Abstract
Recent recommendations by the World Health Organization support treatment for all people living with HIV (PLWH) globally to be initiated at the point of testing. While there has been marked success in efforts to identify and expand treatment for PLWH throughout sub-Saharan Africa, the goal of universal treatment may prove challenging to achieve. The pre-ART phase of the care cascade from HIV testing to HIV treatment initiation includes several social and structural barriers. One such barrier is antiretroviral therapy (ART) treatment refusal, a phenomenon in which HIV-infected individuals choose not to start treatment upon learning their ART eligibility. Our goal is to provide further understanding of why treatment-eligible adults may choose to present for HIV testing but not initiate ART when indicated. In this article, we will discuss factors driving pre-ART loss and present a framework for understanding the impact of decision-making on early losses in the care cascade, with a focus on ART refusal.
Keywords: Antiretroviral medications, Treatment refusal, Global epidemic, HIV/AIDS, HIV prevention, Science of prevention, ART refusal, Point of testing, Review
Introduction
Over the past 15 years [1], there have been significant advances expanding HIV treatment access in sub-Saharan Africa. Recent studies focused on the timing of antiretroviral therapy (ART) initiation have found that early diagnosis and treatment improves outcomes for people living with HIV (PLWH) [2••, 3••] and prevents HIV transmission to their sexual partners [4]. Based upon these results, the World Health Organization (WHO) has now stated it supports a “test and treat” model of care for all PLWH [5], supported by the Vancouver Statement [6]. If this strategy is ultimately adopted across nations in sub-Saharan Africa, it will lead to an expansive effort to include early-stage individuals in care, many of whom will be asymptomatic.
While there has been a substantial focus on the entire care continuum, the pre-ART period—specifically the time between HIV acquisition, testing, and treatment initiation—deserves special attention. This is a time where barriers to treatment initiation are not yet balanced by the motivation to recover from a debilitating and immediately life-threatening illness. The challenge of expanding treatment to people with earlier disease is supported by the observation that CD4+ cell counts at linkage to care and at ART initiation have not appreciably increased during the past decade in sub-Saharan Africa, despite multinational efforts to expand ART availability [7].
In this article, we will discuss factors driving pre-ART loss and present a framework for understanding the impact of pre-ART decision-making on early losses in the care cascade, with a focus on ART refusal. Our goal is to provide further understanding of why treatment-eligible adults may choose to present for HIV testing, yet may not initiate ART when indicated. By defining the stages of pre-ART decision-making, we hope to guide future empirical study of the factors related to refusal and corresponding interventions to increase treatment acceptance.
Identifying the Scope of Loss in the Pre-ART Care Continuum
As of 2014, there were an estimated 36.9 million PLWH. The vast majority of these individuals live in sub-Saharan Africa. Unfortunately, fewer than half (15 million) are currently receiving ART [8], despite widespread voluntary testing and free treatment. This is largely due to losses of PLWH whose CD4+ counts are too high to render them able to start treatment [9–13] and failure to initiate treatment promptly among those who are already ART eligible at HIV diagnosis [7, 14]. There is a growing body of literature focused on pre-ART losses and the need to understand both structural and socio-behavioral barriers to ART initiation, in order to inform effective combination interventions [15•, 16–18].
Structural barriers to ART initiation exist at every level of the pre-ART care cascade, with continual losses through all stages, from the period prior to testing through entry into care and ART initiation [19–22]. Such barriers can include distance to the testing and treatment center [23–25], costs involved in transport and time lost from work [26, 27], and perceived poor quality of care [28•]. Evidence indicates that decreasing structural barriers to testing through programs such as home-based counseling and testing [29–37] and point-of-care diagnostics [38] may be highly effective in improving rates of treatment initiation. In addition, shifting models of care from centralized top-down programs to more decentralized treatment structures [39, 40], including community-based adherence clubs [41, 42], may decrease barriers to initiating treatment and staying in care [43].
Social factors may also impact the pre-ART care continuum. A particular area of focus has been on the impact of psychological consequences of an HIV diagnosis (e.g., depression [44] and internalized stigma [45–49]) and how it may delay ART initiation [50, 51•, 52]. Other forces, including challenges associated with disclosure [53], fatalistic beliefs [54], and a lack of social support [46] may influence the decision to avoid testing, or treatment initiation. In addition, concerns about medication side effects [55] or a belief that ART is only reserved for the “sick” may also result in treatment avoidance [51•]. Interventions focused on addressing stigma through alternate means of testing [56, 57] and optimizing disclosure (e.g., through couple’s based counseling) [58] may mitigate some of the challenges associated with test and treat strategies and lead to improved uptake of services in pre-ART care cascade.
Four Stages of ART Decision-Making—Developing a Framework for Understanding Its Impact on Early Losses in Care
Pre-ART losses can occur at any point prior to testing, between testing and the receipt of CD4+ results, and between the receipt of CD4+ results and ART initiation. As outlined above, social and structural forces may derail PLWH at any of these steps along the continuum. Prior work has focused on losses prior to testing, and between testing and the receipt of CD4+ results; therefore, we have developed a framework to more clearly define components of treatment initiation.
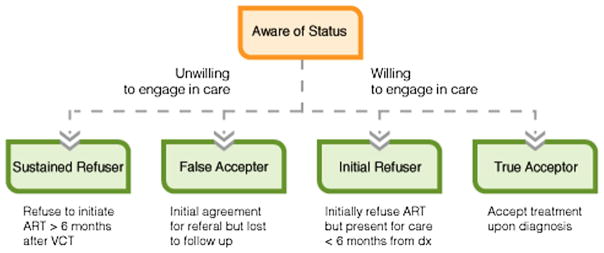
We have previously explained how ART-related decision-making at the point of testing can be framed in the context of risk aversion and that the pros of known health benefits need to be weighed against the feared risks of medication-associated disclosure and potentially disfiguring side effects of treatment [59]. Based on our research and a growing body of literature focused on developing interventions to reduce pre-ART losses [60], we have refined our understanding of the pre-ART care continuum to encompass four distinct patterns of decision-making among individuals who present for voluntary counseling and testing (VCT) (Fig. 1). While described as four distinct groups, individuals may initially be part of one group and move into another over time.
Fig. 1.
Four categories of ART initiation
Individuals may choose to initiate or refuse ART upon learning their eligibility. If they initially refuse and continue to refuse for at least 6 months, they can be classified as “Sustained Refusers.” This group readily acknowledges they are unwilling to start treatment from the time of testing, and then continues to be unwavering in this belief, often despite multiple attempts by clinic providers to help engage them in care, including phone calls, text messages, and home visits. While this population presents considerable challenges for care providers, they are often forthright in their desire to forgo treatment from an early stage in the pre-ART care cascade and likely present unique intervention opportunities at the time of testing.
Conversely, those who refuse to initiate upon learning their CD4+ but change their mind within 6 months are labeled “Initial Refusers.” Initial and sustained refusal may be influenced by different factors. Specifically, concerns about treatment initiation at the point of testing may be related to a sense of wellness and a feeling that they are not yet “sick enough” to start treatment [61]. Whereas those who refuse treatment over a prolonged period tend to express concerns about unwanted disclosure, having a fatalistic worldview, low social support, and fears that medication-associated side effects may render them unable to adhere to a prescribed regimen [50, 51•, 62•, 63•].
Unfortunately, exaggerated beliefs related to ART side effects may have unwittingly been fueled by governmental policies designed to restrict treatment (e.g., the use of CD4+ cutoffs to guide treatment availability), the usage of medications with severe toxicities early in the HIV epidemic due to cost constraints, and ongoing mandatory pre-adherence counseling sessions [64]. Motivational interviewing may be effective in this population as a way to provide goal-directed, client-centered counseling to elicit behavior change [59, 65–69].
Conversely, individuals may convey a willingness to start treatment at the point of learning of their eligibility. They may then go on to initiate treatment (“True Acceptors”) or fail to start within 6 months (“False Acceptors”). Research on the latter group has shown that structural barriers, including distance from clinic and economic constraints [70], may play a larger role in impeding ART initiation than with “sustained refusers.” Psychosocial factors likely exist, however, including stigma, or poor emotional health, as well as a social desirability bias in reporting intended behavior [28•, 70–72]. Rates of “False Acceptors” may be well over 20 % in certain key populations [13, 73, 74] and will likely benefit from combination interventions targeting both structural and psychosocial factors through peer navigation and peer support.
A new long-term strategy for marketing ART should promote more tolerable regimens, and the importance of preventing both long-term complications of HIV inflammation, as well as the benefits of avoiding HIV transmission. Interventions will need to be developed at several levels from public information campaigns to restructuring counseling messages for HIV testing and treatment, to programs that provide direct support for those considering treatment initiation.
Conclusion
The time between HIV acquisition and ART initiation is a critical period in the treatment continuum. To date, few studies have focused on this vulnerable period [75]. Issues of recruitment and retention remain challenging in this population, and measurement of ART initiation may lack clarity and rigor that has now been well developed and applied in ART adherence research [21, 76, 77]. Yet, it is clear that the cascade of refusal in the pre-ART period remains a critical concern, particularly as treatment is expanded to PLWH earlier in the course of their infection. If a “test and treat” strategy of care is to be adopted in sub-Saharan Africa, there will not only be economic and structural barriers to manage but also deeply held beliefs that ART is associated with illness and even death [51•]. Research in this area, which focuses on socio-behavioral intervention design and development, will be critical to the success of treatment as prevention in the time ahead.
Acknowledgments
Funding This publication was made possible with funding from US National Institute for Mental Health K23 MH097667 (Katz). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Ingrid T. Katz and David R. Bangsberg declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Joint United Nations Programme on HIV/AIDS (UNICEF) How AIDS changed everything—MDG 6: 15 years, 15 lessons of hope from the AIDS response. Geneva, Switzerland: 2015. [Accessed 22 August 2015]. http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf. [Google Scholar]
- 2••.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Eng J Med. 2015 doi: 10.1056/NEJMoa1506816. A trial of over 4000 patients in 35 countries, showed that the risk of death, a serious AIDS-related event, or a serious non–AIDS-related event was 57% lower among those treated early than among those treated when the CD4+ cell count decreased to 350 cells per cubic millimeter. INSIGHTand TEMPRANO confirm previous observational studies and add important new evidence that advances our knowledge of the risks and benefits of early ART in patients with CD4+ cell counts of more than 500 cells per cubic millimeter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Eng J Med. 2015 doi: 10.1056/NEJMoa1507198. This study involved over 2000 patients in the Ivory Coast, and showed that early ART initiation was associated with a 44% lower risk of death or severe HIV-related illness than was ART initiated according to prevailing World Health Organization criteria. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: 2015. Available from: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1. Accessed: Accessed. [PubMed] [Google Scholar]
- 6.Beyrer C, Birx DL, Bekker L-G, Barré-Sinoussi F, Cahn P, Dybul MR, et al. The Vancouver Consensus: antiretroviral medicines, medical evidence, and political will. Lancet. 2015;386(9993):505–7. doi: 10.1016/S0140-6736(15)61458-1. [DOI] [PubMed] [Google Scholar]
- 7.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis. 2015;60(7):1120–7. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piot P, Abdool Karim SS, Hecht R, Legido-Quigley H, Buse K, Stover J, et al. Defeating AIDS—advancing global health. Lancet. 2015;386(9989):171–218. doi: 10.1016/S0140-6736(15)60658-4. [DOI] [PubMed] [Google Scholar]
- 9.Bassett IV, Wang B, Chetty S, Mazibuko M, Bearnot B, Giddy J, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51(2):135–9. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassett IV, Regan S, Chetty S, Giddy J, Uhler LM, Holst H, et al. Who starts antiretroviral therapy in Durban, South Africa?… not everyone who should. Aids. 2010;24(Suppl 1):S37–44. doi: 10.1097/01.aids.0000366081.91192.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Losina E, Bassett IV, Giddy J, Chetty S, Regan S, Walensky RP, et al. The “ART” of linkage: pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS One. 2010;5(3):e9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tayler-Smith K, Zachariah R, Massaquoi M, Manzi M, Pasulani O, van den Akker T, et al. Unacceptable attrition among WHO stages 1 and 2 patients in a hospital-based setting in rural Malawi: can we retain such patients within the general health system? Trans R Soc Trop Med Hyg. 2010;104(5):313–9. doi: 10.1016/j.trstmh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingle SM, May M, Uebel K, Timmerman V, Kotze E, Bachmann M, et al. Outcomes in patients waiting for antiretroviral treatment in the Free State Province, South Africa: prospective linkage study. Aids. 2010;24(17):2717–25. doi: 10.1097/QAD.0b013e32833fb71f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Geng EH, Bwana MB, Muyindike W, Glidden DV, Bangsberg DR, Neilands TB, et al. Failure to initiate antiretroviral therapy, loss to follow-up and mortality among HIV-infected patients during the pre-ART period in Uganda. J Acquir Immune Defic Syndr. 2013;63(2):e64–71. doi: 10.1097/QAI.0b013e31828af5a6. This study used a sampling-based approach to understand uptake of ART among eligible patients at a scale-up clinic in Uganda. The authors found the rate and total uptake of ARTwas neither optimally fast nor complete. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gwadz M, Cleland CM, Applegate E, Belkin M, Gandhi M, Salomon N, et al. Behavioral intervention improves treatment outcomes among HIV-infected individuals who have delayed, declined, or discontinued antiretroviral therapy: a randomized controlled trial of a novel intervention. AIDS Behav. 2015;19(10):1801–17. doi: 10.1007/s10461-015-1054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNairy ML, Gachuhi AB, Lamb MR, Nuwagaba-Biribonwoha H, Burke S, Ehrenkranz P, et al. The Link4Health study to evaluate the effectiveness of a combination intervention strategy for linkage to and retention in HIV care in Swaziland: protocol for a cluster randomized trial. Implement Sci. 2015;10:101. doi: 10.1186/s13012-015-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siedner MJ, Santorino D, Lankowski AJ, Kanyesigye M, Bwana MB, Haberer JE, et al. A combination SMS and transportation reimbursement intervention to improve HIV care following abnormal CD4 test results in rural Uganda: a prospective observational cohort study. BMC Med. 2015;13:160. doi: 10.1186/s12916-015-0397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox MP, Shearer K, Maskew M, Meyer-Rath G, Clouse K, Sanne I. Attrition through multiple stages of pre-treatment and ART HIV care in South Africa. PLoS One. 2014;9(10):e110252. doi: 10.1371/journal.pone.0110252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kranzer K, Zeinecker J, Ginsberg P, Orrell C, Kalawe NN, Lawn SD, et al. Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PLoS One. 2010;5(11):e13801. doi: 10.1371/journal.pone.0013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mugglin C, Estill J, Wandeler G, Bender N, Egger M, Gsponer T, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. 2012;17(12):1509–20. doi: 10.1111/j.1365-3156.2012.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGrath N, Glynn JR, Saul J, Kranzer K, Jahn A, Mwaungulu F, et al. What happens to ART-eligible patients who do not start ART? Dropout between screening and ART initiation: a cohort study in Karonga, Malawi. BMC Public Health. 2010;10:601. doi: 10.1186/1471-2458-10-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lankowski AJ, Siedner MJ, Bangsberg DR, Tsai AC. Impact of geographic and transportation-related barriers on HIV outcomes in sub-Saharan Africa: a systematic review. AIDS Behav. 2014;18(7):1199–223. doi: 10.1007/s10461-014-0729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siedner MJ, Lankowski A, Tsai AC, Muzoora C, Martin JN, Hunt PW, et al. GPS-measured distance to clinic, but not self-reported transportation factors, are associated with missed HIV clinic visits in rural Uganda. Aids. 2013;27(9):1503–8. doi: 10.1097/QAD.0b013e32835fd873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: a qualitative study. AIDS Behav. 2010;14(4):778–84. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chimbindi N, Bor J, Newell ML, Tanser F, Baltusen R, Hontelez J, et al. Time and money: the true costs of health care utilization for patients receiving ‘free’ HIV/TB care and treatment in rural KwaZulu-Natal. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen S, Ketlhapile M, Sanne I, DeSilva MB. Cost to patients of obtaining treatment for HIV/AIDS in South Africa. S Afr Med J. 2007;97(7):524–9. [PubMed] [Google Scholar]
- 28•.Ware NC, Wyatt MA, Geng EH, Kaaya SF, Agbaji OO, Muyindike WR, et al. Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: a qualitative study. PLoS Med. 2013;10(1):e1001369. doi: 10.1371/journal.pmed.1001369. Researchers indentified people to interview by using “tracking lists” from HIV/AIDS care clinics in the three countries and found the main unintentional reason for missing clinic visits was a conflicting demand on the patients’ time, which was often unexpected and for complex reasons, such as caring for a dying relative, going to a funeral, or traveling to work. These findings indicate that absences may be unintentional as well as intentional and that the reasons are complex and can change over time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naik R, Doherty T, Jackson D, Tabana H, Swanevelder S, Thea DM, et al. Linkage to care following a home-based HIV counselling and testing intervention in rural South Africa. J Int AIDS Soc. 2015;18:19843. doi: 10.7448/IAS.18.1.19843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negin J, Wariero J, Mutuo P, Jan S, Pronyk P. Feasibility, acceptability and cost of home-based HIV testing in rural Kenya. Trop Med Int Health. 2009;14(8):849–55. doi: 10.1111/j.1365-3156.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 31.Menzies N, Abang B, Wanyenze R, Nuwaha F, Mugisha B, Coutinho A, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. Aids. 2009;23(3):395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 32.Naik R, Tabana H, Doherty T, Zembe W, Jackson D. Client characteristics and acceptability of a home-based HIV counselling and testing intervention in rural South Africa. BMC Public Health. 2012;12:824. doi: 10.1186/1471-2458-12-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabapathy K, Van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of home-based voluntary HIV testing in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Med. 2012;9(12):e1001351. doi: 10.1371/journal.pmed.1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helleringer S, Kohler HP, Frimpong JA, Mkandawire J. Increasing uptake of HIV testing and counseling among the poorest in sub-Saharan countries through home-based service provision. J Acquir Immune Defic Syndr. 2009;51(2):185–93. doi: 10.1097/QAI.0b013e31819c1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mutale W, Michelo C, Jurgensen M, Fylkesnes K. Home-based voluntary HIV counselling and testing found highly acceptable and to reduce inequalities. BMC Public Health. 2010;10:347. doi: 10.1186/1471-2458-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekandi JN, Sempeera H, List J, Mugerwa MA, Asiimwe S, Yin X, et al. High acceptance of home-based HIV counseling and testing in an urban community setting in Uganda. BMC Public Health. 2011;11:730. doi: 10.1186/1471-2458-11-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalal W, Feikin DR, Amolloh M, Ransom R, Burke H, Lugalia F, et al. Home-based HIV testing and counseling in rural and urban Kenyan communities. J Acquir Immune Defic Syndr. 2013;62(2):e47–54. doi: 10.1097/QAI.0b013e318276bea0. [DOI] [PubMed] [Google Scholar]
- 38.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378(9802):1572–9. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 39.Sanne I, Orrell C, Fox MP, Conradie F, Ive P, Zeinecker J, et al. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet. 2010;376(9734):33–40. doi: 10.1016/S0140-6736(10)60894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fairall L, Bachmann MO, Lombard C, Timmerman V, Uebel K, Zwarenstein M, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. 2012;380(9845):889–98. doi: 10.1016/S0140-6736(12)60730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luque-Fernandez MA, Van Cutsem G, Goemaere E, Hilderbrand K, Schomaker M, Mantangana N, et al. Effectiveness of patient adherence groups as a model of care for stable patients on antiretroviral therapy in Khayelitsha, Cape Town, South Africa. PLoS One. 2013;8(2):e56088. doi: 10.1371/journal.pone.0056088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimsrud A, Lesosky M, Kalombo C, Bekker LG, Myer L. Community-based Adherence Clubs for the management of stable antiretroviral therapy patients in Cape Town, South Africa: a cohort study. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 43.Holmes CB, Sanne I. Changing models of care to improve progression through the HIV treatment cascade in different populations. Curr Opin HIV AIDS. 2015;10(6):447–50. doi: 10.1097/COH.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 44.Ramovha RKL, Lebese RT, Shilubane HN. The psychological experience of HIVand AIDS by newly diagnosed infected patients at hospital A of Vhembe District, Limpopo Province. J AIDS Clinic Res. 2012;S1:006. doi: 10.4172/2155-6113.S1-006. [DOI] [Google Scholar]
- 45.Abdool Karim SS. Stigma impedes AIDS prevention. Nature. 2011;474(7349):29–31. doi: 10.1038/474029a. [DOI] [PubMed] [Google Scholar]
- 46.Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(3 Suppl 2):18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilbert L, Walker L. ‘My biggest fear was that people would reject me once they knew my status…’: stigma as experienced by patients in an HIV/AIDS clinic in Johannesburg, South Africa. Health Soc Care Community. 2010;18(2):139–46. doi: 10.1111/j.1365-2524.2009.00881.x. [DOI] [PubMed] [Google Scholar]
- 48.Tsai AC, Bangsberg DR, Kegeles SM, Katz IT, Haberer JE, Muzoora C, et al. Internalized stigma, social distance, and disclosure of HIV seropositivity in rural Uganda. Ann Behav Med. 2013;46(3):285–94. doi: 10.1007/s12160-013-9514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takada S, Weiser SD, Kumbakumba E, Muzoora C, Martin JN, Hunt PW, et al. The dynamic relationship between social support and HIV-related stigma in rural Uganda. Ann Behav Med. 2014;48(1):26–37. doi: 10.1007/s12160-013-9576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katz IT, Essien T, Marinda ET, Gray GE, Bangsberg DR, Martinson NA, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. Aids. 2011;25(17):2177–81. doi: 10.1097/QAD.0b013e32834b6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Curran K, Ngure K, Shell-Duncan B, Vusha S, Mugo NR, Heffron R, et al. If I am given antiretrovirals I will think I am nearing the grave’: Kenyan HIV serodiscordant couples’ attitudes regarding early initiation of antiretroviral therapy. Aids. 2014;28(2):227–33. doi: 10.1097/QAD.0000000000000025. Researchers interviewed serodiscordant couples in Kenya to understand attitudes toward initiation of ART at higher CD4+ cell counts and highlights the fact that many participants felt ART was the final stage before death. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gamble TMM, Talley J, Dusara P, Maliwichi M, Kumarasamy N, Batani J, Matchere F, Calvet G, Brum T, Dhayarkar S, Chariyalertsak S, Lira RA, Mills LA, Govender S, Badal-Faesen S, Li X, Cohen M. Acceptance of ART in the delay arm after notification of interim study results: data from HPTN 052. 20th Conference on Retrovirus and Opportunistic Infections; Atlanta. 2013. [Google Scholar]
- 53.Abdool Karim Q, Dellar RC, Bearnot B, Werner L, Frohlich JA, Kharsany AB, et al. HIV-positive status disclosure in patients in care in rural South Africa: implications for scaling up treatment and prevention interventions. AIDS Behav. 2015;19(2):322–9. doi: 10.1007/s10461-014-0951-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ojikutu B, Nnaji C, Sithole-Berk J, Bogart LM, Gona P. Barriers to HIV testing in black immigrants to the U.S. J Health Care Poor Underserved. 2014;25(3):1052–66. doi: 10.1353/hpu.2014.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Houston E, McKirnan DJ, Cervone D, Johnson MS, Sandfort TG. Assessing treatment motivation among patients receiving antiretroviral therapy: a multidimensional approach. Psychol Health. 2012;27(6):674–87. doi: 10.1080/08870446.2011.618536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jurgensen M, Sandoy IF, Michelo C, Fylkesnes K, Group ZS. Effects of home-based voluntary counselling and testing on HIV-related stigma: findings from a cluster-randomized trial in Zambia. Soc Sci Med. 2013;81:18–25. doi: 10.1016/j.socscimed.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Coates TJ, Kulich M, Celentano DD, Zelaya CE, Chariyalertsak S, Chingono A, et al. Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. Lancet Glob Health. 2014;2(5):e267–77. doi: 10.1016/S2214-109X(14)70032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matthews LT, Moore L, Crankshaw TL, Milford C, Mosery FN, Greener R, et al. South Africans with recent pregnancy rarely know partner’s HIV serostatus: implications for serodiscordant couples interventions. BMC Public Health. 2014;14:843. doi: 10.1186/1471-2458-14-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katz IDJ, Bogart LM, Leone D, Courtney I, Tshabalala G, Fitzmaurice GM, Bangsberg DR, Orrell C, Gray G. Treatment refusal in South Africa in an era of expanded antiretroviral therapy availability—a cross-sectional multi-site study. International Association of Providers of AIDS Care; Miami, FL: 2015. [Google Scholar]
- 60.Okeke NL, Ostermann J, Thielman NM. Enhancing linkage and retention in HIV care: a review of interventions for highly resourced and resource-poor settings. Curr HIV/AIDS Rep. 2014;11(4):376–92. doi: 10.1007/s11904-014-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen L, Condit CM, Wright L. The psychometric property and validation of a fatalism scale. Psychol Health. 2009;24(5):597–613. doi: 10.1080/08870440801902535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Katz IT, Dietrich J, Tshabalala G, Essien T, Rough K, Wright AA, et al. Understanding treatment refusal among adults presenting for HIV-testing in Soweto, South Africa: a qualitative study. AIDS Behav. 2015;19(4):704–14. doi: 10.1007/s10461-014-0920-y. This is our first study defining treatment refusal in a South African context. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Christopoulos KA, Olender S, Lopez AM, Lekas HM, Jaiswal J, Mellman W, et al. Retained in HIV care but not on antiretroviral treatment: a qualitative patient-provider dyadic study. PLoS Med. 2015;12(8):e1001863. doi: 10.1371/journal.pmed.1001863. This manuscript is a qualitative study focused on understanding ART refusal. Specifically, ART as a signifier of AIDS and approaching death is viewed as negative, not a life saver. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siedner MJ, Lankowski A, Haberer JE, Kembabazi A, Emenyonu N, Tsai AC, et al. Rethinking the “pre” in pre-therapy counseling: no benefit of additional visits prior to therapy on adherence or viremia in Ugandans initiating ARVs. PLoS One. 2012;7(6):e39894. doi: 10.1371/journal.pone.0039894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vansteenkiste M, Sheldon KM. There’s nothing more practical than a good theory: integrating motivational interviewing and self-determination theory. Br J Clin Psychol. 2006;45(Pt 1):63–82. doi: 10.1348/014466505X34192. [DOI] [PubMed] [Google Scholar]
- 66.Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65(11):1232–45. doi: 10.1002/jclp.20638. [DOI] [PubMed] [Google Scholar]
- 67.Goggin K, Gerkovich MM, Williams KB, Banderas JW, Catley D, Berkley-Patton J, et al. A randomized controlled trial examining the efficacy of motivational counseling with observed therapy for anti-retroviral therapy adherence. AIDS Behav. 2013;17(6):1992–2001. doi: 10.1007/s10461-013-0467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moyers TB, Miller WR, Hendrickson SM. How does motivational interviewing work? Therapist interpersonal skill predicts client involvement within motivational interviewing sessions. J Consult Clin Psychol. 2005;73(4):590–8. doi: 10.1037/0022-006X.73.4.590. [DOI] [PubMed] [Google Scholar]
- 69.Catley D, Harris KJ, Goggin K, Richter K, Williams K, Patten C, et al. Motivational Interviewing for encouraging quit attempts among unmotivated smokers: study protocol of a randomized, controlled, efficacy trial. BMC Public Health. 2012;12:456. doi: 10.1186/1471-2458-12-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drain PK, Losina E, Parker G, Giddy J, Ross D, Katz JN, et al. Risk factors for late-stage HIV disease presentation at initial HIV diagnosis in Durban, South Africa. PLoS One. 2013;8(1):e55305. doi: 10.1371/journal.pone.0055305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abaynew Y, Deribew A, Deribe K. Factors associated with late presentation to HIV/AIDS care in South Wollo ZoneEthiopia: a case-control study. AIDS Res Ther. 2011;8:8. doi: 10.1186/1742-6405-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelly CA, Soler-Hampejsek E, Mensch BS, Hewett PC. Social desirability bias in sexual behavior reporting: evidence from an interview mode experiment in rural Malawi. Int Perspect Sex Reprod Health. 2013;39(1):14–21. doi: 10.1363/3901413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peltzer K, Ramlagan S, Khan MS, Gaede B. The social and clinical characteristics of patients on antiretroviral therapy who are ‘lost to follow-up’ in KwaZulu-Natal, South Africa: a prospective study. SAHARA J. 2011;8(4):179–86. doi: 10.1080/17290376.2011.9725002. [DOI] [PubMed] [Google Scholar]
- 74.McNairy ML, Lamb MR, Abrams EJ, Elul B, Sahabo R, Hawken MP, et al. Use of a comprehensive HIV care cascade for evaluating HIV program performance: findings from 4 sub-Saharan African countries. J Acquir Immune Defic Syndr. 2015;70(2):e44–51. doi: 10.1097/QAI.0000000000000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Plazy M, Orne-Gliemann J, Dabis F, Dray-Spira R. Retention in care prior to antiretroviral treatment eligibility in sub-Saharan Africa: a systematic review of the literature. BMJ Open. 2015;5(6):e006927. doi: 10.1136/bmjopen-2014-006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haberer JE, Musinguzi N, Boum Y, 2nd , Siedner MJ, Mocello AR, Hunt PW, et al. Duration of antiretroviral therapy adherence interruption is associated with risk of virologic rebound as determined by real-time adherence monitoring in rural Uganda. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mugo PM, Sanders EJ, Mutua G, van der Elst E, Anzala O, Barin B, et al. Understanding adherence to daily and intermittent regimens of oral HIV pre-exposure prophylaxis among men who have sex with men in Kenya. AIDS Behav. 2015;19(5):794–801. doi: 10.1007/s10461-014-0958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]