Abstract
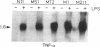
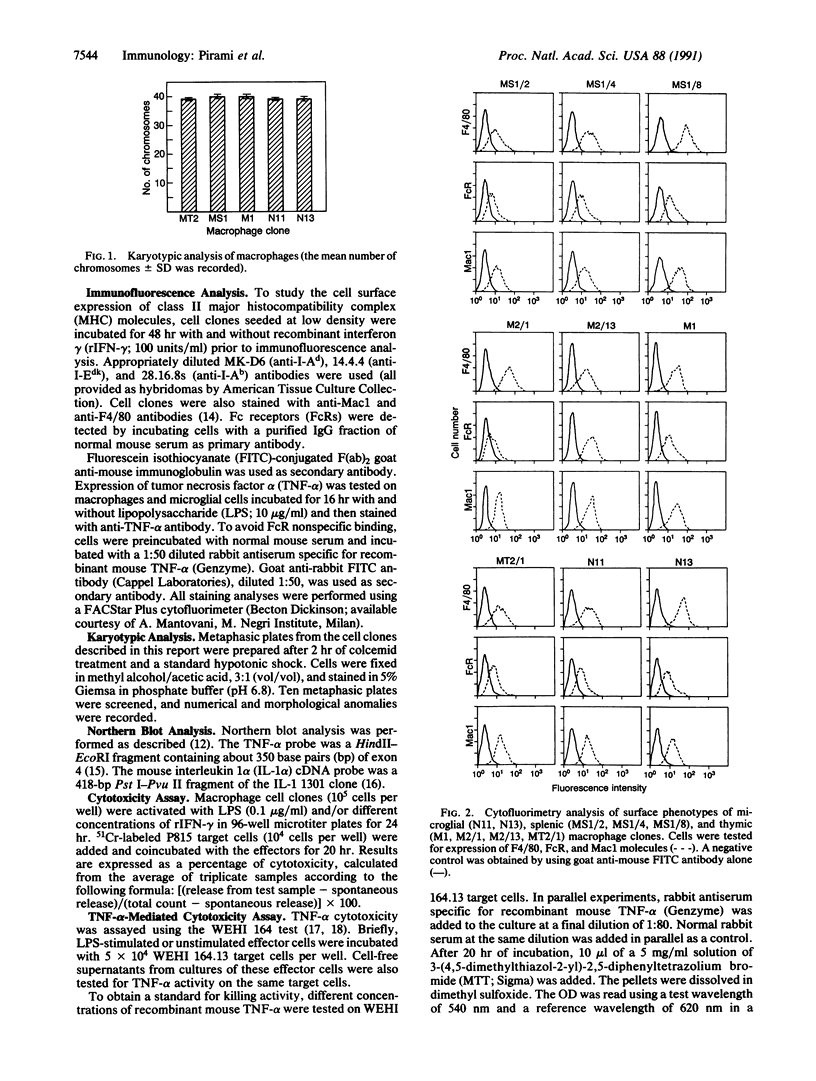
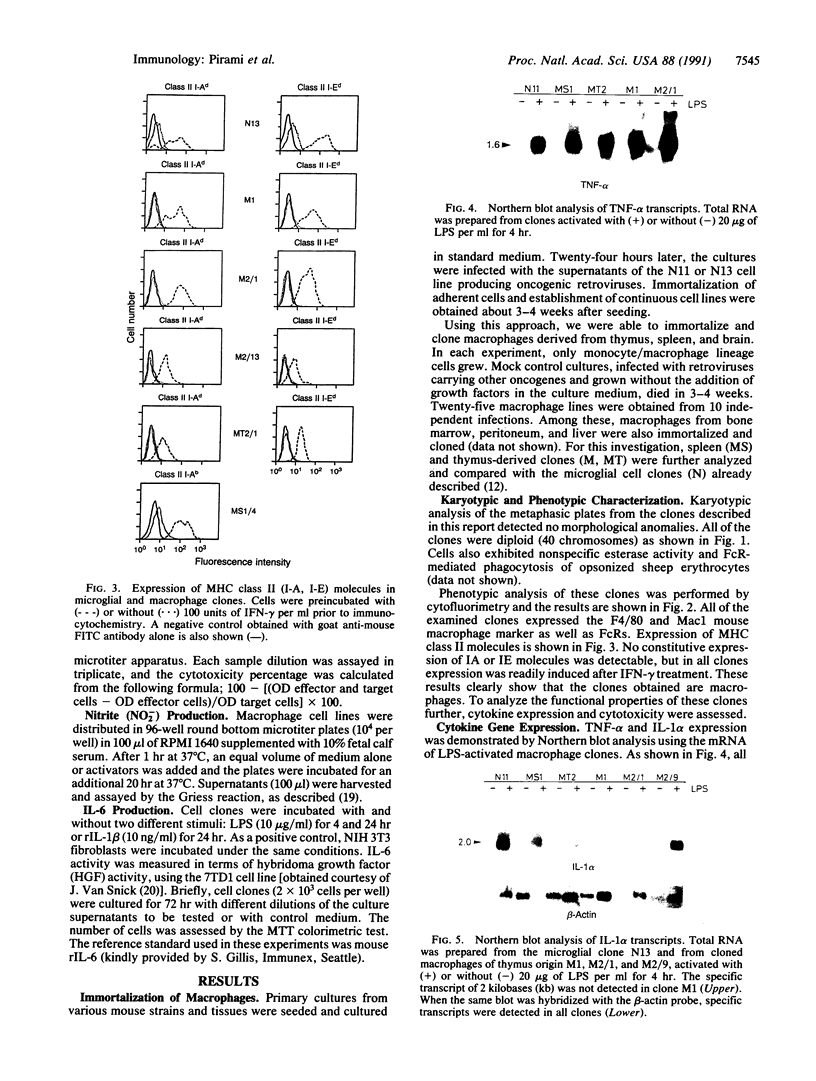
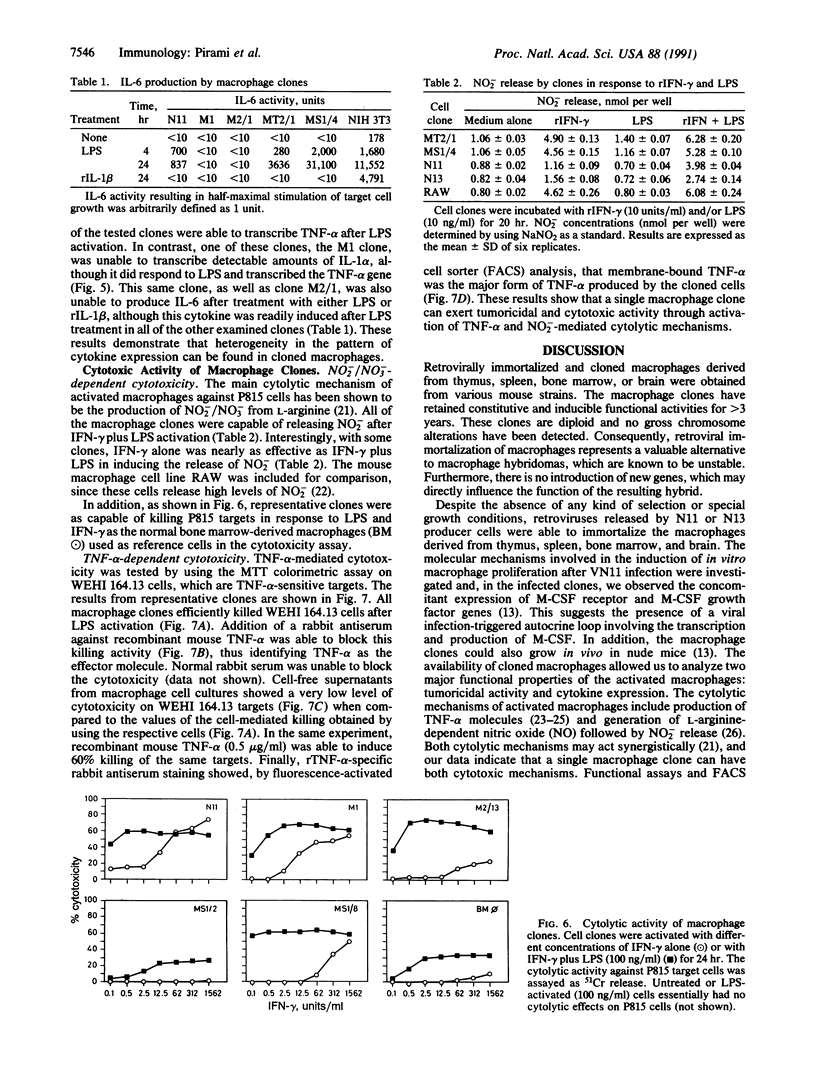
Murine macrophage clones were generated from thymus, spleen, brain, and bone marrow by in vitro immortalization with recombinant retroviruses carrying an avian v-myc oncogene. The cloned cell lines express F4/80 molecules, exert phagocytosis, have nonspecific esterase activity, and express class II molecules after interferon gamma activation. The macrophage clones are diploid and their karyotypes have remained stable for greater than 3 years in culture. After the macrophage clones were activated, their pattern of cytokine production was investigated. Functional heterogeneity in cytokine transcription was demonstrated: one of six liposaccharide-activated macrophages was unable to transcribe interleukin 1 alpha, whereas all of the liposaccharide-activated clones were able to transcribe tumor necrosis factor alpha. Interleukin 6 production was detected in three of six clones. The production of nitrite and tumor necrosis factor alpha as effector molecules of cytotoxicity was detected in all clones, thus showing that a single macrophage can exert more than one cytotoxic mechanism. The results indicate that immortalized and cloned macrophages have a differentially regulated expression of cytokine genes, adding further evidence for the existence of functional heterogeneity among cloned macrophages. This heterogeneity seems to derive from differentiation-related mechanisms rather than from external constraints.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bakouche O., Ichinose Y., Heicappell R., Fidler I. J., Lachman L. B. Plasma membrane-associated tumor necrosis factor. A non-integral membrane protein possibly bound to its own receptor. J Immunol. 1988 Feb 15;140(4):1142–1147. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The history, properties, and biological effects of cachectin. Biochemistry. 1988 Oct 4;27(20):7575–7582. doi: 10.1021/bi00420a001. [DOI] [PubMed] [Google Scholar]
- Blasi E., Mathieson B. J., Varesio L., Cleveland J. L., Borchert P. A., Rapp U. R. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature. 1985 Dec 19;318(6047):667–670. doi: 10.1038/318667a0. [DOI] [PubMed] [Google Scholar]
- Colotta F., Peri G., Villa A., Mantovani A. Rapid killing of actinomycin D-treated tumor cells by human mononuclear cells. I. Effectors belong to the monocyte-macrophage lineage. J Immunol. 1984 Feb;132(2):936–944. [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Gifford G. E. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987 Feb 1;138(3):957–962. [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Karck U., Peters T., Decker K. Comparative study of cytotoxicity, tumor necrosis factor, and prostaglandin release after stimulation of rat Kupffer cells, murine Kupffer cells, and murine inflammatory liver macrophages. J Leukoc Biol. 1989 Feb;45(2):139–146. doi: 10.1002/jlb.45.2.139. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Gordon S., Keshav S., Chung L. P. Mononuclear phagocytes: tissue distribution and functional heterogeneity. Curr Opin Immunol. 1988 Sep-Oct;1(1):26–35. doi: 10.1016/0952-7915(88)90047-7. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Higashi N., Taki H., Osawa T. Cytolytic mechanisms of activated macrophages. Tumor necrosis factor and L-arginine-dependent mechanisms act synergistically as the major cytolytic mechanisms of activated macrophages. J Immunol. 1990 Feb 15;144(4):1425–1431. [PubMed] [Google Scholar]
- Kinkhabwala M., Sehajpal P., Skolnik E., Smith D., Sharma V. K., Vlassara H., Cerami A., Suthanthiran M. A novel addition to the T cell repertory. Cell surface expression of tumor necrosis factor/cachectin by activated normal human T cells. J Exp Med. 1990 Mar 1;171(3):941–946. doi: 10.1084/jem.171.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Leenen P. J., Slieker W. A., Melis M., Van Ewijk W. Murine macrophage precursor characterization. I. Production, phenotype and differentiation of macrophage precursor hybrids. Eur J Immunol. 1990 Jan;20(1):15–25. doi: 10.1002/eji.1830200104. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nedospasov S. A., Hirt B., Shakhov A. N., Dobrynin V. N., Kawashima E., Accolla R. S., Jongeneel C. V. The genes for tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) are tandemly arranged on chromosome 17 of the mouse. Nucleic Acids Res. 1986 Oct 10;14(19):7713–7725. doi: 10.1093/nar/14.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez C., Albert I., DeFay K., Zachariades N., Gooding L., Kriegler M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell. 1990 Oct 19;63(2):251–258. doi: 10.1016/0092-8674(90)90158-b. [DOI] [PubMed] [Google Scholar]
- Righi M., Mori L., De Libero G., Sironi M., Biondi A., Mantovani A., Donini S. D., Ricciardi-Castagnoli P. Monokine production by microglial cell clones. Eur J Immunol. 1989 Aug;19(8):1443–1448. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- Righi M., Pierani A., Boglia A., De Libero G., Mori L., Marini V., Ricciardi-Castagnoli P. Generation of new oncogenic murine retroviruses by cotransfection of cloned AKR and MH2 proviruses. Oncogene. 1989 Feb;4(2):223–230. [PubMed] [Google Scholar]
- Righi M., Sassano M., Valsasnini P., Shammah S., Ricciardi-Castagnoli P. Activation of the M-CSF gene in mouse macrophages immortalized by retroviruses carrying a v-myc oncogene. Oncogene. 1991 Jan;6(1):103–111. [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Cerami A. Cachectin: a hormone that triggers acute shock and chronic cachexia. J Infect Dis. 1988 Mar;157(3):413–420. doi: 10.1093/infdis/157.3.413. [DOI] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. S. Origins of macrophage diversity: functional and phenotypic analysis of cloned populations of mouse splenic macrophages. Cell Immunol. 1987 Jul;107(2):417–432. doi: 10.1016/0008-8749(87)90249-8. [DOI] [PubMed] [Google Scholar]