and securely entered into a web based data entry system. A kappa of 0.8 was a priori considered adequate between center reliability. Supplementary trainings were conducted for kappa values <0.8 until the goal was achieved. All centers achieved excellent inter-rater agreement (kappa > 0.8).15–17
Patient Outcomes
The primary outcome measures were in-hospital mortality and discharge GOS (alive). The GOS ranges from 1 (death) to 5 (baseline status). A dichotomous measure of discharge GOS (alive) was used to categorize “poor” (GOS 2–3; vegetative state- major impairment) versus “favorable” (GOS 4–5; minor-moderate impairment-baseline functioning).18
Definitions
Early care was defined as the care provided in the prehospital (PH) or emergency department (ED) locations across 5 study centers. Prehospital location includes emergency medical services (EMS) and index hospital if patient was transferred from scene before admission to the trauma center. We adopted the definitions for hypotension and hypoxia from the 2003 BTF guidelines.10 “Hypotension was defined as systolic blood pressure (SBP) below the 5th percentile for age which is estimated by SBP < 70 + 2 (age in years) and hypoxia was defined as PaO2 < 60 mmHg or oxygen saturation < 90%”. Timely treatment was defined as treatment within 30 minutes of a documented episode.
Statistical Analyses
Clinical characteristics and discharge GOS were examined in univariate analyses and categorized by the presence of early hypotension using χ2 tests for categorical variables and t-tests for continuous variables. All analyses were clustered within hospital. Statistical significance was defined as p < 0.05. All multiple regression analyses were adjusted for age, sex, maximum head AIS score, maximum non-head AIS score, and motor GCS at admission. Multivariable modified Poisson regression (Poisson regression with a robust error variance to estimate the relative risk directly)19, 20 was used to examine the effect of hypotension and hypoxia treatment across the PH and ED locations on mortality and discharge GOS. Data were analyzed using Stata v. 1121 and SAS 9.3.
RESULTS
Clinical and Outcome Characteristics of Children with Severe Pediatric TBI
Table 1 describes the study cohort. Most children were male, and had extracranial injuries. All 236 children had documented blood oxygen saturation levels and 234/236 children had blood pressure data recorded during early care13.
Table 1.
Clinical and Outcome Characteristics of 234 Children with Severe Traumatic Brain Injury across Five Study Centers Categorized by Early (pre-hospital or emergency department) Hypotension. Hypotension is defined as systolic blood pressure (SBP) < 70 + 2 (Age) according to 2003 BTF guidelines.10 Data expressed as column percent. Two children had blood pressure missing from early care documentation.
| Clinical Characteristics | Early Hypotension (N = 60) | No Early Hypotension N=174 N (%) |
|
|---|---|---|---|
| No treatment N=5 N (%) |
Treatment N=55 N (%) |
||
| Age (years) [SD] | 5.8 [6.9] | 10.6 [6.5] | 7.4 [6.0] |
| Gender | |||
| Male | 3 (60.0) | 34 (61.8) | 125 (71.8) |
| Injury Severity Score (mean) [SD] | 28.6 [7.1] | 35.7 [15.7] | 26.1 [11.0] |
| Head Abbreviated Injury Severity (AIS) Score | |||
| 3 | 0 (0.0) | 5 (9.1) | 22 (12.6) |
| 4 | 2 (40.0) | 17 (30.9) | 60 (34.5) |
| 5 | 3 (60.0) | 31 (56.4) | 91 (52.3) |
| 6 | 0 (0.0) | 2 (3.6) | 1 (0.6) |
| Highest Non-Head Abbreviated Injury Severity (AIS) Score | |||
| 0 | 0 (0.0) | 5 (9.1) | 48 (27.6) |
| 1 | 3 (60.0) | 12 (21.8) | 43 (24.7) |
| 2 | 0 (0.0) | 6 (10.9) | 30 (17.2) |
| 3 | 2 (40.0) | 17 (30.9) | 40 (23.0) |
| 4 | 0 (0.0) | 10 (18.2) | 12 (6.9) |
| 5 | 0 (0.0) | 5 (9.1) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 1 (0.6) |
| Admit Glasgow Coma Scale (GCS) score (motor) | |||
| 1, paralyzed | 0 (0.0) | 36 (65.4) | 61 (35.1) |
| 1, not paralyzed | 1 (20.0) | 3 (5.5) | 16 (9.2) |
| 2 | 1 (20.0) | 1 (1.8) | 8 (4.6) |
| 3 | 1 (20.0) | 5 (9.1) | 15 (8.6) |
| 4 | 0 (0.0) | 6 (10.9) | 37 (21.3) |
| 5 | 2 (40.0) | 4 (7.3) | 33 (19.0) |
| 6 | 0 (0.0) | 0 (0.0) | 4 (2.3) |
| Extracranial Injuries | 5 (100.0) | 46 (83.6) | 118 (67.8) |
| Extracranial Injury location | |||
| Face | 5 (100.0) | 28 (50.9) | 73 (42.0) |
| Neck | 1 (20.0) | 4 (7.3) | 5 (2.9) |
| Thorax | 2 (40.0) | 28 (50.9) | 36 (20.7) |
| Abdomen | 2 (40.0) | 20 (36.4) | 26 (14.9) |
| Spine | 0 (0.0) | 9 (16.4) | 11 (6.3) |
| Extremities | 3 (60.0) | 30 (54.6) | 79 (45.4) |
| All Head Computed Tomography Diagnoses | |||
| Epidural Hematoma | 1 (20.0) | 9 (16.4) | 30 (17.2) |
| Subdural Hematoma | 4 (80.0) | 34 (61.8) | 117 (67.2) |
| Subarachnoid Hemorrhage | 2 (40.0) | 32 (58.2) | 73 (42.0) |
| Intracerebral Hemorrhage | 0 (0.0) | 20 (36.4) | 53 (30.5) |
| Intraventricular Hemorrhage | 2 (40.0) | 11 (20.0) | 34 (19.5) |
| Cerebral Edema | 5 (100.0) | 31 (56.4) | 90 (51.7) |
| Cerebral Infarction | 2 (40.0) | 3 (5.4) | 32 (18.4) |
| Contusion | 2 (40.0) | 23 (41.8) | 69 (39.7) |
| Diffuse Axonal | 3 (60.0) | 20 (36.4) | 47 (27.0) |
| Any High Intracranial Pressure | 5 (100.0) | 42 (76.4) | 145 (83.3) |
| Any Surgery | 4 (80.0) | 35 (63.6) | 111 (63.8) |
| Outcome Characteristics | Early Hypotension (N = 60) |
No Early Hypotension N=174 N (%) |
|
|
No treatment N=5 N (%) |
Treatment N=55 N (%) |
||
| ICU Length of Stay (days) mean[SD] | 17.2 [13.9] | 15.4 [13.4] | 14.5 [11.8] |
| In-hospital Mortality | 3 (60.0) | 11 (20.0) | 15 (8.6) |
| Discharge Glasgow outcome scale (GOS) score (alive) | N = 2 | N = 44 | N = 159 |
| Poor (vegetative state/major impairment) | 2 (100.0) | 32 (72.7) | 99 (62.3) |
| Favorable (minor-moderate. impairment/baseline functioning) | 0 (0.0) | 12 (27.3) | 60 (37.7) |
Hemodynamic Resuscitation during Early Care
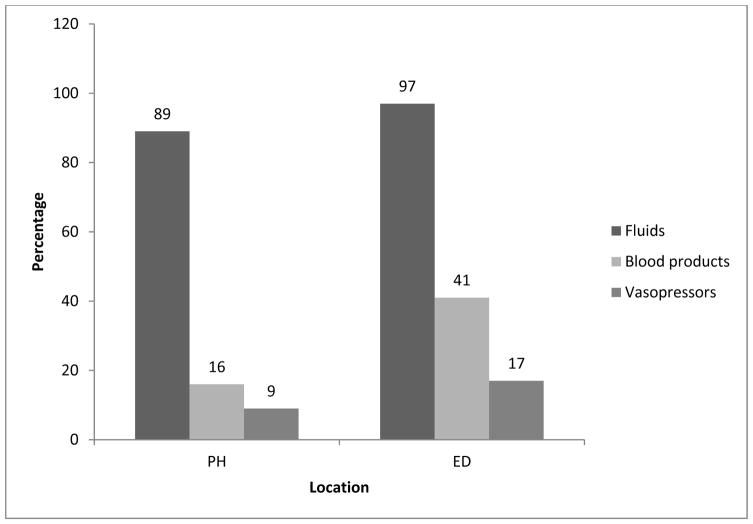
Twenty six percent (60/234; Table 1) had hypotension in PH (21%) and/or ED (13%) locations and 7% of children had hypotension in both PH and ED locations. Of the 60 hypotensive children, 55 (92%) received timely hemodynamic resuscitation treatment with fluids (92%), blood products (28%) or vasopressors (13%). Intravenous fluids were the mainstay of treatment of hypotension during early care, especially in the PH location (Figure). Intravenous epinephrine was the most common vasopressor used (87.5%).
Figure.
Timely (within 30 minutes of documented episode) Treatment of Hypotension (N = 60/234; SBP < 70 + 2 [age]) During Early Care by Treatment Location.
PH = prehospital (emergency medical services [EMS] and index hospital) and ED = emergency department.
Timely Hypotension Treatment and Outcomes
In univariate analysis, hypotension during early care was associated with in-hospital mortality (23.3% vs 8.6%; p = 0.01). Table 2 shows that adjusted for age, sex, and markers of injury severity (head AIS score, motor GCS score, and maximum non-head AIS score), timely treatment of hypotension during early care was associated with reduced in-hospital mortality (aRR 0.46; 95% CI 0.24, 0.90). Children who did not have hypotension in the PH and/or ED location were 77% less likely to die in-hospital (aRR 0.23; 95% CI 0.06, 0.87) than children whose hypotension was not treated in a timely manner.
Table 2.
Timely (within 30 minutes of documented episode) Treatment of Hypotension (SBP < 70 + 2 [age]) During Early Care (pre-hospital or emergency department) and Discharge Outcomes in 234 Children with Severe Traumatic Brain Injury. No timely treatment refers to treatment provided but beyond 30 minutes of episode occurrence.
| In-hospital Mortality | aRR (95% CI) |
|---|---|
| No timely treatment | Reference group |
| Timely treatment | 0.46 (95% CI 0.24, 0.90) |
| No hypotension | 0.23 (95% CI 0.06, 0.87) |
| Discharge Glasgow Outcome Scale (GOS) Score (alive) | aRR (95% CI) |
| No timely treatment | Reference group |
| Timely treatment | 0.54 (95% CI 0.39, 0.76) |
| No hypotension | 0.56 (95% CI 0.43, 0.73) |
All models adjusted for age, sex, head abbreviated injury severity (AIS) score, motor Glasgow coma scale (GCS) score, and maximum non-head AIS score.
After adjusting for confounders of age, sex, head AIS score, motor GCS score, and maximum non-head AIS score, timely treatment of hypotension during early care was significantly associated with discharge GOS (alive) (aRR 0.54; 95% CI 0.39, 0.76; Table 2). Children who did not have hypotension during early care were 44% less likely to have poor GOS (aRR 0.56; 95% CI 0.43, 0.73) at discharge than children with hypotension not treated in a timely manner.
Early Hypoxia and Treatment
Seventeen percent of children had hypoxia in PH and/or ED, 15% of children had hypoxia in the PH and 6% had hypoxia in the ED. All patients received timely treatment with 100% supplemental oxygen during early care.
DISCUSSION
The main findings of our study are that for children with severe TBI who received care at five leading regional PTCs: 1) Early hypoxia was common but all patients received timely treatment with oxygen, 2) Early hypotension was common and was associated with in-hospital mortality, 3) Timely treatment of hypotension during early care was associated with better discharge survival and GOS. These data provide added evidence that timely hemodynamic resuscitation in the PH and ED locations benefits children with severe TBI.
Although the primary impact TBI dictates the outcome in some pediatric patients, many patients die of secondary injury caused by increased intracranial pressure, severe multiple trauma, or presence of hypotension, hypoxia, or hypercarbia.22 Since children have an increased incidence of elevated intracranial pressure and more frequent association of TBI with multiple trauma including subsequent hypotension, hypoxia, and hypercarbia, secondary insults are extremely important to prevent and treat expediently. Therefore, early (PH and/or ED) hemodynamic resuscitation aimed at preventing secondary injuries can have a significant effect on outcomes. Our findings show that there should be continued efforts to reduce delayed treatment of hypotension in children with severe TBI. In this study, timely treatment of hypoxia occurred consistently, suggesting that the focus may need to be prevention of hypoxia after severe pediatric TBI.
Zebrack’s previous single center study documented an early (PH and/or ED) hypotension rate of 39% and an attempt to treat rate of 48%.12 In the current multicenter study, hypotension occurred less frequently (26%) and the early treatment rate with isotonic crystalloids was significantly higher (92%) than previously reported, possibly suggesting better hemodynamic resuscitation and higher adherence to evidence based guideline care over time.10 Data from our study show that the frequency of hypotension is higher in PH location compared to the ED. This may speak to either the complexity of care required immediately post injury or to needed improvements in PH care. In this study, we specified timely treatment of hypotension during early care as treatment within 30 minutes of a documented hypotensive/hypoxic episode, and while this may not be the optimal benchmark for time, it gives us a starting point to examine time as an important metric for hemodynamic resuscitation in this group of patients. Few patients in our study did not receive timely treatment during early care. While the reasons for this are not known, explanations may include paucity of resources, delayed recognition of hypoxia or hypotension or competing priorities during the early care of these patients.
The majority of the children with hypotension received intravenous fluids followed by blood products and vasopressors, consistent with the 2003 BTF recommendations on priorities of treatment during early care.10 Less than 37% of included patients received blood products or vasopressors during early care, and while this may be consistent with best practice, these data also suggest an opportunity to study timing of adjunctive resuscitation strategies to achieve timely hemodynamic resuscitation in these patients.
While the 2012 BTF guidelines do not include a recommendation on early resuscitation in severe pediatric TBI, there is Level III evidence supporting a minimum cerebral perfusion pressure (CPP) of 40mmHg.11 Since intracranial pressure (ICP) is not commonly monitored in PH and ED settings, achieving SBP targets become important to helping maintain CPP. As this study suggests, timely hemodynamic resuscitation of these patients during early care may be critical to ensuring timely cerebral hemodynamic resuscitation that may positively impact outcomes.
Our study has some limitations worth discussing. First, this is a retrospective analysis and we relied on recorded data. We could not examine the patients who died within 48 hours of admission to ICU as they did not satisfy the inclusion criteria of the parent PEGASUS study, which might have led to us excluding patients with non-life sustaining trauma in whom early and timely resuscitation may improve outcomes. We could not sufficiently examine the relationship between vasopressor choice and outcomes. As all of our patients received treatment for hypoxia, we could not examine the effect of timely treatment of hypoxia on outcomes. There were only few children in the reference group (who did not receive timely resuscitation), but we wanted to examine potential role of timely treatment in clinical decision making and hence generate preliminary data on this topic. There may be residual confounding by patient or treatment factors, despite adjusting for potential confounders. Despite these limitations, this is the first study to examine the association of timely resuscitation during early care on outcomes among children with severe TBI.
In summary, the burden of secondary insults such as hypoxia and hypotension remain high among children with severe TBI. Yet, our study shows that timely hemodynamic resuscitation during early care is important and associated with better discharge outcomes. Achieving hemodynamic stability within the first 30 minutes of a documented episode in the PH or ED locations may also help achieve better cerebral hemodynamic conditions with downstream higher discharge survival and better neurological outcomes among children with severe TBI.
Acknowledgments
Sources of Support: The source of support for this work including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript was NINDS R01 NS072308-05.
Source of Funding: All phases of this study were supported by an NIH grant, NINDS R01 NS072308-05 (Vavilala)
Footnotes
The work was performed at University of Washington’s Harborview Medical Center, Seattle, WA (lead and data coordinating center); Children’s Hospital of Pittsburgh, Pittsburgh, PA; Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL; Harbor University of California, Los Angeles Medical Center, Torrance, CA; and Nationwide Children’s Hospital, Columbus, OH
The following staff have contributed to data collection reported in the manuscript and were compensated as project staff and we have obtained permission from them, and they have been sent the manuscript under review: Rachelle Bell, RN University of Pittsburgh Medical Center, Pittsburgh, PA; Kristi Schmidt, MD, Ann & Robert H. Lurie Children’s Hospital, Chicago, IL; Alma Ramirez, Los Angeles BioMedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA; Sheila Giles, RN, Nationwide Children’s Hospital, Columbus, OH.
Financial Disclosure: The authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of Interest: There are no conflicts of interests, including relevant financial interests, activities, relationships, and affiliations. For all the authors none were declared.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Faul M, Xu L, Wald MM, et al. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta, GA: Centers for Disease Control, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 3.Pigula FA, Wald SL, Shackford SR, Vane DW. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatr Surg. 1993;28(3):310–316. doi: 10.1016/0022-3468(93)90223-8. [DOI] [PubMed] [Google Scholar]
- 4.Kokoska ER, Smith GS, Pittman T, Weber TR. Early hypotension worsens neurological outcome in pediatric patients with moderately severe head trauma. J Pediatr Surg. 1998;33(2):333–338. doi: 10.1016/s0022-3468(98)90457-2. [DOI] [PubMed] [Google Scholar]
- 5.Chiaretti A, De Benedictis R, Della Corte F, et al. The impact of initial management on the outcome of children with severe head injury. Child’s Nerv Syst. 2002;18:54–60. doi: 10.1007/s00381-001-0533-4. [DOI] [PubMed] [Google Scholar]
- 6.Chiaretti A, Piastra M, Pulitano S, et al. Prognostic factors and outcome of children with severe head injury: an 8-year experience. Childs Nerv Syst. 2002;18(3–4):129–136. doi: 10.1007/s00381-002-0558-3. [DOI] [PubMed] [Google Scholar]
- 7.Vavilala MS, Bowen A, Lam AM, et al. Blood pressure and outcome after severe pediatric traumatic brain injury. J Trauma. 2003;55(6):1039–1044. doi: 10.1097/01.TA.0000101759.23607.57. [DOI] [PubMed] [Google Scholar]
- 8.Coates BM, Vavilala MS, Mack CD, et al. Influence of definition and location of hypotension on outcome following severe pediatric traumatic brain injury. Crit Care Med. 2005;33(11):2645–2650. doi: 10.1097/01.ccm.0000186417.19199.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samant UB, Mack CD, Koepsell T, et al. Time of hypotension and discharge outcome in children with severe traumatic brain injury. Journal of Neurotrauma. 2008;25(5):495–502. doi: 10.1089/neu.2007.0491. [DOI] [PubMed] [Google Scholar]
- 10.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 4 – Resuscitation of blood pressure and oxygenation and prehospital brain-specific therapies for the severe pediatric traumatic brain injury patient. Pediatr Crit Care Med. 2003;4(3 suppl):S12–S18. [PubMed] [Google Scholar]
- 11.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents-second edition. Pediatr Crit Care Med. 2012;13(1 suppl):S1–82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 12.Zebrack M, Dandoy C, Hansen K, et al. Early resuscitation of children with moderate-to-severe traumatic brain injury. Pediatrics. 2009;124(1):56–64. doi: 10.1542/peds.2008-1006. [DOI] [PubMed] [Google Scholar]
- 13.Vavilala MS, Kernic MA, Wang J, et al. Acute care clinical indicators associated with discharge outcomes in children with severe traumatic brain injury. Crit Care Med. 2014;42(10):2258–2266. doi: 10.1097/CCM.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Classification of Diseases 9th Revision Clinical Modification for Physicians. Contexo Media. 2010; v.2
- 15.Altman DG. Practical Statistics For Medical Research. London: Chapman and Hall; 1991. [Google Scholar]
- 16.Cohen J. A Coefficient Of Agreement For Nominal Scales. Educational And Psychological Measurement. 1960;20:37–46. [Google Scholar]
- 17.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3. Hoboken, NJ: John Wiley & Sons; 2003. [Google Scholar]
- 18.Jennett B, Teasdale G, Braakman R, Minderhoud J, Knill-Jones R. Predicting outcome in individual subjects after severe head injury. Lancet. 1976;1(7968):1031–1034. doi: 10.1016/s0140-6736(76)92215-7. [DOI] [PubMed] [Google Scholar]
- 19.Zou G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 20.McNutt LA, Wu C, Xue X, et al. Estimating the Relative Risk in Cohort Studies and Clinical Trials in Common Outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 21.Stata Statistical Software. College Station, TX: StataCorp LP; 2009. (computer program). Release 11. [Google Scholar]
- 22.Mayer TA, Walker ML. Pediatric head injury: the critical role of the emergency physician. Ann Emerg Med. 1985;14(12):1178–1184. doi: 10.1016/s0196-0644(85)81025-8. [DOI] [PubMed] [Google Scholar]