Abstract
In asthma, mucus hypersecretion is thought to be a prominent pathological feature associated with widespread mucus plugging. However, the current treatments for mucus hypersecretion are often ineffective or temporary. The potential therapeutic targets of mucus hypersecretion in asthma remain unknown. Here, we show that Lyn is a central effector of endoplasmic reticulum stress (ER stress) and mucous hypersecretion in asthma. In Lyn-transgenic mice (Lyn-TG) and wild-type (WT) C57BL/6J mice exposed to ovalbumin (OVA), Lyn overexpression attenuates mucus hypersecretion and ER stress. Interleukin 13 (IL-13) induced MUC5AC expression by enhancing ER stress in vitro. Lyn serves as a negative regulator of IL-13-induced ER stress and MUC5AC expression. We further find that an inhibitor of ER stress, which is likely involved in the PI3K p85α/Akt pathway and NFκB activity, blocked MUC5AC expression in Lyn-knockdown cells. Furthermore, PI3K/Akt signaling is required for IL-13-induced ER stress and MUC5AC expression in airway epithelial cells. The ER stress regulation of MUC5AC expression depends on NFκB in Lyn-knockdown airway epithelial cells. Our studies indicate not only a concept of mucus hypersecretion in asthma that involves Lyn kinase but also an important therapeutic candidate for asthma.
Keyword: Mucus secretion, Lyn kinase, Endoplasmic reticulum stress, MUC5AC, Asthma
Highlights
-
•
ER stress may be critical for the pathogenesis of bronchial asthma, especially the steroid-resistant neutrophilic asthma.
-
•
We confirmed that Lyn serves as a negative regulator of ER stress and MUC5AC through PI3K p85α/Akt/NFκB pathway in asthma.
-
•
This indicates not only a concept of mucus hypersecretion that involves Lyn but also a therapeutic candidate for asthma.
1. Introduction
Asthma is one of the most common chronic airway inflammatory diseases. Type 2 inflammation is an important molecular mechanism of asthma (Fahy, 2015). The type 2 immune response results in goblet cell metaplasia, mucus hypersecretion, leukocyte infiltration (predominantly eosinophils) and collagen deposition in the airways (Balenga et al., 2015). Mucus hypersecretion is prominent pathological feature in severe asthma patients coupled with widespread mucus plugging (Kuyper et al., 2003). MUC5AC is a major component of airway mucins and is implicated in pulmonary diseases with mucus hypersecretion. Epithelial tethering of MUC5AC-rich mucus causes mucostasis and is likely a major cause of mucus plugging in asthma (Bonser et al., 2016). IL-13 induces mucus production and goblet cell hyperplasia in airway epithelial cells. Dexamethasone at therapeutic concentrations did not inhibit the effects of IL-13 on goblet cell differentiation (Kanoh et al., 2011). The activation of MEK1/2, phosphatidylinositol-3 kinase (PI3K), sphingosine kinase 1 (SPhk1), and MAPK14 (p38α-MAPK) are critical for IL-13-induced mucus production (Alevy et al., 2012). Transcriptional control of Muc5ac gene expression through regulators such as SPDEF (SAM pointed domain-containing Ets transcription factor), Notch, and Hypoxia Inducible Factor-1 is not currently targetable (Evans et al., 2015). However, further investigation is critical for identifying potential therapeutic targets for mucus hypersecretion in asthma.
Endoplasmic reticulum stress (ER stress) causes the activation of activating transcription factor 6 (ATF6), CCAAT/enhancer-binding protein homologous protein (CHOP) and X-box binding protein 1 (XBP1) and the phosphorylation of protein kinase-like ER kinase (PERK) (Koh et al., 2013). Previous studies have shown that oxidative stress and ER stress have a direct and pathogenic impact on mucus secretion in asthma. The house dust mite allergen (HDM) induces oxidative injury to the airway epithelial cells (Li et al., 2012). Asthmatic patients with severe exacerbations exhibit increased oxidative stress damage and NFκB phosphorylation (Lan et al., 2014). HDM induces ER stress in airway epithelial cells (Hoffman et al., 2013). Aspergillus fumigatus is also associated with steroid-resistant eosinophilic allergic lung inflammation via ER stress. Phosphoinositide 3-kinase-δ (PI3K-δ) regulates fungus-induced allergic lung inflammation via endoplasmic reticulum stress (Lee et al., 2016). Furthermore, oxidative stress causes mucin synthesis via the transactivation of epidermal growth factor receptor (EGFR), but MUC5AC synthesis is not inhibited by antioxidants (Takeyama et al., 2000). Mucin maturation is achieved by posttranslational modifications initiated in the endoplasmic reticulum (ER) before they traffic to the Golgi. Allergen induced mucus overproduction is impaired in the absence of ER of specialized ER proteins such as AGR2 and IRE-1beta (Schroeder et al., 2012, Martino et al., 2013).
Src homology 2-containing protein tyrosine phosphatase-2 acts as a negative regulator for H2O2- induced mucus overproduction and hypersecretion in human airway epithelial cells (Song et al., 2013). Lyn kinase, a member of the Src family of tyrosine kinases, modulates mucus production in asthma. Lyn deficiency resulted in the mucous hypersecretion in a mouse model of asthma (Li et al., 2013). Lyn kinase may be one of the most important targets for the treatment of asthma. However, the effects of Lyn kinase on ER stress in asthma are less clear. In this study, we investigated the contribution of Lyn kinase to mucus hypersecretion and ER stress in asthma. Lyn regulated ER stress and MUC5AC expression in a murine model and in our in vitro experiments on airway epithelial cells, resulting in a phenotype associated with PI3K, Akt and NFκB signals.
2. Methods
2.1. Reagents
The following antibodies for histology and cell staining were purchased from Santa Cruz Biotechnology: anti-MUC5AC (Santa Cruz, CA, sc-16903, AB_649616), anti-phospho-PI3K p85α (Tyr467: Santa Cruz, CA, sc-293115, AB_10844180), anti-PI3K p85α (Santa Cruz, CA, sc-31970, AB_2268186), anti-phospho-Akt1 (Thr308: Santa Cruz, CA, sc-135650, AB_2224730), anti-Akt1 (Santa Cruz, CA, sc-1618, AB_630849), anti-phospho-NFκB p65 (Ser536: Santa Cruz, CA, sc-33020, AB_2179018), anti-NFκB p65 (Santa Cruz, CA, sc-109, AB_632039), anti-Lyn (Santa Cruz, CA, sc-15, AB_2281450), and anti-β-actin (Santa Cruz, CA, sc-130656, AB_2223228). The following antibodies for histology were purchased from Abcam Biotechnology: anti-BIP (Abcam, ab21685, AB_2119834), anti-CHOP (Abcam, ab11419, AB_298023), anti-histone H3 (Abcam, ab1791, AB_302613), and anti-IL-13 (Abcam, ab133353, AB_11157609). Anti-phospho-Lyn (Tyr416: Cell Signaling Technology, #2101, AB_331697) was purchased from Cell Signaling Technology. Anti-IL13 (R&D Systems, AF-413-NA, and AB_2124173) and IL-13 ELISA reagents (R&D Systems, M1300CB) were purchased from R&D Systems. IL-13 (PeproTech, #200-13), 4-Phenylbutyric acid (4-PBA, Sigma-Aldrich, P21005), PI3K Inhibitor PI-103 (Selleck, S1038) were purchased as indicated. A nonsilencing siRNA control and a Lyn-specific siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
2.2. Human lung tissue
From May 2015 to July 2016, patients with asthma and controls were recruited from the Affiliated Hospital of Southwest Medical University (Sichuan, China). The diagnosis of asthma was based on the asthma guidelines according to our previous describes (Li et al., 2013). Human lung tissues were collected according to our previous procedures (Xie et al., 2015). This study was approved by the Southwest Medical University Ethics Committee (Assurance Number: KY2015008) according to the principle of the Helsinki Declaration II. Participants gave written informed consent to their inclusion in the study.
2.3. Animals and experimental protocol
Transgenic (TG) mice overexpressing Lyn were generated by the Cyagen Biosciences, Inc. (Guangzhou, China). In brief, the Lyn gene was cloned in a transgene vector linking the gene to the EF1α promoter, creating pRP.ExSi-EF1α-mLYN. The transgene DNA was linearized and microinjected into the pronuclei of fertilized eggs of C57BL/6J mice to generate transgenic mice. Stable Lyn-transgenic mice were obtained by crossing founder mice with wild type C57BL/6J mice. The analysis and maintenance for Lyn-transgenic mice was performed in the background of C57BL/6J. There is no differentiation of growth and development in Lyn transgenic mice compared to that in wild type mice in natural lifespan.
Wild type C57BL/6J mice that were 8 to 10 weeks of age and free of murine-specific pathogens were obtained from Tengxin Biotechnology Co., Ltd. (Chongqing, China). The Lyn-transgenic mice and wild type C57BL/6J mice, aged 8–10 weeks, were used for all of the subsequent experiments, which were maintained under specific pathogen-free conditions in the Animal Experimental Center of Southwest Medical University. All animal experiments in this study were approved by and performed in accordance with the guidelines of the Committee of Animal Experiments Center of Southwest Medical University and the National Institute of Health guidelines on the care and use of animals.
Lyn-TG and WT mice were intraperitoneally sensitized on days 1 and 14 with 20 μg of ovalbumin (OVA) (Sigma-Aldrich,) and 1 mg of aluminum hydroxide. From week 3 to week 8 after the initial sensitization, the mice were challenged with an intranasal administration of 10 μg of OVA in 50 μl of normal saline (NS) 2 times per week for duration of 5 weeks, as previously described (Li et al., 2013). Control groups of Lyn-TG mice and WT mice were given NS alone. 4-phenylbutyric acid (4-PBA, 1 g/kg body weight per day; Sigma-Aldrich) diluted in phosphate-buffered saline (PBS) was given to each animal by intragastric administration 2 h before the challenge with OVA and 6 h after the last challenge in OVA-4-PBA mice. Twenty-four hours after the final intranasal challenge, the mice were killed with an intraperitoneal injection of 40 mg/kg ketamine. The trachea was carefully intubated with a 20-gauge catheter, and the lungs were slowly lavaged four times with 1.0 ml of pre-warmed PBS. The total cell numbers were counted. Smears of the bronchoalveolar lavage (BAL) cells were fixed and stained as described previously to examine cell differentials (Li et al., 2013).
2.4. Histology
The lungs of mice were filled intratracheally with 10% neutral-buffered formalin and then removed. Specimens were embedded in paraffin. For histological examination, sections (5 μm) of the lung specimens were stained with standard hematoxylin-eosin staining (H&E) methods and periodic acid–Schiff (PAS) reagent. The stained slides were analyzed with a DM4000 Leica light microscope (Leica, Germany). Histological review was performed in a blinded fashion by two independent pulmonary observers. The severity of peribronchial and perivascular inflammation was scored using previously described methods. Each bronchus observed was scored from 0 to 3, with approximately 10 areas scored in total (Xie et al., 2015).
2.5. Cell culture, viral infection and transfection
Human airway epithelial cells (16HBE) were obtained from American Type Culture Collection (ATCC; Manassas, USA) and kept in our lab. 16HBEs were used in vitro according to previous report (Sweerus et al., 2016). 16HBEs were seeded in culture dishes with DMEM culture medium containing 10% fetal bovine serum (FBS) at 37 °C in with 5% CO2 and grown to 70% confluence. The pLV.ExBi.P/Puro-EF1α-IRES-eGFP/pLV.Des3d.P/Puro vector was constructed and validated in our lab (data not shown). The lentiviral vector was transfected into human embryo kidney cells (293 T cells) to create infectious lentiviral vector-containing particles. After the 16HBE cells reached 85% confluence, the medium was replaced with serum-free DMEM culture medium. The lentiviral vector expressing Lyn was used to transfected 16HBE cells in the 6-well plates (MOI = 20). For Lyn knockdown, cells were transfected with Lyn small interfering RNA (Lyn siRNA, 20 μM) using Lipofectamine 2000 according to the manufacturer's instructions. Lyn expression was analyzed by Western blotting and immunofluorescence using an SP5 Leica confocal microscope (Leica, German). The medium was then replaced with a new medium containing PBS, PI-103 (10 ng/ml) or 4-PBA (10 mM). After 2 h of treatment with the inhibitor PI-103 (10 ng/ml) or 4-PBA, the medium containing IL-13 (10 ng/ml) was added to incubate overnight (24 h) at 37 °C.
2.6. Immunohistochemical staining for Lyn, IL-13, BIP, CHOP, MUC5AC and NFκB
For immunohistochemical staining of Lyn and IL-13, sections (5 μm) of the lung specimens embedded in paraffin were stained using Envision detection kit (Dako, K5007) with Lyn and IL-13 antibody. The stained slides were analyzed with a DM4000 Leica light microscope (Leica, Germany). Immunofluorescence staining was done as described previously (Li et al., 2012, Li et al., 2013, Xie et al., 2015). Frozen lung tissues embedded in optimal cutting temperature (OCT) compound were sectioned, and immunohistochemical staining was performed on glass slides using standard histological methods. Tissues or cells were fixed with ice-cold methanol and permeabilized in PBS containing 0.25% Triton X-100 for 10 min at room temperature and washed 3 times with PBS. Nonspecific binding was blocked for 1 h with 1% BSA (Sigma-Aldrich) in PBS containing 0.05% Tween 20. Specimens were then incubated with antibodies against IL-13, BIP (Immunoglobulin heavy chain binding protein), CHOP, Muc5ac, and NFκB. FITC-conjugated or TRITC-conjugated secondary Abs was used to probe the primary Abs. After the specimens were washed, nuclei were stained with 4′-6-diamidino-2-phenylindole dihydrochloride (DAPI, Invitrogen). The specimens were analyzed by immunofluorescence using an SP5 Leica confocal microscope with Leica Application Suite Software (Version number: 14.0.0.162, Leica, German). Human lung tissue from asthmatic patients and healthy subjects were kept in our lab. Biopsy specimens were collected as described previously (Xie et al., 2015). A mouse isotype control (Abcam, ab18428), a rabbit isotype control (Abcam, ab27478) or a goat isotype control (Santa Cruz, sc-2028) was replaced the primary antibody (Ab) as the negative control.
2.7. RNA isolation and real-time PCR
Total RNA was isolated from lung tissue using the TRIzol RNA Reagent (Invitrogen, Thermo Scientific, USA). RNA was quantified using an Epoch multi-volume spectrophotometer system (Biotech, USA). The total RNA preparation was reverse-transcribed using PrimeScript™ RT Master Mix (Takara, China). Quantitative real-time RT-PCR analysis was performed using SYBR Advantage qPCR Premix (Clontech, USA). The △cycle threshold method was used to calculate the relative differences in mRNA levels in the Light Cycler 480 Multiple Plate Analysis Software (Roche Diagnostics, USA). The primer sequences were as follows: Gapdh primer (sense, 5′-AAGAAGGTGGTGAAGCAGG-3′; antisense, 5′-GAAGGTGGAAGAGTGGGAGT-3′; Muc5ac primer (sense, 5′-TCTACCACTCCCTGCTTCT-3′; antisense, 5′-TGACTAACCC TCTTGACCAC-3′).
2.8. Lung tissue homogenization and cytosolic or nuclear protein extraction
Lung tissue or cells were homogenized with radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate) with an optional protease inhibitor cocktail (Roche or Fisher scientific). For cytosolic or nuclear protein extraction, cells were collected and washed twice with PBS before nuclear and cytosolic fractionation. Nuclear and cytoplasmic fractionation was carried out with a nuclear extract kit (Cat: 40410, active motif, Carlsbad, CA, USA) according to the manufacturer's instructions. Each separated protein fraction was analyzed by Western blot.
2.9. Measurement of cytokine levels
Protein concentrations were quantified using an Epoch multi-volume spectrophotometer system (Biotech, USA). The IL-13 levels were determined in triplicate in the total lung lysates from each animal using an ELISA according to the manufacturer's instructions. The lower detection limit is 7.8 pg/ml.
2.10. Western blot analysis
Protein concentrations were determined by an Epoch multi-volume spectrophotometer system (Biotech, USA). Samples (20 μg/lane) were loaded into an SDS-PAGE gel. After electrophoresis at 100 V for 90 min, proteins were transferred to a microporous polyvinylidene difluoride (PVDF) membrane with 100 mA current for 2 h using a wet transfer method. Nonspecific sites were blocked with 5% skim milk in Tris-buffered saline/Tween 20 for 1 h. The blots were then incubated overnight at 4 °C with antibodies against phospho-PI3K p85α, PI3K p85α, phospho-Akt1, Akt1, phospho-NFκB p65 (Ser536), NFκB p65, Lyn, β-actin, BIP, CHOP, histone H3, and phospho-Lyn (Tyr416). Anti-rabbit, anti-mouse, or anti-rat horseradish peroxidase-conjugated IgG secondary antibodies (Cell Signaling Technologies) were used to detect antibody binding, and the bands were visualized using the Pierce ECL Western Blotting kit (Pierce Biotechnology, USA).
2.11. Statistical analysis
All statistical analyses were done using the SPSS 17.0 software (Chicago, IL, USA). All data are presented as the mean ± s.d. Differences among multiple groups were analyzed using a one-way ANOVA. Significant differences between two groups were analyzed by using the Tukey-Kramer post-test or Dunnett's T3. Statistical significance was defined as a P value < 0.05.
3. Results
3.1. Lyn ameliorated airway inflammation and ER stress in allergic airway inflammation disease
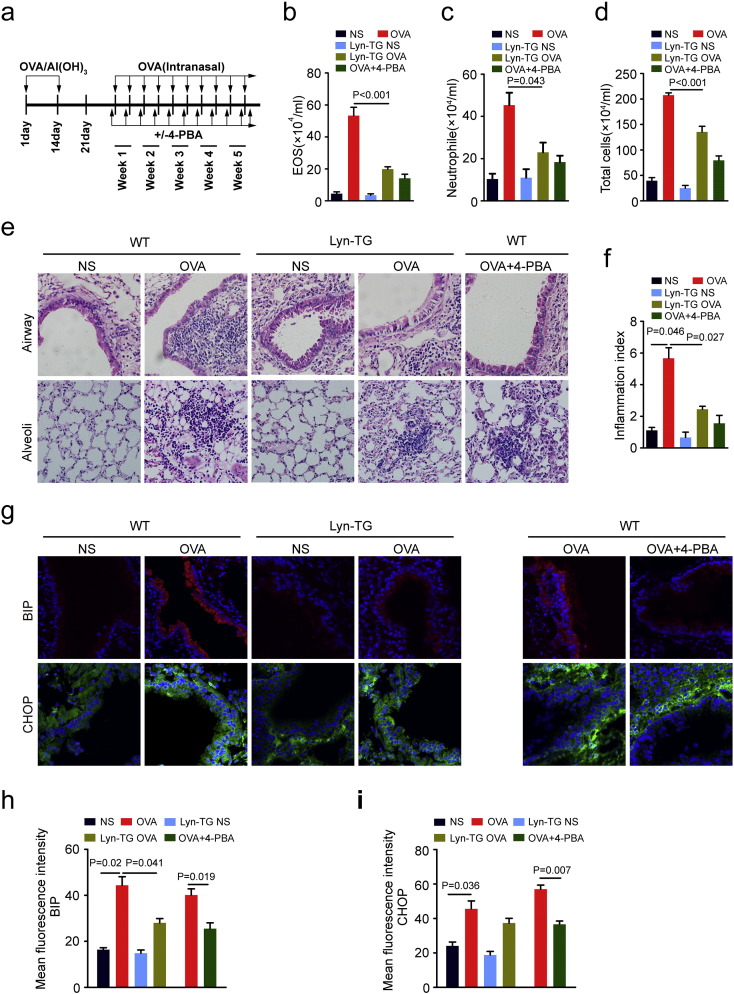
Previous studies have shown that ER stress may be critical for the pathogenesis of bronchial asthma, especially the steroid-resistant neutrophilic asthma (Kim et al., 2013). Nonetheless, we found that Lyn modulates the development of airway remodeling (Li et al., 2013), raising the question of whether Lyn may affect the ER stress implicated in asthma. To examine the effects of Lyn on ER stress, Lyn-TG mice and WT mice were sensitized using an intraperitoneal (i.p.) injection and challenged by repeated OVA intranasal administrations to induce ER stress (Fig. 1a). 4-Phenylbutyric acid (4-PBA) was evaluated as a positive control for ER stress in the WT mouse model of OVA-induced chronic airway inflammation. In present studies, immunohistochemical staining showed that Lyn-TG mice showed increased expression of Lyn the airway epithelium, alveolar epithelial cell and inflammatory cells in lung compared with WT mice (Supplementary Fig. S1 online). OVA induced the characteristic features of allergic airway inflammation: an increased number of total cells, neutrophils and eosinophils in the bronchoalveolar lavage fluid (BALF); lung inflammation, and increased inflammatory index. In OVA-induced Lyn-TG mice, the features of allergic airway inflammation were mitigated: the number of total cells, neutrophils and eosinophils in the BALF were decreased, and lung inflammation and the inflammatory index were both decreased. As expected, 4-PBA also significantly suppressed the characteristic features of OVA-induced chronic airway inflammation shown above in positive control mice compared with that in normal WT mice (Fig. 1b–f).
Fig. 1.
Lyn regulated airway inflammation and ER stress in allergic airway inflammation disease. (a) Experimental design for the sensitization and challenge of WT mice and Lyn-TG mice with OVA. (b–d) Total cell numbers and cell differentials in the BALF were determined. (e–f) Representative H&E–stained sections of the lungs of WT mice and Lyn-TG mice (original magnification: 200 ×). The degree of peribronchial and perivascular inflammation was scored (Kruskal-Wallis test). (g) Representative immunohistochemical staining of BIP or CHOP in the lung tissue of WT mice and Lyn-TG mice. (h–i) Quantitation of the fluorescence intensity of BIP or CHOP per micrometer in 10 random fields. All data are presented as the mean ± s.d. (n = 8 each group, one-way ANOVA with Tukey-Kramer post-test.).
To investigate the effect of Lyn on ER stress in airway epithelial cells in the mouse model of allergic airway inflammation, the localization of ER stress markers expressed in lung tissue was determined by immunofluorescence staining. Confocal microscope analyses revealed that OVA induced the features of ER stress, increasing the expression of CHOP and BIP in the lung tissue of OVA-induced WT mice. The expression of BIP and CHOP predominantly occurred in the cytoplasmic areas of the airway epithelium in OVA-induced WT mice. OVA-induced mice exhibited lower fluorescence intensities corresponding to BIP and CHOP in the lung tissue than OVA-induced WT mice (Fig. 1h–i). 4-PBA also substantially suppressed BIP and CHOP in OVA-induced WT mice (Fig. 1g). The immunofluorescence intensities of BIP and CHOP in 4-PBA-treated positive control WT mice were significantly decreased by 39.2 (14.63/40.13) and 34.5 (19.66/57.03) percent, respectively, compared with those in OVA-induced WT mice (Fig. 1h–i, P < 0.05). Taken together, these results indicated that Lyn overexpression repressed the OVA-induced chronic airway inflammation and ER stress in mice exposed to OVA. The amelioration of airway inflammation caused by Lyn overexpression may be associated with the inhibition of ER stress.
3.2. Lyn inhibited mucus hypersecretion and ER stress associated with PI3K, Akt and NFκB
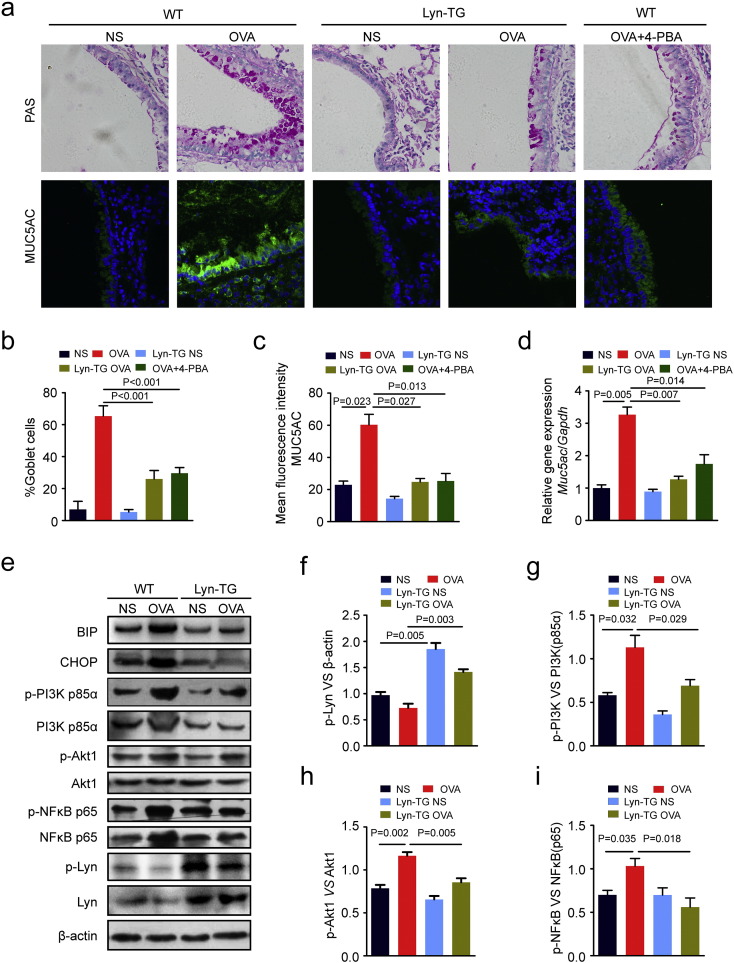
The role of ER stress in airway mucus production has been established. Our previous studies have found that Lyn modulates mucus hypersecretion and goblet cell hyperplasia in mice exposed to house dust mite allergen (Li et al., 2013). However, it remains unclear whether Lyn-regulated mucus secretion is associated with ER stress. To investigate whether Lyn suppresses ER stress or unfolded protein response (UPR) in the airway epithelium, 4-PBA was evaluated as a positive control for ER stress in WT mice exposed to OVA. We observed a direct correlation among goblet cell hyperplasia, Muc5ac expression and ER stress. 4-PBA significantly suppresses goblet cell hyperplasia and Muc5ac expression in positive control WT mice exposed to OVA. Similarly, we observed that Lyn kinase regulates mucus secretion and Muc5ac expression in Lyn-TG mice exposed to OVA (Fig. 2a). The number of goblet cells present in the airway epithelium of Lyn-TG mice exposed to OVA decreased by 60.2% (39.33/65.33) compared with that in WT mice exposed to OVA (Fig. 2b, P < 0.001). The immunofluorescence intensities of Muc5ac in the airway epithelium of Lyn-TG mice exposed to OVA were reduced by 59.0% (35.57/60.29) compared to those in WT mice exposed to OVA (Fig. 2c, P = 0.027). The mRNA levels of Muc5ac in the lung tissue were measured by quantitative real-time PCR. We observed a robust decrease in Muc5ac transcript levels in the Lyn-TG mice exposed to OVA compared with WT mice exposed to OVA (61.1% decrease (2.00/3.27); Fig. 2d; P = 0.007).
Fig. 2.
Lyn inhibited mucus hypersecretion and the ER stress-associated pathway in allergic airway inflammation disease. (a) Representative PAS staining of the lung tissue of WT mice and Lyn-TG mice. Representative immunohistochemical staining of Muc5ac in the lung tissue of WT mice and Lyn-TG mice (original magnification: 200 ×). (b–c) The goblet cell percentage was quantified in 10 random fields. The fluorescence intensity of Muc5ac was quantified per micrometer in 10 random fields. (d) RT-PCR of Muc5ac mRNA expression. (e) Representative Western blots of phospho-PI3K p85α, PI3K p85α, phospho-Akt1, Akt1, phospho-NFκB p65 (Ser536), NFκB p65, Lyn, β-actin, BIP, CHOP, and phospho-Lyn (Tyr416) in the lung tissue. (f–i) Relative changes in the density of phospho-Lyn and β-actin, phospho-PI3K p85α and PI3K p85α, phospho-Akt1 and Akt1, and phospho-NFκB p65 (Ser536) and NFκB p65, as measured by Western blot. Data are representative of three experiments. Bars represent the mean ± s.d (n = 8 each group, one-way ANOVA with Tukey-Kramer post-test).
Previous studies showed that PI3K-δ regulates Aspergillus fumigatus-induced allergic lung inflammation through ER stress (Lee et al., 2016). To investigate whether Lyn is involved in ER stress in the pathogenesis of bronchial asthma, we also measured the levels of ER stress markers and PI3K/Akt/NFκB in the lung tissue of WT mice and Lyn-TG mice exposed to OVA. The levels of BIP and CHOP showed a 1.8-fold (1.32/0.72) and 1.7-fold (1.25/0.72) increase, respectively, in mice exposed to OVA compared to those in mice exposed to normal saline (NS). We further observed a robust decrease in the levels of BIP and CHOP in Lyn-TG mice exposed to OVA compared to those in WT mice exposed to OVA (Fig. 2e), suggesting a link between Lyn and ER stress in asthma.
ER stress-mediated NFκB activation and PI3K pathway-regulated mucus hypersecretion are associated with bronchial asthma pathogenesis (Lee et al., 2016, Kim et al., 2013). In positive control mice, 4-PBA also suppressed the phosphorylation levels of PI3K p85, Akt and NFκB p65 (Supplementary Fig. s2). However, it remains unclear whether Lyn-modulated ER stress is associated with the PI3K/Akt/NFκB pathway in the pathogenesis of bronchial asthma. PI3K p110α and PI3K p110δ were also measured in mice (Supplementary Fig. s3). We showed that PI3K p110α and PI3K p110δ were not critical effectors in the Lyn signaling pathways. More importantly, Western blot analyses showed that the levels of PI3K p85, Akt and NFκB in the lungs of WT mice exposed to OVA were significantly higher than the levels in normal control WT mice. The phosphorylation levels of PI3K p85, Akt and NFκB in the lungs of WT mice exposed to OVA were also significantly higher than the levels in normal control WT mice. Lyn overexpression significantly inhibited the expression levels and phosphorylation of PI3K p85, Akt and NFκB in the lungs of Lyn-TG mice exposed to OVA compared with that in WT mice exposed to OVA (Fig. 2e). The phosphorylation levels of PI3K p85 and Akt decreased by approximately 38.9% (0.44/1.13) and 26.5% (0.31/1.16), respectively, in Lyn-TG mice exposed to OVA compared with WT mice exposed to OVA (Fig. 2g–h, P = 0.029, P = 0.005). We also found that phosphorylation levels of NFκB p65 showed an approximately 45.6% (0.47/1.03) decrease in the lungs of Lyn-TG mice exposed to OVA compared with the levels in WT mice exposed to OVA (Fig. 2i, P = 0.018). These results suggested that the Lyn kinase-mediated PI3K p85/Akt pathways inhibited mucus overproduction by modulating allergen induced-ER stress and NFκB activation.
3.3. Lyn regulated IL-13-induced ER stress and MUC5AC involved in the PI3K p85/Akt/NFκB pathway
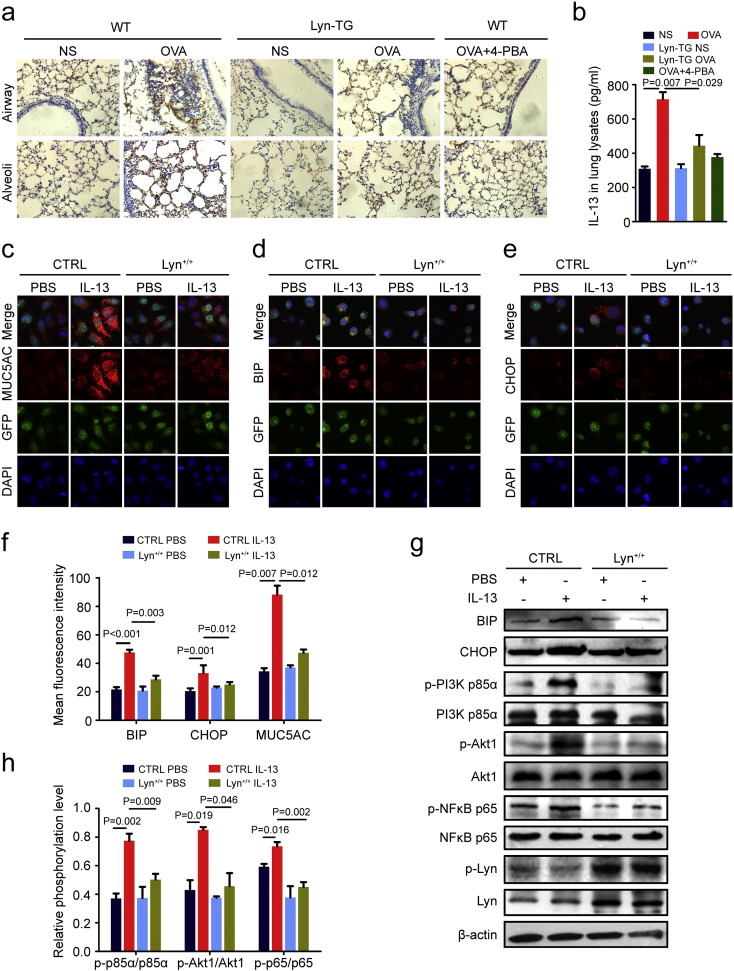
IL-4, IL-5 and IL-13 is related to disease progression in patients with a type 2 allergic phenotype (Trivedi et al., 2016). Its signaling promotes the differentiation of goblet cells. IL-13 severely impairs mucus transport by airway epithelial cells (Tyner et al., 2006, Yan et al., 2014, Bonser et al., 2016)and induces ROS formation and ER stress in airway smooth muscle (Delmotte and Sieck, 2015). Consistent with previous results showing an increase in IL-13 in the lungs of mice exposed to OVA, we found that Lyn and 4-PBA suppressed IL-13 in mice exposed to OVA (Fig. 3a, b). Aside from IL-13, we have also found that Lyn over-expression impacted on IL-4 and IL-5 in asthma mice models. Lyn over-expression and 4-PBA suppressed the levels of IL-4 and IL-5 in mice exposed to OVA (Supplementary Fig. S4a and S4b online).
Fig. 3.
Lyn regulated IL-13-induced ER stress and MUC5AC expression. (a) Representative immunohistochemical staining for IL-13 in the lungs (original magnification: 200 ×). (b) IL-13 levels in the lung tissue were determined by ELISA in triplicate lung lysate samples from each animal. (c–e) 16HBE cells were stably transfected with control (CTRL) lentiviral or Lyn lentiviral, respectively. Representative confocal laser immunofluorescence photomicrographs of MUC5AC, BIP and CHOP expression in cells (original magnification: 400 ×). (f) Quantitation of the fluorescence intensity of MUC5AC, BIP and CHOP in 10 random fields. (g) Representative Western blots of phospho-PI3K p85α, PI3K p85α, phospho-Akt1, Akt1, phospho-NFκB p65 (Ser536), NFκB p65, Lyn, β-actin, BIP, CHOP, and phospho-Lyn (Tyr416) in cells. (h) Relative change in density of phospho-Lyn and β-actin, phospho-PI3K p85α and PI3K p85α, and phospho-Akt1 and Akt1, as measured by Western blot. Data are representative of three experiments. Bars represent the mean ± s.d (n = 8 each group, one-way ANOVA with Tukey-Kramer post-test).
To investigate the involvement of Lyn in IL-13-induced ER stress and MUC5AC expression in airway epithelial cells, we measured the levels of MUC5AC, BIP and CHOP in airway epithelial cell lines (16HBE) using lentiviral Lyn overexpression vectors. Similar results were seen for the IL-13 levels, and Lyn over-expression also reduced IL-13-induced MUC5AC expression in 16HBE cells (Fig. 3c, f). Notably, confocal microscopic analyses revealed that BIP and CHOP were predominantly in the cytoplasm of 16HBE cells after addition of IL-13 (Fig. 3d–e). The overexpression of Lyn in 16HBE cells led to a dramatic decrease in BIP and CHOP after the addition of IL-13 compared with control cells (16HBE cells with stable GFP expression). The fluorescence intensities of BIP and CHOP decreased significantly by 39.7% (18.91/47.59) and 24.4% (8.07/33.12), respectively, in Lyn+/+ cells (16HBE cells with stable Lyn and GFP expression) after the addition of IL-13 compared with their intensities in control cells (Fig. 3f). The BIP and CHOP protein levels in 16HBE cells increased markedly after the addition of IL-13 compared with their levels after the addition of PBS (Fig. 3g, h), supporting the idea that IL-13-induced ER stress may be involved in mucus overproduction. Thus, Lyn plays a critical role in IL-13-induced ER stress and MUC5AC expression in airway epithelial cells.
In animal experiments, our studies found that Lyn-mediated ER stress and mucus hypersecretion were involved in the PI3K p85/Akt pathways in the mouse model of allergic lung inflammation. To investigate how Lyn mediates ER stress and mucus hypersecretion in airway epithelial cells, we measured the levels of PI3K p85/Akt/NFκB in lung tissue using Western blotting. A marked decrease was observed in the phosphorylation levels of PI3K p85, Akt and NFκB in Lyn+/+ cells after the addition of IL-13 compared with the levels in control cells (Fig. 3g-h). Collectively, these studies suggest that IL-13-mediated PI3K p85/Akt/NFκB pathways can be inhibited by overexpression of Lyn.
3.4. Lyn repressed IL-13-induced NFκB activity in airway epithelial cells
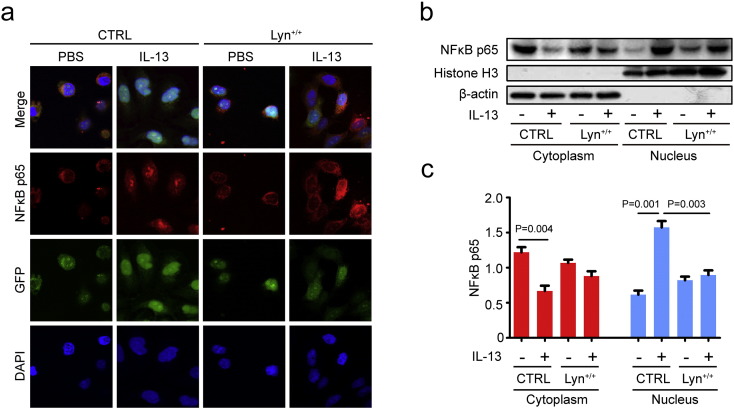
Previous studies suggested that the regulation of MUC5AC expression is intimately linked to NFκB-based transcriptional mechanisms that are mediated by the binding of NFκB to a specific area within the MUC5AC promoter (Koeppen et al., 2013). We found that the nuclear translocation of NFκB was critical for MUC5AC induction (Xie et al., 2015). To examine the effects of IL-13 on NFκB nuclear translocation in airway epithelial cells, the expression localization of NFκB was determined by immunofluorescence staining. Confocal microscope analysis revealed that IL-13 induced significant NFκB nuclear translocation (Fig. 4a). In addition, Western blot analysis revealed that the NFκB p65 levels in cytoplasmic extracts of cells was significantly decreased in IL-13-induced control cells compared with the levels in PBS-treated control cells. The NFκB p65 levels in nuclear extracts of cells was significantly increased in IL-13-induced control cells compared with the levels in PBS-treated control cells. The levels of NFκB p65 in the nuclear extracts of cells increased approximately 2.57-fold (1.57/0.61) in IL-13-induced control cells compared with the levels in PBS-treated controls (Fig. 4b, c, P = 0.001). The results indicated that IL-13 can induce NFκB activity.
Fig. 4.
Lyn regulated IL-13-induced NFκB activity in vitro. (a) NFκB nuclear translocation was assessed by immunofluorescence staining with the NFκB p65 antibody. Nuclei were counterstained with DAPI (blue), TRICT-conjugated secondary Abs for NFκB (red); eGFP or Lyn-eGFP (green). (b) Representative Western blot of NFκB in the cytosolic or nuclear protein extract. (c) Relative change in density of NFκB in the nuclear protein extracts, as measured by Western blot. Data are representative of three experiments. Bars represent the mean ± s.d (n = 8 each group, one-way ANOVA with Tukey-Kramer post-test).
To investigate the effect of Lyn in IL-13-induced NFκB activity in airway epithelial cells, we next examined NFκB activity in cells infected with the Lyn-expressing lentivirus or control vector. Interestingly, confocal microscope analysis also revealed that Lyn substantially reduced the increase in NFκB levels in the nucleus of IL-13-induced Lyn+/+ cells compared with the NFκB levels in IL-13-induced control cells (Fig. 4a). The NFκB p65 levels in the nuclear extracts of cells were significantly decreased in IL-13-induced Lyn+/+ cells compared with the levels in IL-13-induced control cells. The levels of NFκB p65 in the nuclear extracts of cells showed a decrease of approximately 43.1% (0.68/1.57) in the IL-13-induced Lyn+/+ cells compared with the levels in IL-13-induced control cells (Fig. 4b, c, P = 0.003). Together, these data suggest that the overexpression of Lyn suppresses IL-13-induced NFκB activity in airway epithelial cells.
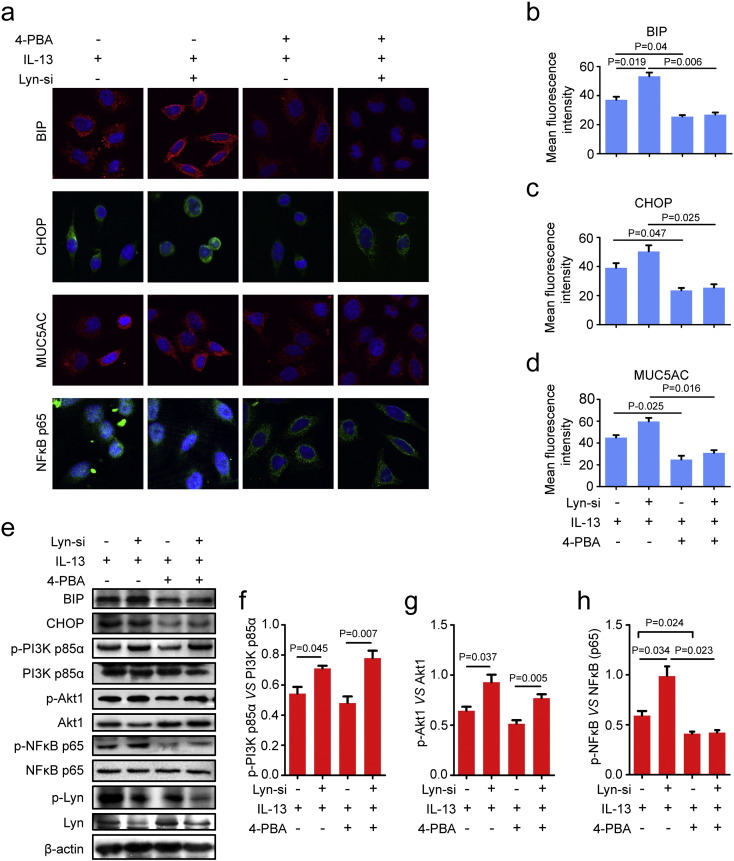
3.5. ER stress regulated the activity of NFκB, not PI3K p85/Akt in Lyn-knockdown airway epithelial cells
In our present studies, we found that 4-PBA decreased mucus secretion in OVA-induced WT mice (Fig. 2a), raising the question of whether 4-PBA also affects MUC5AC in Lyn-knockdown cells (transfected with Lyn siRNA). To further examine the effects of 4-PBA on MUC5AC in Lyn-knockdown airway epithelial cells, we observed MUC5AC and the expression of BIP and CHOP in Lyn siRNA-transfected 16HBE cells in the presence of IL-13 and 4-PBA. Consistent with the in vivo results, Lyn-knockdown cells showed an approximately 0.44-fold (17.21/37.04) and 0.29-fold (11.31/39.15) increase in the fluorescence intensities of BIP and CHOP, respectively, compared to their intensities in siCTRL cells (transfected with a nonsilencing siRNA control) in the presence of IL-13 (Fig. 5a–c). The fluorescence intensity of MUC5AC increased by approximately 0.33-fold (14.80/44.95) in Lyn-knockdown cells compared to its intensity in siCTRL cells in the presence of IL-13. We observed a direct correlation between the fluorescence intensities of MUC5AC and 4-PBA, detecting an approximately 48.2% (28.81/59.76) decrease in the fluorescence intensity of MUC5AC in Lyn-knockdown cells treated with 4-PBA compared to the intensity in cells not exposed to 4-PBA (Fig. 5a, d, P = 0.016). Furthermore, 4-PBA suppresses IL-13-induced nuclear translocation of NFκB in Lyn-knockdown cells (Fig. 6a).
Fig. 5.
ER stress regulated the activity of NFκB in Lyn-knockdown airway epithelial cells. (a) Confocal laser immunofluorescence photomicrographs of MUC5AC, BIP, CHOP and NFκB p65 showed the expression of MUC5AC, BIP, and CHOP and the localization of NFκB p65. (b–d) Quantitation of the fluorescence intensity of MUC5AC, BIP and CHOP in 10 random fields. (e) Representative Western blots of phospho-PI3K p85α, PI3K p85α, phospho-Akt1, Akt1, phospho-NFκB p65 (Ser536), NFκB p65, Lyn, β-actin, BIP, CHOP, and phospho-Lyn (Tyr416) in cells. (f-h) Relative change in density of phospho-PI3K p85α and PI3K p85α, phospho-NFκB p65 (Ser536) and NFκB p65, and phospho-Akt1and Akt1, as measured by Western blot. Data are representative of three experiments. Bars represent the mean ± s.d (n = 8 each group, one-way ANOVA with Tukey-Kramer post-test).
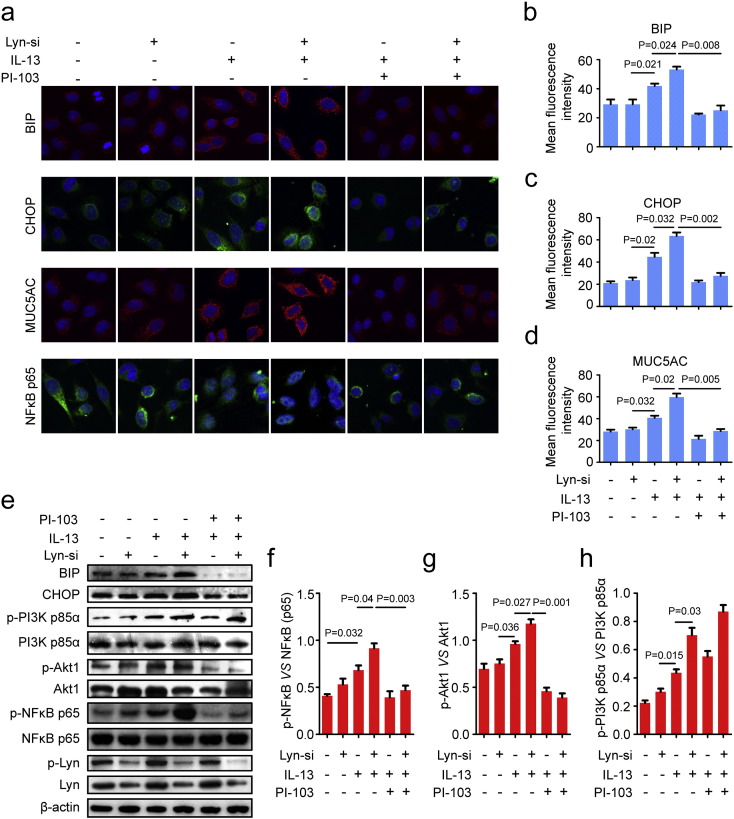
Fig. 6.
The PI3K/Akt pathway regulated IL-13-induced ER stress and mucus overproduction in Lyn-knockdown airway epithelial cells. (a) Confocal laser immunofluorescence photomicrographs of MUC5AC, BIP, CHOP and NFκB p65 in 16HBE. Original magnification: 400 ×. (b–d) Quantitation of the fluorescence intensities of MUC5AC, BIP and CHOP in 10 random fields. (e) Representative Western blots of phospho-Akt1, Akt1, phospho-NFκB p65 (Ser536), NFκB p65, Lyn, β-actin, BIP, CHOP, and phospho-Lyn (Tyr416) in cells. (f–g) Relative change in density of phospho-NFκB p65 (Ser536) and NFκB p65 and relative of Phospho-Akt1 and Akt1 on Western blot. Data are representative of three experiments. Bars represent the mean ± s.d (n = 8 each group, one-way ANOVA with Tukey-Kramer post-test).
Consistent with our previous results, 4-PBA decreased the expression of BIP and CHOP in WT mice after exposure to OVA. 4-PBA decreased the levels of phospho-PI3K p85α and phospho-NFκB p65 (Ser536) in WT mice exposed to OVA (Supplementary Fig. s2 online). To confirm these observations, we analyzed the expression of BIP and CHOP in Lyn-knockdown airway epithelial cells in the presence of IL-13. 4-PBA decreased the expression of BIP and CHOP in siCTRL cells and Lyn-knockdown cells in the presence of IL-13 (Fig. 5e). Furthermore, we analyzed the activity of PI3K p85, Akt and NFκB in Lyn-knockdown airway epithelial cells in the presence of IL-13. Lyn deficiency increased the phosphorylation of PI3K and Akt in the presence of IL-13. Lyn-knockdown 16HBE cells showed approximately 0.31-fold (0.17/0.54) and 0.44-fold (0.28/0.64) increases in the levels of p-PI3K p85/PI3K p85 and p-Akt/Akt, respectively, compared with the levels in siCTRL cells in the presence of IL-13 (Fig. 5f–g, P = 0.045 and P = 0.037). Interestingly, 4-PBA did not significantly inhibit the levels of p-PI3K p85/PI3K p85 and p-Akt/Akt compared with the levels in cells that were not treated with 4-PBA in Lyn-knockdown cells in the presence of IL-13 (Fig. 5f–g). Lyn deficiency significantly increased the phosphorylation of NFκB in the presence of IL-13 in Lyn-knockdown cells compared to that in siCTRL cells (Fig. 5h, P = 0.034). Consistent with previous reports (Kim et al., 2013), 4-PBA significantly inhibited the levels of p-NFκB/NFκB compared to levels in cells that were not treated with 4-PBA in Lyn-knockdown cells in the presence of IL-13 (Fig. 6H, P = 0.023).
3.6. The PI3K p85/Akt pathway was critical for IL-13-induced ER stress and MUC5AC in Lyn- knockdown cells
PI-103, a PI3K inhibitor, has been shown to have a direct inhibitory effect on PI3K and Akt (Bagci-Onder et al., 2011) and is considered one of the most potent compounds in blocking the phosphorylation of Akt (Fan et al., 2006). To determine whether PI3K directly influences IL-13-induced ER stress and mucus overproduction in asthma, we examined the effect of PI-103 on ER stress markers in airway epithelial cells. Consistent with our previous results, the fluorescence intensities of BIP and CHOP increased by approximately 0.27-fold (11.31/41.86) and 0.42-fold (18.72/44.81), respectively, in IL-13-treated Lyn-knockdown (treated with Lyn siRNA) 16HBE cells compared to the intensities in siCTRL cells (treated with a nonsilencing siRNA control) (Fig. 6a–c, P = 0.024 and P = 0.032). The data demonstrate that Lyn knockdown increased the IL-13-induced ER stress in airway epithelial cells. Furthermore, the fluorescence intensities of BIP and CHOP showed robust decreases of approximately 52.7% (28.00/53.17) and 56.6% (35.94/63.53), respectively, in IL-13-treated Lyn siRNA cells in the presence of PI-103 compared with the intensities in the PBS controls (Fig. 6a–c, P = 0.008 and P = 0.002).
To further evaluate the role of PI3K in mucus overproduction in asthma, we also examined the effect of PI-103 on MUC5AC in airway epithelial cells. In the presence of PI-103, confocal microscope analyses revealed that Lyn-knockdown 16HBE cells showed an approximately 52.1% (31.18/59.78) decrease in the fluorescence intensity of MUC5AC after addition of IL-13 (Fig. 6a, d, P = 0.005). Hence, PI-103 significantly decreases the fluorescence intensities of NFκB p65 in Lyn-knockdown 16HBE cells after the addition of IL-13.
Western blot analysis showed that protein levels of BIP and CHOP were higher in IL-13-treated Lyn siRNA-transfected 16HBE cells than in control siRNA transfected cells. PI-103 significantly inhibited the increase in the BIP and CHOP protein levels in Lyn-knockdown 16HBE cells after the addition of IL-13 (Fig. 6e). Consistent with these observations, Lyn knockdown significantly increased the phosphorylation of Akt in Lyn siRNA-transfected 16HBE cells compared with that in control siRNA transfected cells after the addition of IL-13. PI-103 blocked the phosphorylation of Akt after the addition of IL-13 to Lyn siRNA-transfected 16HBE cells. After the addition of IL-13, the phosphorylation levels of Akt showed an approximately 66.8% (0.79/1.18) decrease in the presence of PI-103 in Lyn siRNA-transfected 16HBE cells compared to those in cells grown in the absence of PI-103 (Fig. 6e, g, P = 0.001). Lyn knockdown also significantly increased the IL-13-induced phosphorylation of NFκB p65 in Lyn siRNA-transfected 16HBE cells compared to that in control siRNA transfected cells after the addition of IL-13. In the presence of PI-103, the phosphorylation levels of NFκB p65 showed an approximately 56.1% (0.54/0.97) decrease in Lyn siRNA-transfected 16HBE cells after the addition of IL-13 (Fig. 6f, P = 0.003). These results indicate that PI3K/Akt signaling is required for IL-13-induced ER stress and mucus overproduction in airway epithelial cells. Thus, the ER stress regulation of mucus secretion depends on NFκB in Lyn-knockdown airway epithelial cells.
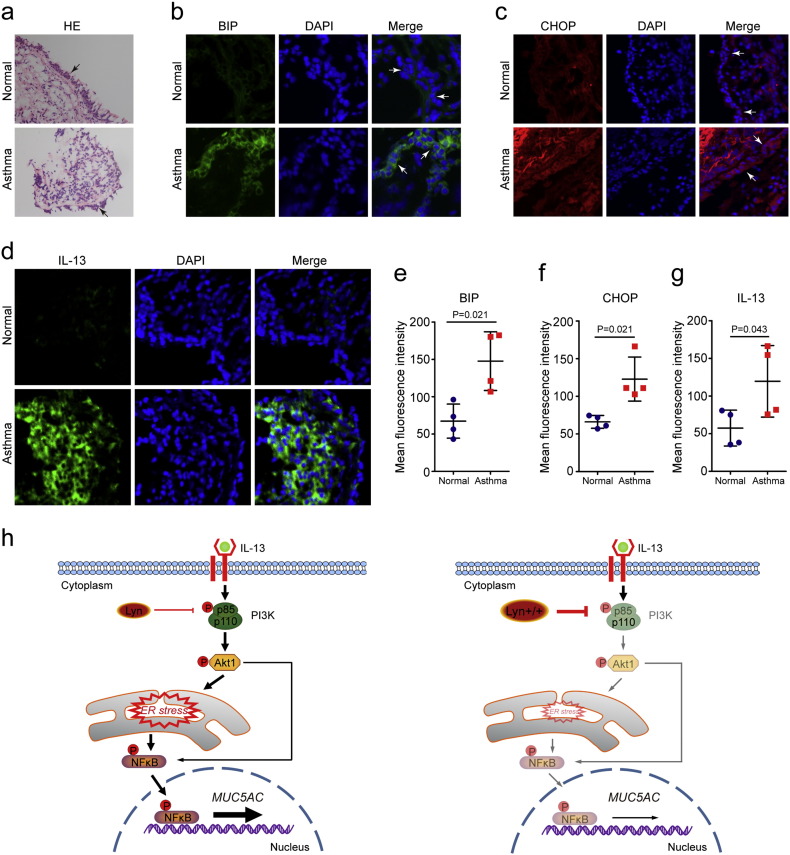
To explore the potential clinical value of this newly revealed molecular pathway, we analyzed the expression levels of BIP, CHOP and IL-13 in the lung tissue of asthmatic patients. Patients with asthma exhibited infiltration of inflammatory cells (Fig. 7a). We also found that immunohistochemical staining for BIP, CHOP and IL-13 in the lung tissue of asthmatic patients was significantly increased compared to that in healthy subjects (Fig. 7b–g). It was negative that the images from isotype staining of human lung showed in Supplementary Fig. s5 online. Fig. 7h illustrates a model delineating the role of Lyn in IL-13-induced mucus secretion related to the PI3K p85/Akt/ER stress/NFκB pathways. Lyn overexpression decreased IL-13-induced mucus secretion via the PI3K p85/Akt/ER stress/NFκB pathways.
Fig. 7.
ER stress in asthmatic patients and a graphical abstract of Lyn-regulated ER stress and mucus secretion in asthma. Biopsy specimens were collected from the right middle lobar bronchi. Ages of asthmatic patients (years, mean ± s.d), 44.35 ± 7.21; control subjects, 39.47 ± 6.19. Forced expiratory volume in one second (FEV1%) in patients, 75.13 ± 6.28; Control subjects, 92 ± 4.26.(a)Representative H&E–stained sections of biopsy specimens (original magnification, × 100). (b–d) Confocal laser immunofluorescence photomicrographs of BIP, CHOP and IL-13 in asthmatic patients (original magnification, × 200). Four patients (males) with asthma and four control subjects (males). (e–g) The fluorescence intensity of BIP, CHOP and IL-13 were quantified in epithelial cells. Bars represent the mean ± s.d (n = 4 each group, Mann-Whitney U test was used for Statistical analysis). (h) Graphical abstract of Lyn-regulated ER stress and mucus secretion in asthma.
4. Discussion
Asthma is an increasingly common chronic disease that is characterized by underlying airway inflammation and airflow obstruction and is caused by the interaction of various genetic and environmental factors. The prevalence of asthma has continued to rise over the past 50 years. The epithelium plays an important role in asthma and shows evidence of damage along with the presence of more mucus-producing cells in asthma (Grainge and Davies, 2013). Previous studies have shown that house dust mite-induced oxidative stress damage and DNA repair modulate asthma-associated pathophysiology (Chan et al., 2016). ER stress is generally defined by the unfolded protein response (UPR). UPR regulates the expression of numerous genes that maintain homeostasis in the ER (Walter and Ron, 2011). 4-PBA reduces ER stress by acting as a chemical chaperone (Chen et al., 2016). Molecular chaperones participate in the folding process of protein through recognizing, retaining and targeting mis-folded proteins for their eventual degradative pathway (Welch and Brown, 1996). Chemical chaperone 4-PBA prevents the aggregation of mis-folded proteins and corrects folding of proteins to get their right conformation, which is commonly used to alleviate ER stress (Wang et al., 2016). 4-PBA is also a histone deacetylase inhibitor. Previous studies indicated that 4-PBA mediated ER stress-induced neuronal cell death through the chemical chaperone activity rather than inhibition of histone deacetylase (Mimori et al., 2013). Chemical chaperone 4-PBA prevented cigarette smoke-induced mucociliary clearance disruption (Jochems et al., 2015). ER stress has also been implicated in the pathogenesis of bronchial asthma through the modulation of NFκB. Inflammation of human bronchial epithelia and activates the ER stress transducer inositol-requiring enzyme 1 (IRE1) α, resulting in airway epithelial mucin production (Martino et al., 2013). 4-PBA alleviated ER stress in the murine model of asthma (Kim et al., 2013). Here, we show that 4-PBA significantly suppressed the characteristic features of OVA-induced chronic airway inflammation and mucus secretion in positive control mice. Our data reveal that 4-PBA decreased goblet cell hyperplasia and Muc5ac expression was accompanied by inhibition of ER stress, suggesting that ER stress was important in mediating these effects. However, it remains possible that the decreased mucus secretion is caused by other activities of 4-PBA such as HDAC inhibition in our murine model of asthma.
Lyn kinase participates in the downstream signaling pathways of a variety of receptors on the plasma membrane, serving as a signaling platform under oxidative stress (Matsuda et al., 2006). In our previous studies, we observed chronic house dust mite exposure-induced mucous hypersecretion in Lyn-knockout mice (Li et al., 2013). In the current study, we tested the hypothesis that Lyn plays a role in mucus hypersecretion by regulating ER stress in asthma. Notably, using Lyn transgenic mice, the expression of Lyn increased in the airway epithelium, alveolar epithelial cell and inflammatory cells. The over-expression of Lyn in epithelial cells and inflammatory cells may be associated with the phenotype of allergen-challenged Lyn-TG mice. We confirmed that Lyn overexpression suppressed OVA-induced chronic airway inflammation and mucus hypersecretion in mice exposed to OVA. Lyn ameliorated the pathological features of asthma but also showed evidence of decreasing ER stress in an asthmatic murine model. We observed a robust decrease in the levels of BIP and CHOP in Lyn-TG mice compared with the levels in WT mice exposed to OVA. Our data reveal a role for Lyn in ER stress in a mouse model of allergic airway inflammation. We further note that decreased mucus secretion is associated with decreased ER stress in Lyn-overexpressing mice despite chronic exposure to OVA.
Lyn tyrosine kinase regulates PI3K activity (Kannan et al., 2008). The activity of Lyn is crucial for the activation of PI3K. Lyn deficiency increased basal and inducible PI3K activity in the hyper-responsive phenotype of B cells (Xu et al., 2012). PI3K/Akt signaling at the mitochondria-associated endoplasmic reticulum membranes regulate mitochondrial physiology (Betz et al., 2013). The present study indicates that PI3K p85 and Akt are phosphorylated when mice are exposed to OVA. These findings are consistent with previous studies showing that PI3K/Akt are critical in regulating allergic lung inflammation (Liu et al., 2015, Bonifazi et al., 2010). We found that phosphorylation of PI3K p85 and Akt was decreased in Lyn-overexpressing mice exposed to OVA and inhibited ER stress in the lungs. ER stress triggers the activation of NFκB, a key molecule in the onset of inflammation (Cantero-Recasens et al., 2010). Previous studies have indicated that NFκB-based transcriptional mechanisms modulate the transcriptional regulation of MUC5AC during acute lung injury (Koeppen et al., 2013). In support of this, our results revealed that the enhanced phosphorylation of NFκB that accompanies an increased mucus hypersecretion in response to ER stress in the lung was suppressed in Lyn-TG mice exposed to OVA.
Previous studies confirmed that interleukin-13 directly affect epithelial cells to cause airway hyper-reactivity and mucus overproduction in asthma (Kuperman et al., 2002). IL-13 has been shown to significantly enhance the ER stress activated by lipopolysaccharide in microglia (Liu et al., 2010). ER stress is able to influence inflammatory signaling through modulation of the JNK and NFκB pathways. Altered UPR signaling is differentially sensitive to the various biological effects of IL-4 and IL-13 (Arensdorf and Rutkowski, 2013). IL-13 is highly expressed in Lyn-deficient mast cells. Lyn, as a negative regulator of allergen-stimulated bone marrow-derived mast cells, regulated the expression of the IL-13 gene (Hernandez-Hansen et al., 2005). The binding of IL-13 triggers the recruitment of several molecules, such as PI3K, Akt, and Src kinase, and enables activation of PI3K (Bartolome et al., 2015). We further found that Lyn overexpression significantly repressed IL-13 expression in the lungs of mice exposed to OVA. Interestingly, we also found that in the presence of IL-13, Lyn overexpression can help prevent ER stress and decrease MUC5AC expression. Lyn deficiency enhanced ER stress and increased MUC5AC expression in airway epithelial cells. We further found that treatment with 4-PBA blocked MUC5AC expression in Lyn-knockdown cells, which are implicated in the PI3K p85/Akt pathway and NFκB activity. Our studies provide further insight into Lyn-mediated effects on mucus production through ER stress.
ER stress leads to the activation of the PI3K/Akt signaling pathway. Akt promotes NFκB activity by increasing NFκB nuclear translocation and the transcription of its target genes (Hamanaka et al., 2009, Bellacosa et al., 2004). Early phosphorylation of Akt possibly mediated the activation of mTOR complex 1 (mTORC1) by ER stress. Increased activation of mTORC1 triggers a negative feedback loop for the PI3K/Akt pathway, leading to the suppression of Akt (Kato et al., 2012, Inoki et al., 2005). PI-103 is an inhibitor of PI3K and mTOR. PI-103 caused a depletion of the phosphorylated form of Akt and down-regulate PI3K signaling (Djuzenova et al., 2016). PI3K inhibitors PI-103 potently enhanced IgE switch in low dose range (0.1–2 μmol/l), however, they inhibited IgE production at > 2 μmol/l PI-103 in murine model of asthma. The switch of IgE phenotype may be associated with inhibiting the mTOR at these doses (Zhang et al., 2008). In present studies, our in vivo results show that the protein expression of the ER stress markers (BIP and CHOP) and MUC5AC in airway epithelial cells was also blocked by PI-103, even in Lyn-knockdown cells. The increase in the activation of NFκB was significantly attenuated by inhibiting PI3K p85/Akt with PI-103 in Lyn-knockdown cells. Our findings suggest that defects in PI3K p85/Akt could play a critical role in the effects of Lyn on ER stress in mucus overproduction, although those effects of PI-103 in mTOR cannot be excluded.
In summary, we have uncovered the role of Lyn in mucus hypersecretion in a model of experimental bronchial asthma in mice as well as in airway epithelial cells in vitro. Our results show that Lyn overexpression attenuates chronic airway inflammation and ER stress, which are associated with the PI3K p85/Akt pathway and NFκB activity. Our results also show that IL-13 induces MUC5AC expression by enhancing ER stress in vitro. Lyn plays a critical role in IL-13-induced ER stress and MUC5AC expression in airway epithelial cells. We further demonstrate that an inhibitor of ER stress blocked MUC5AC expression in Lyn-knockdown cells, which are implicated in the PI3K p85/Akt pathway and NFκB activity. Moreover, PI-103 decreases IL-13-induced ER stress and mucus overproduction in airway epithelial cells. PI-103 also decreases the activity of NFκB p65. Our studies elucidate not only a concept of mucus hypersecretion in asthma involving Lyn kinase but also an important therapeutic candidate for asthma.
Competing interests
The authors declare that they have no competing financial interests.
Authors' contributions
Conceived and designed the study: Guoping Li, Min Wu and Nanshan Zhong. Performed the experiments: Xing Wang, Juan Wu, Xiaoyun Wang, Yun Zhang, Xi Dai, Yin Li, Xiefang Yuan, Anjie Xiong, and Xiaoqiong Yang. Wrote the paper: Guoping Li, Yin Li, and Min Wu. Analyzed the data: Xing Wang, Xiaoyun Wang, Yin Li, and Zhigang Liu. All authors read and approved the final manuscript.
Acknowledgements
This project was supported by grant from the Chinese National Science Foundation (81170032, 81600024) and project grants from National Institute of Health (AI109317-01A1 and AI101973-01).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.12.010.
Contributor Information
Nanshan Zhong, Email: Nanshan@vip.163.com.
Min Wu, Email: min.wu@med.und.edu.
Guoping Li, Email: lzlgp@163.com.
Appendix A. Supplementary data
Supplementary content
References
- Alevy Y.G., Patel A.C., Romero A.G., Patel D.A., Tucker J., Roswit W.T., Miller C.A., Heier R.F., Byers D.E., Brett T.J., Holtzman M.J. IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J. Clin. Invest. 2012;122:4555–4568. doi: 10.1172/JCI64896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arensdorf A.M., Rutkowski D.T. Endoplasmic reticulum stress impairs IL-4/IL-13 signaling through C/EBPbeta-mediated transcriptional suppression. J. Cell Sci. 2013;126:4026–4036. doi: 10.1242/jcs.130757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagci-Onder T., Wakimoto H., Anderegg M., Cameron C., Shah K. A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer Res. 2011;71:154–163. doi: 10.1158/0008-5472.CAN-10-1601. [DOI] [PubMed] [Google Scholar]
- Balenga N.A., Klichinsky M., Xie Z., Chan E.C., Zhao M., Jude J., Laviolette M., Panettieri R.A., Jr., Druey K.M. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun. 2015;6:6763. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolome R.A., Garcia-Palmero I., Torres S., Lopez-Lucendo M., Balyasnikova I.V., Casal J.I. IL13 receptor alpha2 signaling requires a scaffold protein, FAM120A, to activate the FAK and PI3K pathways in colon cancer metastasis. Cancer Res. 2015;75:2434–2444. doi: 10.1158/0008-5472.CAN-14-3650. [DOI] [PubMed] [Google Scholar]
- Bellacosa A., Testa J.R., Moore R., Larue L. A portrait of AKT kinases: human cancer and animal models depict a family with strong individualities. Cancer Biol. Ther. 2004;3:268–275. doi: 10.4161/cbt.3.3.703. [DOI] [PubMed] [Google Scholar]
- Betz C., Stracka D., Prescianotto-Baschong C., Frieden M., Demaurex N., Hall M.N. Feature article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc. Natl. Acad. Sci. U. S. A. 2013;110:12526–12534. doi: 10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifazi P., D'angelo C., Zagarella S., Zelante T., Bozza S., De Luca A., Giovannini G., Moretti S., Iannitti R.G., Fallarino F., Carvalho A., Cunha C., Bistoni F., Romani L. Intranasally delivered siRNA targeting PI3K/Akt/mTOR inflammatory pathways protects from aspergillosis. Mucosal Immunol. 2010;3:193–205. doi: 10.1038/mi.2009.130. [DOI] [PubMed] [Google Scholar]
- Bonser L.R., Zlock L., Finkbeiner W., Erle D.J. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J. Clin. Invest. 2016;126:2367–2371. doi: 10.1172/JCI84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero-Recasens G., Fandos C., Rubio-Moscardo F., Valverde M.A., Vicente R. The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress. Hum. Mol. Genet. 2010;19:111–121. doi: 10.1093/hmg/ddp471. [DOI] [PubMed] [Google Scholar]
- Chan T.K., Loh X.Y., Peh H.Y., Tan W.N., Tan W.S., Li N., Tay I.J., Wong W.S., Engelward B.P. House dust mite-induced asthma causes oxidative damage and DNA double-strand breaks in the lungs. J. Allergy Clin. Immunol. 2016;138:84–96. doi: 10.1016/j.jaci.2016.02.017. (e1) [DOI] [PubMed] [Google Scholar]
- Chen Y., Wu Z., Zhao S., Xiang R. Chemical chaperones reduce ER stress and adipose tissue inflammation in high fat diet-induced mouse model of obesity. Sci. Rep. 2016;6:27486. doi: 10.1038/srep27486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmotte P., Sieck G.C. Interaction between endoplasmic/sarcoplasmic reticulum stress (ER/SR stress), mitochondrial signaling and Ca(2 +) regulation in airway smooth muscle (ASM) Can. J. Physiol. Pharmacol. 2015;93:97–110. doi: 10.1139/cjpp-2014-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuzenova C.S., Fiedler V., Katzer A., Michel K., Deckert S., Zimmermann H., Sukhorukov V.L., Flentje M. Dual PI3K- and mTOR-inhibitor PI-103 can either enhance or reduce the radiosensitizing effect of the Hsp90 inhibitor NVP-AUY922 in tumor cells: the role of drug-irradiation schedule. Oncotarget. 2016;7:38191–38209. doi: 10.18632/oncotarget.9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.M., Raclawska D.S., Ttofali F., Liptzin D.R., Fletcher A.A., Harper D.N., Mcging M.A., Mcelwee M.M., Williams O.W., Sanchez E., Roy M.G., Kindrachuk K.N., Wynn T.A., Eltzschig H.K., Blackburn M.R., Tuvim M.J., Janssen W.J., Schwartz D.A., Dickey B.F. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat. Commun. 2015;6:6281. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy J.V. Type 2 inflammation in asthma–present in most, absent in many. Nat. Rev. Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q.W., Knight Z.A., Goldenberg D.D., Yu W., Mostov K.E., Stokoe D., Shokat K.M., Weiss W.A. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainge C.L., Davies D.E. Epithelial injury and repair in airways diseases. Chest. 2013;144:1906–1912. doi: 10.1378/chest.12-1944. [DOI] [PubMed] [Google Scholar]
- Hamanaka R.B., Bobrovnikova-Marjon E., Ji X., Liebhaber S.A., Diehl J.A. PERK-dependent regulation of IAP translation during ER stress. Oncogene. 2009;28:910–920. doi: 10.1038/onc.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Hansen V., Bard J.D., Tarleton C.A., Wilder J.A., Lowell C.A., Wilson B.S., Oliver J.M. Increased expression of genes linked to FcepsilonRI Signaling and to cytokine and chemokine production in Lyn-deficient mast cells. J. Immunol. 2005;175:7880–7888. doi: 10.4049/jimmunol.175.12.7880. [DOI] [PubMed] [Google Scholar]
- Hoffman S.M., Tully J.E., Nolin J.D., Lahue K.G., Goldman D.H., Daphtary N., Aliyeva M., Irvin C.G., Dixon A.E., Poynter M.E., Anathy V. Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis. Respir. Res. 2013;14:141. doi: 10.1186/1465-9921-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K., Corradetti M.N., Guan K.L. Dysregulation of the TSC-mTOR pathway in human disease. Nat. Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- Jochems J., Teegarden S.L., Chen Y., Boulden J., Challis C., Ben-Dor G.A., Kim S.F., Berton O. Enhancement of stress resilience through histone deacetylase 6-mediated regulation of glucocorticoid receptor chaperone dynamics. Biol. Psychiatry. 2015;77:345–355. doi: 10.1016/j.biopsych.2014.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S., Audet A., Huang H., Chen L.J., Wu M. Cholesterol-rich membrane rafts and Lyn are involved in phagocytosis during Pseudomonas aeruginosa infection. J. Immunol. 2008;180:2396–2408. doi: 10.4049/jimmunol.180.4.2396. [DOI] [PubMed] [Google Scholar]
- Kanoh S., Tanabe T., Rubin B.K. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin. Exp. Allergy. 2011;41:1747–1756. doi: 10.1111/j.1365-2222.2011.03852.x. [DOI] [PubMed] [Google Scholar]
- Kato H., Nakajima S., Saito Y., Takahashi S., Katoh R., Kitamura M. mTORC1 serves ER stress-triggered apoptosis via selective activation of the IRE1-JNK pathway. Cell Death Differ. 2012;19:310–320. doi: 10.1038/cdd.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.R., Kim D.I., Kang M.R., Lee K.S., Park S.Y., Jeong J.S., Lee Y.C. Endoplasmic reticulum stress influences bronchial asthma pathogenesis by modulating nuclear factor kappaB activation. J. Allergy Clin. Immunol. 2013;132:1397–1408. doi: 10.1016/j.jaci.2013.08.041. [DOI] [PubMed] [Google Scholar]
- Koeppen M., Mcnamee E.N., Brodsky K.S., Aherne C.M., Faigle M., Downey G.P., Colgan S.P., Evans C.M., Schwartz D.A., Eltzschig H.K. Detrimental role of the airway mucin Muc5ac during ventilator-induced lung injury. Mucosal Immunol. 2013;6:762–775. doi: 10.1038/mi.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh H.J., Toyoda T., Didesch M.M., Lee M.Y., Sleeman M.W., Kulkarni R.N., Musi N., Hirshman M.F., Goodyear L.J. Tribbles 3 mediates endoplasmic reticulum stress-induced insulin resistance in skeletal muscle. Nat. Commun. 2013;4:1871. doi: 10.1038/ncomms2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman D.A., Huang X., Koth L.L., Chang G.H., Dolganov G.M., Zhu Z., Elias J.A., Sheppard D., Erle D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- Kuyper L.M., Pare P.D., Hogg J.C., Lambert R.K., Ionescu D., Woods R., Bai T.R. Characterization of airway plugging in fatal asthma. Am. J. Med. 2003;115:6–11. doi: 10.1016/s0002-9343(03)00241-9. [DOI] [PubMed] [Google Scholar]
- Lan N., Luo G., Yang X., Cheng Y., Zhang Y., Wang X., Wang X., Xie T., Li G., Liu Z., Zhong N. 25-Hydroxyvitamin D3-deficiency enhances oxidative stress and corticosteroid resistance in severe asthma exacerbation. PLoS One. 2014;9:e111599. doi: 10.1371/journal.pone.0111599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.S., Jeong J.S., Kim S.R., Cho S.H., Kolliputi N., Ko Y.H., Lee K.B., Park S.C., Park H.J., Lee Y.C. Phosphoinositide 3-kinase-delta regulates fungus-induced allergic lung inflammation through endoplasmic reticulum stress. Thorax. 2016;71:52–63. doi: 10.1136/thoraxjnl-2015-207096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Yuan K., Yan C., Fox J., III, Gaid M., Breitwieser W., Bansal A.K., Zeng H., Gao H., Wu M. 8-Oxoguanine-DNA glycosylase 1 deficiency modifies allergic airway inflammation by regulating STAT6 and IL-4 in cells and in mice. Free Radic. Biol. Med. 2012;52:392–401. doi: 10.1016/j.freeradbiomed.2011.10.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Fox J., III, Liu Z., Liu J., Gao G.F., Jin Y., Gao H., Wu M. Lyn mitigates mouse airway remodeling by downregulating the TGF-beta3 isoform in house dust mite models. J. Immunol. 2013;191:5359–5370. doi: 10.4049/jimmunol.1301596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.H., Yang C.N., Pan H.C., Sung Y.J., Liao K.K., Chen W.B., Lin W.Z., Sheu M.L. IL-13 downregulates PPAR-gamma/heme oxygenase-1 via ER stress-stimulated calpain activation: aggravation of activated microglia death. Cell. Mol. Life Sci. 2010;67:1465–1476. doi: 10.1007/s00018-009-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.N., Zha W.J., Ma Y., Chen F.F., Zhu W., Ge A., Zeng X.N., Huang M. Galangin attenuates airway remodelling by inhibiting TGF-beta1-mediated ROS generation and MAPK/Akt phosphorylation in asthma. Sci. Rep. 2015;5:11758. doi: 10.1038/srep11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino M.B., Jones L., Brighton B., Ehre C., Abdulah L., Davis C.W., Ron D., O'neal W.K., Ribeiro C.M. The ER stress transducer IRE1beta is required for airway epithelial mucin production. Mucosal Immunol. 2013;6:639–654. doi: 10.1038/mi.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda D., Nakayama Y., Horimoto S., Kuga T., Ikeda K., Kasahara K., Yamaguchi N. Involvement of Golgi-associated Lyn tyrosine kinase in the translocation of annexin II to the endoplasmic reticulum under oxidative stress. Exp. Cell Res. 2006;312:1205–1217. doi: 10.1016/j.yexcr.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Mimori S., Ohtaka H., Koshikawa Y., Kawada K., Kaneko M., Okuma Y., Nomura Y., Murakami Y., Hamana H. 4-Phenylbutyric acid protects against neuronal cell death by primarily acting as a chemical chaperone rather than histone deacetylase inhibitor. Bioorg. Med. Chem. Lett. 2013;23:6015–6018. doi: 10.1016/j.bmcl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Schroeder B.W., Verhaeghe C., Park S.W., Nguyenvu L.T., Huang X., Zhen G., Erle D.J. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am. J. Respir. Cell Mol. Biol. 2012;47:178–185. doi: 10.1165/rcmb.2011-0421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K.S., Choi J.K., Ahn D.W. Src homology 2-containing protein tyrosine phosphatase-2 acts as a negative regulator for MUC5AC transcription via the inhibition of the ERK1/2 MAPK signalling pathway in the airway. Acta Physiol. (Oxford) 2013;208:245–250. doi: 10.1111/apha.12104. [DOI] [PubMed] [Google Scholar]
- Sweerus K., Lachowicz-Scroggins M., Gordon E., Lafemina M., Huang X., Parikh M., Kanegai C., Fahy J.V., Frank J.A. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J. Allergy Clin. Immunol. 2016 doi: 10.1016/j.jaci.2016.02.035. (pii: S0091-6749(16)30089-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeyama K., Dabbagh K., Jeong Shim J., Dao-Pick T., Ueki I.F., Nadel J.A. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J. Immunol. 2000;164:1546–1552. doi: 10.4049/jimmunol.164.3.1546. [DOI] [PubMed] [Google Scholar]
- Trivedi A., Pavord I.D., Castro M. Bronchial thermoplasty and biological therapy as targeted treatments for severe uncontrolled asthma. Lancet Respir. Med. 2016;4:585–592. doi: 10.1016/S2213-2600(16)30018-2. [DOI] [PubMed] [Google Scholar]
- Tyner J.W., Kim E.Y., Ide K., Pelletier M.R., Roswit W.T., Morton J.D., Battaile J.T., Patel A.C., Patterson G.A., Castro M., Spoor M.S., You Y., Brody S.L., Holtzman M.J. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J. Clin. Invest. 2006;116:309–321. doi: 10.1172/JCI25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang Z., Huang Y., Cheng Y., Tan Y., Wu F., Wu J., Shi H., Zhang H., Yu X., Gao H., Lin L., Cai J., Zhang J., Li X., Cai L., Xiao J. Endoplasmic reticulum stress-induced neuronal inflammatory response and apoptosis likely plays a key role in the development of diabetic encephalopathy. Oncotarget. 2016 doi: 10.18632/oncotarget.12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W.J., Brown C.R. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., Luo G., Zhang Y., Wang X., Wang X., Wu M., Li G. Rho-kinase inhibitor fasudil reduces allergic airway inflammation and mucus hypersecretion by regulating STAT6 and NFkappaB. Clin. Exp. Allergy. 2015;45:1812–1822. doi: 10.1111/cea.12606. [DOI] [PubMed] [Google Scholar]
- Xu Y., Huntington N.D., Harder K.W., Nandurkar H., Hibbs M.L., Tarlinton D.M. Phosphatidylinositol-3 kinase activity in B cells is negatively regulated by Lyn tyrosine kinase. Immunol. Cell Biol. 2012;90:903–911. doi: 10.1038/icb.2012.31. [DOI] [PubMed] [Google Scholar]
- Yan F., Li W., Zhou H., Wu Y., Ying S., Chen Z., Shen H. Interleukin-13-induced MUC5AC expression is regulated by a PI3K-NFAT3 pathway in mouse tracheal epithelial cells. Biochem. Biophys. Res. Commun. 2014;446:49–53. doi: 10.1016/j.bbrc.2014.02.051. [DOI] [PubMed] [Google Scholar]
- Zhang T.T., Okkenhaug K., Nashed B.F., Puri K.D., Knight Z.A., Shokat K.M., Vanhaesebroeck B., Marshall A.J. Genetic or pharmaceutical blockade of p110delta phosphoinositide 3-kinase enhances IgE production. J. Allergy Clin. Immunol. 2008;122:811–819. doi: 10.1016/j.jaci.2008.08.008. (e2) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary content