Abstract
Despite the significant global burden of gastroenteritis and resulting sequelae, there is limited evidence on risk factors for sequelae development. We updated and extended previous systematic reviews by assessing the role of antibiotics, proton pump inhibitors (PPI) and symptom severity in the development of sequelae following campylobacteriosis and salmonellosis. We searched four databases, including PubMed, from 1 January 2011 to 29 April 2016. Observational studies reporting sequelae of reactive arthritis (ReA), Reiter's syndrome (RS), irritable bowel syndrome (IBS) and Guillain-Barré syndrome (GBS) following gastroenteritis were included. The primary outcome was incidence of sequelae of interest amongst cases of campylobacteriosis and salmonellosis. A narrative synthesis was conducted where heterogeneity was high. Of the 55 articles included, incidence of ReA (n = 37), RS (n = 5), IBS (n = 12) and GBS (n = 9) were reported following campylobacteriosis and salmonellosis. A pooled summary for each sequela was not estimated due to high level of heterogeneity across studies (I2 > 90%). PPI usage and symptoms were sparsely reported. Three out of seven studies found a statistically significant association between antibiotics usage and development of ReA. Additional primary studies investigating risk modifying factors in sequelae of GI infections are required to enable targeted interventions.
Keywords: Gastroenteritis, Campylobacter, Salmonella, Sequelae, Antibiotics, Acid suppression
Highlights
-
•
There is no clear direction of the association between antibiotics and gastroenteritis triggered reactive arthritis.
-
•
Precision of genomic methods and increased use of record linkage techniques may provide clarity.
Antibiotics are known to change the gut flora but little is known of their potential to cause complications in patients who have gastroenteritis. We conducted a systematic review of the existing evidence to assess the potential association of antibiotic usage in patients with gastroenteritis and the occurrence of complications such as reactive arthritis (ReA). The available evidence did not indicate a clear direction in the association of antibiotics and ReA. The lack of clarity in the association of antibiotics and ReA raises a call for further primary research on the role of medications in the development of complications of gastroenteritis.
1. Introduction
Campylobacter and non-typhoidal Salmonella enterica (NTS) are important agents of human bacterial gastroenteritis, representing over 30% (174.3 million) of diarrhoeal illnesses globally in 2010. While Campylobacter was the most common cause of bacterial gastroenteritis, NTS accounted for most of the deaths caused by a bacterial foodborne agent (over 59,000) and with the highest rank for disability adjusted life years amongst foodborne disease hazards in 2010 (Havelaar et al., 2015). Globally, foodborne disease burden is not equally distributed amongst the World Health Organisation (WHO) sub regions, with the greatest burden falling on the sub regions in Africa. Nevertheless, both Campylobacter and NTS (henceforth gastrointestinal (GI) infections) still pose a significant disease and economic burden in developed countries (Scallan et al., 2011, Majowicz et al., 2010).
Gastroenteritis caused by Campylobacter jejuni/coli and most serotypes of NTS are characterised by a self-limiting illness without the need for medical intervention. Yet, a subset of patients develop sequelae such as reactive arthritis (ReA), Reiter's Syndrome (RS), irritable bowel syndrome (IBS), Guillain-Barré Syndrome (GBS), Inflammatory Bowel Disease (IBD), Crohn's disease (CD) and ulcerative colitis (UC) (Ajene et al., 2013, Keithlin et al., 2014, Keithlin et al., 2015).
Evidence on the factors predisposing some patients to sequelae development is limited, with only one study assessing the factors for development of IBS following enteric infection (Thabane et al., 2007). The authors found that young age, prolonged fever, anxiety and depression were risk factors for post-infectious IBS, but they did not stratify those factors by the infecting pathogen. This is a drawback for burden of disease studies, as estimates of pathogen specific sequelae development are required for prioritization of public health interventions.
In a systematic review to assess the proportion of patients who develop chronic sequelae following GI infection, the authors found that study-level factors, such as diagnosis method for complications, follow-up period from infection to sequelae development, and study size, contribute to the reported incidence of ReA and IBS following Campylobacter and NTS infection (Keithlin et al., 2014, Keithlin et al., 2015). However, the association of clinical factors such as proton pump inhibitors (PPI) usage and antibiotics in the development of chronic sequelae were not investigated. These drugs, which commonly increase risk of gastroenteritis, may also have a role in sequelae development due to changes to the gut microbiome and gastric pH that can favour pathogenic organisms (Doorduyn et al., 2008).
In light of the existing gap in the evidence of factors contributing to sequelae development in patients with GI infections, this systematic review extends the previous reviews to assess the study- and patient-level risk factors associated with the development of complications following Campylobacter and NTS infections. Specifically, we assess whether use of PPI, treatment with antibiotics and clinical symptoms such as duration of diarrhea and fever are risk factors for the development of ReA, RS, IBS, GBS, IBD, CD and UC in adults and children with a Campylobacter or NTS infection.
2. Methods
This systematic review and meta-analysis was conducted in line with the ‘Meta-analysis of Observational Studies in Epidemiology’ (MOOSE) guidelines (Stroup et al., 2000). The protocol was registered on PROSPERO (CRD 42015026042).
2.1. Search Strategy and Selection Criteria
We searched four electronic databases, PubMed, Agricola[http://agricola.nal.usda.gov/], EMBASE [OvidSP] (1974–2016 April 27) and CabDirect [OvidSP] (2000 to 2016 Week 15) for studies reporting sequelae of ReA, RS, IBS, GBS, IBD, CD and UC following gastrointestinal infections (Campylobacter and NTS). The search strategies consisted of a combination of relevant subject headings and free-text words in title and abstract for exposure and outcome. We restricted our search to studies published between 01 January 2011 and 29 April 2016 as this was an update and extension of the previous reviews with searches up to July 2011 (Keithlin et al., 2014, Keithlin et al., 2015). The detailed search strategies and results for each of the databases are presented in Supplementary Table S1. Additional eligible studies on GI infections and associated sequelae were sought by reviewing the reference lists of identified articles. No language restrictions were applied during the search.
Two reviewers independently screened titles and abstracts for relevance. Studies were included if they were cohort (prospective or retrospective), case-control, surveillance report or cross-sectional studies, or outbreak investigations of people with Campylobacter or NTS infection, and reported the number or proportion of people who developed the sequelae of interest following Campylobacter or NTS infection. Studies were excluded if they: reported sequelae without evidence of a past exposure with Campylobacter or NTS infection; reported sequelae with only serological evidence of past exposure to pathogens; reported sequelae for multiple foodborne infections without a breakdown of the proportion/numbers by pathogen and sequelae; were case reports, case series or experimental studies such as randomised controlled trials and laboratory based studies. We added data for the period before July 2011 from the previous systematic reviews (Keithlin et al., 2014, Keithlin et al., 2015) and extracted additional variables as required.
2.2. Data Extraction and Bias Assessment
Data were extracted independently by two reviewers (OE and MP) using a standardized form. Inconsistencies were resolved through a consensus process, with any disagreement resolved by a third reviewer, TF. Data coding and categorisation was in accordance with the previous reviews or otherwise stated (Supplementary Table S2) (Keithlin et al., 2014, Keithlin et al., 2015). The primary outcome was the number of cases who developed the specific sequelae of interest divided by the total number of Campylobacter or NTS cases.
The Joanna Briggs Institute Prevalence Critical Appraisal Tool was adapted to evaluate the quality of each study (Munn et al., 2014). This tool was selected because of its flexibility to address the risk of bias across a variety of study designs, as commonly found in the study of incidence and prevalence. Initially a calibration exercise was performed by review members using a random sample of three studies. The items in the tool were applied to the selected studies to ensure consistency across reviewers and validity in assessing the risk of bias with this tool. Following this exercise two additional questions were included (Supplementary Table S3). Inconsistency was resolved through a consensus process.
2.3. Statistical Analysis
We performed meta-analysis in STATA version 13 (StataCorp LP) using “metaprop_one”, a user written command for meta-analysing proportions (Nyaga et al., 2014, Freeman and Tukey, 1950). Heterogeneity was quantified using the I2 measure (Higgins et al., 2003). Where heterogeneity was high (I2 over 50%), no summary estimate was calculated.
It was possible to have multiple outcomes per study. Briefly, some studies reported multiple diagnostic methods for both pathogen and complication or multiple pathogen serotype/species. Each combination of pathogen diagnosis or sequelae diagnosis was considered as a separate outcome measure. Meta-analysis was performed using the most rigorous outcome measure based on the reference standard. For instance, where a study reported both laboratory-confirmed and probable diagnosis for a pathogen with multiple diagnostic methods for the sequelae of interest, such as self-reported diagnosis and further diagnosis by a specialist (rheumatologist for reactive arthritis), the combination of Campylobacter/NTS cases with a laboratory-confirmed diagnosis and the sequelae assessed by a specialist was used.
A priori subgroups to explore potential sources of heterogeneity were investigated based on relevant methodological characteristics (study design, study size, follow-up period and sequelae diagnosis) (Keithlin et al., 2014, Keithlin et al., 2015) and clinical characteristics (healthcare facility visited, symptoms of GI infections, reported PPI and antibiotic usage), if data were available.
3. Results
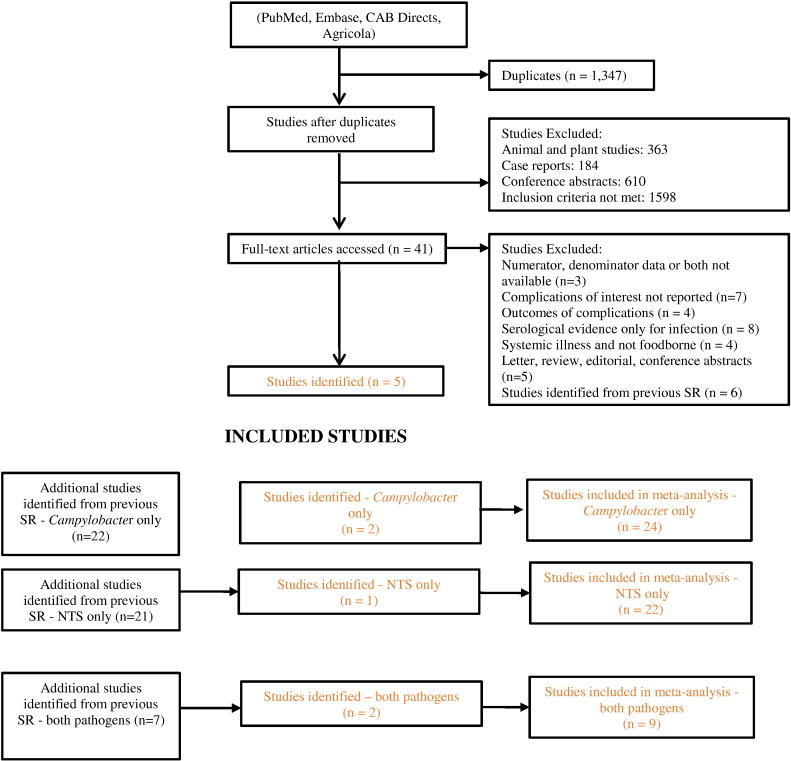
Primary searches identified 4133 references. On removal of duplicates and after screening, five studies met the inclusion criteria reporting ReA, RS, IBS and GBS following Campylobacter infection (n = 4) and ReA, RS and IBS following NTS infection (n = 3) (Baker et al., 2012, Uotila et al., 2014, Porter et al., 2013a, Porter et al., 2013b, Tuompo et al., 2013). Data was extracted from all five studies and additional studies from the previous systematic reviews on ReA, RS, IBS and GBS following GI infections (n = 50). Hence 55 studies were included in the analysis (Fig. 1 and Table 1). No eligible studies on IBD, CD and UC were identified by our database search, so these sequelae are not considered further in this review.
Fig. 1.
Flowchart of included studies.
Results of all database searches, screening of titles and abstracts, full-text screening, additional references and selected studies.
Table 1.
Included studies by pathogen and sequelae reported.
Table 2 shows the characteristics of the included studies. The number of patients with GI infections varied widely (range 6 to 57,425 with Campylobacter and 24 to 34,664 with NTS infection) and represented all age groups. Studies reported diagnoses of the pathogen and complication according to standard practices for all cases of GI infections (87%, 48/55 and 75%, 41/55 respectively). Only 18% (10/55) reported adequate sample size calculation and, where response rate was low (49%, 27/55), only 15% (n = 4) adjusted for possible response bias in their analysis. An overall risk of bias score was not assigned as it was possible to have a high score (> 70%, 8.5/12) without using a reliable method of pathogen and diagnosis for all patients.
Table 2.
Study characteristics for complications following Campylobacter and non-typhoidal Salmonella infection.
| First author, year | Country | Study design | Data source | Outbreak source | Date_Data collection | Season | Age | % Female | Complication |
|---|---|---|---|---|---|---|---|---|---|
| Campylobacter only | |||||||||
| Baker et al. (2012) | New Zealand | Prospective population surveillance | Hospital records | N.A. | 1995–2008 | All | All ages | N.R. | GBS |
| Bremell et al. (1991) | Sweden | Prospective outbreak | Outbreak in community | Unknown | 1981 | Autumn | Adults | 56% | ReA |
| Dunlop et al. (2003) | England | Prospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 1999–2002 | Various | Adult | N.R. | IBS |
| Eastmond et al. (1983) | Scotland | Retrospective outbreak | Outbreak in community | Food - dairy | 1979 | Winter | N.R. | N.R. | ReA |
| Gardner et al. (2011) | Canada | Prospective outbreak | Outbreak in community | Food - vegetable | 2008 | Autumn | All ages | 51% | GBS |
| Gumpel et al. (1981) | England | Retrospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 1978 | All | All ages | N.R. | ReA |
| Hannu et al. (2002a) | Finland | Prospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 1997–1998 | All | All ages | 59% | ReA |
| Kosunen et al. (1981) | Finland | Unknown | Surveillance of population (sporadic or outbreak) | N.A. | 1978–1979 | All | N.R. | N.R. | ReA |
| Locht and Krogfelt (2002) | Denmark | Retrospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 1997–2000 | All | Adults | 57% | ReA |
| McCarthy et al. (1999) | Sweden | Retrospective outbreak | Outbreak in community | Waterborne | 1980, 1994, 1995 | Various | N.R. | N.R. | GBS |
| McCarthy and Giesecke (2001) | Sweden | Retrospective population surveillance | Disease registry | N.A. | 1987–1995 | All | All ages | N.R. | GBS |
| Melby et al. (1990) | Norway | Retrospective outbreak | Outbreak in community | Waterborne | Pre-1990 | Spring/summer | All ages | 48% | ReA |
| Moss-Morris and Spence (2006) | New Zealand | Prospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 2002–2003 | Various | Adults | N.R. | IBS |
| Pitkanen et al. (1983) | Finland | Prospective hospital surveillance | Hospital records | N.A. | 1978–1981 | All | All ages | 47% | ReA |
| Pitkanen et al. (1981) | Finland | Prospective hospital surveillance | Hospital records | N.A. | 1978–1980 | All | All ages | 46% | ReA |
| Ponka et al. (1984) | Finland | Prospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 1978–1981 | All | N.R. | N.R. | ReA |
| Schoenberg-Norio et al. (2010) | Finland | Cross sectional | Surveillance of population (sporadic or outbreak) | N.A. | 2002 | Summer | All ages | 48% | ReA |
| Short et al. (1982) | UK | Prospective | Hospital records | N.A. | 1979 | Various | N.R. | N.R. | ReA |
| Spence and Moss-Morris (2007) | New Zealand | Prospective | Surveillance of population (sporadic or outbreak) | N.A. | Pre-2006 | All | Adults | N.R. | IBS |
| Spiller et al. (2000) | UK | Prospective population surveillance | Hospital records | N.A. | Pre-2000 | All | Adult | N.R. | IBS |
| Tam et al. (2006) | UK | Retrospective population surveillance | Disease registry | N.A. | 1991–2001 | All | N.R. | N.R. | GBS |
| Thornley et al. (2001) | UK | Prospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 1997 | Spring/summer | Adults | N.R. | IBS |
| Uotila et al. (2014) | Finland | Retrospective outbreak | Outbreak in community | Waterborne | 2007 | Winter | All ages | 73% | ReA |
| Wang et al. (2008) | China | Retrospective population surveillance | Hospital records | N.A. | 2000–2006 | All | Children | 30% | GBS |
| NTS only | |||||||||
| Arnedo-Pena et al. (2010) | Spain | Prospective outbreak | Outbreak in community | Food - meat | 2005 | Summer | All ages | 49.7% | ReA |
| Buxton et al. (2002) | Canada | Prospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 1999–2000 | All | All ages | 53.0% | ReA |
| Dworkin et al. (2001) | USA | Prospective outbreak | Outbreak in community | Food - meat | 1994 | Autumn/winter | Adults | 58.5% | ReA, Reiter's |
| Eastmond (1983) | Scotland | Prospective outbreak | Outbreak in community | Food - dairy | 1981 | Autumn | All ages | 47.8% | ReA |
| Ekman et al. (2000) | Finland | Prospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 1998–1999 | All | NR | 53.0% | ReA |
| Hakansson et al. (1976) | Sweden | Retrospective outbreak | Outbreak in community | 1974 | N.R. | Adults | N.R. | ReA | |
| Hannu et al. (2002b) | Finland | Prospective outbreak | Outbreak in community | Unknown | 1999 | Spring/summer | Adults & Children | 56.9% | ReA |
| Lee et al. (2005) | Australia | Retrospective outbreak | Outbreak in community | Food - vegetable | 1999 | Various | All ages | 48.3% | ReA |
| Locht et al. (1993) | Finland | Retrospective outbreak | Outbreak in community | Food - other | 1990 | Spring | Adults | 44.4% | ReA |
| Locht et al. (2002) | Denmark | Prospective outbreak | Outbreak in community | Food - other | 1999 | Winter | Adults | 56.0% | ReA |
| Mattila et al. (1994) | Finland | Prospective outbreak | Outbreak in community | Food - vegetable | 1992 | Autumn | All ages | 62.2% | ReA |
| Mattila et al. (1998) | Finland | Prospective outbreak | Outbreak in community | Food - vegetable | 1994 | Spring | All ages | 68.1% | ReA & Reiter's |
| McColl et al. (2000) | Australia | Prospective outbreak | Outbreak in community | Food - meat | 1997 | Spring | All ages | 51.0% | ReA |
| McKendrick and Read (1994) | UK | Prospective outbreak | Outbreak in community | Food- other | Pre-1994 | N.R. | N.R. | 65.8% | IBS |
| Mearin et al. (2005) | Spain | Prospective outbreak | Outbreak in community | Food - dairy | 2002 | Summer | Adults | 55.3% | IBS |
| Rohekar et al. (2008) | Canada | Retrospective outbreak | Outbreak in community | Food - vegetable | 2005 | Autumn/winter | Adults | 71.2% | ReA |
| Rudwaleit et al. (2001) | Germany | Prospective outbreak | Outbreak in community | Food - dairy | 1998 | Winter | Children | N.R. | ReA |
| Samuel et al. (1995) | USA | Retrospective outbreak | Outbreak in community | Unknown | 1993 | Summer | NR | N.R. | ReA |
| Thomson et al. (1994) | Canada | Retrospective outbreak | Outbreak in community | Food - meat | 1990 | Spring | NR | N.R. | ReA |
| Thomson et al. (1992) | Canada | Prospective outbreak | Outbreak in community | Food - meat | Pre-1992 | N.R. | Adults | 94.5% | ReA |
| Tuompo et al. (2013) | Finland | Prospective population surveillance | Surveillance of population (sporadic or outbreak) | NA | 2003–2004 | Various | All ages | 60.1% | ReA |
| Urfer et al. (2000) | Switzerland | Prospective outbreak | Outbreak in community | Food - meat | 1993 | Autumn | All ages | 37.2% | IBS & ReA |
| Campylobacter and NTS | |||||||||
| Doorduyn et al. (2008) | Netherlands | Prospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 2002–2003, 2005 | All | NR | N.R. | GBS, ReA, Reiter's |
| Helms et al. (2006) | Denmark | Retrospective population surveillance | Disease registry | N.A. | 1991–1999 | All | All ages | 52% | GBS, IBS, ReA |
| Petersen et al. (1996) | Denmark | Retrospective hospital surveillance | Hospital records | 1991–1993 | All | All ages | N.R. | ReA | |
| Porter et al. (2013b) | USA | Retrospective population surveillance | Disease registry | N.A. | 1998 to 2009 | All | Adults | N.R. | Reiter's |
| Porter et al. (2013a) | USA | Retrospective population surveillance | Disease registry | N.A. | 1998 to 2009 | All | Adults | N.R. | IBS |
| Saps et al. (2008) | USA & Italy | Prospective population surveillance | Hospital records | N.A. | 2006 | Various | Children | N.R. | IBS |
| Schiellerup et al. (2008) | Denmark | Prospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 2002–2003 | All | Adults | 57% | ReA |
| Ternhag et al. (2008) | Sweden | Retrospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 1997–2004 | All | All ages | Campylobacter - 47% NTS - 50.6% | GBS, IBS, ReA |
| Townes et al. (2008b) | USA | Prospective population surveillance | Surveillance of population (sporadic or outbreak) | N.A. | 2002–2004 | All | All ages | Campylobacter - 46.9% NTS - 56.0% | ReA |
N.A. – not applicable; N.R. – not reported.
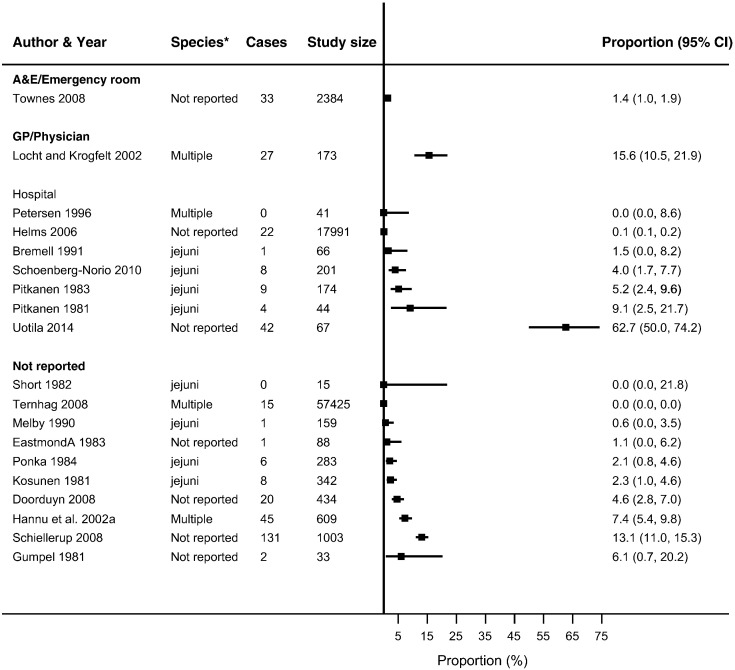
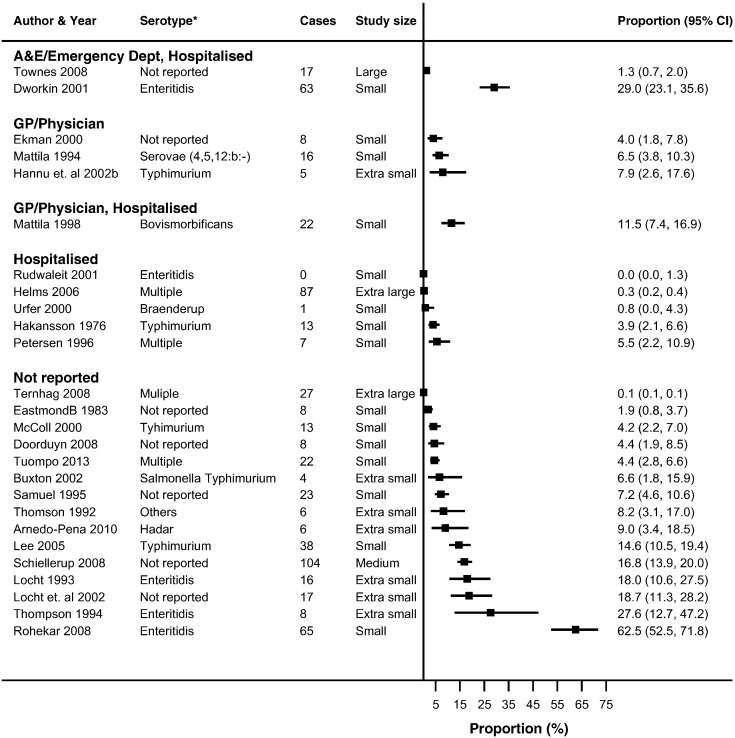
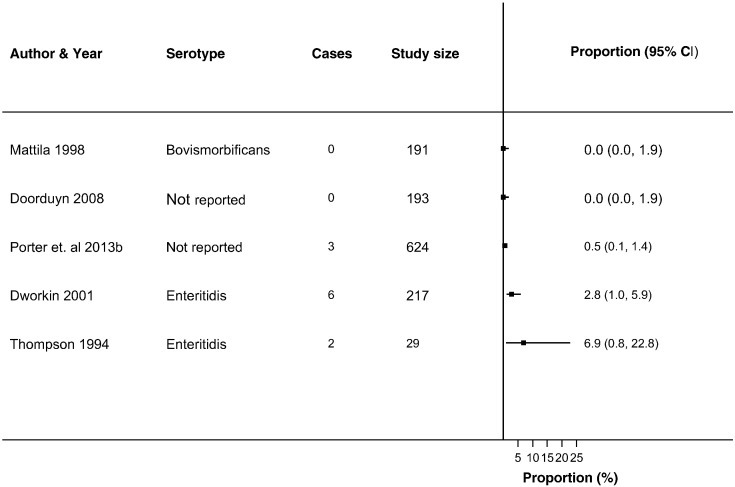
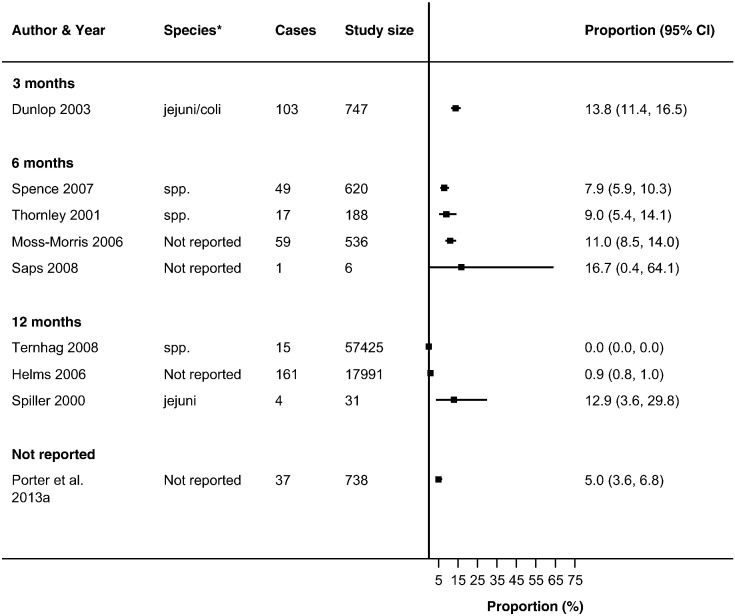
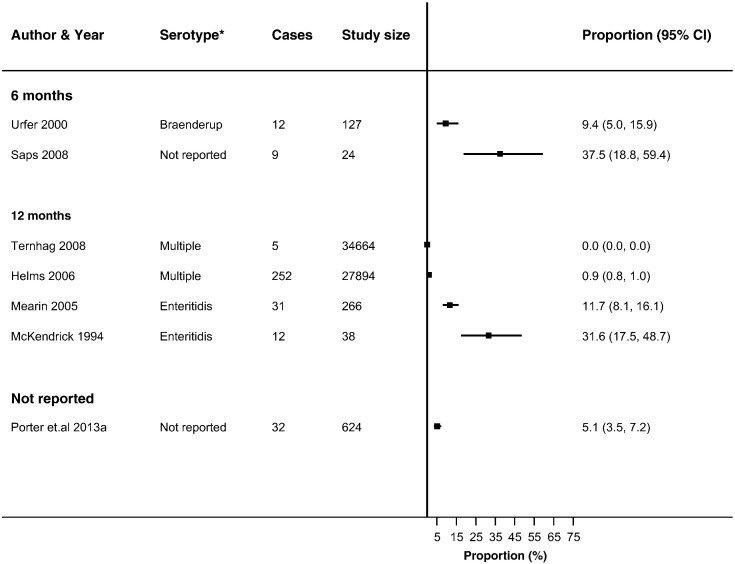
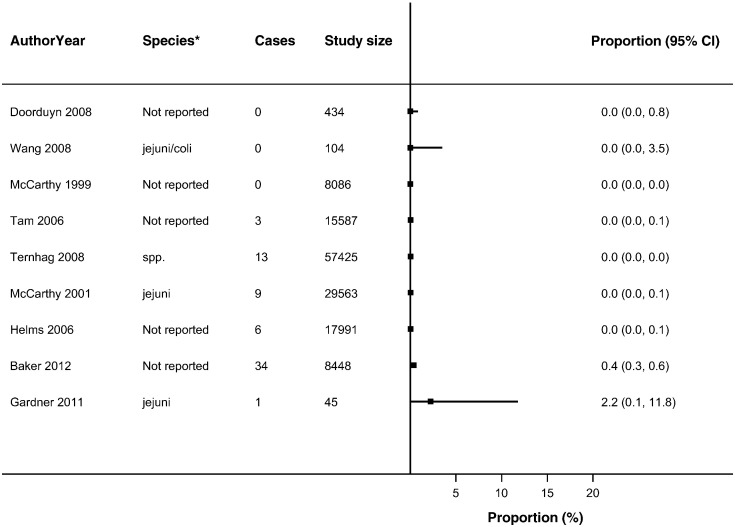
Thirty-seven studies reported ReA following Campylobacter (n = 19) and NTS infections (n = 26) (Buxton et al., 2002, Bremell et al., 1991, Dworkin et al., 2001, Eastmond, 1983, Ekman et al., 2000, Gumpel et al., 1981, Hakansson et al., 1976, Hannu et al., 2002a, Hannu et al., 2002b, Helms et al., 2006, Pitkanen et al., 1981, Lee et al., 2005, Locht et al., 1993, Locht and Krogfelt, 2002, Locht et al., 2002, Mattila et al., 1994, Mattila et al., 1998, McColl et al., 2000, Petersen et al., 1996, Pitkanen et al., 1983, Ponka et al., 1984, Rohekar et al., 2008, Rudwaleit et al., 2001, Samuel et al., 1995, Schiellerup et al., 2008, Schoenberg-Norio et al., 2010, Short et al., 1982, Ternhag et al., 2008, Thomson et al., 1992, Thomson et al., 1994, Townes et al., 2008a, Tuompo et al., 2013, Uotila et al., 2014, Urfer et al., 2000, Doorduyn et al., 2008, Arnedo-Pena et al., 2010, Eastmond et al., 1983, Melby et al., 1990) in up to 63% of patients with either infection (Supplementary Table 4). The majority of studies reported ReA triggered by Campylobacter (n = 14) or NTS infection (n = 18) in < 10% of patients with gastroenteritis. No overall summary of incidence of sequelae following gastroenteritis were calculated, as there was a high level of heterogeneity across studies (I2 > 90%) (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7). The incidences of RS, IBS and GBS were reported in 5, 12, and 9 studies respectively (Fig. 4, Fig. 5, Fig. 6, Fig. 7 and Table S4). < 10% of patients with either infection developed RS in all studies. Incidence of IBS was reported in Campylobacter (0% to 18%) and NTS (0% to 38%) patients. GBS was less frequent with all but one study reporting an incidence of < 2% following Campylobacter infection.
Fig. 2.
Forest plot of studies reporting incidence of Campylobacter triggered ReA stratified by healthcare facility.
Studies reporting the incidence of reactive arthritis following Campylobacter infection stratified by the type of healthcare facility/practitioner visited/utilised. No summary estimate was calculated due to high heterogeneity across all studies (I2 > 90%).
Fig. 3.
Forest plot of studies reporting incidence of NTS triggered ReA stratified by healthcare facility.
Studies reporting the incidence of reactive arthritis following non-typhoidal Salmonella infection stratified by the type of healthcare facility/practitioner visited/utilised. No summary estimate was calculated due to high heterogeneity across all studies (I2 > 90%).
Fig. 4.
Forest plot of studies reporting incidence of RS following NTS infection.
Studies reporting the incidence of Reiter's syndrome following non-typhoidal Salmonella infection. No summary estimate was calculated due to high heterogeneity across all studies (I2 > 90%).
Fig. 5.
Forest plot of studies reporting incidence of IBS following Campylobacter infection stratified by follow-up period.
Studies reporting the incidence of irritable bowel syndrome following Campylobacter infection stratified by the length of follow-up from infection to sequelae. No summary estimate was calculated due to high heterogeneity across all studies (I2 > 90%).
Fig. 6.
Forest plot of studies reporting incidence of IBS following NTS infection stratified by follow-up period.
Studies reporting the incidence of irritable bowel syndrome following non-typhoidal Salmonella infection stratified by the length of follow-up from infection to sequelae. No summary estimate was calculated due to high heterogeneity across all studies (I2 > 90%).
Fig. 7.
Forest plot of studies reporting incidence of GBS following Campylobacter infection.
Studies reporting the incidence of Guillain-Barré syndrome following Campylobacter infection No summary estimate was calculated due to high heterogeneity across all studies (I2 > 90%).
Only studies reporting incidence of ReA considered the use of PPI and antibiotics as potential factors contributing to sequelae development. One study assessing the use of PPI in the development of ReA following Campylobacter and NTS infection found a significant association after adjustment for age, sex and degree of urbanization (adjusted OR 2.9 (95% CI 1.4–6.1)) (Doorduyn et al., 2008).
The prescription/usage of antibiotics was reported in 17 studies (n = 7 for Campylobacter and n = 11 for NTS) (Arnedo-Pena et al., 2010, Buxton et al., 2002, Rudwaleit et al., 2001, Ekman et al., 2000, Schonberg-Norio et al., 2010, Dworkin et al., 2001, Mattila et al., 1998, Hannu et al., 2002b, Townes et al., 2008b, Tuompo et al., 2013, Lee et al., 2005, Pitkanen et al., 1981, Pitkanen et al., 1983, Ponka et al., 1984, Locht and Krogfelt, 2002, Locht et al., 1993, Uotila et al., 2014). Seven studies considered the risk of ReA development following antibiotics usage (Arnedo-Pena et al., 2010, Buxton et al., 2002, Dworkin et al., 2001, Mattila et al., 1998, Hannu et al., 2002b, Locht and Krogfelt, 2002, Townes et al., 2008b). Of these studies, increased risk of ReA development was observed following treatment of infection with antibiotics (class not provided) for Campylobacter and fluoroquinolones for NTS infection (n = 1 and 2, respectively) (Locht and Krogfelt, 2002, Dworkin et al., 2001, Mattila et al., 1998). Three studies found a protective effect in the use of antibiotics for NTS; however the findings were not statistically significant (Arnedo-Pena et al., 2010, Buxton et al., 2002, Hannu et al., 2002b). The remaining study did not find any associated risk with antibiotic usage for either Campylobacter or NTS infection. Moreover the data were not reported in the study (Townes et al., 2008b) (Table 3). Studies assessing association of antibiotic treatment and development of ReA reported visit to a GP/Physician (n = 4) or accident and emergency/hospital (n = 3).
Table 3.
Risk of developing ReA following prescription/usage of antibiotics in Campylobacter and NTS patients stratified by healthcare facility accessed.
| Author_year | Pathogen | No. with pathogen | Antibiotics usage N (%) | Antibiotics | Proportion of ReA vs. non-ReA using antibiotics |
|---|---|---|---|---|---|
| Visited general practitioner/physician | |||||
| Locht and Krogfelt (2002) | Campylobacter | 173 | 56 (32) | N.R. | 56% with ReA vs. 26% non-ReA; p = 0.03a, d |
| Arnedo-Pena et al. (2010) | S. Hadar PT2 | 155 | 57 (38)b | Fluoroquinolones | aRRc 0.43; 95% CI 0.17–1.08 |
| Hannu et al. (2002b) | S. Typhimurium DT193 | 63 | 32 (63)b | Fluoroquinolones | 0% with ReA vs. 16% no ReA; p = 0.056 |
| Mattila et al. (1998) | S. Bovismorbificans | 191 | 78 (41) | Fluoroquinolones | 59% with ReA vs. 35% non-ReA; p = 0.021a, d |
| Visited accident & emergency/hospitalised | |||||
| Dworkin et al. (2001) | S. Enteritidis | 217 | 66 (30) | Fluoroquinolones | RR 1.6; 95% CI 1.1–2.5a |
| Townes et al. (2008b) | NTS | 1356 | 365 (27) | Quinolone, β-lactam and macrolide | No associated risk (data not reported) |
| Townes et al. (2008b) | Campylobacter | 2384 | 1978 (83) | Quinolone, β-lactam and macrolide | No associated risk (data not reported) |
| Visit of healthcare facility not reported | |||||
| Buxton et al. (2002) | S. Typhimurium | 61 | 28 (46) | N.R. | OR 0.29; 95% CI 0.07–1.18 |
Antibiotics information not available for all with gastroenteritis.
Adjusted relative risk.
Significant results.
Chi-squared test in comparison of proportion.
Clinical symptoms were scarcely reported by studies and, where available, studies used different definitions and thresholds for diarrhea and fever (data not shown). This prevented further analysis of the role of symptom severity in sequelae development.
For the methodological subgroup analysis, only consultation with a rheumatologist statistically reduced heterogeneity of studies within the “specialist” in Campylobacter and NTS triggered ReA (Table 4A, Table 4B, Table 4C, Table 4D, Fig. 2, Fig. 3). Follow-up may have contributed to heterogeneity in Campylobacter triggered IBS as studies within the 6-month follow-up stratum had a statistically significant reduction in heterogeneity (I2 = 24.2%) (Table 4A, Table 4B, Table 4C, Table 4D, Fig. 6). The only clinical characteristic considered in a subgroup analysis was the healthcare facility visited for the GI infection in those developing ReA and IBS. Studies reporting a “GP/Physician” visit for NTS infection were fairly homogenous (I2 = 2.0%), nonetheless high heterogeneity remained in this stratum for patients with Campylobacter triggered ReA (Fig. 2, Fig. 3).
Table 4A.
Subgroup meta-analysis for studies reporting development of reactive arthritis following Campylobacter infection by sequelae diagnosis, follow-up period, study size and healthcare facility visited.
| Variable | I2 | Number of studies |
|---|---|---|
| Sequelae diagnosis | ||
| Physician/medical records | 95.6% | 6 |
| Self-reported disease status | 97.0% | 6 |
| Self-reported disease status based on a validated scale | – | 1 |
| Specialista | 48.6% | 2 |
| Combination | 0.0% | 3 |
| Not reported | – | 1 |
| Follow-up period | ||
| < 3 months | 97.0% | 8 |
| 3 months | – | 1 |
| > 3 months < 1 year | – | – |
| 1 year | 95.6% | 3 |
| > 1 year | 97.8% | 2 |
| Not reported | 56.4% | 5 |
| Study size | ||
| Extra small (n < 100) | 95.3% | 7 |
| Small (101–500) | 86.3% | 7 |
| Medium (501–1000) | – | 1 |
| Large (1001–10,000) | 97.70% | 2 |
| Extra-large (> 10.000) | 99.20% | 2 |
| Healthcare facility visited | ||
| GP/physician | – | 1 |
| GP/hospitalised | – | – |
| A&E/hospitalised | – | 1 |
| Hospitalised | 97.8% | 7 |
| Not reported | 98.8% | 10 |
Heterogeneity significantly reduced (I2 < 50%).
Table 4B.
Subgroup meta-analysis for studies reporting development of reactive arthritis following NTS infection by sequelae diagnosis, follow-up period, study size and healthcare facility visited.
| Variable | I2 | Number of studies |
|---|---|---|
| Sequelae diagnosis | ||
| Physician/medical records | 97.7% | 9 |
| Self-reported disease status | 96.8% | 9 |
| Self-reported disease status based on a validated scale | – | 1 |
| Specialista | 41.2% | 6 |
| Combination | – | 1 |
| Not reported | – | 1 |
| Follow-up period | ||
| < 3 months | 97.3% | 10 |
| 3 months | 81.3% | 5 |
| > 3 months < 1 year | 93.5% | 4 |
| 1 year | 97.8% | 2 |
| > 1 year | 95.3% | 2 |
| Not reported | 97.7% | 4 |
| Study size | ||
| Extra small (n < 100) | 58.8% | 7 |
| Small (101–500) | 95.6% | 16 |
| Medium (501–1000) | 96.1% | 1 |
| Large (1001–10,000) | – | 1 |
| Extra-large (> 10.000) | 99.80% | 1 |
| Healthcare facility visited | ||
| GP/physiciana | 2.0% | 3 |
| GP/hospitalised | – | 1 |
| A&E/hospitalised | 99.9% | 2 |
| Hospitalised | 91.7% | 5 |
| Not reported | 98.8% | 20 |
Heterogeneity significantly reduced (I2 < 50%).
Table 4C.
Subgroup meta-analysis for studies reporting development of irritable bowel syndrome following Campylobacter infection by sequelae diagnosis, follow-up period, study size and healthcare facility visited.
| Variable | I2 | Number of studies |
|---|---|---|
| Sequelae diagnosis | ||
| Physician/medical records | 99.6% | 3 |
| Self-reported disease status based on a validated scale | 63.2% | 6 |
| Follow-up period | ||
| 3 months | – | 1 |
| > 3 months < 1 year⁎ | 24.2% | 4 |
| 1 year | 99.4% | 3 |
| Not reported | – | 1 |
| Study size | ||
| Extra small (n < 100) | 87.7% | 3 |
| Small (101–500) | – | 1 |
| Medium (501–1000) | 92.2% | 4 |
| Extra large (> 10.000) | 99.90% | 2 |
Heterogeneity significantly reduced (I2 < 50%).
Table 4D.
Subgroup meta-analysis for studies reporting development of irritable bowel syndrome following NTS infection by sequelae diagnosis, follow-up period, study size and healthcare facility visited.
| Variable | I2 | Number of studies |
|---|---|---|
| Sequelae diagnosis | ||
| Physician/medical records | 99.6% | 3 |
| Self-reported disease status | – | 1 |
| Self-reported disease status based on a validated scale | 87.3% | 3 |
| Follow-up period | ||
| > 3 months < 1 year | 99.8% | 2 |
| 1 year | 99.5% | 4 |
| Not reported | – | 1 |
| Study size | ||
| Extra small (n < 100) | 87.7% | 3 |
| Small (101–500) | – | 1 |
| Medium (501–1000) | – | 1 |
| Extra-large (> 10.000) | 99.20% | 2 |
| Healthcare facility | ||
| Hospitalised | 99.70% | 2 |
| Not reported | 98.8% | 5 |
4. Discussion
Previous systematic reviews considering incidence of ReA, RS, IBS and GBS conducted literature searches up until 2011 without assessing factors contributing to sequelae development. Five years after these searches were conducted, we only identified five new studies based on our inclusion criteria. We found that use of PPI and antibiotics may be possible factors associated with the development of ReA following GI infections. These factors were sparsely reported by studies and where information was available high heterogeneity (I2 > 90%) prevented the pooling of data.
Despite the over prescription of PPI in both primary and secondary settings (Forgacs and Loganayagam, 2008), only one study reported an association of PPI usage and ReA development in patients with gastroenteritis (Doorduyn et al., 2008). The authors demonstrated that PPI usage was independent of a single nucleotide polymorphism (SNP) in interferon gamma (IFN-γ) in cases with Campylobacter and NTS infections and the development of reactive arthritis (Doorduyn et al., 2008). IFN-γ is a cytokine crucial in the immune response against enteric infections. The combination of a SNP in IFN-γ with PPI usage could lead to increased susceptibility to enteric infections and subsequent prolonged or repeated episodes of GI infection. These sequential events may increase the susceptibility to reactive arthritis (Doorduyn et al., 2008).
Of all the sequelae considered, only risk of reactive arthritis was assessed following antibiotic usage in cases of Campylobacter and NTS infection. The associated risk is not clear as the studies report elevated, decreased or no risk of ReA following GI infection. The association may be dependent on the dose and type of antibiotics, as evidenced in a systematic review (Agger et al., 2015) evaluating the risk of hemolytic uremic syndrome following the use of antibiotics in patients with shiga toxin producing Escherichia coli (STEC) infections. Agger et al. (2015) showed that protein and cell wall synthesis class of antibiotics may be protective and improve recovery time and proposed a review of guidelines on contraindication of antibiotics for STEC infections.
Most gastroenteritis cases do not require treatment with antibiotics unless they are severe and occur in at risk groups i.e. elderly, children and those with underlying comorbidities. Due to insufficient information in the studies, we could not assess the potential reason for antibiotic treatment or the risk associated with sequelae development following antibiotic usage in a meta-analysis. Moreover, information on duration of treatment, dose of antibiotics, age and gender of all cases who received antimicrobial treatment were not available in all of the studies, thereby limiting any further comparisons or pooling of data.
Heterogeneity could not be explained by most of the subgroup analysis considered, except in the reported method of diagnosing reactive arthritis complication following Campylobacter and NTS infection, the type of healthcare facility visited for an NTS infection and follow-up period in Campylobacter triggered IBS. In studies reporting a visit to the GP/Physicians in cases of NTS infection the heterogeneity was significantly reduced (I2 = 2%). The three studies were all conducted in Finland, reported specialist diagnosis for reactive arthritis, had laboratory confirmation of the NTS infection and two assessed use of antibiotics in development of ReA in NTS patients. A GP/physician consultation may lead to a laboratory confirmed diagnosis of infection, prescription of antibiotics, and referral to a specialist; hence influencing the reported sequelae incidence. The small number of studies with inconsistent follow-up period from NTS infection to ReA development limits further interpretation of this finding.
Follow-up period may be crucial in the reported incidence of IBS following campylobacteriosis, due to a statistically significant reduction in heterogeneity in studies reporting a 6-month follow-up period, despite using different versions of Rome I, II and III criteria for diagnosis. The current gold-standard for IBS diagnosis is Rome III classification, which is symptom-based, requiring patients to be symptomatic both at 3 and 6 months after initial symptom onset (Longstreth et al., 2006) was used by only one study (Spence and Moss-Morris, 2007). Potential risk modifying factors in the development of IBS following gastroenteritis were not evaluated due to insufficient reporting and primary aim of included studies.
Data source may be a potential source of unexplained heterogeneity even though it was not significant in our subgroup analysis (data not shown). Using a combination of studies reporting outbreaks, population surveillance and hospital surveillance may have introduced additional heterogeneity. Outbreaks are usually caused by a single strain, while population surveillance identifies the circulating strains. In one of the studies included in this review, Tuompo et al. (2013) used phenotypic methods to determine potential differences in the O antigens of different NTS serotypes circulating in a population surveillance. However no significant differences in the arthritogenicity of the serotypes to trigger ReA were identified (Tuompo et al., 2013). The precision of genomic methods, such as whole genome sequencing, may provide further insights into differing potential for bacterial strains to trigger sequelae.
Our study has a number of major strengths. Firstly, our study addresses an existing knowledge gap by assessing the available evidence on factors contributing to the development of sequelae following Campylobacter and NTS infection. This is important in understanding factors amenable to intervention. Secondly, our review highlights the need for investigators to consider remediable risk factors in sequelae development following GI infections.
The reliance of observational studies on “natural experiments” such as outbreaks makes them prone to low study quality and high level of bias and heterogeneity. Most outbreak investigations are set up as a rapid response to an emergency situation to determine the cause of the outbreak. Time constraints limits the opportunity to address secondary factors that may improve the overall evidence on GI triggered sequelae. Therefore, it was possible for a study to have a high quality score for the primary research but missing key items such as method of diagnosis of pathogen and sequelae, crucial for risk factor study. Thus, a quality criteria was not adopted for inclusion of studies for meta-analysis. Although heterogeneity was significantly reduced for studies reporting a specialist consultation for diagnosis of ReA following either Campylobacter or NTS infection, we did not report a pooled estimate. Specialist diagnosis was preceded by self-reported symptoms. Not all patients with symptoms were seen by a specialist. Further, the studies reported different follow-up times indicating different definitions of ReA were adopted.
Other limitations of our study include inconsistencies in the reporting of key features, such as age, definition of sequelae, and follow-up of gastroenteritis cases in included studies. The risk factors we considered were also sparsely reported. Further, unexplained sources of heterogeneity prevented the reporting of a summary statistic for factors contributing to sequelae development. These limit the application of estimates to the general population and made interpretation of results difficult.
In conclusion, the findings of this systematic review show that the factors contributing to sequelae development following GI infections remain unclear. This is a challenge for policy makers in targeting interventions to reduce the overall burden of GI infections. Researchers should consider reporting information that will improve the overall evidence of sequelae of GI infections when observational studies are conducted. Applying record linkage in following up patients' health journey to study sequelae of GI infections may improve reporting of observational studies.
Furthermore, primary research in risk modifiers of gastroenteritis triggered sequelae and the potential for different bacterial strains to cause sequelae may address the knowledge gap in the relationship between pathogen and host.
Conflict of Interest
Prof. Perera received grants from UK NIHR, during the conduct of the study.
Author Contributions
OE wrote the manuscript. OE conceived the initial idea for the study, NM, MV, RP and TF critically appraised the protocol, manuscript and also contributed to its development by revising different versions. NR assisted with the database searches and ran the updated searches. OE and OvH screened all abstracts; OE and DC completed the full text screening; OE and MP performed all data extraction and risk of bias was completed by OE, MP and TF. All authors approved the final version and take responsibility for its content.
Acknowledgements
The research was funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) (NIHR HPRU-2012-10038) in Gastrointestinal Infections at the University of Liverpool in partnership with Public Health England (PHE), in collaboration with the University of East Anglia, University of Oxford and the Institute of Food Research. Oluwaseun Esan is based at the University of Oxford. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England’.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.12.006.
Appendix A. Supplementary data
Supplementary material
References
- Agger M., Scheutz F., Villumsen S., Molbak K., Petersen A.M. Antibiotic treatment of verocytotoxin-producing Escherichia coli (VTEC) infection: a systematic review and a proposal. J. Antimicrob. Chemother. 2015;70:2440–2446. doi: 10.1093/jac/dkv162. [DOI] [PubMed] [Google Scholar]
- Ajene A.N., Fischer Walker C.L., Black R.E. Enteric pathogens and reactive arthritis: a systematic review of Campylobacter, Salmonella and Shigella-associated reactive arthritis. J. Health Popul. Nutr. 2013;31:299–307. doi: 10.3329/jhpn.v31i3.16515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnedo-Pena A., Beltran-Fabregat J., Vila-Pastor B., Tirado-Balaguer M.D., Herrero-Carot C., Bellido-Blasco J.B., Romeu-Garcia M.A., Safont-Adsuara L., Pac-Sa M.R., Guillen-Grima F. Reactive arthritis and other musculoskeletal sequelae following an outbreak of Salmonella Hadar in Castellon, Spain. J. Rheumatol. 2010;37:1735–1742. doi: 10.3899/jrheum.091250. [DOI] [PubMed] [Google Scholar]
- Baker M.G., Kvalsvig A., Zhang J., Lake R., Sears A., Wilson N. Declining Guillain-Barre syndrome after campylobacteriosis control, New Zealand, 1988–2010. Emerg. Infect. Dis. 2012;18:226–233. doi: 10.3201/eid1802.111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremell T., Bjelle A., Svedhem A. Rheumatic symptoms following an outbreak of Campylobacter enteritis: a five year follow up. Ann. Rheum. Dis. 1991;50:934–938. doi: 10.1136/ard.50.12.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton J.A., Fyfe M., Berger S., Cox M.B., Northcott K.A. Reactive arthritis and other sequelae following sporadic Salmonella typhimurium infection in British Columbia, Canada: a case control study. J. Rheumatol. 2002;29:2154–2158. [PubMed] [Google Scholar]
- Doorduyn Y., Van Pelt W., Siezen C.L., Van Der Horst F., Van Duynhoven Y.T., Hoebee B., Janssen R. Novel insight in the association between salmonellosis or campylobacteriosis and chronic illness, and the role of host genetics in susceptibility to these diseases. Epidemiol. Infect. 2008;136:1225–1234. doi: 10.1017/S095026880700996X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop S., Jenkins D., Neal K., Spiller R. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in post infectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Dworkin M.S., Shoemaker P.C., Goldoft M.J., Kobayashi J.M. Reactive arthritis and Reiter's syndrome following an outbreak of gastroenteritis caused by Salmonella enteritidis. Clin. Infect. Dis. 2001;33:1010–1014. doi: 10.1086/322644. [DOI] [PubMed] [Google Scholar]
- Eastmond C.J. Gram-negative bacteria and B27 disease. Br. J. Rheumatol. 1983;22:67–74. doi: 10.1093/rheumatology/xxii.suppl_2.67. [DOI] [PubMed] [Google Scholar]
- Eastmond C., Rennie J., Reid T. An outbreak of Campylobacter enteritis–a rheumatological follow-up survey. J. Rheumatol. 1983;10:107–108. [PubMed] [Google Scholar]
- Ekman P., Kirveskari J., Granfors K. Modification of disease outcome in Salmonella-infected patients by HLA-B27. Arthritis Rheum. 2000;43:1527–1534. doi: 10.1002/1529-0131(200007)43:7<1527::AID-ANR17>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Forgacs I., Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336:2–3. doi: 10.1136/bmj.39406.449456.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M.F., Tukey J.W. Transformations related to the angular and the square root. Ann. Math. Stat. 1950:607–611. [Google Scholar]
- Gardner T., Fitzgerald C., Xavier C., Klein R., Pruckler J., Stroika S., McLaughlin J. Outbreak of campylobacteriosis associated with consumption of raw peas. Clin. Infect. Dis. 2011;53:26–32. doi: 10.1093/cid/cir249. [DOI] [PubMed] [Google Scholar]
- Gumpel J., Martin C., Sanderson P. Reactive arthritis associated with Campylobacter enteritis. Ann. Rheum. Dis. 1981;40:64–65. doi: 10.1136/ard.40.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson U., Eitrem R., Low B., Winblad S. HLA-antigen b27 in cases with joint affections in an outbreak of salmonellosis. Scand. J. Infect. Dis. 1976;8:245–248. doi: 10.3109/inf.1976.8.issue-4.05. [DOI] [PubMed] [Google Scholar]
- Hannu T., Mattila L., Rautelin H., Pelkonen P., Lahdenne P., Siitonen A., Leirisalo-Repo M. Campylobacter-triggered reactive arthritis: a population-based study. Rheumatology. 2002;41:312–318. doi: 10.1093/rheumatology/41.3.312. [DOI] [PubMed] [Google Scholar]
- Hannu T., Mattila L., Siitonen A., Leirisalo-Repo M. Reactive arthritis following an outbreak of Salmonella typhimurium phage type 193 infection. Ann. Rheum. Dis. 2002;61:264–266. doi: 10.1136/ard.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J., Praet N., Bellinger D.C., de Silva N.R., Gargouri N., Speybroeck N., Cawthorne A., Mathers C., Stein C., Angulo F.J., Devleesschauwer B. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12:e1001923. doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms M., Simonsen J., Molbak K. Foodborne bacterial infection and hospitalization: a registry-based study. Clin. Infect. Dis. 2006;42:498–506. doi: 10.1086/499813. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithlin J., Sargeant J., Thomas M.K., Fazil A. Systematic review and meta-analysis of the proportion of Campylobacter cases that develop chronic sequelae. BMC Public Health. 2014;14:1203. doi: 10.1186/1471-2458-14-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithlin J., Sargeant J.M., Thomas M.K., Fazil A. Systematic review and meta-analysis of the proportion of non-typhoidal Salmonella cases that develop chronic sequelae. Epidemiol. Infect. 2015;143:1333–1351. doi: 10.1017/S0950268814002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosunen T., Ponka A., Kauranen O., Martio J., Pitkanen T., Hortling L., Aittoniemi S., Penttila O., Koskimies S. Arthritis associated with Campylobacter jejuni/enteritis. Scand. J. Rheumatol. 1981;10:77–80. doi: 10.3109/03009748109095276. [DOI] [PubMed] [Google Scholar]
- Lee A.T., Hall R.G., Pile K.D. Reactive joint symptoms following an outbreak of Salmonella typhimurium phage type 135a. J. Rheumatol. 2005;32:524–527. [PubMed] [Google Scholar]
- Locht H., Krogfelt K. Comparison of rheumatological and gastrointestinal symptoms after infection with Campylobacter jejuni/coli and enterotoxigenic Escherichia coli. Ann. Rheum. Dis. 2002;61:448–452. doi: 10.1136/ard.61.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht H., Kihlstrom E., Lindstrom F.D. Reactive arthritis after Salmonella among medical doctors–study of an outbreak. J. Rheumatol. 1993;20:845–848. [PubMed] [Google Scholar]
- Locht H., Molbak K., Krogfelt K.A. High frequency of reactive joint symptoms after an outbreak of Salmonella enteritidis. J. Rheumatol. 2002;29:767–771. [PubMed] [Google Scholar]
- Longstreth G.F., Thompson W.G., Chey W.D., Houghton L.A., Mearin F., Spiller R.C. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Majowicz S.E., Musto J., Scallan E., Angulo F.J., Kirk M., O'Brien S.J., Jones T.F., Fazil A., Hoekstra R.M. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- Mattila L., Leirisalo-Repo M., Koskimies S., Granfors K., Siitonen A. Reactive arthritis following an outbreak of Salmonella infection in Finland. Br. J. Rheumatol. 1994;33:1136–1141. doi: 10.1093/rheumatology/33.12.1136. [DOI] [PubMed] [Google Scholar]
- Mattila L., Leirisalo-Repo M., Pelkonen P., Koskimies S., Granfors K., Siitonen A. Reactive arthritis following an outbreak of Salmonella Bovismorbificans infection. J. Infect. 1998;36:289–295. doi: 10.1016/s0163-4453(98)94243-8. [DOI] [PubMed] [Google Scholar]
- McCarthy N., Giesecke J. Incidence of Guillain-Barre syndrome following infection with Campylobacter jejuni. Am. J. Epidemiol. 2001;153:610–614. doi: 10.1093/aje/153.6.610. [DOI] [PubMed] [Google Scholar]
- McCarthy N., Andersson Y., Jormanainen V., Gustavsson O., Giesecke J. The risk of Guillain-Barre syndrome following infection with Campylobacter jejuni. Epidemiol. Infect. 1999;122:15–17. doi: 10.1017/s0950268898001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl G.J., Diviney M.B., Holdsworth R.F., McNair P.D., Carnie J., Hart W., McCluskey J. HLA-B27 expression and reactive arthritis susceptibility in two patient cohorts infected with Salmonella Typhimurium. Aust. NZ J. Med. 2000;30:28–32. doi: 10.1111/j.1445-5994.2000.tb01050.x. [DOI] [PubMed] [Google Scholar]
- McKendrick M.W., Read N.W. Irritable bowel syndrome–post Salmonella infection. J. Infect. 1994;29:1–3. doi: 10.1016/s0163-4453(94)94871-2. [DOI] [PubMed] [Google Scholar]
- Mearin F., Perez-Oliveras M., Perello A., Vinyet J., Ibanez A., Coderch J., Perona M. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129:98–104. doi: 10.1053/j.gastro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Melby K., Dahl O., Crisp L., Penner J. Clinical and serological manifestations in patients during a waterborne epidemic due to Campylobacter jejuni. J. Infect. 1990;21:309–316. doi: 10.1016/0163-4453(90)94125-j. [DOI] [PubMed] [Google Scholar]
- Moss-Morris R., Spence M. To “lump” or to “split” the functional somatic syndromes: can infectious and emotional risk factors differentiate between the onset of chronic fatigue syndrome and irritable bowel syndrome? Psychosom. Med. 2006;68:463–469. doi: 10.1097/01.psy.0000221384.07521.05. [DOI] [PubMed] [Google Scholar]
- Munn Z., Moola S., Riitano D., Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int. J. Health Policy Manag. 2014;3:123–128. doi: 10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch. Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.M., Nielsen S.V., Meyer D., Ganer P., Ladefoged K. Bacterial gastroenteritis among hospitalized patients in a Danish County, 1991–93. Scand. J. Gastroenterol. 1996;31:906–911. doi: 10.3109/00365529609052000. [DOI] [PubMed] [Google Scholar]
- Pitkanen T., Pettersson T., Ponka A., Kosunen T. Clinical and serological studies in patients with Campylobacter fetus/ssp./jejuni infection: clinical findings. Infection. 1981;9:274–278. doi: 10.1007/BF01640990. [DOI] [PubMed] [Google Scholar]
- Pitkanen T., Ponka A., Peterson T., Kosunen T. Campylobacter enteritis in 188 hospitalized patients. Arch. Intern. Med. 1983;143:215–219. doi: 10.1001/archinte.1983.00350020033007. [DOI] [PubMed] [Google Scholar]
- Ponka A., Pitkanen T., Sarna S., Kosunen T. Infection due to Campylobacter jejuni: a report of 524 outpatients. Infection. 1984;12:175–178. doi: 10.1007/BF01640893. [DOI] [PubMed] [Google Scholar]
- Porter C.K., Choi D., Cash B., Pimentel M., Murray J., May L., Riddle M.S. Pathogen-specific risk of chronic gastrointestinal disorders following bacterial causes of foodborne illness. BMC Gastroenterol. 2013;13:46. doi: 10.1186/1471-230X-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C.K., Choi D., Riddle M.S. Pathogen-specific risk of reactive arthritis from bacterial causes of foodborne illness. J. Rheumatol. 2013;40:712–714. doi: 10.3899/jrheum.121254. [DOI] [PubMed] [Google Scholar]
- Rohekar S., Tsui F.W., Tsui H.W., Xi N., Riarh R., Bilotta R., Inman R.D. Symptomatic acute reactive arthritis after an outbreak of salmonella. J. Rheumatol. 2008;35:1599–1602. [PubMed] [Google Scholar]
- Rudwaleit M., Richter S., Braun J., Sieper J. Low incidence of reactive arthritis in children following a salmonella outbreak. Ann. Rheum. Dis. 2001;60:1055–1057. doi: 10.1136/ard.60.11.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M.P., Zwillich S.H., Thomson G.T., Alfa M., Orr K.B., Brittain D.C., Miller J.R., Phillips P.E. Fast food arthritis–a clinico-pathologic study of post-Salmonella reactive arthritis. J. Rheumatol. 1995;22:1947–1952. [PubMed] [Google Scholar]
- Saps M., Pensabene L., Di Martino L., Staiano A., Wechsler J., Zheng X., Di Lorenzo C. Post-infectious functional gastrointestinal disorders in children. J. Pediatr. 2008;152:812–816. doi: 10.1016/j.jpeds.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Scallan E., Hoekstra R., Angulo F., Tauxe R., Widdowson M., Roy S., Jones J., Griffin P. Foodborne illness acquired in the United States - major pathogens. Emerg. Infect. Dis. 2011;17:7. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiellerup P., Krogfelt K., Locht H. A comparison of self-reported joint symptoms following infection with different enteric pathogens: effect of HLA-B27. J. Rheumatol. 2008;35:480–487. [PubMed] [Google Scholar]
- Schoenberg-Norio D., Mattila L., Lauhio A., Katila M., Kaukoranta S., Koskela M., Pajarre S., Uksila J., Eerola E., Sarna S., Rautelin H. Patient-reported complications associated with Campylobacter jejuni infection. Epidemiol. Infect. 2010;138:1004–1011. doi: 10.1017/S0950268809991099. [DOI] [PubMed] [Google Scholar]
- Schonberg-Norio D., Mattila L., Lauhio A., Katila M.L., Kaukoranta S.S., Koskela M., Pajarre S., Uksila J., Eerola E., Sarna S., Rautelin H. Patient-reported complications associated with Campylobacter jejuni infection. Epidemiol. Infect. 2010;138:1004–1011. doi: 10.1017/S0950268809991099. [DOI] [PubMed] [Google Scholar]
- Short C., Klouda P., Smith L. Campylobacter jejuni/enteritis and reactive arthritis. Ann. Rheum. Dis. 1982;41:287–288. doi: 10.1136/ard.41.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence M., Moss-Morris R. The cognitive behavioral model of irritable bowel syndrome: a prospective investigation of patients with gastroenteritis. Gut. 2007;56:1066–1071. doi: 10.1136/gut.2006.108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller R., Jenkins D., Thornley J., Hebden J., Wright T., Skinner M., Neal K. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Tam C., Rodrigues L., Petersen I., Islam A., Hayward A., O'Brien S. Incidence of Guillain-Barre syndrome among patients with Campylobacter infection: a general practice research database study. J. Infect. Dis. 2006;194:95–97. doi: 10.1086/504294. [DOI] [PubMed] [Google Scholar]
- Ternhag A., Torner A., Svensson A., Ekdahl K., Giesecke J. Short- and long-term effects of bacterial gastrointestinal infections. Emerg. Infect. Dis. 2008;14:143–148. doi: 10.3201/eid1401.070524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabane M., Kottachchi D., Marshall J. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment. Pharmacol. Ther. 2007;26:535–544. doi: 10.1111/j.1365-2036.2007.03399.x. [DOI] [PubMed] [Google Scholar]
- Thomson G.T., Chiu B., De Rubeis D., Falk J., Inman R.D. Immunoepidemiology of post-Salmonella reactive arthritis in a cohort of women. Clin. Immunol. Immunopathol. 1992;64:227–232. doi: 10.1016/0090-1229(92)90204-2. [DOI] [PubMed] [Google Scholar]
- Thomson G.T., Alfa M., Orr K., Thomson B.R., Olson N. Secretory immune response and clinical sequelae of Salmonella infection in a point source cohort. J. Rheumatol. 1994;21:132–137. [PubMed] [Google Scholar]
- Thornley J., Jenkins D., Neal K., Wright T., Brough J., Spiller R. Relationship of Campylobacter toxigenicity in vitro to the development of post infectious irritable bowel syndrome. J. Infect. Dis. 2001;184:606–609. doi: 10.1086/322845. [DOI] [PubMed] [Google Scholar]
- Townes J., Deodhar A., Laine E., Smith K., Krug H., Barkhuizen A., Thompson M., Cieslak P., Sobel J. Reactive arthritis following culture-confirmed infections with bacterial enteric pathogens in Minnesota and Oregon: a population-based study. Ann. Rheum. Dis. 2008;67:1689–1696. doi: 10.1136/ard.2007.083451. [DOI] [PubMed] [Google Scholar]
- Townes J.M., Deodhar A.A., Laine E.S., Smith K., Krug H.E., Barkhuizen A., Thompson M.E., Cieslak P.R., Sobel J. Reactive arthritis following culture-confirmed infections with bacterial enteric pathogens in Minnesota and Oregon: a population-based study. Ann. Rheum. Dis. 2008;67:1689–1696. doi: 10.1136/ard.2007.083451. [DOI] [PubMed] [Google Scholar]
- Tuompo R., Hannu T., Mattila L., Siitonen A., Leirisalo-Repo M. Reactive arthritis following Salmonella infection: a population-based study. Scand. J. Rheumatol. 2013;42:196–202. doi: 10.3109/03009742.2012.739201. [DOI] [PubMed] [Google Scholar]
- Uotila T., Korpela M., Vuento R., Laine J., Lumio J., Kuusi M., Virtanen M.J., Mustonen J., Antonen J. Joint symptoms after a faecal culture positive Campylobacter infection associated with a waterborne gastroenteritis outbreak: a questionnaire study. Scand. J. Rheumatol. 2014;43:524–526. doi: 10.3109/03009742.2014.920916. [DOI] [PubMed] [Google Scholar]
- Urfer E., Rossier P., Mean F., Krending M.J., Burnens A., Bille J., Francioli P., Zwahlen A. Outbreak of Salmonella Braenderup gastroenteritis due to contaminated meat pies: clinical and molecular epidemiology. Clin. Microbiol. Infect. 2000;6:536–542. doi: 10.1046/j.1469-0691.2000.00148.x. [DOI] [PubMed] [Google Scholar]
- Wang S., Chang L., Hsueh P., Lu C., Lee P., Shao P., Hsieh Y., Yen F., Lee C., Huang L. Campylobacter enteritis in children in northern Taiwan-a 7-year experience. J. Microbiol. Immunol. Infect. 2008;41:408–413. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material