Abstract
Objective
This present study aims to address the gap in the literature regarding the severity and underlying neural correlates of psychotic symptoms in frontotemporal dementia with and without the C9orf72 gene expansion.
Methods
Fifty-six patients with behavioural variant frontotemporal dementia (20 with concomitant amyotrophic lateral sclerosis) and 23 healthy controls underwent neuropsychological assessments, detailed clinical interview for assessment of psychosis symptoms, brain MRI and genetic testing. Carers underwent a clinical interview based upon the neuropsychiatric inventory. Patients were assessed at ForeFront, the Frontotemporal Dementia Research Group at Neuroscience Research Australia or at the Brain and Mind Centre, between January 2008 and December 2013.
An index of psychosis was calculated, taking into account the degree and severity of psychosis in each case. Voxel-based morphometry analyses were used to explore relationships between the psychosis index and grey matter changes.
Results
Thirty-four percent of frontotemporal dementia patients showed psychotic features. C9orf72 expansion cases were more likely to exhibit psychotic symptoms than non-carriers (64% vs. 26%; p = 0.006), which were also more severe (psychotic index 23.1 vs. 8.1; p = 0.002). Delusions comprised persecutory, somatic, jealous and grandiose types and were present in 57% of C9orf72 carriers and 19% of non-carriers (p = 0.006). Auditory, visual or tactile hallucinations were present in 36% of C9orf72 carriers and 17% of non-carriers (p = 0.13). Increased psychotic symptoms in C9orf72 expansion carriers correlated with atrophy in a distributed cortical and subcortical network that included discrete regions of the frontal, temporal and occipital cortices, as well as the thalamus, striatum and cerebellum.
Conclusions
This study underlines the need to consider and assess for psychotic symptoms in the frontotemporal dementia-amyotrophic lateral sclerosis continuum particularly in those with C9orf72 gene expansions. The network of brain regions identified in this study is strikingly similar to that identified in other psychotic disorders such as schizophrenia, which suggests that treatment strategies in psychiatry may be beneficial for the management of psychotic symptoms in frontotemporal dementia.
Keywords: Frontotemporal dementia, Amyotrophic lateral sclerosis, C9orf72 expansion, Psychosis, Schizophrenia, Neuroimaging
Highlights
-
•
This study has confirmed a high rate of psychotic symptoms in C9orf72 carriers (64%).
-
•
Psychotic symptoms are also common in C9orf72 non-carriers (26%).
-
•
Psychotic symptoms are more severe in C9orf72 carriers than non-carriers.
-
•
A distributed cortical and subcortical network is associated with increased psychotic symptoms in C9orf72 carriers.
-
•
The network of affected brain regions is very similar to that seen in other psychotic disorders such as schizophrenia.
1. Introduction
With the discovery and clinical descriptions of the C9orf72 gene expansion, it has become clear that such carriers may develop psychotic features and in some instances present with florid delusions or hallucinations (Snowden et al., 2012; Devenney et al., 2014). As such, we have seen renewed interest in psychosis in the Frontotemporal dementia-Amyotrophic lateral sclerosis (FTD-ALS) continuum, with recent systematic reviews of the literature suggesting that the prevalence is approximately 10–25% (Hall and Finger, 2015, Shinagawa et al., 2013). Neuroimaging studies of the C9orf72 expansion in both FTD and ALS have highlighted an excess of subcortical atrophy in comparison to non-carriers, and have led researchers to postulate that perhaps subcortical structures play a role in the generation of psychotic symptoms in C9orf72 cases but up until now this theory has not been explored (Bede et al., 2013b, Downey et al., 2014, Mahoney et al., 2012). The largest body of evidence regarding psychosis and associated brain abnormalities comes from the literature in schizophrenia and related psychotic disorders, where abnormal changes in the volume, connectivity and function of the frontal and temporal cortices, thalamus and cerebellum have been reported (Andreasen et al., 1996, Byne et al., 2009, Fusar-Poli et al., 2012). These findings may be relevant to psychosis in FTD and FTD-ALS where similar cortical and subcortical regions are involved (Lillo et al., 2012).
The present study aimed to improve our understanding of psychosis across a well-characterised cohort of FTD and FTD-ALS patients, including a subset of C9orf72 carriers, by collecting prospective data using a validated behavioural tool in combination with a clinical interview. We then determined the underlying neural correlates of psychosis using structural neuroimaging techniques. It was hypothesised that, similar to other psychotic disorders, and in line with imaging studies of C9orf72 patients, an extended network of cortical and subcortical regions would play a role in the generation of psychosis in FTD.
2. Methods
2.1. Participants
In total 79 participants were included in the study; 36 consecutive patients with behavioural variant FTD (bvFTD) and 20 consecutive patients with bvFTD in combination with ALS (FTD-ALS) were matched by sex-, age- and education history to 23 healthy controls. Patients were assessed at ForeFront, the Frontotemporal Dementia Research Group at Neuroscience Research Australia (NeuRA) and the Brain and Mind Centre, between 2008 and 2013. Healthy controls were selected from a volunteer panel at NeuRA.
Diagnosis of bvFTD was made by an experienced clinical neurologist based on international diagnostic criteria for bvFTD (Rascovsky et al., 2011). ALS was diagnosed by an experienced clinical neurologist according to the El Escorial and Awaji diagnostic criteria (Brooks et al., 2000, Nodera et al., 2007). Global cognitive function was measured using the Addenbrooke's Cognitive Examination-Revised (ACE-R) (Mioshi et al., 2006). Disease staging was assessed with the FTD Functional Rating Scale (FTD-FRS) (Mioshi et al., 2010).
In each participant psychotic features began within a 10-year period prior to meeting consensus criteria for FTD. We intended to exclude participants if they were diagnosed with schizophrenia or another delusional disorder by a psychiatrist > 10 years prior to presentation but this did not apply to these cases. Exclusion criteria also included a past history of traumatic brain injury, drug or alcohol abuse and cerebrovascular disease. None of the patients in the study were taking anti-psychotic medication or psychosis-inducing medication at the time of their initial assessments. Patients were not systematically assessed for hypoxia however this study was conducted at first presentation and at this stage none of the patients required non-invasive ventilation.
Ethical approval was obtained from the South Eastern Sydney and Illawarra Area Health Service and the University of New South Wales ethics committees. All participants, or their responsible person, provided informed written consent in accordance with the Declaration of Helsinki.
2.2. Genetic status
All participants underwent blood sampling for the C9orf72 expansion. The repeat primed PCR was performed using the procedure described previously (Dobson-Stone et al., 2012), based on the protocol of Renton and colleagues (Renton et al., 2011). A patient's DNA sample was deemed positive for the C9orf72 repeat expansion if it contained an allele with > 30 repeats. Patients with a family history were also screened for other common genetic mutations (GRN, MAPT) by Sanger sequencing of genomic DNAs corresponding to all coding exons (Schofield et al., 2010, Stanford et al., 2003).
2.3. Presence of delusions and hallucinations
A delusion was defined as a false belief based on incorrect inference about external reality that is firmly sustained despite what almost everybody else believes and despite what constitutes incontrovertible and obvious proof or evidence to the contrary. The belief is not ordinarily accepted by other members of the person's culture or subculture. A hallucination was defined as a perception of an external stimulus when none is present but which the person believes to be real (American Psychiatric Association, 2013).
The presence of delusions and hallucinations was determined in two ways. Firstly, the delusions and hallucinations subscale of the Neuropsychiatric Inventory (NPI) (Cummings et al., 1994) was completed with the carer. The subscales assess delusions and hallucinations separately with regards to frequency and severity of symptoms. Frequency is scored from 0 to 4; 0 = never, 1 = rarely – less than once per week, 2 = sometimes – about once per week, 3 = often – a few times per week, 4 = frequently – once or more per day. Severity is scored from 0 to 3; with 0 representing never, 1 = mild – produces little distress, 2 = moderate – more disturbing to the patient but can be redirected by the caregiver, 3 = severe – very disturbing to the patient and difficult to redirect.
During the visit, the nature of abnormal behaviours was clarified with the carer and patient, and re-scored according to the subscales of the NPI. A revised score was derived from the carer and patient interview. A total score was then generated for psychosis to reflect severity of psychotic symptoms by combining delusions (maximum 12) and hallucinations (maximum 12) scores for each participant and then expressing it as a percentage of the total score for delusions and hallucinations (maximum 24), referred to as the ‘psychosis index’.
2.4. Image acquisition and pre-processing
All participants underwent whole-brain T1 imaging using a 3T Philips scanner with standard quadrature head coil. The 3D T1-weighted sequences were acquired as follows: coronal orientation, matrix 256 × 256, 200 slices, 1 mm2 in-plane resolution, 1 mm slice thickness, echo time/repetition time = 2.6/5.8 ms, flip angle 8°. MRI scans were obtained within 2 days of the clinical interview.
Three-dimensional T1-weighted sequences were analyzed with FSL-VBM (http://www.fmrib.ox.ac.uk/fsl/fslvbm/index.html) (Ashburner and Friston, 2000). Structural images were brain-extracted using BET and tissue segmentation was carried out using FMRIB's Automatic Segmentation Tool (FAST) (Zhang et al., 2001). The resulting grey matter partial volume maps were then aligned to the Montreal Neurological Institute standard space (MNI 152) using the non-linear registration approach (FNIRT) (Andersson et al., 2007) using a b-spline representation of the registration warp field (Rueckert et al., 1999). A study-specific template was created and the native grey matter images were non-linearly re-registered. The registered partial volume maps were modulated (to correct for local expansion or contraction) by dividing them by the Jacobian of the warp field. The modulated images were then smoothed with an isotropic Gaussian kernel with a standard deviation of 3 mm (full-width at half-maximum: 8 mm).
2.5. Statistical analyses
Statistical analyses compared patients with controls irrespective of genetic status (bvFTD and FTD-ALS). Further comparisons between patients groups were carried out according to genetic status (C9orf72 carriers vs. non-carriers) and presence or absence of psychotic features.
2.5.1. Behavioural analyses
Data were analyzed using SPSS 22.0 statistical package. Kolmogorov-Smirnoff tests were applied to determine if clinical and demographic variables were normally distributed. Parametric variables were analyzed using univariate ANOVA, with post hoc analyses comparing differences across groups, using Sidak correction for multiple comparisons. Non-parametric data was analyzed using Mann-Whitney and the Kruskal-Wallis tests, and categorical data were compared with Chi-Square tests.
2.5.2. Voxel-based morphometry analyses
First, atrophy analyses were carried out to identify differences in grey matter intensity between patients groups and healthy controls according to clinical diagnosis (bvFTD and FTD-ALS) and genotype (C9orf72 carriers vs. non-carriers). Voxel-wise general linear models and t-tests were applied using permutation-based, non-parametric statistics, with 5000 permutations per contrast (Nichols and Holmes, 2002). Significant clusters were defined at a t-threshold corrected for family-wise error of p < 0.05 with a minimum cluster size of 50 voxels.
Next, correlations between psychosis index scores and grey matter intensity were investigated using an unbiased whole-brain approach. First, to uncover the neural correlates of psychosis in FTD, correlations between demeaned psychosis index scores and grey matter intensity were assessed combining all patients together (bvFTD, FTD-ALS). Then, correlations between psychosis index and grey matter intensity were investigated, using the same analyses described above, in C9orf72 expansion carriers to identify neural correlates of psychosis specific to this genetic expansion. For all analyses, the statistical threshold was set at p < 0.005 uncorrected for multiple comparisons with a conservative cluster extent threshold of 25 voxels. This approach is designed to minimize Type I error while balancing the risk of Type II error (Lieberman and Cunningham, 2009). Anatomical locations of significant results were overlaid on the Montreal Neurological Institute (MNI) standard brain within the mricron software (http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html), with maximum coordinates provided in MNI stereotaxic space. Anatomical labels were determined with reference to the Harvard-Oxford probabilistic cortical and subcortical atlases.
3. Results
3.1. Demographics, cognitive and behavioural screening measures
A comparison of the patient groups (bvFTD and FTD-ALS) with healthy controls revealed a higher ACE-R score for controls compared to patients. Otherwise there were no significant differences across the groups for age, sex and education years. Within the patient groups bvFTD patients scored lower in the FRS, indicating more functional impairment. Of the 56 patients included in the study 25% were C9orf72 expansion carriers. No patients carried GRN or MAPT mutations. Comparison between C9orf72 carriers and non-carriers revealed no significant differences across demographic, cognitive and functional measures (all p > 0.05) (Table 1).
Table 1.
Demographics and clinical characteristics in bvFTD, FTD-ALS and healthy controls, and C9orf72 carriers and non-carriers.
| bvFTD (n = 36) |
FTD-ALS (n = 20) |
HC (n = 23) |
p value | Post-hoc |
C9orf72 carriers (n = 14) |
C9orf72 non-carriers (n = 42) |
p value | |
|---|---|---|---|---|---|---|---|---|
| C9orf72 positive, n (%) | 9 (25%) | 5 (25%) | 0 | – | – | – | – | – |
| Sex (M:F) | 26:12 | 13:7 | 14:9 | 0.65 | – | 11:3 | 28:14 | 0.5 |
| Age (years) | 59 ± 7.2 | 60.6 ± 6.6 | 62.5 ± 3.9 | 0.37 | – | 61.2 ± 5.9 | 59.1 ± 7.1 | 0.4 |
| Education (years) | 12.8 ± 3.4 | 12.6 ± 3.0 | 12.6 ± 2.9 | 0.47 | – | 12.6 ± 2.8 | 12.8 ± 3.5 | 0.83 |
| Disease duration (years) | 3.7 ± 2.4 | 2.5 ± 1.2 | – | 0.08 | – | 4.2 ± 2.6 | 3.1 ± 2.0 | 0.2 |
| ACE-R (max 100) | 73.5 ± 13.4 | 68.7 ± 12.3 | 94.1 ± 3.8 | < 0.001⁎⁎ | HC > bvFTD, FTD-ALS | 73.8 ± 17.1 | 71.7 ± 12.5 | 0.3 |
| FRS Rasch scorea | − 0.7 ± 1.3 | 0.7 ± 1.7 | – | 0.007⁎ | – | − 0.03 ± 1.7 | − 0.34 ± 1.6 | 0.72 |
Values are expressed as mean ± standard deviation. bvFTD = behavioural variant frontotemporal dementia; FTD-ALS = frontotemporal dementia – amyotrophic lateral sclerosis; HC = healthy controls; ACE-R = Addenbrooke's Cognitive Examination – Revised; FRS = Functional dementia Rating Scale.
The FRS provides logit scores ranging from 4.12 (very mild) to − 4.99 (very severe).
Denotes significant differences at the p < 0.05 level.
Denotes significant differences at the p < 0.001 level.
3.2. Psychotic features
3.2.1. Neuropsychiatric inventory
Of the 56 patients, 34% showed psychotic features; 28% experienced delusions while 25% experienced hallucinations. There were no significant differences between patients with and without psychosis for all demographic variables (all p > 0.05; Table e1). Furthermore, there were no significant differences between patients with and without psychosis, for NPI scores of disinhibition, apathy, depression, anxiety, agitation, elation, irritability, appetite or sleep (all p > 0.1).
3.2.2. Psychosis index
Exactly 64% of C9orf72 expansion carriers exhibited psychotic symptoms compared to 26% of non-carriers (p = 0.006). The psychosis index was 12 in the overall patient cohort and was significantly higher in the C9orf72 positive cohort than the C9orf72 negative cohort (p = 0.002; Table 2).
Table 2.
Psychosis scores and characterisation of psychotic symptoms in C9orf72 positive and negative patients.
| C9orf72 positive (n = 14) | C9orf72 negative (n = 42) | p value | |
|---|---|---|---|
| Psychosis score | 5.3 ± 5.3 | 1.9 ± 4.6 | 0.002⁎ |
| Psychosis index | 24.3 ± 22 | 7.7 ± 19.5 | 0.002⁎ |
| Psychotic symptoms, n (%) | 9 (64) | 11 (26) | 0.006⁎ |
| Delusions | 8 (57) | 8 (19) | 0.006⁎ |
| Persecutory | 4 (29) | 6 (14) | 0.04⁎ |
| Somatic | 3 (21) | 2 (5) | 0.06 |
| Jealous | 2 (14) | 1 (2) | 0.08 |
| Grandiose | 2 (14) | 2 (5) | 0.23 |
| Hallucinations | 5 (36) | 9 (17) | 0.133 |
| Auditory | 3 (21) | 2 (5) | 0.06 |
| Visual | 2 (14) | 3 (7) | 0.42 |
| Somatic | 1 (7) | 1 (2) | 0.41 |
Values are expressed as mean ± standard deviation.
Denotes significant differences at the p < 0.05 level.
3.2.3. Characterisation of psychosis
Delusions were characterised according to their content and following accepted criteria (Kiran and Chaudhury, 2009). Persecutory delusions were the most common and were present in 63% of all affected patients. Somatic delusions (31%), delusions of a jealous nature (19%) and grandiose delusions (25%) were also present. In total, 38% of patients had a mixture of the above delusion types.
Hallucinations were characterised according to modality. Auditory hallucinations were all in the second person and of a negative and persecutory nature. Visual hallucinations were either in the form of people, both alive and dead, or animals. Tactile hallucinations were of human touch in one and of insects crawling under the skin in another.
Hallucinations and delusions in the C9orf72 cohort were similar to that seen in the patient cohort as a whole (Table 2), however there were no significant differences in the rate of hallucinations between carriers and non-carriers. Of note, somatic delusions were common in C9orf72 carriers and included medically unexplained sensory disturbances and abdominal pains.
3.3. Neuroimaging results
3.3.1. Atrophy analyses
Group comparisons between clinical diagnoses and controls revealed the characteristic profiles of brain atrophy previously reported in bvFTD and FTD-ALS (Lillo et al., 2012). These results are presented in Supplementary Fig. e1. In brief, both patient groups showed extensive overlap of grey matter density loss with widespread atrophy predominantly in frontal and temporal regions including the anterior insula, orbitofrontal cortex, striatum, thalamus and temporal poles. Parietal and occipital regions and the cerebellum were also involved. Direct comparisons between bvFTD and FTD-ALS revealed no significant regions of greater grey matter loss in either group.
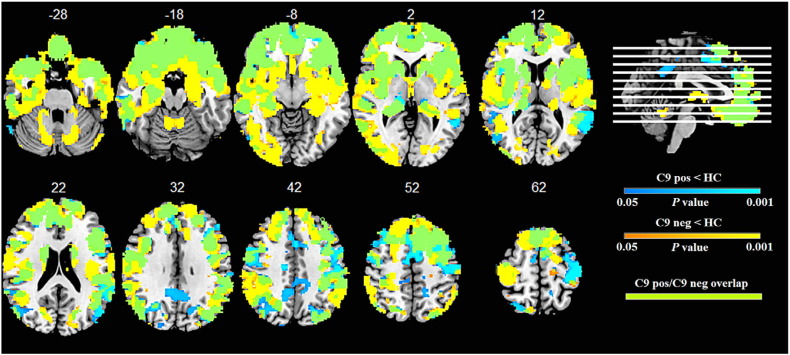
Consistent with previous studies in both FTD and ALS, patients with and without C9orf72 expansions showed overlapping but distinct atrophy patterns (Bede et al., 2013a, Boeve et al., 2012, Mahoney et al., 2012, Whitwell et al., 2012). C9orf72 expansion carriers showed atrophy in bilateral anterior cingulate, dorsolateral and orbitofrontal prefrontal cortex, insular cortices and lateral parietal cortices, striatum and bilateral thalamus compared to healthy controls (Fig. 1). Non-carriers exhibited similar but more extensive bilateral atrophy in the frontotemporal, insular, cingulate and striatal regions. C9orf72 expansion carriers showed atrophy in bilateral precuneus and posterior cingulate cortex (not affected in C9orf72 non-carriers), whereas C9orf72 non-carriers (but not C9orf72 expansion carriers) showed atrophy in the cerebellum. Direct comparisons between C9orf72 carriers and non-carriers did not reveal regions of significant differences between groups.
Fig. 1.
Regions of brain atrophy in C9orf72 carriers and non-carriers compared to healthy controls.
Group results from voxel-based morphometry analyses demonstrating areas of decreased grey matter density in C9orf72 positive (blue) and C9orf72 negative (yellow) relative to healthy controls. Patient groups showed extensive overlapping atrophy (green). Significant clusters were defined at a t-threshold corrected for family-wise error of p < 0.05 with a minimum cluster size of 50 voxels. No significant clusters were identified in direct comparisons between negative and positive C9orf72 patients. The statistical maps are superimposed on the Montreal Neurological Institute template brain. Images are displayed in radiological convention (the left side of images corresponds to the right side of the brain). C9 pos = C9orf72 positive; C9 neg = C9orf72 negative; HC = healthy controls.
3.3.2. Neural correlates of psychosis
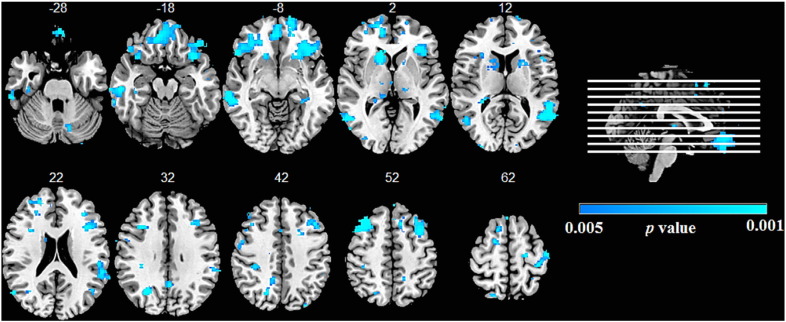
A higher psychosis score for all participants combined (bvFTD and FTD-ALS) was associated with volume loss in a network of cortical and subcortical regions. The regions implicated included the bilateral medial prefrontal and occipital cortices, and the right thalamus and the left cerebellum (Fig. 2 and Supplementary Table e2).
Fig. 2.
Neural correlates of psychosis in the FTD-ALS continuum.
Results from voxel-based morphometry analyses demonstrating correlations between psychosis index and areas of grey matter density in the whole FTD-ALS cohort. The statistical maps are superimposed on the Montreal Neurological Institute template brain. Coloured voxels show regions that were significant in the analyses (p < 0.005 uncorrected). Images are displayed in radiological convention (the left side of image corresponds to the right side of the brain).
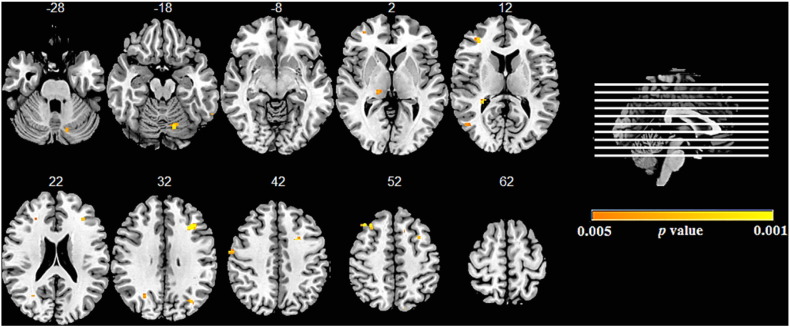
In C9orf72 expansion carriers, higher psychosis scores correlated with grey matter volume loss in a broader network of regions including bilateral medial frontal cortex, anterior cingulate cortex and orbitofrontal cortex, bilateral insula, caudate, putamen and thalamic nuclei, middle, inferior and superior temporal gyrus, temporal fusiform gyrus, lateral occipital cortex and right cerebellum (Fig. 3 and Supplementary Table e3).
Fig. 3.
Neural correlates of psychosis in C9orf72 expansion carriers.
Results from voxel-based morphometry analyses demonstrating correlations between psychosis index and areas of grey matter density in C9orf72 expansion carriers. The statistical maps are superimposed on the Montreal Neurological Institute template brain. Coloured voxels show regions that were significant in the analyses (p < 0.005 uncorrected). Images are displayed in radiological convention (the left side of image corresponds to the right side of the brain).
4. Discussion
Delusions and hallucinations in the FTD-ALS continuum have received renewed interest following the discovery of the C9orf72 expansion as the most common genetic abnormality in FTD and FTD-ALS (DeJesus-Hernandez et al., 2011, Renton et al., 2011). First, by means of a prospective study design, this study has confirmed that psychosis is common and can be more severe than previously recognised in patients with FTD. The rate of psychotic features was higher in C9orf72 carriers than non-carriers and these symptoms were more marked in such cases as reflected by the significantly higher psychosis index. Of interest, this is the first study to explore the patterns of brain atrophy associated with psychosis in C9orf72 FTD-ALS. A distributed network of cortical and subcortical regions was identified, that included discrete regions of the frontal temporal and occipital cortices, as well as the thalamus, striatum and cerebellum. These patterns are strikingly similar to the changes seen in grey matter in schizophrenia and other psychotic disorders.
Over one-third of the study cohort exhibited a degree of psychosis at first presentation, in contrast to previous studies where lower rates have been reported, and also slightly higher than recent systematic reviews have suggested (Hall and Finger, 2015, Shinagawa et al., 2013), but in line with a recent study which identified psychotic symptoms in 32% of FTD patients by means of a retrospective chart review. This discrepancy may be partly explained by case selection; in our cohort we included only those with bvFTD and FTD-ALS and in these cohorts psychosis is generally considered to be more common (Lillo et al., 2010). The use of a face-to-face focused carer and patient interview allowed for better delineation of symptoms. Of course the nature of referral patterns and referral bias to tertiary centres must always be taken into consideration and these results may not be readily transferrable to the population as a whole. Nonetheless, these results highlight the extent of psychosis in FTD and particularly the need for more effective treatments for these symptoms.
Overall, the delusions and hallucinations in these patients were largely negative in nature, and were not in keeping with the patients' previous life experiences. It could be argued that these delusions represent confabulation related to frontal inhibitory dysfunction and ‘filling in the gaps’ of memory loss which occurs in FTD (Mendez et al., 2008). The relative lack of grandiose delusions plus the pervasive nature of the delusions makes this unlikely. It is also difficult to differentiate true somatic hallucinations from medically unexplained symptoms, which often have a complex psychosocial basis. Moreover, abnormalities in perception, specifically auditory and temperature perception, related to underlying brain atrophy have been documented in these conditions (Fletcher et al., 2015, Hailstone et al., 2011). In the absence of more sensitive measures of psychosis it is difficult to determine the relationship between psychotic symptoms, medically unexplained symptoms and perceptual changes. Of interest is also the finding that the rate of hallucinations did not differ between carriers and non-carriers. This might merely reflect the relatively small numbers of patients with hallucinations but also suggests a disassociation between the mechanisms involved in the generation of delusions and hallucinations in FTD-ALS and warrants further study in a larger cohort. It has recently been reported that patients with ALS can experience non-psychotic primary psychiatric disorders years before the first diagnosis of ALS and while anecdotally a similar pattern is seen in patients with the C9orf72 expansion, this has not yet been systematically reviewed but might offer some insight into the underlying neurobiology which renders some patients more susceptible to developing psychiatric symptoms (Turner et al., 2016).
Until now the neural correlates of psychosis in FTD have been relatively unexplored. A recent study utilized pathological data to correlate regions of atrophy at post-mortem to psychotic symptoms reported at any time during life, and found an association with predominantly right-sided brain degeneration but failed to find any link with subcortical atrophy (Landqvist Waldö et al., 2015). Methodological differences may explain the divergent findings between this recent study and the current study. Although the current study did not have the benefit of pathological disease confirmation, a major strength is that after detailed clinical phenotyping each patient underwent MRI scanning within 2 days, therefore ensuring that the patterns of brain atrophy best reflected the symptoms experienced by the patient at that time. Furthermore, in the current cohort there were no differences between patients who experienced psychotic symptoms and those who did not in terms of other abnormal behaviours, such as disinhibition and apathy, suggesting that the results are not driven by other behavioural factors.
The association of a network of cortical and subcortical atrophy with increased psychosis scores in C9orf72 is new and a key finding of this study. The findings are exploratory however they do converge with the hypothesis of Downey and colleagues who showed that C9orf72 carriers have an altered body schema (Downey et al., 2014). These authors suggested that altered body schema deficits might contribute to the development of psychosis generation in C9orf72 carriers through alterations in thalamo-cortico-cerebellar networks. The regions of atrophy identified here are remarkably similar to those identified consistently in meta-analyses of VBM studies of schizophrenia and other psychotic disorders including schizoaffective disorder and first-episode psychosis (Amann et al., 2016, Bora et al., 2011, Glahn et al., 2008). Similar to our findings, these meta-analyses repeatedly show atrophy of key temporal lobe structures including the superior temporal gyrus, which has also been linked with positive symptoms and in particular auditory hallucinations in schizophrenia (Aguayo, 1990). Atrophy of the insular and anterior cingulate cortex are associated with higher psychosis scores in our cohort and again these regions, which are key structures of the salience network with links to the superior temporal pole, the dorsolateral prefrontal cortex, thalamus, and the striatum (Seeley et al., 2007), are shown to be atrophied across multiple VBM studies in psychotic syndromes (Amann et al., 2016, Bora et al., 2011, Glahn et al., 2008). The salience network is involved in detection, analysis and integration of emotionally salient stimuli with respect to the internal environment and is implicated in symptom generation in both FTD and schizophrenia (Seeley et al., 2007, Zhou and Seeley, 2014). Similarly, and consistent with the findings from this study, within the frontal cortex the medial frontal region is characteristically abnormal in psychotic disorders. That the thalamus has been repeatedly implicated in psychotic disorders converges well with previous imaging findings in C9orf72 carriers, which showed thalamic atrophy, and thalamic involvement in functional networks (Lee et al., 2014, Mahoney et al., 2012).
This study has limitations. Replication in a larger cohort is necessary to increase statistical power and confirm the findings, although given the relative rarity of these conditions this can be difficult, which in turn points to the need for multicentre collaborations. The neuroimaging findings, although novel, are preliminary and further neuroimaging projects should include methods of analysing network connectivity to confirm if dysfunctions within large-scale brain networks are responsible for psychosis in FTD. It is also important to note that subregions within subcortical structures, such as the thalamus, have distinct functions and map to specific cortical region. Analysis of these subregions was beyond the scope of this study but should be considered for future projects. Furthermore, analysis of delusions and hallucinations separately would have been ideal, as it is possible that these two symptoms may have different neural circuitry. However the small sample size restricted further analysis and therefore was outside the scope of this project but should be performed in a larger cohort in future studies.
In conclusion, psychotic symptoms are common in the FTD-ALS continuum, and should be assessed by means of a detailed carer and patient interview. The commonalities between primary psychotic disorders and the FTD-ALS continuum in terms of underlying neural substrates are notable, and in line with current views that brain network degeneration may be responsible for shared behavioural symptoms between schizophrenia and FTD (Zhou and Seeley, 2014), which further suggests that we may be able to incorporate patient management strategies from psychiatry. Finally, we suggest that an objective measure to characterise psychotic symptoms will be useful as well as targeted studies using functional MRI to gain further insight into the brain networks involved.
Disclosures & conflicts of interest
Dr. E. Devenney is supported by a UNSW PhD scholarship and the Motor Neuron Disease Association UK. Dr. M. Irish is supported by an Australian Research Council Discovery Early Career Researcher Award (DE130100463). Dr. M. Hornberger is supported by Alzheimer's Research UK and the Isaac Newton Trust. Dr. E. Mioshi is supported by Alzheimer's Society UK and Alzheimer's Association USA. Dr. R. Landin-Romero is supported by the ARC Centre of Excellence in Cognition and its Disorders Memory Node (CE11000102). Professor G. Halliday is supported by a NHMRC Senior Principal Research Fellowship (#1079679). The authors report no financial interests or potential conflicts of interest.
Acknowledgments
We are grateful to the research participants involved with the ForeFront research studies. Genetic screening was performed in the laboratory of A/Prof John Kwok (in association with Dr. Carol Dobson-Stone) and in the ForeFront biomarker research laboratory (Glenda Halliday, Olivier Piguet, Lauren Bartley, Yue Huang, Mia MacMillan, Sahar Lateef).
This work was supported by funding to Forefront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the National Health and Medical Research Council of Australia program grant (#1037746) and the Australian Research Council Centre of Excellence in Cognition and its Disorders Memory Node (#CE110001021). The funding source had no involvement in study design, data collection, analysis and interpretation of the data, in writing the report or in the decision to submit the article for publication.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.11.028.
Appendix A. Supplementary data
Supplementary material
References
- Aguayo J. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am. J. Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Amann B., Canales-Rodríguez E., Madre M., Radua J., Monte G., Alonso-Lana S., Landin-Romero R., Moreno-Alcázar A., Bonnin C., Sarró S. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta Psychiatr. Scand. 2016;133:23–33. doi: 10.1111/acps.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Andersson J.L., Jenkinson M., Smith S. FMRIB Technical Report TR07JA1. University of Oxford FMRIB Centre; Oxford, UK: 2007. Non-linear optimisation. [Google Scholar]
- Andreasen N.C., O'Leary D.S., Cizadlo T., Arndt S., Rezai K., Ponto L.L., Watkins G.L., Hichwa R.D. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc. Natl. Acad. Sci. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bede P., Bokde A.L.W., Byrne S., Elamin M., McLaughlin R.L., Kenna K., Fagan A.J., Pender N., Bradley D.G., Hardiman O. Multiparametric MRI study of ALS stratified for the C9orf72 genotype. Neurology. 2013;81:361–369. doi: 10.1212/WNL.0b013e31829c5eee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bede P., Elamin M., Byrne S., McLaughlin R.L., Kenna K., Vajda A., Pender N., Bradley D.G., Hardiman O. Basal ganglia involvement in amyotrophic lateral sclerosis. Neurology. 2013;81:2107–2115. doi: 10.1212/01.wnl.0000437313.80913.2c. [DOI] [PubMed] [Google Scholar]
- Boeve B.F., Boylan K.B., Graff-Radford N.R., DeJesus-Hernandez M., Knopman D.S., Pedraza O., Vemuri P., Jones D., Lowe V., Murray M.E., Dickson D.W., Josephs K.A., Rush B.K., Machulda M.M., Fields J.A., Ferman T.J., Baker M., Rutherford N.J., Adamson J., Wszolek Z.K., Adeli A., Savica R., Boot B., Kuntz K.M., Gavrilova R., Reeves A., Whitwell J., Kantarci K., Jack C.R., Jr., Parisi J.E., Lucas J.A., Petersen R.C., Rademakers R. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. 2012;135:765–783. doi: 10.1093/brain/aws004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E., Fornito A., Radua J., Walterfang M., Seal M., Wood S.J., Yücel M., Velakoulis D., Pantelis C. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr. Res. 2011;127:46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Brooks B.R., Miller R.G., Swash M., Munsat T.L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Byne W., Hazlett E.A., Buchsbaum M.S., Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathol. 2009;117:347–368. doi: 10.1007/s00401-008-0404-0. [DOI] [PubMed] [Google Scholar]
- Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The neuropsychiatric inventory comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenney E., Hornberger M., Irish M., Mioshi E., Burrell J., Tan R., Kiernan M.C., Hodges J.R. Frontotemporal dementia associated with the C9ORF72 mutation: a unique clinical profile. JAMA Neurol. 2014;71:331–339. doi: 10.1001/jamaneurol.2013.6002. [DOI] [PubMed] [Google Scholar]
- Dobson-Stone C., Hallupp M., Bartley L., Shepherd C.E., Halliday G.M., Schofield P.R., Hodges J.R., Kwok J.B.J. C9ORF72 repeat expansion in clinical and neuropathologic frontotemporal dementia cohorts. Neurology. 2012;79:995–1001. doi: 10.1212/WNL.0b013e3182684634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey L.E., Fletcher P.D., Golden H.L., Mahoney C.J., Agustus J.L., Schott J.M., Rohrer J.D., Beck J., Mead S., Rossor M.N. Altered body schema processing in frontotemporal dementia with C9ORF72 mutations. J. Neurol. Neurosurg. Psychiatry. 2014;85:1016–1023. doi: 10.1136/jnnp-2013-306995. (jnnp-2013-306995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.D., Downey L.E., Golden H.L., Clark C.N., Slattery C.F., Paterson R.W., Rohrer J.D., Schott J.M., Rossor M.N., Warren J.D. Pain and temperature processing in dementia: a clinical and neuroanatomical analysis. Brain. 2015;138:3360–3372. doi: 10.1093/brain/awv276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Radua J., McGuire P., Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr. Bull. 2012;38:1297–1307. doi: 10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn D.C., Laird A.R., Ellison-Wright I., Thelen S.M., Robinson J.L., Lancaster J.L., Bullmore E., Fox P.T. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol. Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailstone J.C., Ridgway G.R., Bartlett J.W., Goll J.C., Buckley A.H., Crutch S.J., Warren J.D. Voice processing in dementia: a neuropsychological and neuroanatomical analysis. Brain. 2011;134:2535–2547. doi: 10.1093/brain/awr205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D., Finger E.C. Psychotic symptoms in frontotemporal dementia. Curr. Neurol. Neurosci. Rep. 2015;15:1–8. doi: 10.1007/s11910-015-0567-8. [DOI] [PubMed] [Google Scholar]
- Kiran C., Chaudhury S. Understanding delusions. Ind. Psychiatry J. 2009;18:3–18. doi: 10.4103/0972-6748.57851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landqvist Waldö M., Gustafson L., Passant U., Englund E. Psychotic symptoms in frontotemporal dementia: a diagnostic dilemma? Int. Psychogeriatr. 2015;27:531–539. doi: 10.1017/S1041610214002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.E., Khazenzon A.M., Trujillo A.J., Guo C.C., Yokoyama J.S., Sharon J.S., Takada L.T., Karydas A.M., Block N.R., Coppola G. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain. 2014;137:3047–3060. doi: 10.1093/brain/awu248. (awu248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. (nsp052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillo P., Garcin B., Hornberger M., Bak T.H., Hodges J.R. Neurobehavioral features in frontotemporal dementia with amyotrophic lateral sclerosis. Arch. Neurol. 2010;67:826–830. doi: 10.1001/archneurol.2010.146. [DOI] [PubMed] [Google Scholar]
- Lillo P., Mioshi E., Burrell J.R., Kiernan M.C., Hodges J.R., Hornberger M. Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney C.J., Beck J., Rohrer J.D., Lashley T., Mok K., Shakespeare T., Yeatman T., Warrington E.K., Schott J.M., Fox N.C., Rossor M.N., Hardy J., Collinge J., Revesz T., Mead S., Warren J.D. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135:736–750. doi: 10.1093/brain/awr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M.F., Shapira J.S., Woods R.J., Licht E.A., Saul R.E. Psychotic symptoms in frontotemporal dementia: prevalence and review. Dement. Geriatr. Cogn. Disord. 2008;25:206–211. doi: 10.1159/000113418. [DOI] [PubMed] [Google Scholar]
- Mioshi E., Dawson K., Mitchell J., Arnold R., Hodges J.R. The Addenbrooke's cognitive examination revised (ACE-R): a brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry. 2006;21:1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- Mioshi E., Hsieh S., Savage S., Hornberger M., Hodges J.R. Clinical staging and disease progression in frontotemporal dementia. Neurology. 2010;74:1591–1597. doi: 10.1212/WNL.0b013e3181e04070. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodera H., Izumi Y., Kaji R. New diagnostic criteria of ALS (Awaji criteria) Brain Nerve. 2007;59:1023–1029. [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G., Onyike C.U., Hillis A.E., Josephs K.A., Boeve B.F., Kertesz A., Seeley W.W., Rankin K.P., Johnson J.K., Gorno-Tempini M.L., Rosen H., Prioleau-Latham C.E., Lee A., Kipps C.M., Lillo P., Piguet O., Rohrer J.D., Rossor M.N., Warren J.D., Fox N.C., Galasko D., Salmon D.P., Black S.E., Mesulam M., Weintraub S., Dickerson B.C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T.W., Manes F., Grafman J., Cappa S.F., Freedman M., Grossman M., Miller B.L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton A.E., Majounie E., Waite A., Simón-Sánchez J., Rollinson S., Gibbs J.R., Schymick J.C., Laaksovirta H., Van Swieten J.C., Myllykangas L. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert D., Sonoda L.I., Hayes C., Hill D.L., Leach M.O., Hawkes D.J. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans. Med. Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Schofield E.C., Halliday G.M., Kwok J., Loy C., Double K.L., Hodges J.R. Low serum progranulin predicts the presence of mutations: a prospective study. J. Alzheimers Dis. 2010;22:981–984. doi: 10.3233/JAD-2010-101032. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa S., Nakajima S., Plitman E., Graff-Guerrero A., Mimura M., Nakayama K., Miller B.L. Psychosis in frontotemporal dementia. J. Alzheimers Dis. 2013;42:485–499. doi: 10.3233/JAD-140312. [DOI] [PubMed] [Google Scholar]
- Snowden J.S., Rollinson S., Thompson J.C., Harris J.M., Stopford C.L., Richardson A.M., Jones M., Gerhard A., Davidson Y.S., Robinson A., Gibbons L., Hu Q., DuPlessis D., Neary D., Mann D.M., Pickering-Brown S.M. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135:693–708. doi: 10.1093/brain/awr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford P.M., Shepherd C.E., Halliday G.M., Brooks W.S., Schofield P.W., Brodaty H., Martins R.N., Kwok J.B.J., Schofield P.R. Mutations in the tau gene that cause an increase in three repeat tau and frontotemporal dementia. Brain. 2003;126:814–826. doi: 10.1093/brain/awg090. [DOI] [PubMed] [Google Scholar]
- Turner M.R., Goldacre R., Talbot K., Goldacre M.J. Psychiatric disorders prior to amyotrophic lateral sclerosis. Ann. Neurol. 2016;80:935–938. doi: 10.1002/ana.24801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J.L., Weigand S.D., Boeve B.F., Senjem M.L., Gunter J.L., DeJesus-Hernandez M., Rutherford N.J., Baker M., Knopman D.S., Wszolek Z.K. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain. 2012;135:794–806. doi: 10.1093/brain/aws001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhou J., Seeley W.W. Network dysfunction in Alzheimer's disease and frontotemporal dementia: implications for psychiatry. Biol. Psychiatry. 2014;75:565–573. doi: 10.1016/j.biopsych.2014.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material