Abstract
Aim To evaluate the effect of alirocumab on frequency of standard apheresis treatments [weekly or every 2 weeks (Q2W)] in heterozygous familial hypercholesterolaemia (HeFH).
Methods and results ODYSSEY ESCAPE (NCT02326220) was a double-blind study in 62 HeFH patients undergoing regular weekly or Q2W lipoprotein apheresis. Patients were randomly assigned (2:1, respectively) to receive alirocumab 150 mg (n = 41) or placebo (n = 21) Q2W subcutaneously for 18 weeks. From day 1 to week 6, apheresis rate was fixed according to the patient’s established schedule; from weeks 7 to 18, apheresis rate was adjusted based on the patient’s low-density lipoprotein cholesterol (LDL-C) response in a blinded fashion. Apheresis was not performed when the LDL-C value was ≥30% lower than the baseline (pre-apheresis) value. The primary efficacy endpoint was the rate of apheresis treatments over 12 weeks (weeks 7–18), standardized to number of planned treatments. In the alirocumab group the least square (LS) mean ± SE (95% confidence interval [CI]) per cent change in pre-apheresis LDL-C from baseline at week 6 was −53.7 ± 2.3 (−58.2 to − 49.2) compared with 1.6 ± 3.1 (–4.7 to 7.9) in the placebo group. The primary efficacy endpoint showed statistically significant benefit in favour of alirocumab (Hodges–Lehmann median estimate of treatment difference: 0.75; 95% CI 0.67–0.83; P < 0.0001). Therefore, alirocumab-treated patients had a 0.75 (75%) additional reduction in the standardized rate of apheresis treatments vs. placebo-treated patients. During this period, 63.4% of patients on alirocumab avoided all and 92.7% avoided at least half of the apheresis treatments. Adverse event rates were similar (75.6% of patients on alirocumab vs. 76.2% on placebo).
Conclusions Lipoprotein apheresis was discontinued in 63.4% of patients on alirocumab who were previously undergoing regular apheresis, and the rate was at least halved in 92.7% of patients. Alirocumab was generally safe and well tolerated.
Keywords: Alirocumab, Familial hypercholesterolaemia, Low-density lipoprotein cholesterol, Monoclonal antibody, Proprotein convertase subtilisin/kexin type 9, Low-density lipoprotein receptor
Introduction
Familial hypercholesterolaemia is characterized by raised low-density lipoprotein cholesterol (LDL-C) and premature onset of cardiovascular disease. Without effective lipid-lowering treatment (LLT), adults with heterozygous familial hypercholesterolaemia (HeFH) have a very high risk of a coronary event by age 50. Statins can reduce this risk, but patients still have an approximately two-fold excess of coronary heart disease mortality vs. the general population.1
Lipoprotein apheresis removes apoproteinB100-containing lipoproteins from the blood.2 It is generally regarded as a last-resort option for patients with progressive cardiovascular disease and persistently elevated LDL-C.2 Apheresis is expensive and can only be performed in specialized centres,3 the availability of which varies in different countries. As LDL-C concentrations quickly return to pre-treatment levels, patients must undergo a regular weekly or every 2 weeks (Q2W) apheresis regimen, depending on the patient’s LDL-C value and local practice.4 Lipoprotein apheresis acutely lowers the LDL-C concentration by 50–75%, but the time-averaged reduction approximates 30%.
Alirocumab is a fully human monoclonal antibody that binds PCSK9 and inhibits PCSK9-mediated hepatic catabolism of the low-density lipoprotein receptor. Alirocumab as monotherapy or on a background of LLT reduced LDL-C by 44–58% in patients with and without HeFH.5–9 The 150 mg Q2W dose in combination with statin therapy with or without other LLT reduced LDL-C by 62–72%.10–13 These data showing robust decreases in LDL-C suggested that alirocumab may allow a reduction in the frequency of standard apheresis treatments (weekly or Q2W) or remove the need for apheresis in patients with HeFH. Therefore, the objective of this study was to evaluate the effect of alirocumab 150 mg Q2W vs. placebo on the standardized rate of apheresis treatments in patients with HeFH.
Methods
ODYSSEY ESCAPE (https://www.clinicaltrials.gov/ct2/show/NCT023 26220) was a randomized, double-blind, placebo-controlled, parallel-group, phase 3 study in patients with HeFH undergoing regular weekly or Q2W lipoprotein apheresis. The study was conducted at 14 sites in the United States and Germany; screening started in March 2015 and was completed in September 2015.
The study design has been published.14 The main study criteria are detailed in Supplementary material online, Table S1. Eligible patients had HeFH (diagnosed by genotyping or clinical criteria15,16), had undergone consistent lipoprotein apheresis weekly for ≥4 weeks or Q2W for ≥8 weeks, and had maintained a stable background medical LLT, diet, and exercise level for ≥8 weeks. Patients undergoing all lipoprotein apheresis techniques were recruited (for additional information, see Supplementary material online, Text S1).14 Patients with homozygous familial hypercholesterolaemia (FH) were excluded. Patients continued to take their background LLT and remained on a stable diet and exercise regimen from screening until the end of treatment visits.14
The study was approved by the local institutional review boards, and was conducted according to the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, and applicable local laws and regulations. All patients provided written informed consent.
Intervention
During the 2-week screening period, patients provided informed consent, were checked for eligibility, and underwent a lipid panel and other laboratory testing, safety evaluation, and dietary review.14 LDL-C was calculated using the Friedewald formula (for information on laboratory analyses, as well as the well-being analysis, see Supplementary material online, Text S1).17
Qualifying patients were randomly assigned (2:1, respectively) to receive alirocumab 150 mg or placebo subcutaneously Q2W for 18 weeks during the double-blind treatment period. Randomization was done centrally using an interactive voice or web response system and a permuted-block design to ensure even distribution of treatment assignment. Randomization was stratified by frequency of apheresis procedure (weekly or Q2W) and normal (<30 mg/dL) or elevated (≥30 mg/dL) lipoprotein (a) values. Treatment injections (in 1-mL volume) were administered at the site by research staff using an autoinjector, starting on the day of randomization. The drug or placebo was administered immediately after completion of the apheresis procedure on days when the two coincided.
The double-blind treatment period comprised two intervals: (phase 1) from day 1 to week 6, the apheresis rate was fixed according to the patient’s established schedule (weekly or Q2W); (phase 2) from week 7 to week 18, the apheresis rate was adjusted based on the patient’s LDL-C response to treatment, determined by blinded point-of-care testing. Apheresis was not performed when the LDL-C value at that visit was ≥30% lower than the baseline (pre-apheresis) LDL-C value, as described previously.14 Site personnel and patients were blinded to the LDL-C value and treatment assignment, and only received an alert as to whether apheresis should be performed on the basis of coded and blinded point-of-care LDL-C results.
Patients in Germany who completed the double-blind treatment period were offered an optional open-label extension (to week 86) (results for these patients are not currently available). The extension was not offered to patients in the United States because alirocumab is commercially available, but patients had the option to initiate clinical treatment with alirocumab or evolocumab. Patients who were not eligible or who chose not to enter the extension were followed for 8 weeks after the end of the double-blind treatment period, or after premature discontinuation of study treatment.
Outcome measures
The primary efficacy endpoint was the rate of apheresis treatments over 12 weeks (weeks 7 to 18), standardized according to the number of planned treatments for each individual patient. Key secondary endpoints are listed in Supplementary material online, Table S2.
Safety was assessed based on adverse event reports and laboratory analyses during the treatment-emergent adverse event (TEAE) period (defined as the time from the first dose of double-blind study treatment to the last injection plus 70 days [10 weeks], as alirocumab was anticipated to have a residual effect lasting for 10 weeks after the last injection).
Statistical analyses
We estimated that a sample size of 63 patients (alirocumab 42: placebo 21) would have at least 85% power to detect a 33% difference in mean apheresis rates using a two-sided significance level and assuming a standard deviation (SD) of 40%.14
The primary efficacy analysis population was the primary intent-to-treat (ITT) population, defined as all randomized patients. The secondary efficacy analysis population was based on randomized patients who had atleast one pre-apheresis calculated LDL-C value before the first dose of study drug (or randomization, if the patient did not receive any study drug) and at least one calculated LDL-C value in one of the pre-apheresis analysis windows up to week 6. The statistical analysis was conducted when the last patient completed all efficacy assessments at week 18. LDL-C data were analysed posthoc using the Kroon18 formula (see Supplementary material online, Text S1) to estimate the interval means of LDL-C in weeks 6–18 (after potential withdrawal of apheresis therapy).
The standardized rate of apheresis treatments was analysed through the rate of treatments received during the 12-week period (week 7 to week 18), divided by the number of planned treatments (6 for Q2W and 12 for weekly), using the ranked analysis of covariance (ANCOVA) model.19 The standardized apheresis rate could range from 0 to 1, with 0 indicating that the patient skipped all planned apheresis treatments between week 7 to week 18, and 1 indicating that the patient received all planned treatments. A rate of 0.75 indicated that the patient received 75% of planned apheresis treatments (and skipped 25% of planned treatments). Patient drop-outs were accounted for as described in Supplementary material online, Text S1. The median treatment difference was determined using the Hodges–Lehmann estimation and 95% confidence intervals (CIs) using Moses distribution-free CI (see Supplementary material online, Text S1).14
We used a hierarchical inferential approach to control type I error. As the primary endpoint analysis was significant at the 5% alpha level, key secondary efficacy endpoints were tested sequentially (see Supplementary material online, Table S2).14
Results
Seventy-six patients with HeFH undergoing regular weekly or Q2W lipoprotein apheresis provided consent to participate and were screened; 62 (71.6%) patients were eligible for randomization in the study. Forty-one patients were randomly assigned to alirocumab and 21 to placebo (see Supplementary material online, Figure S1). All randomized patients received double-blind study treatment and were included in the safety population. Sixty patients completed the 6-week double-blind treatment period (40 [97.6%] in the alirocumab group and 20 [95.2%] in the placebo group) (i.e. when apheresis rate was determined by the patient’s established schedule), and 57 completed the 18-week double-blind treatment period (37 [90.2%] and 20 [95.2%], respectively), when the apheresis schedule was determined by LDL-C value previously achieved. Of the five (8.1%) patients who prematurely discontinued study treatment, one (4.8%) was on placebo (withdrawn due to adverse events) and four (9.8%) were on alirocumab (two were withdrawn for adverse events and one for poor compliance, one patient withdrew consent).
The mean ± SD age of the population was 58.7 ± 9.7 years, 36 (58.1%) patients were men, and 60 (96.8%) were white. The median (minimum, maximum) duration of apheresis treatments before entry into the study was 4.9 (0.5, 32.9) years.
The baseline characteristics were balanced between treatment groups (Table 1 and Supplementary material online, Table S3). The mean calculated baseline LDL-C (at study entry) was 4.5 ± 1.4 mmol/L (175.1 mg/dL) in the alirocumab group and 5.0 ± 1.8 mmol/L (191.6 mg/dL) in the placebo group (P = 0.35). Twenty-seven (43.5%) patients followed a weekly apheresis schedule at baseline and 35 (56.5%) patients followed a Q2W schedule. The mean LDL-C value in patients undergoing weekly apheresis was 3.9 ± 1.3 mmol/L (151.3 ± 51.3 mg/dL) vs. 5.3 ± 1.4 mmol/L (204.9 ± 55.7 mg/dL) in patients undergoing Q2W apheresis. Thirty-eight (61.3%) patients had normal baseline lipoprotein (a) levels (<30 mg/dL) and 24 (38.7%) had elevated levels. Thirty-four (54.8%) patients were taking a statin at screening, 19 (55.9%) of whom were on a maximum daily dose. Baseline LDL-C values were 4.0 ± 1.4 mmol/L (155.0 ± 54.6 mg/dL) among statin-treated patients compared with 5.4 ± 1.4 mmol/L (208.0 ± 53.2 mg/dL) in patients not taking a statin. Thirty patients (48.4%) were from Germany and 32 (51.6%) from the United States (see Supplementary material online, Table S4).
Table 1.
Baseline characteristics (randomized patients)a
| Characteristic | Alirocumab (n = 41) | Placebo (n = 21) |
|---|---|---|
| Age (years), mean ± SD | 59.5 ± 9.2 | 57.0 ± 10.5 |
| Men, n (%) | 26 (63.4) | 10 (47.6) |
| Race, n (%)b | ||
| White | 39 (95.1) | 21 (100) |
| Black or African American | 2 (4.9) | 0 |
| Body mass index (kg/m2), mean ± SD | 30.5 ± 5.0 | 30.3 ± 6.2 |
| Country, n (%) | ||
| Germany | 20 (48.8) | 10 (47.6) |
| United States | 21 (51.2) | 11 (52.4) |
| Previous PCI or CABG, n (%) | 35 (85.4) | 14 (66.7) |
| Time from hypercholesterolaemia diagnosis (years), mean ± SD | 13.3 ± 12.9 | 15.2 ± 14.0 |
| Frequency of apheresis, n (%) | ||
| Weekly | 18 (43.9) | 9 (42.9) |
| Every 2 weeks | 23 (56.1) | 12 (57.1) |
| Time from first known apheresis treatment (years), median (IQR) | 4.5 (0.5, 32.9) | 5.3 (0.5, 27.3) |
| Background lipid-modifying therapy at randomization, n (%) | ||
| Any statin | 19 (46.3) | 13 (61.9) |
| High-intensity statinc | 13 (31.7) | 12 (57.1) |
| Any LLTd other than statins (with or without statin) | 26 (63.4) | 16 (76.2) |
| LDL-C at baseline, mean ± SD or median (IQR) (mmol/L) [mg/dL] | ||
| LDL-C (measured formula) | 4.5 ± 1.3 [174.0 ± 51.4] | 5.0 ± 1.7 [195.0 ± 66.9] |
| Range | 1.4–6.8 [56–265] | 2.3–8.1 [90–313] |
| Median (IQR) | 4.6 (3.3:5.7)[176.0 (129.0:219.0)] | 4.7 (3.6:6.2)[180.0 (140.0:240.0)] |
| LDL-C (Friedewald formula) (mmol/L) [mg/dL] | 4.5 ± 1.4 [175.1 ± 54.6] | 5.0 ± 1.8 [191.6 ± 68.9] |
| Range | 1.4–7.1 [53–275] | 2.1–8.2 [81–316] |
CABG, coronary artery bypass graft; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; LLT lipid-lowering therapy; PCI, percutaneous coronary intervention; SD, standard deviation.
There were no clinically or statistically significant between-group differences.
Self-reported.
40–80 mg/day atorvastatin or 20–40 mg/day rosuvastatin.
Colesevelam hydrochloride, colestipol, ezetimibe, fish oil, nicotinic acid, omega 3 fatty acids or omega-3-acid ethyl ester.
The mean ± SD duration of injection exposure was 17.4 ± 2.3 weeks (8.6 ± 1.3 injections) in the alirocumab group and 17.5 ± 3.1 weeks (8.4 ± 1.7 injections) in the placebo group.
Efficacy
The primary efficacy endpoint achieved statistically significant benefit in favour of alirocumab-treated patients, with a Hodges–Lehmann median estimate of the treatment difference vs. placebo of 0.75 (95% CI 0.67–0.83). Therefore, alirocumab-treated patients had a 0.75 (75%) additional reduction in the standardized rate of apheresis treatments vs. placebo-treated patients (P < 0.0001) (Table 2). The median treatment difference in the standardized rate of apheresis treatments from week 7 to week 18, when apheresis treatment was determined by LDL-C concentration previously achieved, was 0.75 (95% CI 0.58–0.92) for patients who had been undergoing weekly apheresis and 0.67 (95% CI 0.50–1.00) for those on a Q2W schedule, in favour of alirocumab.
Table 2.
Primary study endpoint and key secondary endpoint
| Alirocumab (n = 41) | Placebo (n = 21) | Alirocumab vs. placebo |
||
|---|---|---|---|---|
| Hodges–Lehmann estimate of median treatment difference (95% Moses distribution-free CI) | P value | |||
| Primary endpoint: Standardized rate of apheresis treatments over 12 weeks (from weeks 7 to 18),a median (minimum, maximum), % | 0 (0, 100) | 83 (42, 100) | 0.75 (0.67–0.83) | <0.0001 |
| Key secondary endpoint: Standardized rate of apheresis treatments during week 15 to week 18,a median (minimum, maximum), % | 0 (0, 0) | 100 (50, 100) | 0.50 (0.50–1.00) | <0.0001 |
CI, confidence interval; Q2W, every 2 weeks.
The standardized rate of apheresis treatments was analysed through the rate of treatments received during the 12-week period (week 7 to week 18), divided by the number of planned treatments (6 for Q2W and 12 for weekly). One patient on a Q2W schedule was misstratified at the time of randomization; on the basis of the ITT approach, the data for this patient were included in the weekly group.
The median treatment difference in the standardized rate of apheresis treatments over the 4-week period (week 15 to week 18) was 0.50 (95% CI 0.50 – 1.00; P < 0.0001) in favour of alirocumab, indicating a 50% reduction in the standardized rate of apheresis treatments vs. placebo (Table 2). The individual patient reductions in apheresis over the 12-week period are illustrated in Figure 1A. During this period, 63.4% of patients on alirocumab had no apheresis procedures, and 92.7% avoided at least half of the procedures (Figure 1B). The rate of apheresis treatments according to apheresis schedule is illustrated in Supplementary material online, Figure S2.
Figure 1.
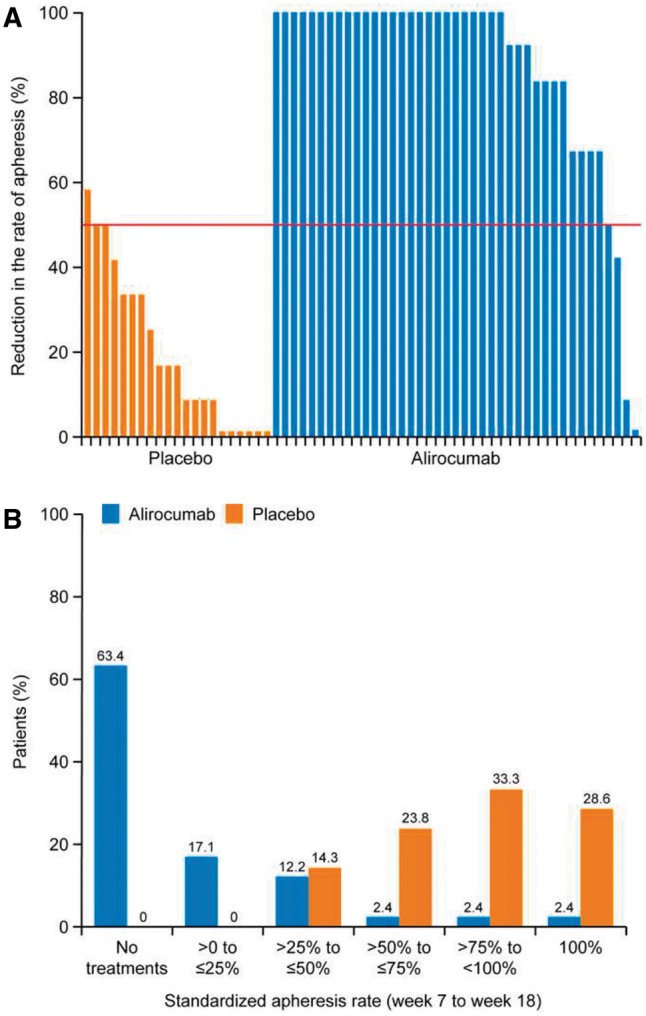
(A) Individual patients’ reductions in standardized rate of apheresis treatments from week 7 to week 18; (B) Standardized rate of apheresis treatments from week 7 to week 18 (intent-to-treat) by randomized treatment. Two patients (one in each randomized treatment group) terminated study treatments before week 6, and their standardized apheresis rates were imputed as 1 (i.e. 100%).
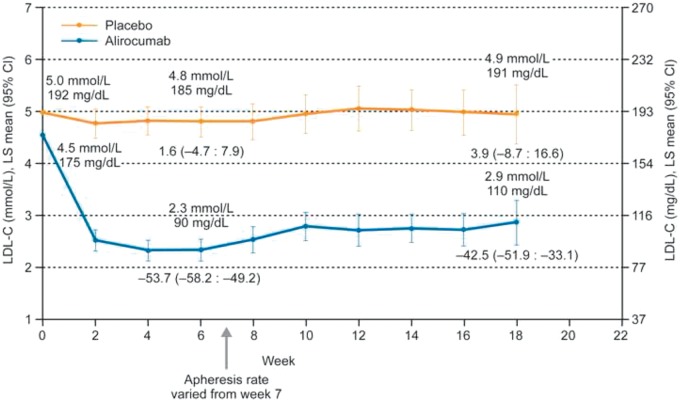
The mean pre-apheresis LDL-C value decreased from 4.5 mmol/L (175 mg/dL) at baseline to 2.3 mmol/L (90 mg/dL) at week 6 in the alirocumab group; corresponding data for patients in the placebo group are 5.0 mmol/L (192 mg/dL) and 4.8 mmol/L (185 mg/dL) (Figure 2).
Figure 2.
Pre-apheresis low-density lipoprotein cholesterol values (mean, with 95% CI) achieved at 2-weekly time-points (intent-to-treat analysis). The arrow indicates the time from which apheresis treatments could be avoided, when a ≥ 30% reduction in low-density lipoprotein cholesterol was achieved vs. baseline (pre-apheresis). Values above the lines indicate LS mean low-density lipoprotein cholesterol values. Values below week 6 and week 18 data points indicate LS mean (95% CI) per cent change from baseline.
The least square (LS) mean ± standard error (SE) (95% CI) per cent change in pre-apheresis LDL-C from baseline at week 6 was –53.7 ± 2.3 (–58.2 to –49.2) in the alirocumab group and 1.6 ± 3.1 (–4.7 to 7.9) in the placebo group (LS mean ± SE per cent difference –55.3 ± 3.9, 95% CI –63.1 to –47.5; P < 0.0001) (Figure 2 and Supplementary material online, Table S5). By week 18, the mean LDL-C value in the alirocumab group had risen slightly, to 2.9 mmol/L (110 mg/dL), compared with 4.9 mmol/L (191 mg/dL) in the placebo group (LS mean ± SE per cent difference –46.4 ± 7.9, 95% CI –62.3 to –30.5; P < 0.0001) (Figure 2 and Supplementary material online, Table S5). Results for other selected key secondary endpoints are shown in Supplementary material online, Table S5. Results from the well-being analysis are given in Supplementary material online, Text S2 and Table S6.
A cross-validation, comparing point-of-care LDL-C values with central laboratory values, showed that both measures were highly correlated (Pearson’s correlation 0.86) (Supplementary material online, Text S3).
Per cent change from baseline to week 18 in lipoprotein (a) was –5.7% for alirocumab vs. –3.0% for placebo among patients with normal baseline values, and 4.9% vs. 7.6%, respectively, in patients with elevated baseline values (Supplementary material online, Table S7). During weeks 7–18, when apheresis treatment could be withdrawn, alirocumab treatment was associated with a lower mean ± SD time-averaged LDL-C value (using the Kroon formula18) over the course of the (potential) apheresis interval of 2.4 ± 1.3 mmol/L (92.7 ± 50.2 mg/dL) vs. 3.8 ± 1.7 mmol/L (146.7 ± 65.6 mg/dL) for placebo (P < 0.0001).
In a posthoc analysis, the time-averaged LDL-C values in the alirocumab-treated patients were consistently lower than those in the placebo-treated patients (Supplementary material online, Figure S3).
Safety
TEAEs were reported by 75.6% of patients in the alirocumab group and by 76.2% of patients in the placebo group, none of which were fatal (Table 3). The rates of serious adverse events (9.8% for alirocumab and 9.5% for placebo) and events leading to treatment discontinuation (4.9% and 4.8%, respectively) were also similar in both groups.
Table 3.
TEAEsaand laboratory parameters (safety population) at 18 weeks
| Alirocumab (n = 41) | Placebo (n = 21) | |
|---|---|---|
| Any TEAE, n (%) | 31 (75.6) | 16 (76.2) |
| Treatment-emergent serious adverse event, n (%) | 4 (9.8) | 2 (9.5) |
| TEAE leading to death, n (%) | 0 | 0 |
| TEAE leading to treatment discontinuation, n (%) | 2 (4.9) | 1 (4.8) |
| TEAEs by system organ class occurring in ≥ 2% of patients in either group, n (%) | ||
| General disorders and administration site conditions | 13 (31.7) | 4 (19.0) |
| Musculoskeletal and connective tissue disorders | 13 (31.7) | 4 (19.0) |
| Infections and infestations | 12 (29.3) | 8 (38.1) |
| Gastrointestinal disorders | 10 (24.4) | 3 (14.3) |
| Injury, poisoning and procedural complications | 8 (19.5) | 3 (14.3) |
| Respiratory, thoracic and mediastinal disorders | 8 (19.5) | 2 (9.5) |
| Nervous system disorders | 7 (17.1) | 1 (4.8) |
| Laboratory investigationsb | 5 (12.2) | 3 (14.3) |
| Cardiac disorders | 4 (9.8) | 3 (14.3) |
| Skin and subcutaneous tissue disorders | 4 (9.8) | 1 (4.8) |
| Blood and lymphatic system disorders | 2 (4.9) | 1 (4.8) |
| Metabolism and nutrition disorders | 2 (4.9) | 1 (4.8) |
| Psychiatric disorders | 2 (4.9) | 1 (4.8) |
| Vascular disorders | 1 (2.4) | 2 (9.5) |
| Immune system disorders | 1 (2.4) | 0 |
| Ear and labyrinth disorders | 1 (2.4) | 0 |
| Reproductive system and breast disorders | 1 (2.4) | 0 |
| Eye disorders | 0 | 1 (4.8) |
| TEAEs occurring in ≥ 5% of patients in either group or TEAEs of interest, n (%) | ||
| Fatigue | 6 (14.6) | 2 (9.5) |
| Nasopharyngitis | 4 (9.8) | 2 (9.5) |
| Diarrhoea | 4 (9.8) | 0 |
| Myalgia | 4 (9.8) | 1 (4.8) |
| Upper respiratory tract infection | 3 (7.3) | 4 (19.0) |
| Headache | 3 (7.3) | 1 (4.8) |
| Arthralgia | 3 (7.3) | 2 (9.5) |
| Nausea | 2 (4.9) | 3 (14.3) |
| Pruritus | 2 (4.9) | 1 (4.8) |
| Back pain | 2 (4.9) | 2 (9.5) |
| Injection site reaction | 1 (2.4) | 0 |
| Palpitations | 0 | 2 (9.5) |
| Laboratory parameters, n (%) | ||
| Alanine aminotransferase > 3 × ULN | 0 | 0 |
| Creatine kinase > 3 × ULN | 3 (7.3) | 0 |
TEAE, treatment-emergent adverse event; ULN, upper limit of normal.
TEAEs are adverse events that developed or worsened or became serious during the TEAE period.
Alanine aminotransferase increased, liver function test abnormal, blood creatine phosphokinase increased.
Three patients (7.3%) in the alirocumab group and none in the placebo group had two consecutive pre-apheresis calculated LDL-C values <0.7 mmol/L (25 mg/dL). Two of these patients had at least one adverse event, one of which had several serious adverse events (pneumonia, acute myocardial infarction, acute respiratory failure, congestive heart failure, sepsis, and aortic valve stenosis). Twenty-seven patients (23 [56.1%] on alirocumab and 4 [19.0%] on placebo) had two consecutive LDL-C values <0.7 mmol/L after apheresis, of which 15 (65.2%) and 2 (50.0%), respectively, had an adverse event. None of the events was serious but one patient (4.3%) discontinued treatment with alirocumab.
Discussion
The ODYSSEY ESCAPE study was conducted in HeFH patients who had been undergoing regular lipoprotein apheresis. The study achieved the primary endpoint in the ITT population of a statistically significant 75% reduction in the standardized rate of apheresis in favour of alirocumab (P < 0.0001). The standardized rate of apheresis was at least halved in 39 of 41 (92.7%) patients on alirocumab, with 63.4% having no apheresis procedures during the 12-week period. Patients receiving alirocumab had lower time-averaged LDL-C values than placebo-treated patients.
At week 6, the pre-apheresis LDL-C concentration was reduced by 53.7% in patients on alirocumab, in line with the anticipated reduction. Consequently, the percentage of patients on apheresis at week 7 dropped markedly in the alirocumab group. Whereas 15 of the 21 placebo-treated patients avoided some of their planned apheresis treatments, three placebo-treated patients achieved a ≥50% reduction in the apheresis rate. The reasons for this reduction in the apheresis rate with placebo are unclear, but may reflect individual variation in LDL-C values, perhaps due to changes in diet or adherence to LLT, the small sample size, and the fact that patients were in a supervised clinical trial.
All of the patients in this study were at high cardiovascular risk and had taken LLT previously, including statins. At screening, only 54.8% of the patients were taking a statin, 55.9% of whom were on a maximally tolerated dose. A large proportion of the overall population reported a history of down-titration of statin treatment due to tolerability issues (43.5%) and 62.9% reported changing to a different statin. Various reasons were given for not taking a statin or for not taking it at the maximum daily dose, ranging from muscle symptoms to anxiety about side-effects, and regional practices/local labelling, indicating that patients with HeFH on apheresis present a diverse and difficult-to-treat population, with limited treatment options.
Patients in the USA showed different characteristics to those in Germany, with higher baseline LDL-C, greater prevalence of statin intolerance, and a less frequent apheresis regimen. In the USA, lipoprotein apheresis for HeFH is often only considered for patients who, after 6 months, do not have an adequate response to maximum tolerated drug therapy and have elevated LDL-C plus other cardiovascular risk factors.20 In Germany, where apheresis centres are more common, apheresis is considered within 12 months of failure of diet and LLT, and at a lower LDL-C threshold, and weekly is preferred to Q2W apheresis.20 Furthermore, recommendations for LDL-C lowering from Europe3 are based on a risk-stratified treat-to-target approach, whereas US guidelines21 advocate a dose-adapted approach. In view of the approach adopted in European guidelines,3 in which the target LDL-C value is <1.8 mmol/L (70 mg/dL), German patients on alirocumab with LDL-C above this target value would still meet the criteria for apheresis. Consequently, LLT with alirocumab may prove complementary to lipoprotein apheresis in patients with very high LDL-C or who fail to meet the target LDL-C value.
A small observational study involving three patients with HeFH on long-term apheresis, who ceased apheresis treatment and received single injections of evolocumab 140 mg Q2W subcutaneously over 7 weeks, reported similar results.22
Limitations
The present study was conducted in two countries, with patients showing markedly different baseline and treatment characteristics. In order to account for these differences, the target level was based on per cent reduction from baseline in LDL-C rather than on achievement of a specific goal (e.g. <1.8 mmol/L[70 mg/dL]) for any given patient. Thus, in some cases apheresis was interrupted despite the fact that specific LDL-cholesterol goals were not achieved. Guideline-recommended threshold values may be achievable in individual patients through treatment with alirocumab alone or in combination with lipoprotein apheresis. Furthermore, the study is limited by a relatively small sample size and the short trial duration. However, there is no reason to believe that longer–term studies or larger sample sizes would provide different results.
Conclusions
Lipoprotein apheresis was discontinued in 63.4% of patients on alirocumab who were previously undergoing regular apheresis every 1 or 2 weeks, and the standardized rate was reduced by at least 50% in an additional 29.3% of patients. PCSK9 inhibition maintained LDL-C at a significantly reduced plateau level as a function of antibody dose over time, whereas LDL-C levels following lipoprotein apheresis started to increase towards pre-procedure levels very rapidly regardless of the frequency of apheresis administration. This difference between PCSK9 inhibition and apheresis translates into a cumulative area under the curve LDL-C benefit in favour of alirocumab, and may be of clinical significance over the long term. Alirocumab was generally safe and well tolerated. The findings from this study suggest a role for alirocumab in the overall management of patients with HeFH undergoing regular lipoprotein apheresis therapy, with the potential to avoid apheresis treatments or delay the requirement for such treatments.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We thank the patients, study coordinators and investigators, and the following persons from the sponsors for their contributions to critical review of the manuscript: Regeneron: William J. Sasiela, PhD, Robert, Pordy, MD, Johanna Mendoza, BSc, and Carol Hudson, BPharm; Sanofi: Jay Edelberg, MD, PhD, L. Veronica Lee, MD, Tu Nguyen, MD, and Michael Howard, MBA. The authors thank Dr Claudia Stefanutti for her assistance in designing the ODYSSEY ESCAPE protocol. Writing support was provided by Sophie K. Rushton-Smith, PhD, funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Funding
Sanofi and Regeneron Pharmaceuticals, Inc. The sponsor was involved in the study design; in the writing of the report; and in the decision to submit the article for publication. Funding to pay the Open Access publication charges for this article was provided by Sanofi and Regeneron Pharmaceuticals, Inc.
Conflict of interest: Dr Moriarty reports grants and personal fees from Regeneron, grants and personal fees from Sanofi, grants and personal fees from Amgen, grants and personal fees from Ionis, grants and personal fees from Genzyme, personal fees from Duke, personal fees from Esperion, personal fees from Eliaz Therapeutics, personal fees from Alexion, grants from Pfizer, grants from Catabasis, grants from Novartis, grants from Kaneka, personal fees from Aegerion, personal fees from Amarin, personal fees from Lilly, outside the submitted work. Dr Parhofer reports grants and personal fees from Sanofi/Regeneron, during the conduct of the study; personal fees from Sanofi-Aventis, grants and personal fees from Regeneron, personal fees from Aegerion, grants and personal fees from Merck, Sharp & Dohme, personal fees from Amgen, personal fees from Boehringer Ingelheim, personal fees from Pfizer, outside the submitted work. Dr Babirak reports other from Sanofi, other from Amgen, during the conduct of the study; other from Sanofi, other from Amgen, outside the submitted work. Dr Cornier reports grants from Regeneron, outside the submitted work. Dr Duell reports grants from Regeneron, during the conduct of the study; personal fees from Regeneron, personal fees from Amgen, personal fees from Kaneka, outside the submitted work. Dr Hohenstein reports personal fees from Amgen GmBH, grants and personal fees from Kaneka Pharma Europe N.V., personal fees from Miltenyi Biotec GmbH, personal fees from Fresenius Medical Care GmbH, grants and personal fees from B. Braun Avitum, personal fees from Sanofi-Aventis, grants and personal fees from Novartis, personal fees from Alexion Pharma, outside the submitted work. Dr Leebmann reports nothing to disclose. Dr Ramlow reports grants and personal fees from Amgen, grants and personal fees from Fresenius, grants and personal fees from Kaneka, personal fees from Aegerion, personal fees from B. Braun, personal fees from Merck Sharp & Dohme, personal fees from Regeneron Pharmaceuticals Inc., personal fees from Sanofi, outside the submitted work. Dr Schettler reports support for lectures from Sanofi-Aventis. Dr Simha reports nothing to disclose. Dr Steinhagen-Thiessen reports nothing to disclose. Dr Thompson reports other from Regeneron, other from Sanofi, other from Amgen, outside the submitted work. Dr Vogt reports other from Sanofi/Regeneron Pharmaceuticals, during the conduct of the study; other from Sanofi/Regeneron Pharmaceuticals, other from Sanofi/Regeneron Pharmaceuticals, outside the submitted work. Dr von Stritzky is an employee of Sanofi Germany. Dr Du is an employee of Regeneron Pharmaceuticals Inc. Dr Manvelian is an employee of Regeneron Pharmaceuticals Inc.
References
- 1. Neil A, Cooper J, Betteridge J, Capps N, McDowell I, Durrington P, Seed M, Humphries SE. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J 2008;29:2625–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosada A, Kassner U, Banisch D, Bender A, Steinhagen-Thiessen E, Vogt A. Quality of life in patients treated with lipoprotein apheresis. J Clin Lipidol 2016;10:323–329.e6. [DOI] [PubMed] [Google Scholar]
- 3. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 4. Robinson JG. Management of familial hypercholesterolemia: a review of the recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Manag Care Pharm 2013;19:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG, Merlet L, Pordy R, Baccara-Dinet MT. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol 2014;176:55–61. [DOI] [PubMed] [Google Scholar]
- 6. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM, ODYSSEY COMBO II Investigators. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J 2015;36:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni-Berthold I, Robinson J, Zhao J, Hanotin C, Donahue S. Alirocumab as add-on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab 2015;100:3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J 2015;169:906–915 e13. [DOI] [PubMed] [Google Scholar]
- 9. Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, Blom D, Civeira F, Krempf M, Lorenzato C, Zhao J, Pordy R, Baccara-Dinet MT, Gipe DA, Geiger MJ, Farnier M. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015;36:2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med 2012;367:1891–1900. [DOI] [PubMed] [Google Scholar]
- 11. McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol 2012;59:2344–2353. [DOI] [PubMed] [Google Scholar]
- 12. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ, ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 13. Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet 2012;380:29–36. [DOI] [PubMed] [Google Scholar]
- 14. Moriarty PM, Parhofer KG, Babirak SP, deGoma E, Duell PB, Hohenstein B, Ramlow W, Simha V, Steinhagen-Thiessen E, Thompson PD, Vogt A, von Stritzky B, Du Y, Manvelian G. Alirocumab in patients with heterozygous familial hypercholesterolemia undergoing lipoprotein apheresis: rationale and design of the ODYSSEY ESCAPE trial. J Clin Lipidol 2016;10:627–634. [DOI] [PubMed] [Google Scholar]
- 15. Scientific Steering Committee on behalf of the Simon Broome Register Group. Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ 1991;303:893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. Familial Hypercholesterolemia (FH). Report of a second WHO Consultation. http://whqlibdoc.who.int/hq/1999/WHO_HGN_FH_CONS_99.2.pdf?ua=1; 1998, 16 (20 July 2016).
- 17. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 18. Kroon AA, Van't Hof MA, Demacker PN, Stalenhoef AF. The rebound of lipoproteins after LDL-apheresis. Kinetics and estimation of mean lipoprotein levels. Atherosclerosis 2000;152:519–526. [DOI] [PubMed] [Google Scholar]
- 19. Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using the SAS System. Cary, NC: SAS Institute Inc.; 2000. [Google Scholar]
- 20. Robinson JG. Management of familial hypercholesterolemia: a review of the recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Manag Care Pharm 2013;19:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- 22. Lappegard KT, Enebakk T, Thunhaug H, Hovland A. Transition from LDL apheresis to evolocumab in heterozygous FH is equally effective in lowering LDL, without lowering HDL cholesterol. Atherosclerosis 2016;251:119–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.