Abstract
Introduction
Subsyndromal delirium (SSD) complicates diagnosis of delirium and dementia, although there is little research comparing their symptom profiles.
Methods
Cross-sectional study of 400 elderly patients' admission to a general hospital or nursing home diagnosed with delirium, SSD, dementia, or no-delirium/no-dementia (NDND). Symptom profiles were assessed using the Delirium Rating Scale-Revised-98 (DRS-R98).
Results
Twenty percent patients had delirium, 19.3% had SSD, 29.8% had dementia-only, and 31% had NDND. Eighty-one percent of subsyndromal and 76% of delirium groups had comorbid dementia. DRS-R98 scores showed ascending severity from NDND < dementia-only < SSD < delirium. DRS-R98 scores for items evaluating the three core symptom domains (cognitive, higher-order thinking, and circadian) distinguished SSD from delirium and both from nondelirium groups. DRS-R98 profiles were essentially the same in delirium and SSD subgroups with or without dementia, although total scale scores were generally higher when in comorbid subgroups.
Discussion
SSD shared characteristic core domain symptoms with delirium, which distinguished each from nondelirium groups, although severity was intermediate in the subsyndromal group. Delirium core symptoms overshadowed the dementia phenotype when comorbid. Milder disturbances of delirium core domain symptoms are highly suggestive of SSD.
Keywords: Delirium, Subsyndromal, Dementia, Elderly, Diagnosis
1. Background
Subsyndromal delirium (SSD) is thought to represent a subthreshold state related to delirium and associated with poor posthospitalization outcomes similar to those associated with delirium [1], [2], [3], [4], [5], [6], [7], [8]. However, it lacks a standardized definition, even in Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) [9] where it is only noted as an “attenuated delirium syndrome” without specific criteria.
Early attempts at categorical delineation of SSD required the presence of fewer symptoms than for syndromal delirium, although the specific symptoms were arbitrarily selected, did not involve severity determinations, and required only one or two symptoms that had not been empirically chosen for specificity to the delirium syndrome [1], [3], [6], [7], [10], [11], [12], [13].
Another approach was dimensional that defined subsyndromal by lower severity score cutoffs than for delirium diagnosis, using the Delirium Rating Scale-Revised-98 (DRS-R98) [14] or the Intensive Care Delirium Screening Checklist [15]. Those ranges for SSD were estimates based on known distributions of scores in studies of delirium but not discerned from direct research [5], [8], [16], [17], [18], [19], [20], [21].
More recent efforts to characterize SSD used an empirical approach to delineate a subsyndromal group. Cluster analysis of DRS-R98 items (n = 859) confirmed an SSD group with intermediate severity between delirium and no-delirium that had a similar pattern of core domain symptoms (cognitive, circadian, and higher-order thinking) previously demonstrated to reflect the characteristic delirium phenotype as the delirium group [22]. To enable clinician diagnosis they proposed criteria where an acute change from baseline accompanied by mild disturbances of sleep-wake cycle, thought process, orientation, attention, and visuospatial ability together would define SSD and distinguish it from nondelirium and delirium.
More recently, Meagher et al. [23] compared two approaches to define SSD in 311 subjects, either by a DRS-R98 total scale score range of 7 to 11 points or by the presence of any two of four features on the Confusion Assessment Method [24]. The Confusion Assessment Method diagnosed more SSD cases than the DRS-R98 (13.2% vs. 7.7%) but with only modest case concordance (50% of DRS-R98 defined cases) between approaches. They proposed SSD clinical criteria as follows: (1) absence of full syndromal delirium, (2) acute or subacute onset, (3) disturbed attention, and (4) evidence of other cognitive and/or neuropsychiatric disturbances not better accounted for by another neuropsychiatric condition.
Better understanding of SSD can inform future revisions of DSM-5 and International Classification of Diseases-10 to increase its clinical recognition. Furthermore, Meagher and Trzepacz [25] have emphasized the need for research to improve phenomenological distinctions between delirium and SSD with dementias. Therefore, the aim of this study was to further examine phenomenology of SSD as defined by the proposed Meagher et al. [23] criteria and to understand the particular features that distinguished it from DSM-5 delirium, dementia with or without comorbid delirium, and subjects with neither delirium nor dementia. We analyzed data from two settings of elderly inpatients that were similarly obtained by our extended delirium research teams.
2. Methods
2.1. Subjects
Subjects derived from two prospective cross-sectional studies that used similar methods of assessment for delirium status and phenomenology obtained by our extended delirium research teams are as follows: (1) a sample of 200 consecutive elderly acute general medical admissions to a university teaching hospital in Sligo (Ireland) [26], and (2) a group of 200 consecutive patients admitted to a skilled nursing home (NH) in Reus (Spain) [27].
All patients aged 70 years and older newly admitted to the general hospital (GH) were asked to participate in the study within the first 72 hours after admission. All patients admitted to the NH were assessed during the first 24 to 48 hours. In both samples, patients were excluded if they (1) had been studied on a previous admission, or (2) had severe communicative problems or did not speak the respective native language (English or Spanish). Of 439 eligible patients, 39 were excluded (see Supplemental Fig. 1). In each setting, patients were recruited consecutively until 200 subjects were included, allowing for a final sample of 400 subjects.
In the GH sample, assessments were conducted by two raters trained in administering the scales with high inter-rater reliability established before the study. Each participant was interviewed by the same rater with the full battery of scales. Patients from the NH sample were evaluated independently by two experienced raters after running a pilot test with 10 patients (not included in the study sample) to evaluate logistic difficulties and possible problems in using research instruments. The first rater evaluated patients to rate DSM-5 criteria, whereas the second (specifically trained in DRS-R98 administration) administered the Spanish DRS-R98. A third researcher contacted the family or a caregiver to administer the Spanish–Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) to determine the dementia status.
Demographic (including gender and age) and clinical data were collected from files and medical records.
2.2. Diagnosis
2.2.1. Delirium
Patients who met DSM-5 criteria for delirium by the clinical research staff were classified as having delirium. We evaluated each patient for delirium using DSM-5 criteria according to all available information from clinical assessment of the patient, discussion with nursing staff, and available collateral sources. In the GH sample, to assess “awareness” for the A criterion, researchers used the relevant item from the Reversible Cognitive Dysfunction Scale [28], [29]. The item has a four-point range, but we assigned a dichotomous (normal/abnormal) score by collapsing the three categories of abnormality into one. In the NH sample, researchers designed a diagnostic criteria checklist to dichotomously rate each item as present or not according to the subjective impression of the assessing clinician, where awareness was based on the patient engaging appropriately with the interviewer and their environment.
2.2.2. Subsyndromal delirium
We applied the Meagher et al. [23] criteria to the nondelirium cases to delineate the SSD group. Criteria were (1) absence of full syndromal delirium, (2) acute or subacute onset, (3) disturbed attention, and (4) evidence of other cognitive and/or neuropsychiatric disturbances not better accounted for by another neuropsychiatric condition. To demonstrate the presence of symptoms for criteria (2) through (4) in a standardized fashion we required a nonzero score on the respective relevant DRS-R98 item. Otherwise, DRS-R98 item or scale score ranges were not used as determinants of SSD.
2.2.3. Dementia
All patients were assessed for dementia. Those having dementia could be comorbid with delirium, SSD, or dementia-only.
In the Irish sample, dementia was defined using DSM-5 criteria (major neurocognitive disorder) according to all available sources (medical files, GP files, collateral history, and evidence from neuroimaging reports).
In the Spanish sample, the Spanish-IQCODE was used to diagnose dementia. This is a structured interview comprising 26 questions posed to an informant about the patient's cognition and function during the preceding 5 years. Scores range from 26 to 130. The validated Spanish version uses a cutoff >85 for possible dementia [30].
2.2.4. No-delirium/no-dementia
Patients who were not classified by the previously described definitions for delirium, SSD, or dementia were classified as the no-delirium/no-dementia (NDND) group.
2.3. Delirium Rating Scale-Revised-98
The DRS-R98 [14] is a widely used, well-validated tool used to evaluate delirium phenomenological severity and profile. This scale includes descriptive anchors for 13 severity (rated from 0 to 3) and three diagnostic items (rated 0 to 2 or 3) where 0 means normal. The DRS-R98 severity scale maximum score is 39 points and DRS-R98 total scale score is 46, with higher scores indicating more severe delirium. Items can be subgrouped to represent symptoms of the three core domains of delirium as follows: cognitive (items 9–13), circadian (items 1, 7, and 8), and higher-order thinking (items 5 and 6). The scale has high validity and inter-rater reliability values in all its versions, including the original English [14] and Spanish translation [31], [32].
2.4. Ethical approval
The study was approved by the institutional review board and the research ethics committees at each center. The procedures and rationale for the study were explained to all patients and relatives but because many patients had cognitive impairment at study entry, it was presumed that some might not be capable of giving informed written consent. Because of the noninvasive nature of the study, ethics committee approval was given to augment patient assent with proxy consent from next of kin (where possible) or a responsible caregiver for all participants, in accordance with the Helsinki Guidelines for Medical Research involving human subjects [33].
2.5. Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS, v 21, IBM, Armonk, NY). Continuous variables were expressed as the means ± standard deviation and discrete variables as frequencies and percentages (%). Chi-square test was used to compare gender, presence of dementia, and frequencies of the four study groups between GH and NH facilities, and t test was used for the comparison of age.
To evaluate internal consistency of DRS-R98 in this combined sample, Cronbach's α was performed for its total and severity scale items in the whole sample and in both GH and NH samples.
For comparison of the DRS-R98 diverse scores among groups, we used Mann-Whitney U test (pairwise comparisons) and Kruskal-Wallis analysis of variance with post hoc pairwise comparisons with Mann-Whitney U test. We used chi-square to compare the frequency of occurrence of scores ≥1 or ≥2 for individual DRS-R98 items.
The P value was set at <.05, except when multiple significance tests were carried out when Bonferroni correction was applied as specified in the corresponding tables.
3. Results
3.1. Sample description
The demographic and general clinical characteristics in the pooled sample and samples by setting are shown in Table 1. The mean age for the whole sample was 79.7 ± 8.5 years, 50.8% were women and 60.8% had pre-existing dementia. The groups differed only for mean age (81.1 ± 6.5 for GH sample and 78.3 ± 9.9 for NH sample, P = .001) but not for gender or occurrence of dementia.
Table 1.
Clinical and demographic characteristics of the sample of 400 patients from a skilled NH and a university GH
| Variable | Whole sample (n = 400) | NH (n = 200) | GH (n = 200) |
|---|---|---|---|
| Age∗ | 79.7 ± 8.5 | 78.3 ± 9.9 | 81.1 ± 6.5 |
| Gender: male | 197 (49.3) | 97 (48.5) | 100 (50.0) |
| Previous dementia | 243 (60.8) | 117 (58.5) | 126 (63.0) |
| DRS-R98 severity scale Cronbach's α | 0.861 | 0.891 | 0.832 |
| DRS-R98 total scale Cronbach's α | 0.892 | 0.917 | 0.861 |
Abbreviations: DRS-R98, Delirium Rating Scale-Revised-98; GH, general hospital; NH, nursing home.
NOTE. Values are expressed as the mean ± standard deviation for age, number (%) for gender and previous cognitive impairment, and Cronbach's α coefficient for internal consistency of DRS-R98 scores between the cohorts.
NH < GH (P = .001).
The internal consistency of DRS-R98 scale scores was excellent in the pooled data set and individual GH and NH settings.
Classification of the pooled sample into diagnostic groups found 80 patients (20.0%) with delirium, 77 (19.3%) with SSD, 119 (29.8%) with dementia-only, and 124 (31%) with NDND. Delirium frequency was significantly higher in the NH group (27% vs. 13%) (P < .001), whereas the GH group had a higher frequency of dementia-only cases (35% vs. 24.5%, P = .02). There were no differences in frequency of SSD or NDND between the two settings.
DRS-R98 severity and total scale scores significantly distinguished all four groups, with a pattern of ascending burden of delirium severity by the diagnostic group: NDND < dementia-only < SSD < delirium (Table 2).
Table 2.
DRS-R98 scores for 400 elderly subjects by diagnostic group: delirium, SSD, dementia-only, and NDND
| DRS-R98 | NDND (n = 124) | Dementia (n = 119) | SSD (n = 77) | Delirium (n = 80) |
|---|---|---|---|---|
| 1. Sleep-wake cycle disturbance∗†‡§‖ | 0.53 ± 0.62 | 0.67 ± 0.75 | 1.17 ± 0.77 | 1.50 ± 0.76 |
| 2. Perceptions and hallucinations∗†§ | 0.07 ± 0.41 | 0.24 ± 0.76 | 0.64 ± 1.11 | 0.41 ± 0.87 |
| 3. Delusions∗† | 0.09 ± 0.44 | 0.24 ± 0.76 | 0.42 ± 0.85 | 0.28 ± 0.71 |
| 4. Lability of affect∗†‡§¶ | 0.04 ± 0.20 | 0.18 ± 0.43 | 0.40 ± 0.61 | 0.53 ± 0.66 |
| 5. Language∗†‡§‖¶ | 0.06 ± 0.33 | 0.18 ± 0.49 | 0.68 ± 0.82 | 1.20 ± 1.07 |
| 6. Thought process abnormalities∗†‡§‖ | 0.10 ± 0.30 | 0.19 ± 0.47 | 0.71 ± 0.76 | 1.28 ± 1.04 |
| 7. Motor agitation∗†‡§ | 0.05 ± 0.22 | 0.11 ± 0.34 | 0.61 ± 0.73 | 0.81 ± 0.90 |
| 8. Motor retardation∗†‡§‖ | 0.12 ± 0.35 | 0.13 ± 0.34 | 0.73 ± 0.81 | 1.16 ± 0.96 |
| 9. Orientation∗†‡§‖¶ | 0.17 ± 0.45 | 0.69 ± 0.84 | 1.27 ± 0.81 | 2.04 ± 0.82 |
| 10. Attention∗†‡§‖ | 0.15 ± 0.38 | 0.26 ± 0.50 | 1.42 ± 0.57 | 1.89 ± 0.78 |
| 11. Short-term memory∗†¶ | 0.43 ± 0.78 | 1.06 ± 1.14 | 1.30 ± 1.19 | 1.50 ± 1.19 |
| 12. Long-term memory∗†‡§¶ | 1.01 ± 1.02 | 1.60 ± 1.09 | 2.21 ± 0.99 | 2.49 ± 0.86 |
| 13. Visuospatial ability∗†‡§‖¶ | 0.31 ± 1.08 | 0.71 ± 0.86 | 1.42 ± 0.85 | 1.96 ± 0.91 |
| 14. Temporal onset of symptoms∗†‡§¶ | 0.06 ± 0.26 | 0.22 ± 0.52 | 1.49 ± 0.66 | 1.65 ± 0.81 |
| 15. Fluctuation of symptom severity∗†‡§‖ | 0.00 ± 0.00 | 0.03 ± 0.18 | 0.44 ± 0.57 | 0.75 ± 0.63 |
| 16. Physical disorder∗†‡§ | 0.27 ± 0.62 | 0.43 ± 0.78 | 1.14 ± 0.88 | 1.45 ± 0.74 |
| DRS-R98 severity∗†‡§‖¶ | 3.11 ± 2.64 | 6.26 ± 3.68 | 12.96 ± 5.41 | 17.10 ± 6.35 |
| DRS-R98 total∗†‡§‖¶ | 3.44 ± 2.90 | 6.94 ± 4.13 | 16.01 ± 6.18 | 20.89 ± 7.25 |
Abbreviations: DRS-R98, Delirium Rating Scale-Revised-98; NDND, no-delirium/no-dementia; SSD, subsyndromal delirium.
NOTE. Scores are expressed as the means ± standard deviation and compared between groups with Kruskal-Wallis analysis of variance (post hoc pairwise comparisons with Mann-Whitney U test). Footnotes describe details of score comparisons. All P values for multiple pairwise comparisons were set at <.0083 according to the Bonferroni correction.
NDND < SSD.
NDND < delirium.
Dementia < delirium.
Dementia < SSD.
SSD < delirium.
NDND < dementia.
3.2. Comparison of DRS-R98 mean item scores by group
Table 2 shows DRS-R98 mean item scores according to diagnostic groups in the pooled sample and after correction for multiple comparisons. These results revealed delirium groups to be largely distinguishable from the two nondelirium groups especially for core domain symptoms, and that the SSD group had intermediate severity of core domain symptoms between the delirium and two nondelirium groups. Noncore items did not have a useful pattern for group discrimination.
Specifically, all core domain items' mean scores distinguished SSD and delirium groups with the exception of motor agitation and memory items. Noncore symptoms (items 2, 3, and 4) did neither discriminate between them nor did temporal onset or physical attribution items.
All core domain symptoms except short-term memory were significantly worse in both delirium groups as compared with the dementia-only group. Specifically, these were orientation, attention, long-term memory; visuospatial ability (cognition), language, and thought process abnormalities (higher-order thinking); and sleep-wake cycle, motor agitation, and motor retardation (circadian) items.
Regarding noncore symptoms, the dementia-only group had less severe lability of affect than both delirium groups and less severe perceptual disturbances than the SSD group.
As expected, greater mean severity for every symptom distinguished both delirium groups from NDND. The latter group also had lower scores than the dementia-only group on language and all cognitive items except attention.
3.3. Comparison of delirium phenomenology by clinical setting
DRS-R98 mean item scores were largely comparable between clinical settings for each group (Table 3). Most notably, short-term memory was more impaired in the GH sample for all groups.
Table 3.
DRS-R98 scores for the 400 subjects according to the clinical setting for each of the four diagnostic groups: NDND, dementia-only, SSD, and delirium
| DRS-R98 | NDND (n = 124) |
Dementia-only (n = 119) |
SSD (n = 77) |
Delirium (n = 80) |
||||
|---|---|---|---|---|---|---|---|---|
| NH (n = 63) | GH (n = 61) | NH (n = 49) | GH (n = 70) | NH (n = 34) | GH (n = 43) | NH (n = 54) | GH (n = 26) | |
| 1. Sleep-wake cycle disturbance | 0.43 ± 0.53 | 0.64 ± 0.68 | 0.37 ± 0.49 | 0.89 ± 0.83∗ | 0.97 ± 0.67 | 1.33 ± 0.81 | 1.61 ± 0.71 | 1.27 ± 0.83 |
| 2. Perceptions and hallucinations | 0.13 ± 0.55 | 0.02 ± 0.13 | 0.33 ± 0.90 | 0.17 ± 0.64 | 0.71 ± 1.12 | 0.58 ± 1.12 | 0.50 ± 0.93 | 0.23 ± 0.71 |
| 3. Delusions | 0.17 ± 0.61 | 0∗ | 0.51 ± 1.04 | 0.06 ± 0.38∗ | 0.71 ± 1.03 | 0.19 ± 0.59∗ | 0.35 ± 0.83 | 0.12 ± 0.33 |
| 4. Lability of affect | 0.06 ± 0.25 | 0.02 ± 0.13 | 0.27 ± 0.53 | 0.13 ± 0.34 | 0.59 ± 0.66 | 0.26 ± 0.54∗ | 0.56 ± 0.69 | 0.46 ± 0.58 |
| 5. Language | 0.10 ± 0.43 | 0.03 ± 0.18 | 0.33 ± 0.66 | 0.09 ± 0.28∗ | 0.79 ± 0.91 | 0.58 ± 0.73 | 1.41 ± 1.02 | 0.77 ± 1.07∗ |
| 6. Thought process abnormalities | 0.19 ± 0.40 | 0∗ | 0.39 ± 0.64 | 0.06 ± 0.23∗ | 1.06 ± 0.73 | 0.44 ± 0.67∗ | 1.43 ± 1.02 | 0.96 ± 1.04 |
| 7. Motor agitation | 0.03 ± 0.18 | 0.07 ± 0.25 | 0.08 ± 0.34 | 0.13 ± 0.34 | 0.68 ± 0.81 | 0.56 ± 0.67 | 0.89 ± 0.90 | 0.65 ± 0.89 |
| 8. Motor retardation | 0.11 ± 0.32 | 0.13 ± 0.39 | 0.16 ± 0.37 | 0.11 ± 0.32 | 0.76 ± 0.89 | 0.70 ± 0.74 | 1.39 ± 0.98 | 0.69 ± 0.74∗ |
| 9. Orientation | 0.25 ± 0.57 | 0.08 ± 0.28 | 1.10 ± 0.85 | 0.40 ± 0.71∗ | 1.50 ± 0.83 | 1.09 ± 0.75∗ | 2.07 ± 0.84 | 1.96 ± 0.77 |
| 10. Attention | 0.19 ± 0.43 | 0.10 ± 0.30 | 0.31 ± 0.55 | 0.23 ± 0.46 | 1.29 ± 0.52 | 1.51 ± 0.59 | 1.91 ± 0.85 | 1.85 ± 0.61 |
| 11. Short-term memory | 0.06 ± 0.30 | 0.80 ± 0.93∗ | 0.37 ± 0.83 | 1.54 ± 1.07∗ | 0.62 ± 1.04 | 1.84 ± 1.02∗ | 1.04 ± 1.10 | 2.46 ± 0.71∗ |
| 12. Long-term memory | 0.89 ± 1.03 | 1.13 ± 0.99 | 1.78 ± 0.98 | 1.47 ± 1.15 | 2.24 ± 1.05 | 2.19 ± 0.96 | 2.33 ± 0.97 | 2.81 ± 0.40∗ |
| 13. Visuospatial ability | 0.11 ± 0.32 | 0.51 ± 1.48∗ | 0.57 ± 0.91 | 0.81 ± 0.82∗ | 1.24 ± 0.99 | 1.56 ± 0.70 | 2.06 ± 1.00 | 1.77 ± 0.65 |
| 14. Temporal onset of symptoms | 0.03 ± 0.18 | 0.08 ± 0.33 | 0.18 ± 0.39 | 0.24 ± 0.60 | 1.41 ± 0.56 | 1.56 ± 0.73 | 1.76 ± 0.80 | 1.42 ± 0.81 |
| 15. Fluctuation of symptoms | 0 | 0 | 0 | 0.06 ± 0.23 | 0.62 ± 0.60 | 0.30 ± 0.51∗ | 0.94 ± 0.56 | 0.35 ± 0.56∗ |
| 16. Physical disorder | 0.13 ± 0.38 | 0.43 ± 0.76∗ | 0.22 ± 0.47 | 0.57 ± 0.91 | 1.38 ± 0.70 | 0.95 ± 0.97 | 1.61 ± 0.56 | 1.12 ± 0.95∗ |
| DRS-R98 severity scale | 2.73 ± 2.52 | 3.52 ± 2.72 | 6.55 ± 3.72 | 6.09 ± 3.68 | 13.21 ± 6.15 | 12.81 ± 4.90 | 17.63 ± 7.05 | 16.00 ± 4.48 |
| DRS-R98 total scale | 2.89 ± 2.71 | 4.03 ± 3.00∗ | 6.96 ± 3.82 | 6.96 ± 4.36 | 16.56 ± 6.62 | 15.63 ± 5.92 | 21.85 ± 7.82 | 18.88 ± 5.51 |
Abbreviations: DRS-R98, Delirium Rating Scale-Revised-98; GH, general hospital; NDND, no-delirium/no-dementia; NH, nursing home; SSD, subsyndromal delirium.
NOTE. Values are expressed as the means ± standard deviation; P values between sites within each group. Pairwise comparisons are made by the Mann-Whitney U test with P values at <.05.
Values with significant difference between the two sites.
3.4. Impact of dementia on DRS-R98 profiles for SSD and delirium
Table 4 compares DRS-R98 mean scores for SSD and delirium subgroups with and without comorbid dementia, where 63 of 77 subjects with SSD (82%) and 61 of 80 with delirium (76%) had comorbid dementia. Overall delirium severities on DRS-R98 total and severity scales were higher when dementia was comorbid with delirium or SSD except for the total scale on the delirium group comparison.
Table 4.
Comparison of DRS-R98 scores for elderly patients with SSD and delirium between subgroups with or without comorbid dementia
| DRS-R98 | SSD without dementia (n = 14) | SSD with dementia (n = 63) | Delirium without dementia (n = 19) | Delirium with dementia (n = 61) |
|---|---|---|---|---|
| 1. Sleep-wake cycle disturbance | 0.93 ± 0.92 | 1.22 ± 0.73 | 1.47 ± 0.77 | 1.51 ± 0.77 |
| 2. Perceptions and hallucinations | 0.43 ± 0.94 | 0.68 ± 1.15 | 0.16 ± 0.37 | 0.49 ± 0.96 |
| 3. Delusions | 0.00 ± 0.00 | 0.51 ± 0.91∗ | 0.11 ± 0.46 | 0.33 ± 0.77 |
| 4. Lability of affect | 0.21 ± 0.43 | 0.44 ± 0.64 | 0.42 ± 0.61 | 0.56 ± 0.67 |
| 5. Language | 0.50 ± 0.65 | 0.71 ± 0.85 | 1.16 ± 0.90 | 1.21 ± 1.13 |
| 6. Thought process abnormalities | 0.43 ± 0.65 | 0.78 ± 0.77 | 1.11 ± 0.88 | 1.33 ± 1.09 |
| 7. Motor agitation | 0.36 ± 0.50 | 0.67 ± 0.76 | 0.58 ± 0.96 | 0.89 ± 0.88 |
| 8. Motor retardation | 0.57 ± 0.85 | 0.76 ± 0.80 | 1.16 ± 0.96 | 1.16 ± 0.97 |
| 9. Orientation | 1.00 ± 0.68 | 1.33 ± 0.82 | 1.74 ± 0.93 | 2.13 ± 0.76 |
| 10. Attention | 1.43 ± 0.65 | 1.41 ± 0.56 | 1.63 ± 0.90 | 1.97 ± 0.73 |
| 11. Short-term memory | 0.93 ± 1.07 | 1.38 ± 1.21 | 0.79 ± 1.03 | 1.72 ± 1.16∗ |
| 12. Long-term memory | 1.86 ± 1.23 | 2.29 ± 0.92 | 1.95 ± 0.97 | 2.66 ± 0.75∗ |
| 13. Visuospatial ability | 1.43 ± 0.85 | 1.41 ± 0.85 | 1.68 ± 0.75 | 2.05 ± 0.94 |
| 14. Temporal onset of symptoms | 1.43 ± 0.65 | 1.51 ± 0.67 | 2.00 ± 0.82 | 1.54 ± 0.79∗ |
| 15. Fluctuation of symptom severity | 0.14 ± 0.36 | 0.51 ± 0.59∗ | 0.79 ± 0.71 | 0.74 ± 0.60 |
| 16. Physical disorder | 1.00 ± 0.96 | 1.17 ± 0.87 | 1.63 ± 0.68 | 1.39 ± 0.76 |
| DRS-R98 severity scale | 10.07 ± 3.91 | 13.63 ± 5.56∗ | 14.05 ± 5.94 | 18.05 ± 6.22∗ |
| DRS-R98 total scale | 12.64 ± 4.20 | 16.79 ± 6.37∗ | 18.37 ± 7.11 | 21.67 ± 7.17 |
Abbreviations: DRS-R98, Delirium Rating Scale-Revised-98; SSD, subsyndromal delirium.
NOTE. Values are expressed as the means ± standard deviation. Pairwise comparisons between subgroups according to dementia status made using Mann-Whitney U test with P values at <.05.
Values with significant difference.
However, DRS-R98 item profiles for core domain symptoms were largely the same in both SSD and delirium groups irrespective of subgroup dementia status, with only a few differences. Memory was more impaired in the comorbid delirium subgroup, but temporal onset was less acute. Also delusions and fluctuation of symptoms were worse in the SSD comorbid subgroup.
3.5. Comparison of dementia patients according to delirium status
Somewhat conversely, Table 5 compares the phenomenological profile of subjects with dementia (n = 243) according to their delirium syndromal status (dementia-only, dementia with SSD, and dementia with delirium). Nearly all items were significantly lower for dementia-only than for comorbid dementia with either SSD or delirium. There was a significant gradient of increasing scores across groups from dementia-only to dementia with SSD to dementia with delirium on DRS-R98 severity and total scales.
Table 5.
DRS-R98 scores for the 243 patients with dementia according to diagnostic groups: full syndromal delirium (delirium), SSD, and dementia-only
| DRS-R98 | Dementia without delirium (n = 119) | Dementia with SSD (n = 63) | Dementia with delirium (n = 61) |
|---|---|---|---|
| 1. Sleep-wake cycle disturbance∗† | 0.67 ± 0.75 | 1.22 ± 0.73 | 1.51 ± 0.77 |
| 2. Perceptions and hallucinations∗† | 0.24 ± 0.76 | 0.68 ± 1.15 | 0.49 ± 0.96 |
| 3. Delusions∗ | 0.24 ± 0.76 | 0.51 ± 0.91 | 0.33 ± 0.77 |
| 4. Lability of affect∗† | 0.18 ± 0.43 | 0.44 ± 0.64 | 0.56 ± 0.67 |
| 5. Language∗†‡ | 0.18 ± 0.49 | 0.71 ± 0.85 | 1.21 ± 1.13 |
| 6. Thought process abnormalities∗†‡ | 0.19 ± 0.47 | 0.78 ± 0.77 | 1.33 ± 1.09 |
| 7. Motor agitation∗† | 0.11 ± 0.34 | 0.67 ± 0.76 | 0.89 ± 0.88 |
| 8. Motor retardation∗† | 0.13 ± 0.34 | 0.76 ± 0.80 | 1.16 ± 0.97 |
| 9. Orientation∗†‡ | 0.69 ± 0.84 | 1.33 ± 0.82 | 2.13 ± 0.76 |
| 10. Attention∗†‡ | 0.26 ± 0.49 | 1.41 ± 0.56 | 1.97 ± 0.73 |
| 11. Short-term memory† | 1.06 ± 1.14 | 1.38 ± 1.21 | 1.72 ± 1.16 |
| 12. Long-term memory∗†‡ | 1.60 ± 1.09 | 2.29 ± 0.92 | 2.66 ± 0.75 |
| 13. Visuospatial ability∗†‡ | 0.71 ± 0.86 | 1.41 ± 0.85 | 2.05 ± 0.94 |
| 14. Temporal onset of symptoms∗† | 0.22 ± 0.52 | 1.51 ± 0.67 | 1.54 ± 0.79 |
| 15. Fluctuation of symptom severity∗† | 0.03 ± 0.18 | 0.51 ± 0.59 | 0.74 ± 0.60 |
| 16. Physical disorder∗† | 0.43 ± 0.78 | 1.17 ± 0.87 | 1.39 ± 0.76 |
| Severity scale∗†‡ | 6.28 ± 3.69 | 13.63 ± 5.56 | 18.05 ± 6.22 |
| Total scale∗†‡ | 6.96 ± 4.13 | 16.79 ± 6.37 | 21.67 ± 7.17 |
Abbreviations: DRS-R98, Delirium Rating Scale-Revised-98; SSD, subsyndromal delirium.
NOTE. Values expressed as the means ± standard deviation. Comparisons made by Kruskal-Wallis analysis of variance, with Mann-Whitney U post hoc pairwise analysis. Significance at P < .0166 according to the Bonferroni correction.
Dementia-only < SSD.
Dementia-only < delirium.
SSD < delirium.
Most DRS-R98 items had a similar ascending pattern when comparing dementia-only to the delirium/dementia comorbid groups, although not significantly for all items. SSD with dementia was significantly worse than dementia-only on all items except for short-term memory. Delirium with dementia was significantly higher than dementia-only on all items except for delusions.
Delirium with dementia was significantly higher than SSD with dementia on language, thought process, orientation, attention, long-term memory, and visuospatial ability but not on any circadian symptoms.
3.6. Comparison of DRS-R98 item score frequencies by group
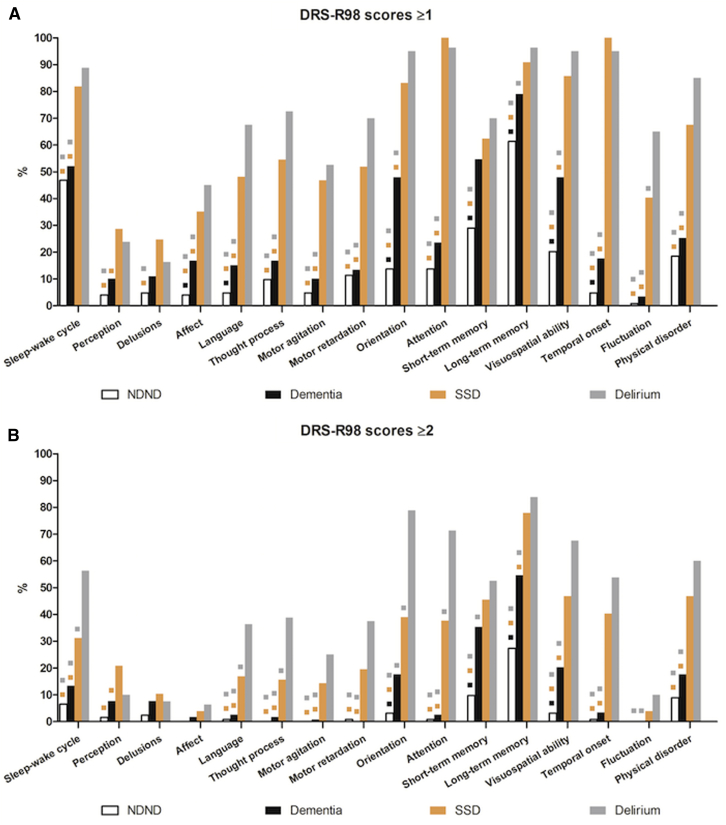
Categorical comparisons for item frequencies for the presence of symptoms at any severity level (DRS-R98 scores ≥1) are shown in bar graphs (Fig. 1A). The two delirium groups were distinguished from the two nondelirium groups by nearly every item except that psychotic items (2 and 3) and memory items (11 and 12) generally did not. All items differentiated SSD from NDND.
Fig.1.
Frequency of symptoms present at any severity (i.e., DRS-R98 scores ≥1 (A)) and at least moderate severity (DRS-R98 scores ≥2 (B)) for 400 elderly subjects by diagnostic group: delirium, subsyndromal delirium (SSD), dementia-only, and no-delirium/no-dementia (NDND). Pairwise comparisons between groups with chi-square, P values at <.0083 according to the Bonferroni correction. Color-coded squares above bars indicate other groups for which the labeled group was statistically different on that DRS-R98 item. Abbreviation: DRS-R98, Delirium Rating Scale-Revised-98.
Using this cutoff score that included mild, moderate, and severe levels of symptoms lumped together was not useful to distinguish SSD from delirium, where only fluctuation of symptoms distinguished them. However, when using a cutoff of ≥2 (Fig. 1B), delirium was more distinguishable from SSD on many items (sleep-wake cycle, language, thought process, orientation, and attention) representing each of the three core domains.
Noncore domain items were not useful to distinguish among the four groups. However, the presence of an attributable physical disorder and acute onset of symptoms was differentiating and reflective of delirium status.
Using the higher threshold, a significantly greater presence of all items representing core domains, except short-term memory, distinguished dementia-only from both delirium groups. Only orientation and short-term memory did not distinguish dementia-only from SSD. Moreover, frequencies for all core domain items were significantly higher in delirium and SSD compared with NDND.
4. Discussion
Given the challenges of detecting SSD when comorbid dementia may be present, we described delirium phenomenological profiles of elderly patients from GH and NH settings. To define the SSD group, we applied the recently proposed Meagher et al. [23] clinical criteria. We found that SSD differed from both dementia-only and NDND groups on severity and frequency of most DRS-R98 items and from delirium for lesser severity of symptoms representing all three core domains. This supports it being a condition distinguishable from both dementia and full syndromal delirium, despite overlapping symptoms. Our finding that SSD is subthreshold to, but characterized by, the same unique core domain symptoms as delirium is consistent with that previously reported by Trzepacz et al. [22] using a cluster analysis non–a priori approach to define symptoms of SSD . However, this is the first report of it being distinguishable from dementia, as noted in two different clinical settings where delirium is common in older persons.
In addition, despite some differences in criteria, we also found support for the more explicit SSD criteria proposed by Trzepacz et al. [22]. The Meagher et al. criteria do not specify the number or type of other neuropsychiatric symptoms besides inattention and do not restrict severities to being mild. In contrast, four symptoms (i.e., sleep-wake cycle, thought process, attention, and visuospatial ability) reflecting three core domains are required to be present at mild severity by the Trzepacz et al. criteria. When we reviewed our results, these four symptoms were present in the majority of our SSD group diagnosed using the Meagher et al. criteria. We found that many core domain items distinguished SSD from delirium when present in at least mild intensity. Specifically, frequencies of scores ≥1 for items representing Trzepacz et al. criteria in the SSD group ranged from 81.8% for sleep-wake cycle disturbance to 100% for attention, with the exception that mild thought process abnormalities were present in only 54.5%. A higher proportion of delirium than SSD cases were rated as at least moderately affected (≥2 points) on symptoms from all three core domains. Therefore, our data support that both criteria could signal to a clinician that an acute change in mental status with mild impairment of core domain symptoms suggests SSD.
The phenomenological profile of DSM-5 delirium and SSD was essentially the same between the two clinical populations, with only minor differences, probably more reflective of underlying characteristics of the different populations than delirium per se. Most notably short-term memory impairment was worse in the GH for both delirium groups.
This study reports the first comparison of SSD occurrence between two settings using DSM-5 criteria, where previous work suggested that the inclusiveness of diagnostic systems can vary according to the setting where they are applied [34], [35]. We found similar incidence of delirium spectrum disorders with some differences in proportion of subsyndromal versus delirium. Delirium occurrence in postacute care services ranges from 6% to 33.3% [4], [36], [37], [38], with higher rates identified in populations with more severe dementia and physical comorbidity [38]. The particular NH in our study has complex patients with a high prevalence of severe cognitive disorders and medical-surgical problems, which may account for the high frequency of delirium (27%) versus our GH (13%). The occurrence of SSD was similar (17% vs. 21.5%), highlighting consistency across clinical settings, at sufficient frequency to deserve better detection.
The severity of delirium symptoms in the SSD group was intermediate between delirium and the nondelirium groups (NDND and dementia-only), which differed mainly in terms of the severity of the three core domain symptoms [39], [40] as previously reported in studies that have defined SSD in different ways in this work. Trzepacz et al. [22] applied binary logistic regression to find that DRS-R98 item scores for five core domain symptoms (disturbances on sleep-wake cycle, thought process, orientation, attention, and visuospatial ability) when taken together at mild severity (item score of 1 point) correctly classified 80% of SSD versus no delirium.
Our study affirmed previous studies that delirium overshadows symptoms of dementia when they are comorbid [41], [42], [43], [44], [45]. Furthermore, we found that the three core domains are specifically affected in delirium subjects, with or without comorbid dementia, in a way that makes them different from nondelirious patients, including those with only dementia [45]. Although our sample of nondemented SSD patients was relatively small, our findings confirm that delirium symptoms overshadow dementia symptoms when they are comorbid. However, noncore symptoms should not be relied on to differentiate SSD from dementia, and among core domain symptoms, memory is less reliable than other core symptoms.
We also found that the five items proposed by Trzepacz et al. [22] criteria distinguished SSD comorbid with dementia from dementia alone and moreover, four of these (thought process, attention, orientation, and visuospatial ability) also differentiated SSD from delirium, which suggests focusing on those four symptoms may be useful when detecting SSD, even among those with dementia. The gradient of increasing scores across dementia-only to the delirium/dementia comorbid groups highlights the cumulative neuropsychiatric burden attributable to delirium.
Our study had a number of limitations. First, evaluations were conducted in two different countries by different researchers and using different language versions of the DRS-R98. However, the DRS-R98 has high inter-rater reliability established in all its validation studies, researchers at both settings were highly expert in delirium assessment, and experienced and specifically trained in the use of the DRS-R98. Furthermore, internal consistency of DRS-R98 items was excellent and similar across these two samples. Also, the DSM-5 criteria were only recently published and discrepancies may exist in the way some criteria are interpreted by different raters [26], [46], [47]. The approach to establish unawareness and the presence of previous cognitive impairment or dementia differed at the two sites (i.e., clinical criteria vs. Spanish-IQCODE for dementia), which could explain some of the differences in the populations' characteristics.
Our cross-sectional design could not differentiate whether SSD represents a stable condition or an evolving or resolving delirium, which warrants future longitudinal studies. Finally, we did not consider the possible impact of other neuropsychiatric conditions (e.g., depression) that are common in elderly patients. However, uniquely, we addressed the impact of dementia on SSD phenomenology.
In summary, when using the Meagher et al. [23] proposed SSD criteria, we largely supported prior literature that SSD's symptoms are subthreshold to that of delirium but distinguishable from dementia-only or nondemented-nondelirious groups, especially when focusing on the three core domain symptoms. Our work contributes more symptom specificity to the understanding of SSD than previous work using total scale score ranges and lends hope that it can be detected even in elderly who carry a high risk for comorbid dementia.
In conclusion, we found that both delirium and SSD are common in elderly patients receiving care in acute GH and NH settings where dementia was commonly comorbid, and both populations had discernible SSD that is worthy of being detected and distinguished from dementia.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations. Subsyndromal delirium (SSD) has been defined in different ways but there are no standardized criteria for it, notwithstanding its clinical relevance and the known difficulties to distinguish it from delirium and dementia.
-
2.
Interpretation: SSD is very prevalent in two elderly populations and has symptoms with intermediate severity between full syndromal delirium and no delirium, including in patients with dementia, centered on the core symptom domains. Also, delirium overshadows dementia symptoms.
-
3.
Future directions: Our work provides a deeper understanding of the phenomenology of SSD and serves a basis for future research on specific criteria requiring the three core delirium symptom domains in its definition.
Acknowledgments
The authors thank the team of the Centro Sociosanitario Monterols in Spain and the Sligo Regional Hospital in Ireland for their help and implication in the collection of clinical data and elaboration of previous reports.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest: P.T.T. holds the copyright for the Delirium Rating Scale-Revised-98 but does not charge a fee for a not-for-profit use. The authors declare that they have no conflicts of interests.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2016.11.002.
Supplementary data
References
- 1.Levkoff S.E., Liptzin B., Cleary P.D., Wetle T., Evans D.A., Rowe J.W. Subsyndromal delirium. Am J Geriatr Psychiatry. 1996;4:320–329. doi: 10.1097/00019442-199622440-00006. [DOI] [PubMed] [Google Scholar]
- 2.Marcantonio E., Ta T., Duthie E., Resnick N.M. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50:850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 3.Cole M., McCusker J., Dendukuri N., Han L. The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760. doi: 10.1046/j.1365-2389.2003.51255.x. [DOI] [PubMed] [Google Scholar]
- 4.Marcantonio E.R., Kiely D.K., Simon S.E., John Orav E., Jones R.N., Murphy K.M. Outcomes of older people admitted to postacute facilities with delirium. J Am Geriatr Soc. 2005;53:963–969. doi: 10.1111/j.1532-5415.2005.53305.x. [DOI] [PubMed] [Google Scholar]
- 5.Ouimet S., Riker R., Bergeron N., Bergeon N., Cossette M., Kavanagh B. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med. 2007;33:1007–1013. doi: 10.1007/s00134-007-0618-y. [DOI] [PubMed] [Google Scholar]
- 6.Cole M.G., McCusker J., Ciampi A., Belzile E. The 6- and 12-month outcomes of older medical inpatients who recover from subsyndromal delirium. J Am Geriatr Soc. 2008;56:2093–2099. doi: 10.1111/j.1532-5415.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 7.Shim J., DePalma G., Sands L.P., Leung J.M. Prognostic significance of postoperative subsyndromal delirium. Psychosomatics. 2015;56:644–651. doi: 10.1016/j.psym.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breu A., Stransky M., Metterlein T., Werner T., Trabold B. Subsyndromal delirium after cardiac surgery. Scand Cardiovasc J. 2015;49:207–212. doi: 10.3109/14017431.2015.1041423. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association . 5th ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and statistical manual of mental disorders (DSM-5TM) [Google Scholar]
- 10.Leonard M., Spiller J., Keen J., MacLullich A., Kamholtz B., Meagher D. Symptoms of depression and delirium assessed serially in palliative-care inpatients. Psychosomatics. 2009;50:506–514. doi: 10.1176/appi.psy.50.5.506. [DOI] [PubMed] [Google Scholar]
- 11.Cole M.G., McCusker J., Voyer P., Monette J., Champoux N., Ciampi A. Subsyndromal delirium in older long-term care residents: incidence, risk factors, and outcomes. J Am Geriatr Soc. 2011;59:1829–1836. doi: 10.1111/j.1532-5415.2011.03595.x. [DOI] [PubMed] [Google Scholar]
- 12.Martínez Velilla N., Alonso Bouzon C., Cambra Contin K., Ibáñez Beroiz B., Alonso Renedo J., Casas Herrero A. Delirium and subsyndromal delirium: prevalence of a disease spectrum. Rev Esp Geriatr Gerontol. 2012;47:158–161. doi: 10.1016/j.regg.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Zuliani G., Bonetti F., Magon S., Prandini S., Sioulis F., D'Amato M. Subsyndromal delirium and its determinants in elderly patients hospitalized for acute medical illness. J Gerontol A Biol Sci Med Sci. 2013;68:1296–1302. doi: 10.1093/gerona/glt021. [DOI] [PubMed] [Google Scholar]
- 14.Trzepacz P., Mittal D., Torres R., Kanary K., Norton J., Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 15.Bergeron N., Dubois M.J., Dumont M., Dial S., Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 16.Franco J.G., Trzepacz P., Mejia M.A., Ochoa S.B. Factor analysis of the Colombian translation of the Delirium Rating Scale (DRS), Revised-98. Psychosomatics. 2009;50:255–262. doi: 10.1176/appi.psy.50.3.255. [DOI] [PubMed] [Google Scholar]
- 17.Ceriana P., Fanfulla F., Mazzacane F., Santoro C., Nava S. Delirium in patients admitted to a step-down unit: analysis of incidence and risk factors. J Crit Care. 2010;25:136–143. doi: 10.1016/j.jcrc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Skrobik Y., Ahern S., Leblanc M., Marquis F., Awissi D.K., Kavanagh B.P. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111:451–463. doi: 10.1213/ANE.0b013e3181d7e1b8. [DOI] [PubMed] [Google Scholar]
- 19.Hakim S.M., Othman A.I., Naoum D.O. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116:987–997. doi: 10.1097/ALN.0b013e31825153cc. [DOI] [PubMed] [Google Scholar]
- 20.Meagher D., Adamis D., Trzepacz P., Leonard M. Features of subsyndromal and persistent delirium. Br J Psychiatry. 2012;200:37–44. doi: 10.1192/bjp.bp.111.095273. [DOI] [PubMed] [Google Scholar]
- 21.Kiely D.K., Bergmann M.A., Murphy K.M., Jones R.N., Orav E.J., Marcantonio E.R. Delirium among newly admitted postacute facility patients: prevalence, symptoms, and severity. J Gerontol A Biol Sci Med Sci. 2003;58:M441–M445. doi: 10.1093/gerona/58.5.m441. [DOI] [PubMed] [Google Scholar]
- 22.Trzepacz P., Franco J.G., Meagher D.J., Lee Y., Kim J.L., Kishi Y. Phenotype of subsyndromal delirium using pooled multicultural Delirium Rating Scale-Revised-98 data. J Psychosom Res. 2012;73:10–17. doi: 10.1016/j.jpsychores.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Meagher D.J., O'Regan N., Ryan D., Connolly W., Boland E., O'Caoimhe R. Frequency of delirium and subsyndromal delirium in an adult acute hospital population. Br J Psychiatry. 2014;205:478–485. doi: 10.1192/bjp.bp.113.139865. [DOI] [PubMed] [Google Scholar]
- 24.Inouye S.K., van Dyck C.H., Alessi C.A., Balkin S., Siegal A.P., Horwitz R.I. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 25.Meagher D., Trzepacz P.T. Phenomenological distinctions needed in DSM-V: delirium, subsyndromal delirium, and dementias. J Neuropsychiatry Clin Neurosci. 2007;19:468–470. doi: 10.1176/jnp.2007.19.4.468. [DOI] [PubMed] [Google Scholar]
- 26.Adamis D., Rooney S., Meagher D., Mulligan O., McCarthy G. A comparison of delirium diagnosis in elderly medical inpatients using the CAM, DRS-R98, DSM-IV and DSM-5 criteria. Int Psychogeriatr. 2015;27:683–689. doi: 10.1017/S1041610214002853. [DOI] [PubMed] [Google Scholar]
- 27.Sepulveda E., Franco J.G., Trzepacz P.T., Gaviria A.M., Meagher D.J., Palma J. Delirium diagnosis defined by cluster analysis of symptoms versus diagnosis by DSM and ICD criteria: diagnostic accuracy study. BMC Psychiatry. 2016;16:167. doi: 10.1186/s12888-016-0878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treloar A.J., Macdonald A.J. Outcome of delirium: part 1. Outcome of delirium diagnosed by DSM-III-R, ICD-10 and CAMDEX and derivation of the Reversible Cognitive Dysfunction Scale among acute geriatric inpatients. Int J Geriatr Psychiatry. 1997;12:609–613. doi: 10.1002/(sici)1099-1166(199706)12:6<609::aid-gps553>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.Treloar A.J., Macdonald A.J. Outcome of delirium: part 2. Clinical features of reversible cognitive dysfunction—are they the same as accepted definitions of delirium? Int J Geriatr Psychiatry. 1997;12:614–618. doi: 10.1002/(sici)1099-1166(199706)12:6<614::aid-gps1553>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 30.Morales J.M., Gonzalez-Montalvo J.I., Bermejo F., Del-Ser T. The screening of mild dementia with a shortened Spanish version of the “Informant Questionnaire on Cognitive Decline in the Elderly”. Alzheimer Dis Assoc Disord. 1995;9:105–111. doi: 10.1097/00002093-199509020-00008. [DOI] [PubMed] [Google Scholar]
- 31.Fonseca F., Bulbena A., Navarrete R., Aragay N., Capo M., Lobo A. Spanish version of the Delirium Rating Scale-Revised-98: reliability and validity. J Psychosom Res. 2005;59:147–151. doi: 10.1016/j.jpsychores.2004.04.373. [DOI] [PubMed] [Google Scholar]
- 32.Sepulveda E., Franco J.G., Trzepacz P.T., Gaviria A.M., Viñuelas E., Palma J. Performance of the Delirium Rating Scale-Revised-98 against different delirium diagnostic criteria in a population with a high prevalence of dementia. Psychosomatics. 2015;56:530–541. doi: 10.1016/j.psym.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 33.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 34.Laurila J.V., Pitkala K.H., Strandberg T.E., Tilvis R.S. The impact of different diagnostic criteria on prevalence rates for delirium. Dement Geriatr Cogn Disord. 2003;16:156–162. doi: 10.1159/000071004. [DOI] [PubMed] [Google Scholar]
- 35.Ryan D.J., O'Regan N.A., Caoimh R.O., Clare J., O'Connor M., Leonard M. Delirium in an adult acute hospital population: predictors, prevalence and detection. BMJ Open. 2013;3:e001772. doi: 10.1136/bmjopen-2012-001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcantonio E.R., Simon S.E., Bergmann M.A., Jones R.N., Murphy K.M., Morris J.N. Delirium symptoms in post-acute care: prevalent, persistent, and associated with poor functional recovery. J Am Geriatr Soc. 2003;51:4–9. doi: 10.1034/j.1601-5215.2002.51002.x. [DOI] [PubMed] [Google Scholar]
- 37.Jones R.N., Kiely D.K., Marcantonio E.R. Prevalence of delirium on admission to postacute care is associated with a higher number of nursing home deficiencies. J Am Med Dir Assoc. 2010;11:253–256. doi: 10.1016/j.jamda.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCusker J., Cole M.G., Voyer P., Monette J., Champoux N., Ciampi A. Prevalence and incidence of delirium in long-term care. Int J Geriatr Psychiatry. 2011;26:1152–1161. doi: 10.1002/gps.2654. [DOI] [PubMed] [Google Scholar]
- 39.Mattoo S.K., Grover S., Chakravarty K., Trzepacz P.T., Meagher D.J., Gupta N. Symptom profile and etiology of delirium in a referral population in northern india: factor analysis of the DRS-R98. J Neuropsychiatry Clin Neurosci. 2012;24:95–101. doi: 10.1176/appi.neuropsych.11010009. [DOI] [PubMed] [Google Scholar]
- 40.Franco J.G., Trzepacz P., Meagher D.J., Kean J., Lee Y., Kim J.L. Three core domains of delirium validated using exploratory and confirmatory factor analyses. Psychosomatics. 2013;54:227–238. doi: 10.1016/j.psym.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Liptzin B., Levkoff S.E., Gottlieb G.L., Johnson J.C. Delirium. J Neuropsychiatry Clin Neurosci. 1993;5:154–160. doi: 10.1176/jnp.5.2.154. [DOI] [PubMed] [Google Scholar]
- 42.Trzepacz P., Mulsant B.H., Dew M.A., Pasternak R., Sweet R.A., Zubenko G.S. Is delirium different when it occurs in dementia? A study using the Delirium Rating Scale. J Neuropsychiatry Clin Neurosci. 1998;10:199–204. doi: 10.1176/jnp.10.2.199. [DOI] [PubMed] [Google Scholar]
- 43.V Laurila J., Pitkala K.H., Strandberg T.E., Tilvis R.S. Delirium among patients with and without dementia: does the diagnosis according to the DSM-IV differ from the previous classifications? Int J Geriatr Psychiatry. 2004;19:271–277. doi: 10.1002/gps.1079. [DOI] [PubMed] [Google Scholar]
- 44.Voyer P., Cole M.G., McCusker J., Belzile E. Prevalence and symptoms of delirium superimposed on dementia. Clin Nurs Res. 2006;15:46–66. doi: 10.1177/1054773805282299. [DOI] [PubMed] [Google Scholar]
- 45.Meagher D.J., Leonard M., Donnelly S., Conroy M., Saunders J., Trzepacz P. A comparison of neuropsychiatric and cognitive profiles in delirium, dementia, comorbid delirium-dementia and cognitively intact controls. J Neurol Neurosurg Psychiatry. 2010;81:876–881. doi: 10.1136/jnnp.2009.200956. [DOI] [PubMed] [Google Scholar]
- 46.Meagher D.J., Morandi A., Inouye S.K., Ely W., Adamis D., Maclullich A.J. Concordance between DSM-IV and DSM-5 criteria for delirium diagnosis in a pooled database of 768 prospectively evaluated patients using the delirium rating scale-revised-98. BMC Med. 2014;12:164. doi: 10.1186/s12916-014-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sepulveda E., Franco J.G. Delirium in the Spanish version of the DSM-5: more confusion? Rev Psiquiatr Salud Ment. 2015;8:242–243. doi: 10.1016/j.rpsm.2015.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.