Abstract
Advax, a delta inulin-derived microparticle, has been developed as an adjuvant for several vaccines. However, its immunological characteristics and potential mechanism of action are yet to be elucidated. Here, we show that Advax behaves as a type-2 adjuvant when combined with influenza split vaccine, a T helper (Th)2-type antigen, but behaves as a type-1 adjuvant when combined with influenza inactivated whole virion (WV), a Th1-type antigen. In addition, an adjuvant effect was not observed when Advax-adjuvanted WV vaccine was used to immunize toll-like receptor (TLR) 7 knockout mice which are unable to respond to RNA contained in WV antigen. Similarly, no adjuvant effect was seen when Advax was combined with endotoxin-free ovalbumin, a neutral Th0-type antigen. An adjuvant effect was also not seen in tumor necrosis factor (TNF)-α knockout mice, and the adjuvant effect required the presences of dendritic cells (DCs) and phagocytic macrophages. Therefore, unlike other adjuvants, Advax potentiates the intrinsic or in-built adjuvant property of co-administered antigens. Hence, Advax is a unique class of adjuvant which can potentiate the intrinsic adjuvant feature of the vaccine antigens through a yet to be determined mechanism.
Keywords: Vaccine, Adjuvant, Particle, Inulin, Macrophage
Highlights
-
•
Advax potentiates built-in adjuvant property of vaccine antigens.
-
•
Advax does not change the T helper immune bias induced by the vaccine antigen.
-
•
Dendritic cells, phagocytic macrophages, and tumor necrosis factor-α play a role in Advax adjuvant activity.
Adjuvants are indispensable agent to maximize the efficacy of vaccines. Most adjuvants consistently impart either T helper (Th)1, Th2 or Th17 bias to the vaccine response regardless of the properties of antigen. For example alum adjuvants consistently impart a Th2 bias regardless of the vaccine antigen. Here we show that a delta inulin-derived microparticle adjuvant, Advax, is an additional class of adjuvant that functions as an amplifier of in-built adjuvant activity within the antigens themselves. Advax enhances different types of adaptive immune response dependent on the antigen's own in-built adjuvant properties, confirming Advax's utility as a general purpose vaccine adjuvant.
1. Introduction
Adjuvants play a critical role in initiating, maximizing and prolonging the immunogenicity, and thereby efficacy, of many vaccines. For most of the last 90 years, aluminum salts (alum) were the only routinely used human adjuvants. Recently, limited additional adjuvant formulations including squalene oil emulsions and the combination of alum with monophosphoryl lipid A have been licensed for human use. Suitable adjuvants should ideally be selected based on desired vaccine properties including the type of immune response best correlated with pathogen protection. Unfortunately the paucity of adjuvants currently approved for human use limits the ability to fine-tune vaccine immune responses, leaving an urgent need for development of different types of adjuvant.
Pathogen recognition receptors (PRRs) such as Toll-like receptor (TLRs), Nod-like receptors (NLRs) or inflammasome receptors targeted adjuvants result in inflammatory cytokine and type I interferon (IFN) production and upregulation of co-stimulatory molecules on antigen presenting cells (APCs) (Olive, 2012). Pathogen-associated molecular patterns (PAMPs) such as microbial nucleic acids, glycolipids or proteins contained in vaccines function as endogenous built-in adjuvants that trigger innate immune responses and consequently shape the type of adaptive immune response induced by a particular antigen (Desmet and Ishii, 2012). For example, viral RNA in influenza WV vaccine activates TLR7 and thereby induces a Th1-biased response to influenza inactivated whole virion (WV) antigens (Geeraedts et al., 2008, Koyama et al., 2010).
Advax is a microparticle polysaccharide adjuvant in which its microparticles are derived from microparticles of polyfructofuranosyl-d-glucose (delta inulin). It has been shown to improve the immunogenicity and efficacy of a wide variety of vaccines including against influenza, hepatitis B, Japanese encephalitis, West Nile virus, Human immunodeficiency virus, anthrax and Listeria (Dolter et al., 2011, Feinen et al., 2014, Honda-Okubo et al., 2012, Larena et al., 2013, Petrovsky et al., 2013, Rodriguez-Del Rio et al., 2015, Saade et al., 2013). Vaccines containing Advax adjuvant have already been evaluated in human clinical trials, including hepatitis B, influenza and insect-sting allergy vaccines (Gordon et al., 2014, Heddle et al., 2013, Nolan et al., 2008).
Although these clinical trials have shown superior immunogenicity and excellent tolerability of Advax-adjuvanted vaccines, the mechanism of action of Advax remains unknown. In this study, we examined the adjuvant effect of Advax with various different vaccine antigens. Unexpectedly, Advax, unlike alum and TLR agonists, did not impart bias to the immune response against the co-administered antigen. Instead, intriguingly, Advax enhanced the immune bias imparted by the vaccine antigen itself, suggesting that Advax functions as an amplifier of in-built adjuvant activity contained within the antigens themselves.
To further explore the mode of action of Advax, the biological properties of Advax itself were examined in vitro and in vivo. This data suggested that phagocytic macrophages are one of the adjuvanticity mediators of Advax and tumor necrosis factor (TNF)-α signaling pathway is important for the adjuvant activity.
2. Materials & Methods
2.1. Mice
Six-week-old female (for all animal experiment except microarray analysis) or male (for microarray analysis) C57BL/6J mice were purchased from CLEA Japan. Tlr7−/− or Il-1r−/− mice were purchased from Oriental BioService and the Jackson Laboratory, respectively. Card9−/− (Hara et al., 2007), Fcrg−/− (Arase et al., 1997) or Dap12−/− (Takai et al., 1994) mice were kindly donated by Dr. Hara, Dr. Saito or Dr. Takai, respectively. Tnfa−/− mice were described previously (Marichal et al., 2011). All animal experiments were approved by the Institutional Animal Care and Use Committee, and performed in accordance with institutional guidelines for the National Institute of Biomedical Innovation, Health and Nutrition animal facility. Mice were monitored at least once daily by technical staff members who were blinded to the study aims.
2.2. Antigens, Antibodies, Adjuvants and Peptides
Ovalbumin was purchased from Seikagaku-kogyo. SV and inactivated WV derived from A/New Caledonia/20/99 strain were a gift from the Institute of Microbial Chemistry (Osaka, Japan). CpG-ODN (5′-ATCGACTCTCGAGCGTTCTC-3′) was synthesized by GeneDesign (Osaka, Japan). CpG-SPG was prepared as previously reported (Kobiyama et al., 2014). Alum (A8222) was purchased from Sigma. Advax and hepatitis B surface antigen (HBs) were provided by Vaxine Pty Ltd. LPS was purchased from Sigma. MHC Class I (ASNENMETM) and class II (ARSALILRGSVAHKSCLPACVYGP) epitope peptides of nucleoprotein were synthesized by Operon Biotechnologies. Cytokine ELISA kits for IFN-γ (DY485), IL-13 (DY413), IL-17 (DY421), TNF-α (DY410) and IL-1β (DY401) were purchased from R&D Systems.
2.3. Immunizations
C57BL/6J mice were immunized twice either intramuscularly (i.m.) or i.d. with 2 week intervals (days 0 and 14). For antigen-specific ELISA, blood samples were taken on days 14 and 28. During vaccination and bleeding, mice were anesthetized with ketamine. Alum-adjuvanted antigen was rotated for more than 1 h before immunization. Advax, alum or CpG-SPG was used for immunization at 1 mg per mouse, 0.67 mg per mouse or 10 μg per mouse, respectively. Control group was administered saline.
2.4. Antibody Titers
For ELISA, 96-well plates were coated with 1 μg/mL SV in carbonate buffer (pH 9.6) for SV- and WV-vaccinated groups, 10 μg/mL OVA for OVA-vaccinated groups, and 1 μg/mL HBs for HBs-vaccinated groups. Wells were blocked with PBS containing 1% bovine serum albumin and diluted sera from immunized mice were incubated on the antigen-coated plate. After washing, goat anti-mouse total IgG, IgG1 or IgG2c conjugated with horseradish peroxidase (Southern Biotech) were added and incubated for 1 h at room temperature. After additional washing, the plates were incubated with TMB substrate for 30 min, the reaction was stopped with 1 N H2SO4 and then the absorbance was measured. Antibody titers were calculated. OD of 0.2 was set as the cut-off value for positive samples. The concentration of total IgE in serum was measured by a total IgE ELISA kit (Bethyl, E90-115).
2.5. Measurement of Antigen-specific Cytokine Responses
Two weeks after the second immunization, spleens were collected from mice and 1 × 106 splenocytes were plated on 96-well plates and stimulated with 20 μg/mL MHC class I or II epitope peptides of nucleoprotein for 2 days. After the stimulation, IFN-γ, IL-13 and IL-17 in supernatant were measured by cytokine ELISA kits.
2.6. Cytokine Production Profile
Several time points after i.p. injection of adjuvant, mice were sacrificed and peritoneal lavage fluids were collected. The cytokines in the fluids were measured by Bio-plex (BioRad, M60009RDPD).
2.7. Activation of DCs
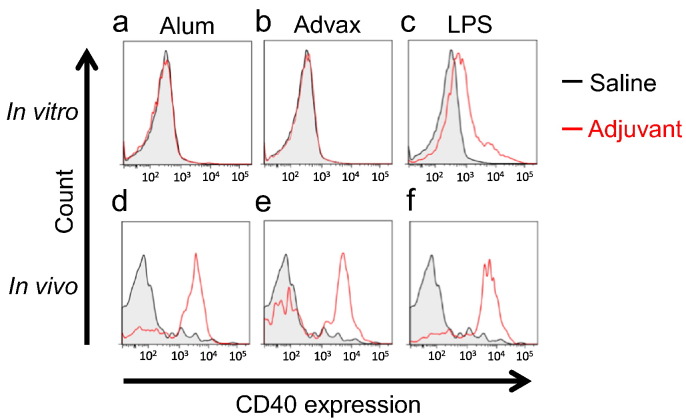
For in vitro experiments, bone marrow-derived DCs were generated by cultivation of bone marrow cells in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 1% Penicillin/Streptomycin solution (NaclaiTesque) and 100 ng/mL of human fms-like tyrosine kinase 3 ligand (Flt3L) (PeproTech, 300-19) for 7 days, stimulated with 1 mg/mL alum, 1 mg/mL Advax or 50 ng/mL LPS (Sigma) for 15 h and then CD40 expression on plasmacytoid DCs (pDCs) was evaluated by FACS. We defined pDC as CD11c+/SiglecH+ cells and cDC as CD11c+/SiglecH− cells.
In vivo experiments performed as described previously (Kobiyama et al., 2014). Briefly, C57BL/6J mice were injected with 0.67 mg alum, 1 mg Advax or 50 ng LPS at the base of tail. Twenty-four hours after the injection, draining lymph nodes were removed, treated with DNaseI and collagenase for 30 min, stained with anti-mCD11c (eBioscience clone N418), mCD8α (eBioscience clone 56-6.7), mPDCA-1 (eBioscience clone JF05-1C2.4.1), mCD40 (eBioscience clone 3/23) antibodies and 7AAD (BioLegend, 420,404) and analyzed by FACS. We defined pDC as CD11c+/mPDCA-1+ cells, CD8α+ DC as CD11c+/CD8α+ cells and CD8α− DC as CD11c+/CD8α−/mPDCA-1− cells.
2.8. In Vitro Stimulation of Macrophages and GM-DCs
For macrophage preparation, mice were i.p. injected with 3 mL of 4% (w/v) thioglycolate (Sigma) solution. Four days later, macrophages were collected from the peritoneal cavity and plated on 96-well plates. Macrophages were primed with 50 ng/mL LPS for 18 h, and stimulated with adjuvants for 8 h. IL-1β in supernatants was measured by ELISA. TNF-α in supernatants was measured by ELISA after stimulation with Advax or alum without priming by LPS.
For GM-DC preparation, mouse bone marrow cells were cultured in RPMI 1640 supplemented with 10% FBS, 1% Penicillin/Streptomycin solution and 20 ng/mL mouse GM-CSF (PeproTech, 315-03) for 7 days. GM-DCs were collected, plated on 96-well plates, primed with 50 ng/mL LPS for 18 h and then stimulated with adjuvants for 8 h. IL-1β in supernatants was measured by ELISA.
2.9. Two Photon Microscopy Analysis
Biotinylated delta inulin particles (1 mg) were pre-mixed with Brilliant Violet 421 Streptavidin (BioLegend, 405225), and then administered i.d. at the tail base of mice. At 30 min before inguinal LN removal, mice were i.d. administered anti-MARCO-phycoerythrin (AbD Serotec clone ED31) or anti-CD169-FITC (BioLegend clone 3D6.112) antibodies. Distributions of Advax particles in the inguinal lymph nodes were examined by two-photon excitation microscopy (FV1000MPE; Olympus, Tokyo, Japan) with Olympus XLPLN25XWMP objective lens (water immersed; numerical aperture, 1.05).
2.10. Clodronate Liposome Injection
Mice were administered 100 μL clodronate liposome (FormMax, F70101C-A) to the base of the tail either 7 or 2 days before immunization. Mice were immunized with WV (1.5 μg) plus adjuvant at the base of the tail on days 0 and 14. Of note, -d2 clodronate treatment depleted both macrophages and DCs at d0. -d7 treatment depleted macrophages but DCs were already recovered at d0. Blood samples were taken on days 14 and 28, and serum antibody titers were measured by ELISA.
2.11. Microarray Analysis
Six hours after administration of 1 mg Advax, the spleen, lung, kidney, lymph node and liver were removed (n = 3), and total RNAs were extracted as described previously (Onishi et al., 2015). After total RNA preparation, the gene expression profiles were obtained using 3′ IVT Express Kit and GeneChip Mouse Genome 430 2.0 Array (Affymetrix, 900496). The expression values were normalized by the median value of each GeneChip. The resulting digital image files were preprocessed using the Affymetrix Microarray Suite version 5.0 algorithm (MAS5.0). The differential expression with P-values by using t-test was computed as the ratio between the mean of the treated samples and control samples. Further customization of the PA (presence and absence) call in MAS5.0 (Pepper et al., 2007) was done as follows. When the ratio is > 1, the PA call is determined by the dominant call of the treated samples. When the ratio is < 1 or = 1, the call is determined by the dominant call of the control vehicle samples. Dominant calls (over half) were applied to a set of samples (e.g. when the ratio < 1 and PA call of control samples are “P”, “P”, and “A”, then the customized PA call of the set is “1”). The MAS5.0 and PA calls analysis were conducted using the Bioconductor Affy package for R (http://www.bioconductor.org). For subsequent analyses, we only included probes where the fold-change between control and stimulated samples was > 2. We excluded probes that were Absent-flagged - i.e. the customized PA call is “0”. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus (Edgar et al., 2002) and are accessible through GEO Series accession number GSE89249 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE89249).
2.12. Cell Population Analysis
The cell population analysis was carried out as follows. We obtained the gene expression profile of various immune cell types from a steady state condition directly from the ImmGen database (http://www.immgen.org/). These expression profiles were used to estimate the cell type origin of the genes. We began by weighting each gene (i) in all ten immune cell types (j):
| (1) |
We assumed that the weight of a cell type of a given gene depended only on the expression level of that gene in that particular cell type independent from the expression in other cell types.
Given the expression profile of each adjuvant sample, we determined the genes based on the fold change (FC > 2), and PA-call threshold (PA = 1). Finally the cell type contribution for sample (k) for cell type (j) was calculated as:
| (2) |
where c is the pseudocount. In Fig. 6b, the above score is represented as the thickness of the ribbon.
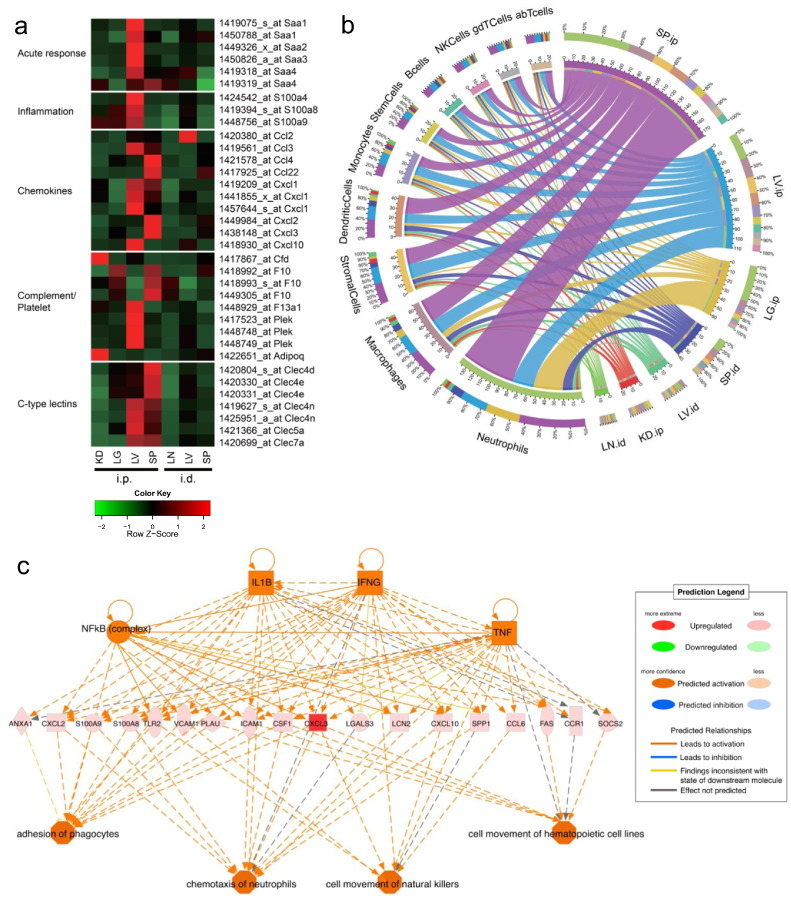
Fig. 6.
Advax alters gene expression of IL-1β, CLRs and TNF-α signaling pathways. Whole organ (lung; LG, liver; LV, spleen; SP, kidney; KD, lymph node; LN) transcriptome of 6 h after Advax administration (i.d. or i.p.) alone was obtained by Affimetrix GeneChip (n = 3). (a) Only selected gene probes (FC > 2 and PA = 1) are displayed. (b) Advax-responding cell population analysis was performed (see Materials & Methods; cell population analysis). The left hemispheres of the figure denote the ten immune cell types and right hemispheres denote the samples. The ribbons in the inner circle denote the cell type score of each sample. The colors in the middle layer rings represent either cell types or samples. The outermost ring denotes the percentage of total contribution for every factor (cell type or samples) viewed from their counterpart factor. For example in SP.ip, neutrophils constitute around 30% of the cell population. (c) IPA upstream regulator analysis of Advax-induced genes in the SP was performed. (see Materials & Methods; upstream regulator analysis in IPA). FC: Fold Change, PA: Presence and Absence. (see Materials & Methods; microarray analysis).
2.13. Upstream Regulator Analysis in IPA
We performed upstream analysis using the IPA Regulator Effects feature by Ingenuity Pathways Analysis (Ingenuity® Systems, http://www.ingenuity.com). The main purpose was to elucidate the upstream regulatory mechanism and their connection to downstream functional impact. For this analysis we selected genes with fa old change > 2 and PA-call = 1. Finally we reported networks with a P-value < 0.001.
2.14. Statistical Analysis
Statistical analyses were performed by GraphPad Prism 5.02. Statistical significance (P < 0.05) between groups was determined by Dunnett's Multiple comparison test or Student t-test.
3. Results
3.1. Advax Adjuvant Enhances Th2 Responses When Combined With a Th2-type Antigen
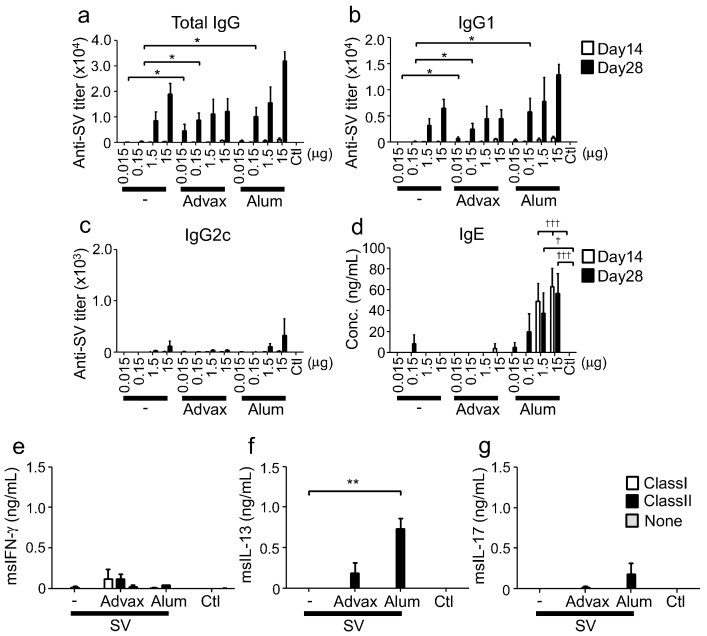
To understand the adjuvant effect of Advax, we initially examined immune responses in mice immunized with Advax plus influenza split vaccine (SV), an antigen previously shown to elicit Th2 immune responses (Kistner et al., 2010). SV immunization alone elicited IgG1 production (a Th2-type IgG subclass) and at higher immunization doses induced IL-13 (Th2-type cytokine) production, consistent with it being a Th2-inducing antigen (Figs. 1a–c and S1a–c). The addition of Advax to SV enhanced IgG1 but not IgG2c antibody production, at lower (0.015 and 0.15 μg) antigen doses with significant enhancement not observed at the higher antigen dose (Fig. 1a–c). Alum, a typical Th2 type adjuvant commonly used for human vaccination also enhanced a IgG1 dominant antibody responses at the lower antigen (0.15 μg) dose. Hence Advax, similarly to alum, induced a Th2 response to SV antigen. Alum is also known to induce IgE production, potentially increasing the risk of vaccine allergy (Nordvall et al., 1982). Therefore, in serum IgE-levels in sera were measured after SV immunization with either Advax or alum. Whereas alum significantly increased IgE production in a dose-dependent manner, Advax did not induce IgE production (Fig. 1d), consistent with Advax only magnifying the antibody subtype response induced by the SV alone.
Fig. 1.
Advax induces Th2 responses when combined with a Th2-type antigen. (a-d) On days 0 and 14, C57BL/6J mice (n = 3) were immunized i.m. with SV and adjuvant. Antigen-specific total IgG, IgG1 and IgG2c titers, and total IgE in sera at days 14 and 28 were measured by ELISA. Mice in control (Ctl) group were administered saline. (e-g) On day 28, splenocytes were prepared from mice immunized with 15 μg SV and adjuvant, and stimulated with MHC class I or II epitope peptides of nucleoprotein. After stimulation, IFN-γ, IL-13 and IL-17 in supernatants were measured by ELISA. Results are representatives of three separate experiments. Median and SEM are shown for each group. Statistical significances are indicated, *P < 0.05, **P < 0.01, ***P < 0.001, †P < 0.05, †††P < 0.001 by Dunnett's Multiple comparison test.
T-cell responses were examined by stimulating splenocytes from immunized mice with MHC class I (CD8 T cell) or class II (CD4 T cell) peptides from the influenza virus nucleoprotein. Interestingly, the effect of Advax to enhance IgG1 subclass antibody production was not associated with increased CD4 T cell IL-13 production, which was not significantly different to SV-alone control mice. By contrast, use of alum adjuvant was associated significantly increased CD4 T cell IL-13 production (Fig. 1e–g). These results demonstrated that while Advax enhanced Th2-type antibody response when combined with SV, unlike alum, this was not associated with increased IL-13 or IgE.
3.2. Advax Adjuvant Enhances Th1 Responses When Combined With a Th1-type Antigen
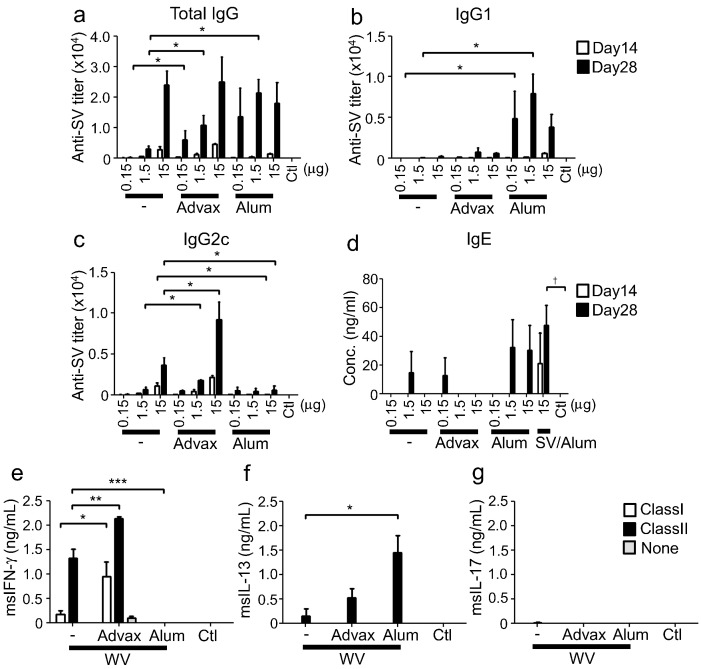
Next, we examined immune responses in mice immunized with inactivated whole virion influenza vaccine (WV) plus either Advax or alum. WV antigen contains viral RNA that induces TLR7 activation and thereby acts as an endogenous adjuvant that induces Th1 responses (Geeraedts et al., 2008, Koyama et al., 2010). When combined with WV, Advax adjuvant only enhanced IgG2c (Th1-type subclass) production with minimal effects on IgG1 production, whereas alum suppressed the WV-induced IgG2c response and significantly increased IgG1 levels (Fig. 2a–d). At the cytokine level, immunization with Advax enhanced IFN-γ (Th1-type cytokine) production by antigen-stimulated CD4 and CD8 T cells when compared to mice immunized with WV alone. By contrast, immunization with alum suppressed IFN-γ production, while significantly increasing IL-13 production by CD4 T cells (Fig. 2e–g). These results demonstrated that Advax functioned as a Th2 adjuvant when combined with SV but a Th1-type adjuvant when combined with WV. By contrast, alum functioned as a Th2-type adjuvant for both SV and WV antigens.
Fig. 2.
Advax induces Th1 responses when combined with Th1-type antigen. (a–d) on days 0 and 14, C57BL/6J mice (n = 3) were immunized i.m. with WV and adjuvant. 15 μg of SV was immunized with alum on day 0 and 14. Antigen-specific total IgG, IgG1 and IgG2c titres, and total IgE in sera at days 14 and 28 were measured by ELISA. (e–g) On day 28, splenocytes were prepared from mice immunized with 15 μg WV and adjuvant, and stimulated with MHC class I or II specific peptides from nucleoprotein. After stimulation, IFN-γ, IL-13 and IL-17 in supernatants were measured by ELISA. Results are representative of three separate experiments. Median and SEM are shown for each group. Statistical significances are indicated, *P < 0.05, **P < 0.01, ***P < 0.001, †P < 0.05 by Dunnett's Multiple comparison test.
3.3. Advax Adjuvant Effect Is Shaped by Endogenous Adjuvant in the Vaccine Antigen
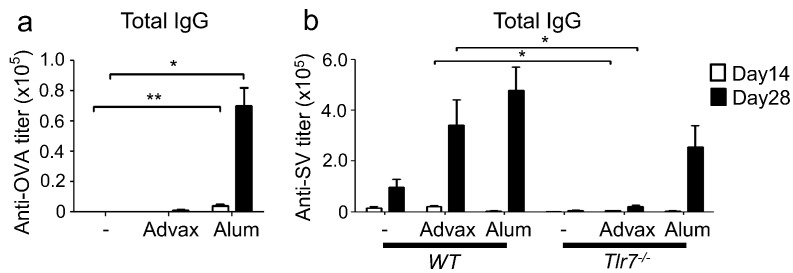
The above findings indicated that whereas the combination of Advax with a Th2-type antigen enhanced Th2 immunity, its combination with a Th1-type antigen enhanced Th1 immunity. This led us to hypothesize that the adjuvant effect of Advax might be mediated by an effect on potentiating the inherent adjuvant property encapsulated within each antigen. By contrast, other adjuvants have a fixed immune bias effect whereby, for example, alum biases to Th2 responses and CpG-ODN to Th1 responses, regardless of the innate properties of the antigen with which they are co-administered. To test this hypothesis, we examined the adjuvant effect of Advax on ovalbumin (OVA) antigen, which is considered a neutral Th0-type antigen. Interestingly, Advax failed to enhance the OVA-specific antibody response whereas alum, as expected, solely enhanced IgG1 production (Figs. 3a, S2a and b).
Fig. 3.
Advax does not enhance antibody responses to OVA, a Th0-type antigen or to WV in Tlr7−/− mice. (a) C57BL/6J mice (n = 4 or 5) or (b) Tlr7−/− mice (n = 5) were i.m. immunized twice (day 0 and 14) with 100 μg OVA and adjuvant, or 1.5 μg WV alone or together with adjuvant, respectively. Antigen-specific total IgG titers in sera at days 14 and 28 were measured by ELISA. Results are representative of two or three separate experiments. Median and SEM are shown for each group. Statistical significances are indicated, *P < 0.05, **P < 0.01 by Dunnett's Multiple comparison test or student t-test.
It has previously been shown that the endogenous built-in adjuvant effect of WV was abolished in Tlr7-deficient mice, establishing the importance of TLR7 signaling in WV immunogenicity (Geeraedts et al., 2008, Koyama et al., 2010). Thus, we tested the adjuvant effect of Advax on WV in Tlr7-deficient mice. Notably, the enhanced WV-antibody response seen with Advax in wild type mice was lost in Tlr7-deficient mice, whereas the enhanced IgG1 response induced by alum was preserved in Tlr7-deficient mice (Figs. 3b, S2c and d). Taken together, these findings suggest that Advax belongs to an additional not previously described class of adjuvant that functions as an amplifier of endogenous built-in adjuvants contained within vaccine antigens without changing their inherent immune-polarizing properties.
3.4. Advax Adjuvant Activates Dendritic Cells In vivo but Not In vitro
Because Advax's adjuvant effect is shaped by co-administered vaccine antigen, the basic biological effect of Advax itself without antigen was examined in vitro and in vivo. Since dendritic cells (DCs) play a central role in adjuvant-induced immune responses, we first investigated the ability of Advax to activate DCs, in vitro and in vivo. Mouse bone marrow-derived DCs were stimulated with Advax, alum or lipopolysaccharide (LPS) for 15 h in vitro and the expression of CD40, an activation marker on DCs, was evaluated by flow cytometry. Whereas LPS as expected increased CD40 expression on pDCs and cDCs in vitro, neither Advax nor alum influenced CD40 expression on both DC populations (Figs. 4a–c and S3a–c). Next we examined the ability of Advax or alum to activate DC CD40 expression, in vivo, by measuring CD40 expression on DCs from draining lymph nodes following local adjuvant administration. In contrast to the in vitro findings, both Advax and alum when administered in vivo increased the frequency of activated pDCs in draining lymph nodes (Fig. 4d–f). Notably, adjuvant dependent activation of CD8α+ DCs and CD8α− DCs were not observed in all adjuvants we tested by this evaluation method (Fig. S4a–f). Effect of adjuvants on CD86 expressions on all DCs populations (pDC, CD8α+ DCs and CD8α− DCs) were similar to effects on CD40 expression (data not shown). These results suggested that Advax activates dendritic cells in vivo by a so far unidentified mechanism that is not a direct effect of Advax on DC themselves as indicated by the lack of in vitro effect.
Fig. 4.
Advax activates DCs in vivo but not in vitro. (a–c) Bone marrow-derived DCs were stimulated with 1 mg/mL alum, 1 mg/mL Advax or 50 ng/mL LPS for 24 h in vitro, and then the expression of CD40 on pDCs was evaluated. (d–f) C57BL/6J mice were injected with 0.67 mg alum, 1 mg Advax or 50 ng LPS at the base of the tail. Twenty-four hours after injection, the inguinal lymph nodes were collected, treated with DNase I and collagenase. Then, the cells were stained and the expression of CD40 on pDCs was analyzed by FACS. Results are representative of three separate experiments.
Alum-induced DC activation in vivo but not in vitro can potentially be explained by alum's effect to induces cell death at the injection site thereby resulting in release of extracellular DNA (Marichal et al., 2011) that then activate DAMP receptors on DCs. We therefore asked whether Advax might similarly induce cell death at the injection site and thereby indirectly activate DCs via DAMP receptors. To test this possibility, cytotoxicity and host DNA/RNA release at the injection site of Advax or alum were evaluated in vivo. After the intraperitoneal (i.p.) administration of Advax or alum, peritoneal lavage fluids were collected, and the number of dead cells and the concentration of DNA/RNA in these fluids were measured. Whereas alum induced cell death and nucleic acid release as expected, injection of Advax induced no cytotoxicity or nucleic acid release, indicating that DAMP signaling pathways are not involved in the ability of Advax to induce DC activation and CD40 or CD86 expression, in vivo (data not shown).
3.5. Phagocytic Macrophages Are Cellular Mediators of the Adjuvant Effect of Advax
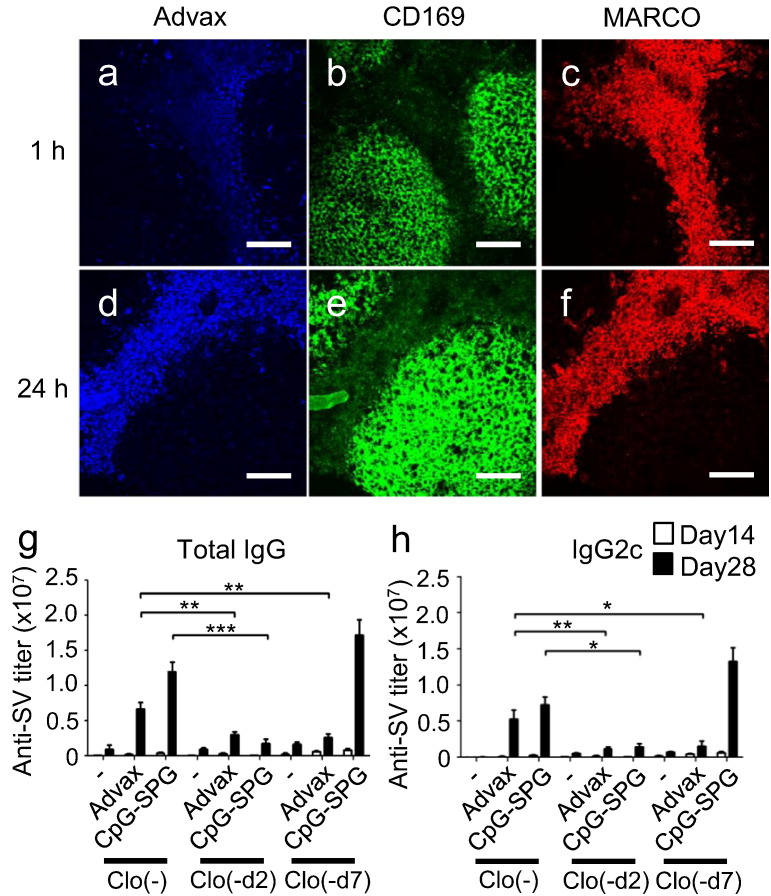
Although the immune complexes of inactivated influenza virus are captured by CD169+ (also called Siglec-1 or MOMA-1) macrophages in draining lymph nodes (DLNs) to induce humoral immune responses (Gonzalez et al., 2010, Suzuki et al., 2009), some particulate adjuvants are efficiently taken up by MARCO+ macrophages (Aoshi et al., 2009, Kobiyama et al., 2014). Thus, the behavior of Advax in DLN was analyzed in vivo using fluorescent-labeled Advax particles. One hour after intradermal (i.d.) administration, a weak Advax signal co-localized with MARCO+ macrophages in the DLN, with this co-localization signal being much higher 24 h later. Almost no co-localization of Advax with CD169+ macrophages was observed in the DLN (Fig. 5a–f), suggesting i.d. administered Advax is taken up by MARCO+ macrophages. Next, to investigate the requirement of Advax uptake into macrophages for its adjuvant effect, we utilized the different recovery kinetics of macrophages and DCs following clodronate liposome injection. This depletes both macrophages and DCs completely by day 2 but subsequently, macrophages do not recover for at least 7 days whereas DCs are mostly recovered by this time (Aoshi et al., 2008, Kobiyama et al., 2014). Interestingly, the adjuvant effect of Advax was significantly reduced with clodronate liposome treatment, irrespective of the time point tested (d2 or d7) (Fig. 5g and h), suggesting Advax adjuvanticity is dependent on the presence of macrophages. By contrast, the adjuvanticity of CpG-SPG was significantly reduced d2 post-clodronate treatment but not at d7, indicating that the adjuvanticity of CpG-SPG was mostly dependent on DCs but not macrophages, as previously reported (Kobiyama et al., 2014).
Fig. 5.
Macrophages are required for Advax's adjuvant effect. Two-photon microscopy analysis of lymph nodes (a–c) 1 h and (d–f) 24 h after i.d. administration of Brilliant Violet 421-labeled Advax delta inulin particles. CD169+ and MARCO+ macrophages were stained by i.d. injection of anti-CD169-FITC and anti-MARCO-phycoerythrin antibodies 30 min before Advax administration. The pictures were taken by FV1000MPE (Olympus) with 25 × lens (XLPLN25XWMP; Olympus). Scale bar indicates 100 μm. (a, d) Blue indicates Advax, (b, e) green indicates CD169+ macrophages and (c, f) red indicates MARCO+ macrophages. (g, h) Phagocytic cells in lymph nodes were depleted by clodronate liposome injection at the indicated days (-d2 and -d7), and then WV plus Advax was i.d. immunized at d0. Antigen-specific total IgG or IgG2c titers in sera at days 14 and 28 were measured by ELISA. Results are representative of three separate experiments. Median and SEM are shown for each group. Statistical significances are indicated, *P < 0.05, **P < 0.01, ***P < 0.001 by Student's t-test.
3.6. Advax Alters the Gene Expression of IL-1β-, C-type Lectin Receptors- and TNF-α-related Signaling Pathways
To understand the biological properties of Advax, its effect on cytokine production in vivo was investigated. At several time points after i.p. administration of Advax or alum, peritoneal lavage fluids were collected, and cytokines in the fluids were analyzed. Alum induced the production of various cytokines including interleukin (IL)-5, IL-10, IL-12, TNF-α, and granulocyte-colony stimulating factor (G-CSF) (Fig. S5a), whereas only a very limited cytokine response involving IL-10, G-CSF and macrophage inflammatory protein (MIP)-1 was observed after Advax administration (Fig. S5b).
To further explore the predominated biological effect of Advax in vivo, gene expression profiles were examined in tissues after local (i.d.) or systemic (i.p.) administration of Advax. Advax administration via i.d. route conferred limited gene expression changes, whereas i.p. administration altered gene expression in several tissues (Fig. 6a). Administration of Advax via i.p. route resulted in differential gene expressions in the liver and spleen at 6 h post-injection associated with the acute phase response, inflammation, chemokines, complement/platelet, and C-type lectin receptor (CLRs)-related responses. Furthermore, analysis of the cell populations corresponding to the differentially regulated genes suggested that Advax activated genes expressed by neutrophils and macrophages in vivo (Fig. 6b). Additionally, upstream regulator analysis in ingenuity pathway analysis (IPA) suggested that four upstream regulators: NF-κB (complex), IL-1β, IFN-γ, and TNF-α were affected by i.p. administration of Advax. This in turn drives the expression of genes that enhance phagocyte adhesion, neutrophil chemotaxis and movement of hematopoietic and natural killer cells (Fig. 6c). Given the short time frame for these gene effects, and the tissue resident nature of Advax inulin particles, these gene effects in liver and spleen possibly represent the indirect effects mediated by soluble signals released from Advax-stimulated cells in the peritoneum.
To identify the signaling pathway related to the adjuvant effect of Advax, we examine the contribution of these biological factors to the adjuvanticity. We first examined the ability of Advax to induce IL-1β production. Peritoneal macrophages or granulocyte-macrophage colony stimulating factor (GM-CSF)–induced bone marrow-derived DCs (GM-DCs) were stimulated with Advax or alum following LPS-priming, and then IL-1β production was examined. However IL-1β production was not detected in these cells stimulated with Advax in vitro, whereas alum significantly induced IL-1β production (Fig. S6a and b). Because some particulate adjuvants require NLRP3 inflammasome activation and subsequent IL-1β production for their adjuvanticity (Eisenbarth et al., 2008, Kuroda et al., 2013), the effect on Advax adjuvanticity of the absence of the NLPR3 inflammasome components, Nlrp3, Caspase1, or IL-1r was examined in vivo using knockout mice. Advax conferred significant adjuvant effects in each of these NLPR3 inflammasome deficient mice (Fig. S7a–i), indicating the adjuvant effect of Advax is independent of the NLPR3 inflammasome/ IL-1β signaling pathway. Next, to examine the involvement of CLR-related signaling pathways in Advax adjuvanticity, its adjuvant effect was examined in mice lacking the CLRs signaling pathway genes, Fcrg−/−, Card9−/−, or Dap12−/−. Absence of these genes did not affect the ability of Advax to enhance antibody responses (Fig. S8a–i), indicating Advax's adjuvant effect is independent of these CLR-related signaling pathways.
3.7. TNF-α Is Essential for the Adjuvant Effect of Advax
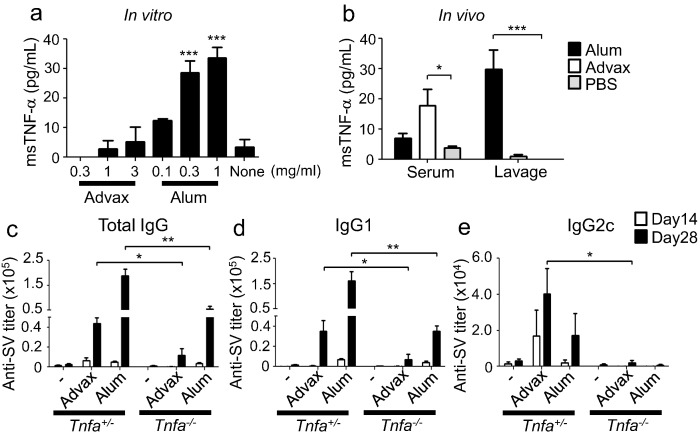
Gene expression analysis indicated that i.p. administration of Advax affected TNF-α-related signaling pathways (Fig. 6c). We thus examined the ability of Advax to generate TNF-α production. Macrophages were stimulated with Advax in vitro and TNF-α measured in the culture supernatant. Advax did not induce TNF-α production whereas stimulation with alum significantly induced macrophage TNF-α production in vitro (Fig. 7a). However, i.p. injection of Advax did result in significantly increased serum TNF-α levels (Fig. 7b), whereas, paradoxically, i.p. alum did not affect serum TNF-α levels. Because i.p. injection of Advax influenced gene expression including TNF-α-signaling pathway-related genes in remote tissues such as the lung and spleen (Fig. 6a and b), this suggests that TNF-α in the serum might be derived from these tissues. Finally, to examine the role of TNF-α in Advax's adjuvant effect, Tnfa−/− mice were immunized with Advax-adjuvanted WV or hepatitis B surface antigen, and the resulting antibody responses were examined (Figs. 7c–e and S9a–c, respectively). The absence of Tnfa significantly decreased the adjuvant effect of Advax on antibody responses, suggesting that intact TNF-α signaling is important for Advax's adjuvant effect on antibody responses.
Fig. 7.
TNF-α is required for the adjuvant effect of Advax. (a) Peritoneal macrophages were stimulated with Advax or alum for 8 h and TNF-α in supernatants was measured by ELISA. (b) One hour after i.p. injection of 1 mg Advax or 0.67 mg alum, sera and peritoneal lavage fluids were collected and TNF-α levels in the sera and fluids were measured by ELISA. (c–e) On days 0 and 14, heterozygous or Tnf−/− mice (n = 5) were immunized i.m. with 1.5 μg WV and adjuvant. Antigen-specific total IgG, IgG1 and IgG2c titers in sera at days 14 and 28 were measured by ELISA. Results are representative of two separate experiments. Median and SEM are shown for each group. Statistical significances are indicated, *P < 0.05, **P < 0.01, ***P < 0.001 by Dunnett's Multiple comparison test or Student's t-test.
4. Discussion
Various particles, such as aluminum salts (alum), poly (lactic-co-glycolic acid) (PLGA) or polystyrene particles, function as vaccine adjuvants (Gupta, 1998, Marrack et al., 2009, Sharp et al., 2009). Antigen loaded on these particles is efficiently taken up by APCs, such as DCs and macrophages, followed by the induction of antigen-specific immune responses (Yan et al., 2013). In addition, antigen-loaded particles also function as an antigen reservoir by the depot effect (Glenny et al., 1926). Size, surface charge, surface hydrophilicity/lipophilicity and antigen-adjuvant binding strength have all been demonstrated to be important for the adjuvant effect of particles (Hayashi et al., 2016a, Yan et al., 2013). Advax is a microparticle adjuvant comprised of delta inulin particles but unlike other particle adjuvants it does not require adsorption with an antigen to mediate its effects, since it still provides an adjuvant effect when administered 24 h prior to administration of the antigen (Saade et al., 2013). By contrast, the typical particulate adjuvant, alum, lost its adjuvant effects when not co-administered with the antigen (Saade et al., 2013). Hence, given the lack of evidence of an antigen binding or depot effect this leaves unanswered the mechanism by which Advax has its adjuvant effect on protein antigens. Hence the current study further provides important new insights into Advax's physiological properties, including contrasting differences between Advax and alum adjuvants. As shown, the combination of Advax with either Th2- or Th1-type antigens enhanced Th2 or Th1 responses, respectively (Figs. 1a–g and 2a–g); whereas its combination with OVA, a Th0-type antigen did not enhance the OVA-specific immune response (Figs. 3a, b and S2a–d). Interestingly, despite enhancing the Th2 response to SV, Advax, unlike alum, did not induce T-cell IL13 production or antigen-specific IgE. This is an important finding as it would suggest that Advax should be safer than alum in respect of the risk of induction of vaccine allergies in immunized subjects. Indeed, Advax has recently been shown in allergic human subjects to be useful for accelerating allergy desensitization therapy (Heddle et al., 2013). It has been reported that when combined with some antigens, Advax also induces modest IL-17 production (Saade et al., 2013), suggesting a Th17 response is also inducible by Advax according to the in-built adjuvant properties of the antigen. By contrast, alum induces Th2 immune responses regardless of the property of the antigen co-administered and other adjuvants such as poly (I:C) or CpG-ODN consistently induce a Th1 response (Coffman et al., 2010). These results suggest that Advax potentiates the inherent immune properties of each antigen.
Although it is very interesting that Advax provides different immune response depending on the type of antigen, this feature makes it difficult to analyze Advax in the presence of antigen. In order to exclude potential confounding immune effects of antigen in-built adjuvants, we examined Advax's basic biological effects without antigen. Both Advax and alum activate DCs in vivo, but not in vitro, suggesting these adjuvants both indirectly activate DCs. However Advax, by contrast with alum, did not induce any cytotoxicity or DNA release at the injection site, suggesting that Advax indirectly activates DCs through a DAMP-independent mechanism. These results are consistent with previous studies that Advax has extremely low local and systemic reactogenicity in animals and humans (Honda-Okubo et al., 2012). Phagocytic clodronate-ingesting macrophages appear indispensable for Advax's adjuvant effect (Fig. 5a–h), with loss of its effects when these cells were depleted by clodronate-loaded liposomes. The exact mechanisms of phagocytic macrophages dependency of Advax's adjuvant effect need to be determined. To what extent this loss of effect is due to lack of antigen presentation in the absence of these phagocytes and to what extent loss of a critical effector population involved in Advax signaling is not yet known. For example, soluble factors secreted by primary phagocytic macrophages that interact with Advax may be responsible for indirect DCs activation by Advax, as well as for the remote effects on gene expression in cells in liver and spleen seen 6 h after Advax i.p. injection.
Despite delta inulin being a semi-crystalline material (Cooper and Petrovsky, 2011), unlike other particle/crystalline adjuvants (Hornung et al., 2008), Advax does not induce IL-1β production from macrophages and DCs in vitro (Fig. S6a and b), and Advax's adjuvant action was IL-1 and inflammasome-independent as confirmed using a variety of gene knockout mice (Fig. S7a–i). While, this study did not fully clarify the exact mechanism of action of Advax, it showed that TNF-α pathways play a role in its adjuvant effect (Figs. 7c–e and S9a–c). As alum also required intact TNF-α signaling for its full adjuvant effect (Fig. 7c–e), TNF-α might be commonly required for particulate adjuvant action, especially on the antibody responses (Marino et al., 1997). However, we have reported that the adjuvant activity of Endocine, a lipid-based mucosal adjuvant, was independent of TNF-α signaling (Hayashi et al., 2016b) suggesting that TNF-α signaling is distinctively involved in a certain type of adjuvant effect. We expect that in addition to TNF-α, many other factors are possibly required for Advax's adjuvant effect. Upstream regulator analysis in IPA suggested that NF-κB or IFN-γ-related signal pathways potentially play a role in Advax's effects (Fig. 6c) and there is consequently a possibility that these signaling pathways may coordinate with TNF-α signaling to mediate Advax's adjuvant effects.
Adjuvants can be categorized as delivery systems (such as alum, Incomplete Freund's Adjuvant, and MF59) or innate immune receptor agonists (many PAMPs adjuvants). In this study, we demonstrated that Advax belongs to additional class of vaccine adjuvant, as its effects do not allow it to be simply categorized into existing adjuvant classes. For example, typical currently known delivery system and PAMPs adjuvants usually showed adjuvant effects on pure (endotoxin free) OVA antigen. However, Advax did not show any adjuvant effect on pure OVA antigen, suggesting Advax is not a typical currently known delivery system adjuvant. On the other hand, our results clearly showed that Advax has the ability to induce DC activation in vivo, but without inducing local cell death and release of DAMPs such as DNA. This suggests that Advax works through yet to be identified receptors, in vivo, possibly via local resident phagocytic macrophages. Interestingly, Advax's adjuvanticity is not affected by the administration routes, being equally effective when administered i.m. (Fig. 1, Fig. 2), i.d. (Figs. S7a–f and S9), or i.p. (data not shown) routes. This may suggest that Advax works through common mechanisms shared by local macrophages and DCs at different injection sites.
In conclusion, our study showed that Advax induces distinct immune response depending on the built-in adjuvant in vaccine antigen. This result also suggested that unlike typical PAMP adjuvants which directly activate DCs, Advax may indirectly activate DCs through phagocytic macrophages with which it interacts at the injection site and DLN, resulting in secretion of soluble signals that are transduced to remote lymphoid tissues. In vivo gene expression analysis and knockout mouse studies suggested that TNF-α signaling is involved in Advax adjuvant action, although exactly how and where is still not known. Further study is required to identify the exact receptor(s) and molecule(s) that mediate Advax's adjuvant activity, for understanding more detailed mechanisms of action of Advax.
Competing Financial Interests Statement
H.M. is an employee of the Mitsubishi Tanabe Pharma Corporation. T.A. is an employee of the Research Foundation of Microbial Diseases of Osaka University (BIKEN). Y. H.O. and N.P. are employees of Vaxine Pty Ltd.
Author Contributions
T.A., C.C., and K.J.I. designed and supervised the research; M.H., T.A., Y.H., and K.K. designed and performed the experiments and analyzed the data; T.A., E.W., N.N., Y.I., D.M.S., and H.Y. designed and performed the bioinformatics research and analyzed the data; Y.H.O., N.P., H.H., T.S., and T.T. contributed new reagents/analytic tools; M.H., T.A., and K.J.I. wrote the manuscript.
Acknowledgements
We thank Akiko Okabe, Mariko Nakamura, Yukiko Fujita, Aki Konishi and Kousaku Murase for excellent technical assistance with animal husbandry and genotyping, as well as the members of the K.J.I. and C.C. laboratories for valuable comments and assistance.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.11.015.
Appendix A. Supplementary data
Supplementary figures.
Supplementary figure legends.
References
- Aoshi T., Zinselmeyer B.H., Konjufca V., Lynch J.N., Zhang X., Koide Y., Miller M.J. Bacterial entry to the splenic white pulp initiates antigen presentation to CD8 + T cells. Immunity. 2008;29:476–486. doi: 10.1016/j.immuni.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Aoshi T., Carrero J.A., Konjufca V., Koide Y., Unanue E.R., Miller M.J. The cellular niche of Listeria monocytogenes infection changes rapidly in the spleen. Eur. J. Immunol. 2009;39:417–425. doi: 10.1002/eji.200838718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase N., Arase H., Park S., Ohno H., Ra C., Saito T. Association with FcRg is essential for activation signal through NKR-P1 (CD161) in natural killer (NK) cells and NK1.1 + T cells. J. Exp. Med. 1997;186:1957–1963. doi: 10.1084/jem.186.12.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P.D., Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising beta-d-[2 → 1] poly(fructo-furanosyl) alpha-d-glucose polymers. Glycobiology. 2011;21:595–606. doi: 10.1093/glycob/cwq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet C.J., Ishii K.J. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat. Rev. Immunol. 2012;12:479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- Dolter K.E., Evans C.F., Ellefsen B., Song J., Boente-Carrera M., Vittorino R., Rosenberg T.J., Hannaman D., Vasan S. Immunogenicity, safety, biodistribution and persistence of ADVAX, a prophylactic DNA vaccine for HIV-1, delivered by in vivo electroporation. Vaccine. 2011;29:795–803. doi: 10.1016/j.vaccine.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Edgar R., Domrachev M., Lash A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;1:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth S.C., Colegio O.R., O'Connor W., Sutterwala F.S., Flavell R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinen B., Petrovsky N., Verma A., Merkel T.J. Advax-adjuvanted recombinant protective antigen provides protection against inhalational anthrax that is further enhanced by addition of murabutide adjuvant. Clin. Vaccine Immunol. 2014;21:580–586. doi: 10.1128/CVI.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraedts F., Goutagny N., Hornung V., Severa M., de Haan A., Pool J., Wilschut J., Fitzgerald K.A., Huckriede A. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by toll-like receptor signalling. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenny A.T., Pope C.G., Waddington H., Wallace U. Immunologicalnotes XVLL–XXIV. J. Pathol. Bacteriol. 1926;29:31–40. [Google Scholar]
- Gonzalez S.F., Lukacs-Kornek V., Kuligowski M.P., Pitcher L.A., Degn S.E., Kim Y.A., Cloninger M.J., Martinez-Pomares L., Gordon S., Turley S.J. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat. Immunol. 2010;11:427–434. doi: 10.1038/ni.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Kelley P., Heinzel S., Cooper P., Petrovsky N. Immunogenicity and safety of Advax, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled phase 1 study. Vaccine. 2014;32:6469–6477. doi: 10.1016/j.vaccine.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R.K. Aluminum compounds as vaccine adjuvants. Adv. Drug Deliv. Rev. 1998;32:155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- Hara H., Ishihara C., Takeuchi A., Imanishi T., Xue L., Morris S.W., Inui M., Takai T., Shibuya A., Saijo S. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and toll-like receptors. Nat. Immunol. 2007;8:619–629. doi: 10.1038/ni1466. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Aoshi T., Kogai Y., Nomi D., Haseda Y., Kuroda E., Kobiyama K., Ishii K.J. Optimization of physiological properties of hydroxyapatite as a vaccine adjuvant. Vaccine. 2016;34:306–312. doi: 10.1016/j.vaccine.2015.11.059. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Aoshi T., Ozasa K., Kusakabe T., Momota M., Haseda Y., Kobari S., Kuroda E., Kobiyama K., Coban C. RNA is an adjuvanticity mediator for the lipid-based mucosal adjuvant, Endocine. Sci. Rep. 2016;6:29165. doi: 10.1038/srep29165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddle R., Russo P., Petrovsky N., Hanna R., Smith A. Immunotherapy – 2076. A controlled study of delta inulin-adjuvanted honey bee venom immunotherapy. World Allergy Organ J. 2013;6:P158. [Google Scholar]
- Honda-Okubo Y., Saade F., Petrovsky N. Advax, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012;30:5373–5381. doi: 10.1016/j.vaccine.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V., Bauernfeind F., Halle A., Samstad E.O., Kono H., Rock K.L., Fitzgerald K.A., Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistner O., Crowe B.A., Wodal W., Kerschbaum A., Savidis-Dacho H., Sabarth N., Falkner F.G., Mayerhofer I., Mundt W., Reiter M. A whole virus pandemic influenza H1N1 vaccine is highly immunogenic and protective in active immunization and passive protection mouse models. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiyama K., Aoshi T., Narita H., Kuroda E., Hayashi M., Tetsutani K., Koyama S., Mochizuki S., Sakurai K., Katakai Y. Nonagonistic Dectin-1 ligand transforms CpG into a multitask nanoparticulate TLR9 agonist. Proc. Natl. Acad. Sci. U. S. A. 2014;111:3086. doi: 10.1073/pnas.1319268111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S., Aoshi T., Tanimoto T., Kumagai Y., Kobiyama K., Tougan T., Sakurai K., Coban C., Horii T., Akira S. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3000759. [DOI] [PubMed] [Google Scholar]
- Kuroda E., Coban C., Ishii K.J. Particulate adjuvant and innate immunity: past achievements, present findings, and future prospects. Int. Rev. Immunol. 2013;32:209–220. doi: 10.3109/08830185.2013.773326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larena M., Prow N.A., Hall R.A., Petrovsky N., Lobigs M. JE-ADVAX vaccine protection against Japanese encephalitis virus mediated by memory B cells in the absence of CD8(+) T cells and pre-exposure neutralizing antibody. J. Virol. 2013;87:4395–4402. doi: 10.1128/JVI.03144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marichal T., Ohata K., Bedoret D., Mesnil C., Sabatel C., Kobiyama K., Lekeux P., Coban C., Akira S., Ishii K.J. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 2011;17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- Marino M.W., Dunn A., Grail D., Inglese M., Noguchi Y., Richards E., Jungbluth A., Wada H., Moore M., Williamson B. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., McKee A.S., Munks M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T., Richmond P.C., Formica N.T., Hoschler K., Skeljo M.V., Stoney T., McVernon J., Hartel G., Sawlwin D.C., Bennet J. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine. 2008;26:6383–6391. doi: 10.1016/j.vaccine.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Nordvall S.L., Grimmer O., Karlsson T., Bjorksten B. Characterization of the mouse and rat IgE antibody responses to timothy pollen by means of crossed radioimmunoelectrophoresis. Allergy. 1982;37:259–264. doi: 10.1111/j.1398-9995.1982.tb01908.x. [DOI] [PubMed] [Google Scholar]
- Olive C. Pattern recognition receptors: sentinels in innate immunity and targets of new vaccine adjuvants. Expert Rev. Vaccines. 2012;11:237–256. doi: 10.1586/erv.11.189. [DOI] [PubMed] [Google Scholar]
- Onishi M., Ozasa K., Kobiyama K., Ohata K., Kitano M., Taniguchi K., Homma T., Kobayashi M., Sato A., Katakai Y. Hydroxypropyl-beta-cyclodextrin spikes local inflammation that induces Th2 cell and T follicular helper cell responses to the coadministered antigen. J. Immunol. 2015;194:2673–2682. doi: 10.4049/jimmunol.1402027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper S.D., Saunders E.K., Edwards L.E., Wilson C.L., Miller C.J. The utility of MAS5 expression summary and detection call algorithms. BMC Bioinforma. 2007;8:273. doi: 10.1186/1471-2105-8-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky N., Larena M., Siddharthan V., Prow N.A., Hall R.A., Lobigs M., Morrey J. An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody. J. Virol. 2013;87:10324–10333. doi: 10.1128/JVI.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Del Rio E., Marradi M., Calderon-Gonzalez R., Frande-Cabanes E., Penades S., Petrovsky N., Alvarez-Dominguez C. A gold glyco-nanoparticle carrying a listeriolysin O peptide and formulated with Advax delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine. 2015;33:1465–1473. doi: 10.1016/j.vaccine.2015.01.062. [DOI] [PubMed] [Google Scholar]
- Saade F., Honda-Okubo Y., Trec S., Petrovsky N. A novel hepatitis B vaccine containing Advax™, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013;31:1999–2007. doi: 10.1016/j.vaccine.2012.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp F.A., Ruane D., Claass B., Creagh E., Harris J., Malyala P., Singh M., O'Hagan D.T., Petrilli V., Tschopp J. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl. Acad. Sci. U. S. A. 2009;106:870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Grigorova I., Phan T.G., Kelly L.M., Cyster J.G. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J. Exp. Med. 2009;206:1485–1493. doi: 10.1084/jem.20090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T., Li M., Sylvestre D., Clynes R., Ravetch J.V. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Yan S., Gu W., Xu Z.P. Re-considering how particle size and other properties of antigen-adjuvant complexes impact on the immune responses. J. Colloid Interface Sci. 2013;395:1–10. doi: 10.1016/j.jcis.2012.11.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.
Supplementary figure legends.