Abstract
In contact with biological fluids diverse type of biomolecules (e.g., proteins) adsorb onto nanoparticles forming protein corona. Surface properties of the coated nanoparticles, in terms of type and amount of associated proteins, dictate their interactions with biological systems and thus biological fate, therapeutic efficiency and toxicity. In this perspective, we will focus on the recent advances and pitfalls in the protein corona field.
Keywords: Protein corona, Nanoparticles, Targeting, Nanotoxicology
1. Introduction
It has been known, for a decade, that the surface of nanoscale materials is masked by a layer composed of various biomolecules (e.g., proteins and metabolomes) after incubation with a biological fluid. This protein-rich layer is the so-called “protein corona” (Mahmoudi et al., 2011a; Monopoli et al., 2012a). The biological fate of nanomaterials are substantially affected by the decoration of protein coronas in terms of type, amount, and conformation of associated proteins (Hamad-Schifferli, 2015). Therefore, protein coronas have recently been the subject of extensive studies (Milani et al., 2012; Monopoli et al., 2012b). In order to accurately interpret the interactions between nanoparticles and cells, it is necessary that the adsorption of protein coronas to nanoparticles be well characterized (Monopoli et al., 2013). Yet, assessment of the absolute number of bound proteins and their exchange dynamics in body fluids is an arduous task. Not to mention that there are a diverse group of biomarkers that cover nanoparticles soon after they come in contact with biological fluids (Maiolo et al., 2014). Proteins can bind to nanoparticles with either high affinity, hence known as a hard corona (HC), or low affinity, hence known as a soft corona (SC). Thus, HC-nanoparticle complexes are stable complexes with a long lifetime and SCs are more dynamic with shorter lifetimes (Maiolo et al., 2014; Hadjidemetriou et al., 2015). In the presence of the corona, cells are protected from the damaging effects that the bare nanoparticle surface can engender until the nanoparticles and protein corona are cleared through phagocytosis (Wang et al., 2013). Understanding these fundamental processes are essential as the unique combination of the nano-bio interphase directs the nature of a corona, which then determines the biological identity of the protein corona-nanoparticle complex (Saie et al., 2015; Jiang et al., 2010).
2. Challenges of the protein corona
Comprehensive understanding of the evolution of protein corona on the surface of nanoparticles should allow for the ability to control and exploit the bio-nano interface. However, a challenge that must be addressed is the potential hazards associated with use of nanoparticles. Numerous protocols and warnings of potential hazards and difficulties of using nanoparticles have been developed as a result of extensive study over the past decade (Monopoli et al., 2013). This encouraged researchers with a guideline to carry out basic experiments to investigate nanoparticle-protein corona complexes. The protein corona has been found to provide multiple protective effects to biological systems. However, interpretations of these beneficial effects can often be unclear due to the variation in reported outcomes. Conflicting reports on cytotoxicity and biological fates, even when identical nanoparticles were studied, have been reported (Sharifi et al., 2012; Mahmoudi et al., 2011b; Mao et al., 2013; Hajipour et al., 2015a). For example, Hadjidemetriou et al. (2015) demonstrated that in vitro plasma incubations could not accurately predict the molecular complexity of the in vivo protein corona, which was formed on clinically developed liposomes. Therefore, the structural characteristics of the corona formed in vitro can be significantly different from the in vivo corona. Investigators must ensure that proper controls and comparisons are being made. There are still many unmet challenges that are required before the design of truly efficient targeted nanoparticles is attained. Additionally, more work is needed to ensure predictable biological and in vivo outcomes (Azhdarzadeh et al., 2015a; Mahon et al., 2012). Should this goal be accomplished, it could potentially revolutionize multiple therapeutic areas and make significant improvements in nanomedicine (Saie et al., 2015).
2.1. Targeting
The ability to target specific cells in vivo using nanoparticles is required for successful therapeutic effects in the field of nanomedicine. Proteins, antibodies, and other biomolecules have been used to functionalize the surface of nanomaterials in order to target specific or overexpressed receptors in targeted cells. However, targeting therapeutic nanoparticles to specific cells in vivo is a major challenge for nanomedicine, because competing protein the functionality of the nanoparticles in biological environments (Maiolo et al., 2015). Salvati et al. (2013) showed that nanoparticles lose their targeting specificity in complex biological media, because the targeting molecules on the surface of nanoparticles are blocked by the protein corona and the interactions of the nanoparticles with other proteins in the medium. Some of the effects the protein corona can cause to specific targeting moieties include: loss of function due to poor orientation or displacement of the targeting moieties, structural or conformational disruption, and obscuring specific surface recognition.
In order to overcome this inherit challenge associated with protein corona, there are two proposed approaches including preparation of free corona particles and directing corona composition for targeting purposes.
2.1.1. Corona free particles
The protein corona plays an important biological role by coating the nanoparticle and masking the surface properties of the particles and can, therefore, make it challenging to distinguish the relationship between chemical functionality of nanoparticles and their biological effects (more specifically on targeting). To overcome this issue, the capability of zwitterionic nanoparticles to inhibit protein absorption to nanoparticles was proposed. The fate of corona-free nanoparticles was demonstrated by Moyano et al. (2014) presenting a series of zwitterionic nanoparticles such that only the formation of soft coronas was facilitated at physiological serum concentrations. Our increasing knowledge of the nano-bio interphase will direct the design of nanoparticles and will lead to the ability to control nanomaterials and biosystems without interference from protein coronas.
2.1.2. Using corona structure as targeting agents
One approach to overcome the masking issue associated with the protein corona to use engineered nanoparticles to direct protein corona composition for active targeting. To this end, our group assessed whether the targeting capabilities of recruited protein ligands taken directly from biological fluid during corona formation could improve the targeting capability of particles (Mirshafiee et al., 2016). To prepare such an engineered nanoparticle, the surface of the silica particles was chemically coated by gamma-globulins. This surface modification could recruit immunoglobulins and complement proteins from biological fluids due to the protein-protein interactions. The adsorption of these specific proteins should enhance the targeting with natural targeting capabilities. For example, the contribution of immunoglobulins and complement proteins should enhance macrophage targeting. Although we had a directed corona composition with an enriched amount of immunoglobulins and complement proteins, we found that the corona proteins with targeting capabilities did not substantially enhance nanoparticle uptake by macrophages (Mirshafiee et al., 2016). This is partly related to the fact that these proteins were not accessible to their partner receptors at the target cell surface. Therefore, one could expect that the functional corona with targeting capabilities should have recruited proteins and must be in a position which could enhance accessibility of their binding motifs to the receptors on the surfaces of the target cells.
In summary, when using the functional corona coated nanoparticles for targeting purposes, researchers should develop strategies that enable controlling both conformations and accessibility of the recruited proteins in the outer layer to facilitate their interactions with their binding partners on the target cells.
2.2. Protein function
Protein coronas are engaged with several biological pathways as they interact with cells and biological barriers. Therefore it is essential to fully study the functional biomolecular motifs at their interface. Kelly et al. (2015) have studied the binding sites on protein coronas and have demonstrated the feasibility of identifying the spatial location of proteins as well as their functional motifs and binding sites. Their findings can be used to describe the biological identity of various nanoparticles at the molecular level. In an in situ study, O’Connell et al. (2015) proposed deployment of protein arrays which are specifically designed to target particular systems and organs in order to study the biomolecular corona. They showed that the protein concentration in plasma and a small quantity of dominant protein–protein interactions are the major factors governing the nanoparticle interactome.
2.3. Modification of in vitro assays
To date, nanoparticles hold great potential in this very new field. Yet the very same qualities of nanoparticles that make them so distinguished in industry also make them toxic and harmful when considered in biological and medical contexts. The progress we have made in understanding and integrating nanotechnology comes to an abrupt stop into the nanomedicine. In order to get nanoparticles in clinical/commercial stage, there is a fundamental question/challenge in the field that needs to be addressed properly. The question is that why there is significant conflict in the biological data (e.g. nanotoxicology) even between identical nanoparticles. According to the findings, there is one main answer to this question: it is that there are several hidden factors at the nanobiointerfaces which had been ignored during the last decade. While it is know that there has been a large pool of overlooked factors, here we focus on only those factors relating to the protein corona.
2.3.1. Modifications of toxicity assays
Most of the toxicological protocols used for nanoparticles were developed decades before the first nano-based publication came out (Azhdarzadeh et al., 2015b). This means that these approaches are not specifically designed for toxicity evaluation of nanoparticles. In other words, essential modifications should be considered when applying these assays to evaluate nanoparticle toxicity. For example, in the MTT assay (a widely used viability assay in nanotoxicology) the presence of nanoparticles can change the structure of the dye into fomazan crystals which are blue in color (Berridge et al., 2005). This assay has a major shortcoming in terms of the negative impact of the protein corona in regards to useful assay results. Due to their high surface to volume ratio, nanoparticles have high energy and can easily interact with the biomolecules in the cell culture media and make a protein corona layer. In this regard, the nanoparticles can easily change the nutrition balance of the media in terms of biomolecules content and consequently the pH; therefore, such the cell culture media itself would have a toxic affect on cells (Mahmoudi et al., 2010). To that end, we have treated the cell culture media with different concentrations of polyvinyl alcohol-coated superparamagnetic iron oxide nanoparticles (Mahmoudi et al., 2010). After spinning and removing the particles, the media was substantially changed in terms of its pH and proteins structure/content. When we incubated the cells with this media and checked their metabolic activity using the MTT assay, we found that the media imposed ~20% toxicity to mouse fibroblast cells. This error in the toxicity evaluation of nanoparticles can occur in the static environment but not in the dynamic culture (like in vivo or bioreactors); so, in order to avoid such error in the toxicity data, we proposed the use of corona coated particles in the assay as follows (Mahmoudi et al., 2010): Introducing the nanoparticles to the culture medium and leaving the solution in contact for a while to get the surface of particles saturated with the preferred biomolecules followed by collection and re-dispersion of the corona-coated particles into a fresh media, and then apply them to the cells in order to get more realistic nanoparticle toxicity evaluation.
Several studies also demonstrated that addressing several factors including the temperature of the target tissue, plasma gradient, cell shape, interfacial effects, and personalized protein corona, could improve consistency of toxicity results between laboratories (Azhdarzadeh et al., 2015a). Thus when these factors are taken into consideration, in vitro assays have the capacity to be reliable predictors of in vivo behavior.
2.3.2. Effect on nanoparticle uptake and cell response
Fate of nanoparticles in biological systems depends highly on all possible parameters that influence the nano-bio interface (Mahmoudi et al., 2013a). An awareness of how the protein corona will interface with the cell surface and biological environment is essential for the development of successful therapies (Pelaz et al., 2015; Maffre et al., 2014). Kah et al. (2014) showed that the properties of the protein corona are influenced by the ligand on the surface of nanorods, which affects the cellular response. For instance, amphiphilic ligands play an intermediary role in the formation and physical characteristics of the protein corona including colloidal stability of the resulting species and, likely, the corona composition. Other studies have shown that silica nanoparticles are internalized very differently depending on the presence of serum in the media (Lesniak et al., 2012). For example, nanoparticles administered to cells in serum-free conditions enter cells more readily than when they are covered by a corona. However, the nanoparticle corona provides a protein layer between the nanoparticle and the cellular environment, mitigating acute cellular toxicity.
Lesniak et al. (2013) demonstrated that nanoparticle uptake is a two-step process, where nanoparticles initially adhere to the cell membrane and then subsequently internalized by the cell via energy dependent pathways. Moreover, they found that protein corona on the nanoparticle surface, strongly reduces nanoparticle adhesion to cellular membranes in comparison to the bare nanomaterial and thereby causes a concomitant decrease in nanoparticle uptake efficiency.
Other studies took advantage of the magnetic properties of silica coated magnetite nanoparticles in order to recover the subcellular fractions of nanoparticles to follow the intracellular trafficking of the nanoparticles (Bertoli et al., 2014). The results suggest that the serum-derived corona is preserved through cellular uptake and corona proteins can allow nanoparticles to reorganize the lipid bilayer (Krpetic et al., 2014). Once bound, these surface-bound proteins can drive activation and inhibition of cell signaling events. Another interesting discovery was reported by Saha et al. (2014) who showed that the presence of the protein corona on the surface of nanoparticles protects red blood cells from both the hydrophilic and hydrophobic nanoparticle-mediated hemolysis.
2.3.3. Effect on drug release
Protein corona on nanoparticle is an important consideration for effective drug release. Thus, the properties of the nanoparticles under investigation and the subsequent conditions for protein corona formation will significantly determine the drug release profile (Behzadi et al., 2014a). Importantly, the protein corona could significantly reduce the burst effect of protein-conjugated nanoparticles or carriers with surface loaded drugs or can be used to load therapeutics. Cifuentes-Rius et al. (2013) showed that the rate of passive release of DNA payloads can be manipulated by varying the corona composition around nanoparticles. They revealed that the effect of buffer strength, human serum concentration, and concentration of cetyltrimethylammonium bromide play an important role in passive release. Kah et al. (2012) showed that the coronas that are formed by serum proteins on gold nanorods capped with cetyltrimethylammonium bromide, can hold molecular therapeutics at a ~5–10-fold greater capacity than covalent conjugation. It has also been shown that the payload capacity varies with assembly strategy, ionic strength, and loading concentration and coronas can be loaded with species of either negative or positive charge.
Our group probed the effect of the protein corona on both synthetized nanocarriers (i.e., superparamagnetic iron oxide nanoparticles (SPIONs) and polymeric nanocapsules) and commercialized nanoparticles (i.e., Abraxane®, albumin-bound paclitaxel drug) (Behzadi et al., 2014b). We found that the protein corona can mask the nanocarriers and, therefore, substantially change their drug release profiles.
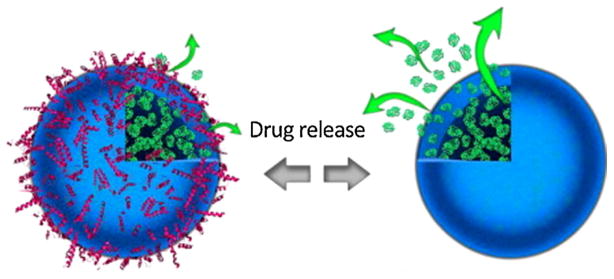
In summary, the protein corona can change the drug release profile (Fig. 1). Therefore, full information with regards to the effect of the protein corona on the drug release profile of each carrier should be carefully obtained in order to reduce not only the conflicts in the nanotoxicology knowledge but also to diminish the gap between in vitro and in vivo results.
Fig. 1.
The protein corona can change the drug release profile of the nanocarriers. Reproduced with permission from reference Behzadi et al. (2014b).
2.3.4. Effect on protein fibrillation
Protein fibrillation occurs when partially or fully unfolded proteins form beta-strand-stacked structures leading to the formation of large insoluble linear amyloid. The protein fibrillation process is the primary cause of several catastrophic neurodegenerative diseases such as Alzheimer’s and Parkinson disease. Nanomaterials demonstrated significant effect on the fibrillation process; some of the particles (e.g., gold nanoparticles) could substantially inhibit the fibrillation process and thus could be considered as promising materials for treatment of these diseases (Rocha et al., 2008; Yokoyama and Welchons, 2007). However, the majority of the performed experiments were done on the surface of pristine coated nanoparticles (Cabaleiro-Lago et al., 2008; Cabaleiro-Lago et al., 2010). This could not predict the in vivo behavior of such particles, as their surfaces are covered by the protein corona. In order to mimic the real effects of such a particle on the fibrillation process in vivo, the fibrillation process should be performed in the presence of corona coated particles and not the bare particles (Mahmoudi et al., 2012).
The effect of the protein corona on the fibrillation process was probed in the presence of a variety of particles (e.g., silica, polystyrene, and carbon nanotube) and it was found that unlike the bare particles (which could accelerate the fibrillation process) the nanoparticles surrounded by a protein corona inhibited fibrillation, depending on the corona composition and plasma concentration (Mahmoudi et al., 2013b). On the contrary, the protein corona on the surface of gold nanoparticles (which had inhibitory effects on the fibrillation process in their pristine state) showed slight acceleratory potencies (Mirsadeghi et al., 2015).
In summary, the effect of the protein corona on the protein fibrillation process is strongly dependent on the type of nanoparticles and protein corona composition in terms of type, amount, and conformation of the associated proteins. There are still many open questions regarding the impact of protein coronas on protein fibrillation. Therefore, more work on the characterization of the role of the protein corona in protein fibrillation is needed.
2.3.5. In vivo protein corona
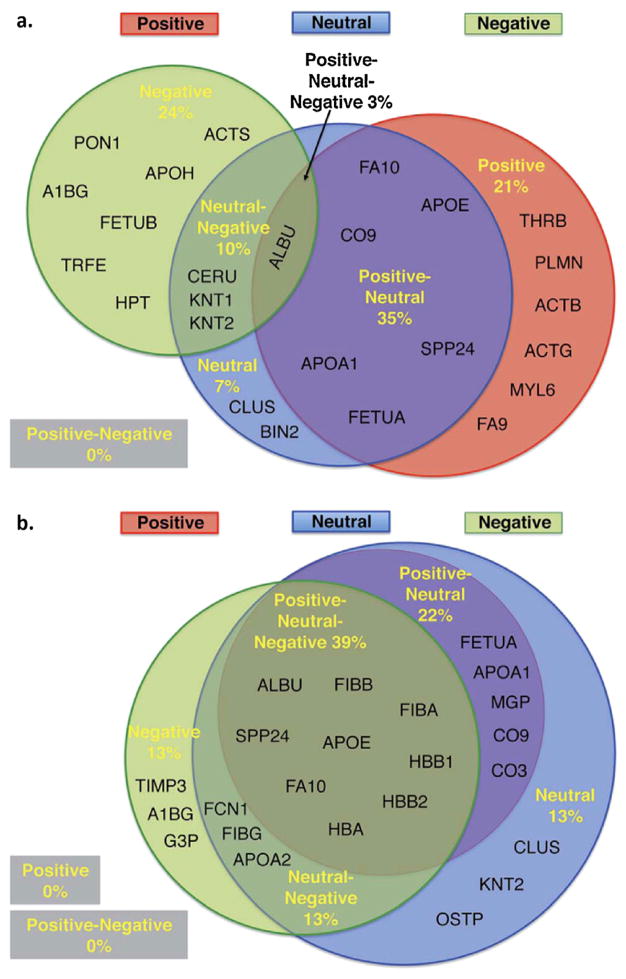
Our ability to comprehend protein corona behavior within the in vivo milieu is a vital step towards the accurate prediction of a particle’s biological fate. While there are numerous reports which describe the protein corona at the surface of nanoparticles in vitro, reports describing the in vivo protein corona are limited. One of the main obstacles limiting the progress in this area is the lack of effective techniques allowing for separation of nanoparticles from the in vivo environment after administration. However, Sakulkhu et al. (2014) were able to overcome this barrier by using polyvinyl-alcohol-coated SPIONs delivered to rats and deployed these nanoparticles to investigate the in vivo protein corona formation. It is noteworthy that the unique magnetic properties of the particles made the particles separation possible using a strong external magnetic field. This study was able to demonstrate that there are substantial differences between the in vitro and in vivo protein corona profiles (see Fig. 2). For example, we found that positive and neutral nanoparticles have 50% proteins in common between in vivo and in vitro conditions, and surprisingly, there were only 8% similarities for the negative particles. Taken together, in order to have an accurate prediction of a nanoparticles fate, new techniques should be developed to extract nanoparticles from the in vivo systems or even to allow noninvasive characterization for the protein corona around nanoparticles in tissue.
Fig. 2.
Prominent associated proteins in the corona composition of particles with various surface charges obtained from (a) in vitro and (b) in vivo environments. Reproduced with permission from reference Sakulkhu et al. (2014).
3. Personalized protein corona
The most progressive protein corona is the one which is tailored for personalized medicine. The source of proteins whether derived from an in vivo or in vitro system, strongly controls the composition of the protein corona on the surface of nanoparticles. Hajipour et al. (2015b) identified the impact of diseases on human plasma as the source of proteins and its consequent effect on corona formation. It was demonstrated that the type of human disease has a crucial role in the protein composition of the nanoparticle corona and humans with specific disease(s) may have specific nanoparticle coronas. Thus personalized protein corona (PPT) is introduced as a new concept in the field of nanobiotechnology. Interestingly, the exact same type of nanoparticles, having various coronas from different diseases, demonstrated substantial different cellular responses (Hajipour et al., 2015a). Caracciolo et al. (2014) were also able to demonstrate disease dependent differential protein bindings and identified relationships between the disease state and nanoparticle bio-identity (Caracciolo et al., 2015). These findings represent a promising new area in protein corona development for both personalized diagnostics and therapeutic treatments.
According to the obtained results on PPT, one could expect that researchers should design efficient and safe, patient-specific nanoparticles in a disease type-specific manner for successful clinical applications.
4. Conclusion
Nanotechnology is now being used in various products related to non-biomedical applications such as displays, automobile parts, clothing, and kitchen appliances. On the contrary, the use of nanomaterials in biomedical applications, particularly as nanotherapeutics, has been relatively limited (number of FDA-approved nanotherapeutics is still less than 30 products). One of the main reasons for this substantial obstacle in nanomedicine are the complications resulting from the protein corona. According to the challenges presented in this perspective, we believe there is an urgent need for systematic debugging of the protein corona issues. Such progresses in the field will not only reduce the conflicts in the nanotoxicology knowledge but will also diminish the gap between in vitro and in vivo results, which will facilitate the use of nanomedicine approaches in clinics.
References
- Azhdarzadeh M, et al. Nanotoxicology: advances and pitfalls in research methodology. Nanomedicine (Lond) 2015a;10:2931–2952. doi: 10.2217/nnm.15.130. http://dx.doi.org/10.2217/nnm.15.130. [DOI] [PubMed] [Google Scholar]
- Azhdarzadeh M, et al. Nanotoxicology: advances and pitfalls in research methodology. Nanomedicine. 2015b;10:2931–2952. doi: 10.2217/nnm.15.130. [DOI] [PubMed] [Google Scholar]
- Behzadi S, et al. Protein corona change the drug release profile of nanocarriers: the “overlooked” factor at the nanobio interface. Colloids Surf B: Biointerfaces. 2014a;123:143–149. doi: 10.1016/j.colsurfb.2014.09.009. http://dx.doi.org/10.1016/j.colsurfb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Behzadi S, et al. Protein corona change the drug release profile of nanocarriers: the “overlooked” factor at the nanobio interface. Colloids Surf B: Biointerfaces. 2014b;123:143–149. doi: 10.1016/j.colsurfb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–152. doi: 10.1016/S1387-2656(05)11004-7. [DOI] [PubMed] [Google Scholar]
- Bertoli F, et al. Magnetic nanoparticles to recover cellular organelles and study the time resolved nanoparticle-cell interactome throughout uptake. Small. 2014;10:3307–3315. doi: 10.1002/smll.201303841. http://dx.doi.org/10.1002/smll.201303841. [DOI] [PubMed] [Google Scholar]
- Cabaleiro-Lago C, et al. Inhibition of amyloid β protein fibrillation by polymeric nanoparticles. J Am Chem Soc. 2008;130:15437–15443. doi: 10.1021/ja8041806. [DOI] [PubMed] [Google Scholar]
- Cabaleiro-Lago C, Quinlan-Pluck F, Lynch I, Dawson KA, Linse S. Dual effect of amino modified polystyrene nanoparticles on amyloid β protein fibrillation. ACS Chem Neurosci. 2010;1:279–287. doi: 10.1021/cn900027u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo G, Caputo D, Pozzi D, Colapicchioni V, Coppola R. Size and charge of nanoparticles following incubation with human plasma of healthy and pancreatic cancer patients. Colloid Surf B. 2014;123:673–678. doi: 10.1016/j.colsurfb.2014.10.008. http://dx.doi.org/10.1016/j.colsurfb.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Caracciolo G, et al. Lipid composition: a “key factor” for the rational manipulation of the liposome-protein corona by liposome design. RSC Adv. 2015;5:5967–5975. http://dx.doi.org/10.1039/c4ra13335h. [Google Scholar]
- Cifuentes-Rius A, de Puig H, Kah JC, Borros S, Hamad-Schifferli K. Optimizing the properties of the protein corona surrounding nanoparticles for tuning payload release. ACS Nano. 2013;7:10066–10074. doi: 10.1021/nn404166q. http://dx.doi.org/10.1021/nn404166q. [DOI] [PubMed] [Google Scholar]
- Hadjidemetriou M, et al. In vivo biomolecule corona around blood-circulating, clinically used and antibody-targeted lipid bilayer nanoscale vesicles. ACS Nano. 2015;9:8142–8156. doi: 10.1021/acsnano.5b03300. http://dx.doi.org/10.1021/acsnano.5b03300. [DOI] [PubMed] [Google Scholar]
- Hajipour MJ, et al. Personalized disease-specific protein corona influences the therapeutic impact of graphene oxide. Nanoscale. 2015a;7:8978–8994. doi: 10.1039/c5nr00520e. http://dx.doi.org/10.1039/C5NR00520E. [DOI] [PubMed] [Google Scholar]
- Hajipour MJ, et al. Personalized disease-specific protein corona influences the therapeutic impact of graphene oxide. Nanoscale. 2015b;7:8978–8994. doi: 10.1039/c5nr00520e. http://dx.doi.org/10.1039/c5nr00520e. [DOI] [PubMed] [Google Scholar]
- Hamad-Schifferli K. Exploiting the novel properties of protein coronas: emerging applications in nanomedicine. Nanomedicine (Lond) 2015;10:1663–1674. doi: 10.2217/nnm.15.6. http://dx.doi.org/10.2217/nnm.15.6. [DOI] [PubMed] [Google Scholar]
- Jiang X, et al. Quantitative analysis of the protein corona on FePt nanoparticles formed by transferrin binding. J R Soc Interface. 2010;7(Suppl 1):S5–S13. doi: 10.1098/rsif.2009.0272.focus. http://dx.doi.org/10.1098/rsif.2009.0272.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kah JC, Chen J, Zubieta A, Hamad-Schifferli K. Exploiting the protein corona around gold nanorods for loading and triggered release. ACS Nano. 2012;6:6730–6740. doi: 10.1021/nn301389c. http://dx.doi.org/10.1021/nn301389c. [DOI] [PubMed] [Google Scholar]
- Kah JC, et al. Protein coronas on gold nanorods passivated with amphiphilic ligands affect cytotoxicity and cellular response to penicillin/streptomycin. ACS Nano. 2014;8:4608–4620. doi: 10.1021/nn5002886. http://dx.doi.org/10.1021/nn5002886. [DOI] [PubMed] [Google Scholar]
- Kelly PM, et al. Mapping protein binding sites on the biomolecular corona of nanoparticles. Nat Nanotechnol. 2015;10:472–479. doi: 10.1038/nnano.2015.47. http://dx.doi.org/10.1038/nnano.2015.47. [DOI] [PubMed] [Google Scholar]
- Krpetic Z, Anguissola S, Garry D, Kelly PM, Dawson KA. Nanomaterials: 2014 impact on cells and cell organelles. Adv Exp Med Biol. 811:135–156. doi: 10.1007/978-94-017-8739-0_8. http://dx.doi.org/10.1007/978-94-017-8739-0_8. [DOI] [PubMed] [Google Scholar]
- Lesniak A, et al. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano. 2012;6:5845–5857. doi: 10.1021/nn300223w. http://dx.doi.org/10.1021/nn300223w. [DOI] [PubMed] [Google Scholar]
- Lesniak A, et al. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J Am Chem Soc. 2013;135:1438–1444. doi: 10.1021/ja309812z. http://dx.doi.org/10.1021/ja309812z. [DOI] [PubMed] [Google Scholar]
- Maffre P, et al. Effects of surface functionalization on the adsorption of human serum albumin onto nanoparticles – a fluorescence correlation spectroscopy study. Beilstein J Nanotechnol. 2014;5:2036–2047. doi: 10.3762/bjnano.5.212. http://dx.doi.org/10.3762/bjnano.5.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi M, et al. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf B: Biointerfaces. 2010;75:300–309. doi: 10.1016/j.colsurfb.2009.08.044. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, et al. Protein-nanoparticle interactions: opportunities and challenges. Chem Rev. 2011a;111:5610. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Hofmann H, Rothen-Rutishauser B, Petri-Fink A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem Rev. 2011b;112:2323–2338. doi: 10.1021/cr2002596. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Akhavan O, Ghavami M, Rezaee F, Ghiasi SM. Graphene oxide strongly inhibits amyloid beta fibrillation. Nanoscale. 2012;4:7322–7325. doi: 10.1039/c2nr31657a. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, et al. Temperature: the “ignored” factor at the NanoBio interface. ACS Nano. 2013a;7:6555–6562. doi: 10.1021/nn305337c. http://dx.doi.org/10.1021/nn305337c. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, et al. The protein corona mediates the impact of nanomaterials and slows amyloid beta fibrillation. Chembiochem. 2013b;14:568–572. doi: 10.1002/cbic.201300007. http://dx.doi.org/10.1002/cbic.201300007. [DOI] [PubMed] [Google Scholar]
- Mahon E, Salvati A, Baldelli Bombelli F, Lynch I, Dawson KA. Designing the nanoparticle-biomolecule interface for “targeting and therapeutic delivery”. J Control Release. 2012;161:164–174. doi: 10.1016/j.jconrel.2012.04.009. http://dx.doi.org/10.1016/j.jconrel.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Maiolo D, Bergese P, Mahon E, Dawson KA, Monopoli MP. Surfactant titration of nanoparticle-protein corona. Anal Chem. 2014;86:12055–12063. doi: 10.1021/ac5027176. http://dx.doi.org/10.1021/ac5027176. [DOI] [PubMed] [Google Scholar]
- Maiolo D, Del Pino P, Metrangolo P, Parak WJ, Baldelli Bombelli F. Nanomedicine delivery: does protein corona route to the target or off road? Nanomedicine (Lond) 2015;10:3231–3247. doi: 10.2217/nnm.15.163. http://dx.doi.org/10.2217/nnm.15.163. [DOI] [PubMed] [Google Scholar]
- Mao HY, et al. Graphene: promises, facts, opportunities, and challenges in nanomedicine. Chem Rev. 2013;113:3407–3424. doi: 10.1021/cr300335p. [DOI] [PubMed] [Google Scholar]
- Milani S, Bombelli FB, Pitek AS, Dawson KA, Radler J. Reversible versus irreversible binding of transferrin to polystyrene nanoparticles: soft and hard corona. ACS Nano. 2012;6:2532–2541. doi: 10.1021/nn204951s. http://dx.doi.org/10.1021/nn204951s. [DOI] [PubMed] [Google Scholar]
- Mirsadeghi S, et al. Protein corona composition of gold nanoparticles/nanorods affects amyloid beta fibrillation process. Nanoscale. 2015;7:5004–5013. doi: 10.1039/c4nr06009a. http://dx.doi.org/10.1039/c4nr06009a. [DOI] [PubMed] [Google Scholar]
- Mirshafiee V, Kim R, Park S, Mahmoudi M, Kraft ML. Impact of protein pre-coating on the protein corona composition and nanoparticle cellular uptake. Biomaterials. 2016;75:295–304. doi: 10.1016/j.biomaterials.2015.10.019. http://dx.doi.org/10.1016/j.biomaterials.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Monopoli MP, Åberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012a;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012b;7:779–786. doi: 10.1038/nnano.2012.207. http://dx.doi.org/10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- Monopoli MP, Pitek AS, Lynch I, Dawson KA. Formation and characterization of the nanoparticle-protein corona. Methods Mol Biol. 2013;1025:137–155. doi: 10.1007/978-1-62703-462-3_11. http://dx.doi.org/10.1007/978-1-62703-462-3_11. [DOI] [PubMed] [Google Scholar]
- Moyano DF, et al. Fabrication of corona-free nanoparticles with tunable hydrophobicity. ACS Nano. 2014;8:6748–6755. doi: 10.1021/nn5006478. http://dx.doi.org/10.1021/nn5006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell DJ, et al. Characterization of the bionano interface and mapping extrinsic interactions of the corona of nanomaterials. Nanoscale. 2015;7:15268–15276. doi: 10.1039/c5nr01970b. http://dx.doi.org/10.1039/c5nr01970b. [DOI] [PubMed] [Google Scholar]
- Pelaz B, et al. Surface functionalization of nanoparticles with polyethylene glycol: effects on protein adsorption and cellular uptake. ACS Nano. 2015;9:6996–7008. doi: 10.1021/acsnano.5b01326. http://dx.doi.org/10.1021/acsnano.5b01326. [DOI] [PubMed] [Google Scholar]
- Rocha S, et al. Influence of fluorinated and hydrogenated nanoparticles on the structure and fibrillogenesis of amyloid beta-peptide. Biophys Chem. 2008;137:35–42. doi: 10.1016/j.bpc.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Saha K, Moyano DF, Rotello VM. Protein coronas suppress the hemolytic activity of hydrophilic and hydrophobic nanoparticles. Mater Horiz. 2014;2014:102–105. doi: 10.1039/C3MH00075C. http://dx.doi.org/10.1039/C3MH00075C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saie AA, Ray M, Mahmoudi M, Rotello VM. Engineering the nanoparticle-protein interface for cancer therapeutics. Cancer Treat Res. 2015;166:245–273. doi: 10.1007/978-3-319-16555-4_11. http://dx.doi.org/10.1007/978-3-319-16555-4_11. [DOI] [PubMed] [Google Scholar]
- Sakulkhu U, et al. Ex situ evaluation of the composition of protein corona of intravenously injected superparamagnetic nanoparticles in rats. Nanoscale. 2014;6:11439–11450. doi: 10.1039/c4nr02793k. http://dx.doi.org/10.1039/c4nr02793k. [DOI] [PubMed] [Google Scholar]
- Salvati A, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. http://dx.doi.org/10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- Sharifi S, et al. Toxicity of nanomaterials. Chem Soc Rev. 2012;41:2323–2343. doi: 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, et al. The biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomes. Nanomedicine. 2013;9:1159–1168. doi: 10.1016/j.nano.2013.04.010. http://dx.doi.org/10.1016/j.nano.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Welchons D. The conjugation of amyloid beta protein on the gold colloidal nanoparticles’ surfaces. Nanotechnology. 2007;18:105101. doi: 10.1088/0957-4484/19/37/375101. [DOI] [PubMed] [Google Scholar]