Abstract
Excessive amounts of heavy metals adversely affect plant growth and development. Whereas some regions naturally contain high levels of heavy metals, anthropogenic release of heavy metals into the environment continuously increases soil contamination. The presence of elevated levels of heavy metal ions triggers a wide range of cellular responses including changes in gene expression and synthesis of metal-detoxifying peptides. To elucidate signal transduction events leading to the cellular response to heavy metal stress we analyzed protein phosphorylation induced by elevated levels of copper and cadmium ions as examples for heavy metals with different physiochemical properties and functions. Exposure of alfalfa (Medicago sativa) seedlings to excess copper or cadmium ions activated four distinct mitogen-activated protein kinases (MAPKs): SIMK, MMK2, MMK3, and SAMK. Comparison of the kinetics of MAPK activation revealed that SIMK, MMK2, MMK3, and SAMK are very rapidly activated by copper ions, while cadmium ions induced delayed MAPK activation. In protoplasts, the MAPK kinase SIMKK specifically mediated activation of SIMK and SAMK but not of MMK2 and MMK3. Moreover, SIMKK only conveyed MAPK activation by CuCl2 but not by CdCl2. These results suggest that plants respond to heavy metal stress by induction of several distinct MAPK pathways and that excess amounts of copper and cadmium ions induce different cellular signaling mechanisms in roots.
Heavy metal ions play essential roles in many physiological processes. In trace amounts, several of these ions are required for metabolism, growth, and development. However, problems arise when cells are confronted with an excess of these vital ions or with nonnutritional ions that lead to cellular damage (Avery, 2001; Schützendübel and Polle, 2002; Gaetke and Chow, 2003; Polle and Schützendübel, 2003). Heavy metal toxicity comprises inactivation of biomolecules by either blocking essential functional groups or by displacement of essential metal ions (Goyer, 1997). In addition, autoxidation of redox-active heavy metals and production of reactive oxygen species (ROS) by the Fenton reaction causes cellular injury (Stohs and Bagchi, 1995).
In response to toxic levels of heavy metals, plants synthesize Cys-rich, metal-binding peptides including phytochelatins and metallothioneins. Therefore, heavy metals can be detoxified by chelation and sequestration in the vacuole (Clemens, 2001; Cobbett and Goldsbrough, 2002), and various membrane transport systems play an important role in metal ion homeostasis and tolerance (Hall and Williams, 2003).
Gene expression patterns change when plants encounter excessive amounts of heavy metals. Cadmium- and copper-responsive genes have been shown to code for signal transduction components, such as the Arabidopsis mitogen-activated protein kinase kinase kinase (MAPKKK) MEKK1, transcription factors, stress-induced proteins, proteins participating in protein folding, and sulfur and glutathione metabolism (Xiang and Oliver, 1998; Suzuki et al., 2001; Louie et al., 2003).
Mitogen-activated protein kinase (MAPK) pathways represent an evolutionary conserved signaling mechanism in eukaryotes (Gustin et al., 1998; Xu, 2000; Pearson et al., 2001; Ichimura et al., 2002; Agrawal et al., 2003). Diverse signal transduction pathways use MAPKs to regulate a variety of cellular functions in response to different extracellular stimuli. MAPKs are part of a phosphorylation cascade that is composed of three sequentially activated protein kinases: MAPKKK, MAPK kinase (MAPKK), and MAPK (Schaeffer and Weber, 1999; Widmann et al., 1999). MAPKKKs are Ser/Thr protein kinases that phosphorylate and thereby activate MAPKKs. MAPKKs in turn are dual-specific kinases that phosphorylate MAPKs on a Thr and Tyr residue. The dual phosphorylation of MAPKs renders the enzymes active. MAPKs are Pro-directed Ser/Thr kinases phosphorylating numerous substrates in different cellular compartments. In this way, information is transduced in the form of a phosphorylation cascade from upstream kinases to downstream targets.
Experimental evidence from different plant species indicates that several MAPK pathways are activated in response to environmental challenges, hormones, and during cell division (Tena et al., 2001; Jonak et al., 2002). For example, in alfalfa (Medicago sativa), the MAPKs SIMK and SAMK are involved in the response to pathogen-associated stimuli as well as to abiotic stresses like mechanical stimulation, wounding, drought, and cold (Bögre et al., 1996, 1997; Jonak et al., 1996; Cardinale et al., 2000). Pathogen-derived elicitors also activate MMK2 and MMK3 (Cardinale et al., 2000). Interestingly, MMK3 plays a role not only in pathogen response but also in cytokinesis (Bögre et al., 1999). Moreover, the ethylene precursor aminocyclopropane-1-carboxylic acid triggers SIMK and MMK3 pathways (Ouaked et al., 2003).
Despite our knowledge regarding ion toxicity and detoxification mechanisms, information about signal transduction induced by heavy metal stress is scarce. In this study, we therefore analyzed protein phosphorylation events in roots of alfalfa in response to excess levels of heavy metals. We report on the identification of several distinct MAPK pathways that are activated in response to copper and cadmium stress.
RESULTS
CuCl2 and CdCl2 Stress Induces Myelin Basic Protein Kinases in Roots
To investigate protein phosphorylation events during the plant response to heavy metal stress we studied heavy metal-induced protein kinase activities in roots of alfalfa. We analyzed activation of protein kinases by CuCl2 as a model for a redox-active micronutrient that is toxic at supraoptimal concentrations and by CdCl2 as a toxic, nonredox, and nonessential metal.
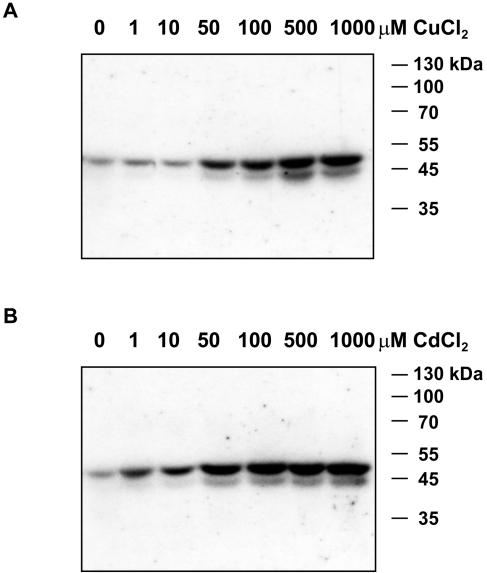
Alfalfa seedlings were challenged for 30 min with increasing concentrations of CuCl2 or CdCl2. Protein extracts from roots of these plants were analyzed for changes in protein kinase activities by in gel-kinase assays using myelin basic protein (MBP) as substrate. As shown in Figure 1, exposure of plants to more than 50 μm CuCl2 activated protein kinases with relative molecular masses of 46 and 44 kD. CdCl2 induced the activity of a 46-kD protein kinase at a concentration of 1 μm, whereas robust activation of protein kinases of 46 and 44 kD were observed at 50 μm CdCl2. These data suggest that heavy metal stress leads to the activation of MBP-phosphorylating protein kinases in roots.
Figure 1.
CuCl2 and CdCl2 induce the activation of 44- and 46-kD protein kinases. Alfalfa roots of hydroponically grown plants were exposed for 30 min to 0, 1, 10, 50, 100, 500, and 1,000 μm CuCl2 (A) or CdCl2 (B). In-gel kinase assays were performed with 20 μg of protein extracts using MBP as substrate.
Distinct MAPKs Are Activated by CuCl2 and CdCl2 in a Dose-Dependent Manner
The sizes of the CuCl2- and CdCl2-responsive MBP-protein kinases are reminiscent of MAPKs. To investigate whether the 46 and 44 kD protein kinases correspond to known MAPKs from alfalfa, immunokinase assays were performed on plants exposed to CuCl2 or CdCl2 using antibodies that are specific for distinct MAPKs. Root protein extracts were prepared from plants exposed to increasing concentrations of CuCl2 or CdCl2 before immunoprecipitation with the antibodies M23, M11, M14, and M24 that specifically recognize SIMK, MMK2, MMK3, and SAMK, respectively (Munnik et al., 1999; Cardinale et al., 2000). Subsequent kinase assays using MBP as substrate revealed that the activity of SIMK, MMK2, MMK3, and SAMK increased in response to CuCl2 or CdCl2. Concentrations higher than 50 μm CuCl2 induced the activity of SIMK, MMK2, and MMK3, and to a lesser extent SAMK (Fig. 2A). Immunokinase assays of CdCl2-treated roots showed similar dose-dependent MAPK activation (Fig. 2B). These results indicate that plants respond to elevated CuCl2 and CdCl2 concentrations by activation of at least four distinct MAPK pathways.
Figure 2.
Concentration-dependent activation of MAPKs by CuCl2 and CdCl2. Seedlings were exposed to increasing concentrations of CuCl2 or CdCl2 for 30 min. Protein extracts from roots were immunoprecipitated with M23, M11, M14, or M24 antibodies that are specific for SIMK, MMK2, MMK3, and SAMK, respectively. Subsequently, kinase activities were determined by in vitro kinase assays with γ-[32P]ATP and MBP as substrate. The experiment was performed independently three times showing similar results.
CuCl2 and CdCl2 Induce MAPK Activation with Different Kinetics
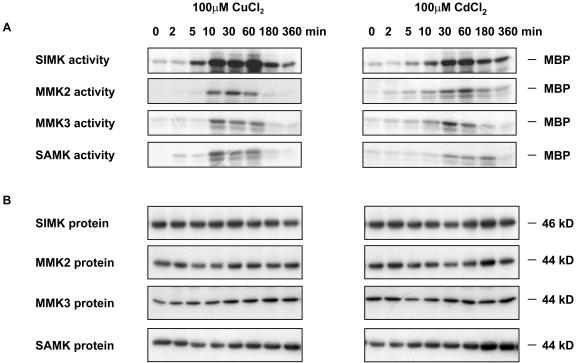
Depending on the plant organ and the extracellular stimulus, the activity of MAPKs can either transiently or constitutively increase. Thus, the kinetics of SIMK, MMK2, MMK3, and SAMK activation was analyzed in response to excess CuCl2 or CdCl2. Application of 100 μm CuCl2 to alfalfa roots rapidly activated SIMK (Fig. 3A, left). SIMK was activated within 5 min and showed maximum activity between 10 and 60 min. Although SIMK activity decreased at later time points, considerable activity was still detected at 180 and 360 min. MMK2, MMK3, and SAMK showed transient activation profiles with a maximum between 10 and 60 min.
Figure 3.
Differential activation of MAPKs by CuCl2 and CdCl2. Seedlings were treated with 100 μm CuCl2 or CdCl2. Roots were harvested at the given time points. A, Immunokinase assays were performed with M23, M11, M14, or M24 antibodies that specifically recognize SIMK, MMK2, MMK3, or SAMK, respectively. B, The same protein extracts were used for protein gel-blot analysis with M23, M11, M14, or M24 antibodies. The experiment was repeated twice revealing similar kinetics in all cases.
When alfalfa seedlings were treated with 100 μm CdCl2, the time course of SIMK activation showed a similar but delayed profile as after treatment with CuCl2 (Fig. 3A, right). While CuCl2 treatment induced maximal SIMK activity within 10 min, maximal activation of SIMK by CdCl2 was observed only at 30 to 60 min after exposure to CdCl2. Similarly, activation of MMK2, MMK3, and SAMK occurred later in response to CdCl2 than to CuCl2. Application of the corresponding amount of water did not activate SIMK, MMK2, MMK3, or SAMK during the experimental period (data not shown).
To determine the MAPK protein levels upon CdCl2 and CuCl2 treatments, aliquots of the same cell extracts that were used for the immunokinase assays (Fig. 3A) were used for immunoblotting. M23, M11, M14, and M24 antibodies detected protein bands of 46, 44, 44, and 44 kD corresponding to the SIMK, MMK2, MMK3, and SAMK proteins, respectively (Fig. 3B). In contrast to the CuCl2- and CdCl2-induced changes in protein kinase activities, the steady-state levels of the proteins remained constant over the experimental period. These data suggest that the CuCl2- and CdCl2-induced activation of SIMK, MMK2, MMK3, and SAMK occurred by posttranslational mechanisms.
SIMKK Mediates the Activation of SIMK and SAMK by CuCl2 in Vivo
MAPKs are activated by distinct MAPKKs in response to different stimuli. To identify the corresponding upstream component of SIMK, MMK2, MMK3, and SAMK in response to excess copper and cadmium, different MAPKKs were tested for their ability to mediate CuCl2- and CdCl2-induced MAPK activation. For this purpose, hemagglutinin (HA)-tagged versions of SIMK, MMK2, MMK3, and SAMK were transiently expressed in Arabidopsis protoplasts in the absence or presence of the three myc-tagged MAPKKs MEK1, PRKK, or SIMKK.
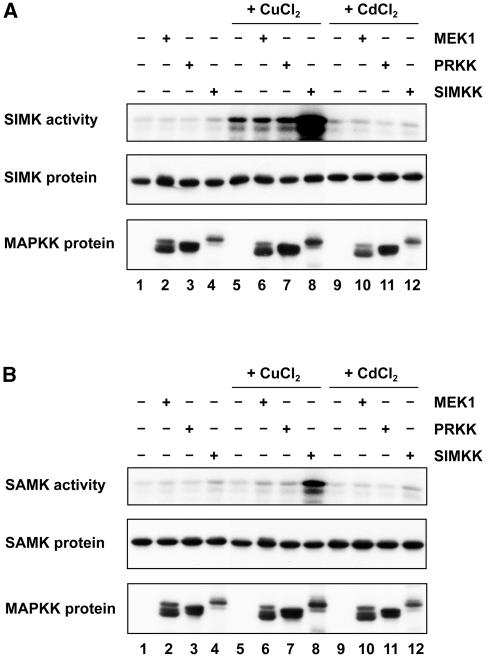
SIMK, MMK2, MMK3, and SAMK activity was measured from control, CuCl2-, and CdCl2-treated protoplasts by immunokinase assays using an anti-HA antibody. In nontreated cells that expressed the MAPKs alone, extremely low kinase activity was detected (Fig. 4, A and B, lane 1). Incubation of protoplasts with 100 μm CuCl2 induced a moderate activation of SIMK (Fig. 4A, lane 5). Coexpression of SIMK with SIMKK strongly enhanced SIMK activation by CuCl2 (Fig. 4A, lane 8), while MEK1 and PRKK did not have any effect on SIMK activity (Fig. 4A, lanes 6 and 7). Analysis of cells coexpressing SAMK and MEK1, PRKK, or SIMKK revealed that CuCl2-induced activation of SAMK is mediated by SIMKK, but not by MEK1 or PRKK (Fig. 4B, lane 8). In contrast, MMK2 and MMK3 activation by copper could not be stimulated by SIMKK, MEK1, or PRKK (data not shown).
Figure 4.
SIMKK enhances the activation of SIMK and SAMK by CuCl2. SIMK-HA (A) and SAMK-HA (B) were either expressed alone (1, 5, and 9) or coexpressed with myc-tagged MEK1 (2, 6, and 10), PRKK (3, 7, and 11), or SIMKK (4, 8, and 12) in protoplasts. The protoplasts were treated either for 30 min with 100 μm CuCl2 (lanes 5–8), 100 μm CdCl2 (lanes 9–12), or as control with equal amount of water (lanes 1–4). Subsequently, SIMK and SAMK activities were determined by immunokinase assays using anti-HA antibodies for immunoprecipitation and MBP as substrate. The same protein extracts were analyzed for the presence of the coexpressed proteins by protein gel-blot analysis with anti-HA or anti-Myc antibodies. Three independent experiments yielded comparable results.
Exposure of protoplasts expressing the MAPKs alone or coexpressing MEK1, PRKK, or SIMKK to 100 μm CdCl2 did not activate SIMK, MMK2, MMK3, or SAMK (Fig. 4, A and B, lanes 9–12; data not shown), indicating that neither MEK1, PRKK, nor SIMKK can convey CdCl2-induced activation of SIMK, MMK2, MMK3, or SAMK.
As shown by protein gel-blot analysis of the same cell extracts that were used for the SIMK and SAMK immunokinase assays (Fig. 4, A and B), equal amounts of SIMK and SAMK were present (Fig. 4, A and B, lower sections), suggesting that SIMK and SAMK were activated by SIMKK in response to CuCl2.
Metal Ion-Specific MAPK Activation
To determine whether MAPK activation is a general response to elevated amounts of metal ions or specific for copper and cadmium, we exposed alfalfa seedlings for 30 min to 100 μm of Al2(SO4)3, CdCl2, CoCl2, CuCl2, FeCl2, Pb(NO3)2, or ZnCl2. Analysis of MAPK activity levels in roots by immunokinase assays showed that SIMK activity was highly induced by CdCl2 and CuCl2 (Fig. 5, lanes 3 and 5, respectively) and slightly induced by FeCl2 and Pb(NO3)2 (Fig. 5, lanes 6 and 7, respectively). Little or no increase in SIMK activity was observed after incubation with Al2(SO4)3, CoCl2, or ZnCl2 (Fig. 5, lanes 2, 4, and 8, respectively). MMK2, MMK3, and SAMK were also most strongly activated by CuCl2 and CdCl2, but showed some differences in response to treatment with the other metals. Whereas MMK2 was also significantly activated by FeCl2, MMK3 and SAMK poorly responded to this metal (Fig. 5, lane 6). The significance of these observations is presently unclear and requires further studies. Taken together, these data indicate that SIMK, MMK2, MMK3, and SAMK are specifically activated in response to excess levels of particular heavy metal ions.
Figure 5.
Ion-specific MAPK activation. Seedlings were exposed to (1) water or 100 μm (2) Al2(SO4)3, (3) CdCl2, (4) CoCl2, (5) CuCl2, (6) FeCl2, (7) Pb(NO3)2, or (8) ZnCl2 for 30 min. Root protein extracts were used to immunoprecipitate SIMK, MMK2, MMK3, and SAMK with M23, M11, M14, or M24 antibodies, respectively. Subsequently, kinase activities were determined using MBP as substrate. Independent repetitions of the experiment showed the same activity profile.
DISCUSSION
Heavy metals are potentially highly toxic to all organisms including animals and plants. Numerous studies on the physiological responses to excess amounts of heavy metal ions indicate that plants have developed various mechanisms to cope with this environmental threat. Until now, however, the cellular mechanisms of heavy metal stress-induced signaling remained elusive. In this study we have analyzed the role of protein kinases in roots that experience heavy metal stress. We demonstrate that in response to elevated levels of copper and cadmium ions four distinct MAPK pathways are activated. Excess of copper ions rapidly activated SIMK, MMK2, MMK3, and SAMK, while activation of the four MAPKs to cadmium ions showed similar but delayed profiles. In transient expression assays copper-induced activation of SIMK and SAMK, but not of MMK2 or MMK3, was specifically mediated by SIMKK.
Copper and cadmium are heavy metals with different physiochemical properties and functions. Copper is a vital micronutrient essential for normal plant growth and development. It is a cofactor for many physiological processes including photosynthesis, respiration, superoxide scavenging, ethylene sensing, and lignification. However, excess levels of copper are harmful due to the production of ROS by autoxidation and Fenton reactions.
Cadmium has no known biological function. It is highly reactive and inactivates various enzymatic processes. Consequently, cadmium is generally toxic for all living cells. Although cadmium does not directly interfere with cellular redox reactions it causes oxidative injury. The displacement and thereby the release of redox-active metal ions from various biomolecules as well as the depletion of the antioxidant system by cadmium disturbs the redox balance of the cell.
Since both copper and cadmium can disturb the redox control of the cell, it could be speculated that MAPK activation by excess copper and cadmium is mediated by ROS. Evidence for activation of MAPKs by ROS has been provided by several studies in different plant species (Kovtun et al., 2000; Desikan et al., 2001; Yuasa et al., 2001; Nakagami et al., 2004; Rentel et al., 2004). A possible explanation for the different kinetics of MAPK activation by copper and cadmium ions might therefore stem from the different cellular reaction mechanisms of these two metals. As a redox-active metal, copper ions directly induce the formation of ROS when present at supraoptimal concentrations. In contrast, cadmium ions do not directly interfere with cellular oxygen metabolism but cause oxidative injury as a secondary effect. These considerations could suggest that the differential rate of ROS production might account for the delayed induction of SIMK, MMK2, MMK3, and SAMK activity by cadmium. Although this is an attractive hypothesis, the possibility that copper- and/or cadmium-induced activation of some MAPKs is independent of ROS production should not be dismissed and requires further investigation.
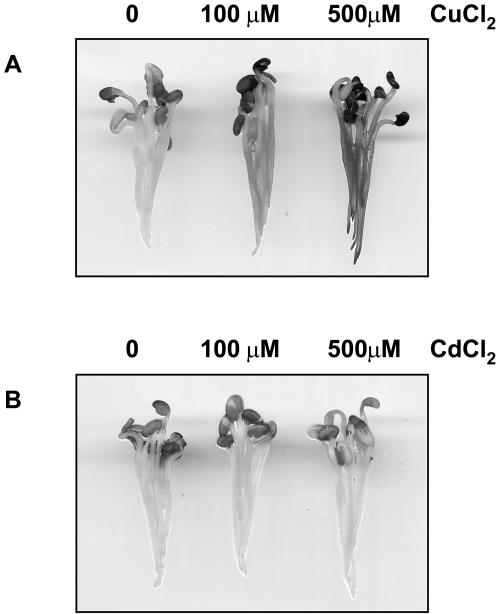
Hydrogen peroxide was reported to accumulate in response to excess levels of copper and cadmium (Schützendübel and Polle, 2002). We used 3,3-diaminobenzidine (DAB) stainings to estimate hydrogen peroxide levels in alfalfa roots after treatment with copper or cadmium. CuCl2 induced a dose-dependent hydrogen peroxide production, whereas even prolonged exposure to high concentrations of CdCl2 did not lead to any detectable DAB staining (Fig. 6), indicating that elevated amounts of copper and cadmium ions induce distinct cellular responses in alfalfa. Although very low levels of hydrogen peroxide cannot be visualized by DAB staining, these data make it likely that SIMK, MMK2, MMK3, and SAMK activation by cadmium ions could be mediated by a ROS-independent mechanism.
Figure 6.
Excess levels of CuCl2 induce hydrogen peroxide accumulation. DAB staining of alfalfa roots exposed to water, 100 μm and 500 μm CuCl2 (A), or 100 μm and 500 μm CdCl2 (B). The experiment was repeated five times showing comparable stainings.
To investigate the possible involvement of upstream factors in the activation of the four alfalfa MAPKs, three different MAPKKs were coexpressed with the MAPKs in protoplasts before heavy metal stress application. The results revealed that in vivo activation of SIMK and SAMK by excess copper is mediated by SIMKK. It is worth noting that SIMKK also mediates SIMK activation in response to ROS (F. Ouaked and H. Hirt, unpublished data). MEK1 and PRKK could not convey activation of any of the four MAPKs by elevated amounts of copper. Interestingly, copper-induced activation of MMK2 and MMK3 is not mediated by SIMKK, suggesting that multiple signaling components are involved in conveying copper-induced MAPK activations.
In our analysis of the involvement of MAPKKs in cadmium-induced activation of MAPKs, SIMKK did not mediate SIMK and SAMK activation by CdCl2. These results suggest that CdCl2-induced MAPK signaling occurs by a mechanism distinct from that for CuCl2. The different kinetics of MAPK activation by CuCl2 and CdCl2 further support the notion that copper and cadmium ions are transduced by different signaling pathways.
MAPK pathways are involved in many processes, including developmental and hormonal responses (Tena et al., 2001; Jonak et al., 2002). So what is the overall significance of MAPK signaling in response to heavy metal stress? Evidence for a central role of MAPKs in heavy metal stress comes from yeast (Schizosaccharomyces pombe) and animals. In fission yeast, the Sty1/Spc1 MAPK pathway is required for tolerance to many adverse stress conditions, including heavy metals (Degols et al., 1996; Degols and Russell, 1997; Smith et al., 2002). Genetic evidence in Caenorhabditis elegans implicates the MAPKK MEK-1 in heavy metal stress tolerance and nematodes carrying a mek-1 deletion are hypersensitive to copper and cadmium ions (Koga et al., 2000). So far, the evidence for an involvement of a MAPK pathway in heavy metal stress is scarce in plants, but a recent screen for cadmium-responsive genes identified the Arabidopsis MAPKKK MEKK1 to be transcriptionally induced by high concentrations of CdCl2 (Suzuki et al., 2001). Further studies with transgenic and mutant plants will verify the role of distinct MAPK modules during the plant response to copper and cadmium stress.
Taken together, heavy metal stress signaling appears to be highly complex and specific for different metal ions. At least four different MAPK pathways are activated in alfalfa roots in response to excess amounts of copper. Even though SIMK, MMK2, MMK3, and SAMK are also activated by CdCl2, copper- and cadmium-induced stress appear to be mediated by distinct signal transduction pathways (Fig. 7).
Figure 7.
Copper and cadmium-induced MAPK signaling pathways. Excess copper and cadmium ions induce distinct MAPK pathways with different kinetics. A, As a redox-active metal ion, copper leads to the production of ROS that might trigger SIMK and SAMK activation via SIMKK. B, Cadmium activates SIMK, MMK2, MMK3, and SAMK. The upstream components mediating MAPK activation by cadmium remain to be identified.
Different biotechnological efforts are undertaken to improve plant heavy metal tolerance and to use plants to extract toxic ions from soil. This new, environment-friendly, plant-based technology is known as phytoremediation and focuses on mechanisms of detoxification and homeostasis of heavy metals (Salt et al., 1998; Meagher, 2000). To further improve this technology it is important to understand cellular signaling induced by heavy metal stress. Our findings that distinct MAPK pathways are activated in response to cadmium and copper stress encourage new strategies for improving plant tolerance to heavy metals and phytoremediation.
MATERIALS AND METHODS
Protein Extraction
Roots of hydroponically grown 4-d-old alfalfa plants (Medicago sativa cv Europa) were exposed to indicated concentrations of heavy metals before shock-freezing them in liquid nitrogen. Cell extracts were prepared in extraction buffer (25 mm Tris-HCl, pH 7.5, 15 mm MgCl2, 15 mm EGTA, 75 mm NaCl, 1 mm dithiothreitol, 1 mm NaF, 0.5 mm NaVO3, 15 mm p-nitophenyl phosphate, 0.1% Tween 20, 15 mm β-glycerophosphate, 0.5 mm phenylmethylsulfonyl fluoride, 5 μg/mL leupeptin, and 5 μg/mL aprotenin). After centrifugation at 20,000g for 45 min the supernatant was immediately used for further experiments.
In-Gel Protein Kinase Assays
For in-gel protein kinase reactions, cell extracts containing 20 μg of total protein/lane were separated by SDS-PAGE. MBP (0.5 mg/mL) was used as a substrate for the kinase reaction and polymerized in the polyacrylamide gel. Protein denaturation, renaturation, and kinase reactions were performed as described (Usami et al., 1995).
Immunokinase Assays
Immunokinase assays were performed with cell extracts containing 100 μg of total protein as described in Cardinale et al. (2000). The antibodies M23, M11, M14, and M24 were used to specifically immunoprecipitate SIMK, MMK2, MMK3, and SAMK, respectively. The specificity of the antibodies was previously demonstrated (Munnik et al., 1999; Cardinale et al., 2000). Immunoprecipitations from protoplast protein extracts were performed with anti-HA antibodies.
Immunoblots
For protein gel-blot analysis 15 μg of total protein extracts were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed, either with M23, M11, M14, and M24 antibodies, at a dilution of 1:10,000 (Cardinale et al., 2000) or with HA (Babco/Convance, Denver) and Myc antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:5,000. Alkaline phosphate-conjugated goat anti-rabbit (Santa Cruz Biotechnology) and anti-mouse IgG (Sigma, St. Louis) were used as secondary antibodies, and the reaction was visualized by fluorography using CDP-Star (Amersham Life Science, Buckinghamshire, UK).
Transient Expression Assays
Cloning of HA-tagged SIMK, MMK2, MMK3, and SAMK and myc-tagged SIMKK into the plant expression vector pRT101 was reported in Kiegerl et al. (2000), Cardinale et al. (2002), and Nakagami et al. (2004). The open reading frames of MEK1 and PRKK were fused at their C termini to a double myc epitope and cloned into the vector pRT101. Transient expression experiments were performed with protoplasts from Arabidopsis cells using polyethylene glycol transformation (Ouaked et al., 2003). Twelve to 16 h after transformation with 5 μg DNA of each construct protoplasts were treated with 100 μm CuCl2 or CdCl2 for 30 min and subjected to biochemical analysis.
Hydrogen Peroxide Detection
Hydrogen peroxide accumulation was visualized with DAB according to a procedure adapted from Thordal-Christensen et al. (1997). Briefly, alfalfa seedlings were vacuum infiltrated for 5 min with 1 mg/mL DAB. Subsequently, different concentrations of CuCl2 or CdCl2 were added and DAB staining was assessed visually.
This work was supported by the Austrian Science Foundation.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.045724.
References
- Agrawal GK, Iwahashi H, Rakwal R (2003) Rice MAPKs. Biochem Biophys Res Commun 302: 171–180 [DOI] [PubMed] [Google Scholar]
- Avery SV (2001) Metal toxicity in yeasts and the role of oxidative stress. Adv Appl Microbiol 49: 111–142 [DOI] [PubMed] [Google Scholar]
- Bögre L, Calderini O, Binarova P, Mattauch M, Till S, Kiegerl S, Jonak C, Pollaschek C, Barker P, Huskisson NS, et al (1999) A MAP kinase is activated late in plant mitosis and becomes localized to the plane of cell division. Plant Cell 11: 101–113 [PMC free article] [PubMed] [Google Scholar]
- Bögre L, Ligterink W, Heberle-Bors E, Hirt H (1996) Mechanosensors in plants. Nature 383: 489–490 [DOI] [PubMed] [Google Scholar]
- Bögre L, Ligterink W, Meskiene I, Barker PJ, Heberle-Bors E, Huskisson NS, Hirt H (1997) Wounding induces the rapid and transient activation of a specific MAP kinase pathway. Plant Cell 9: 75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale F, Jonak C, Ligterink W, Niehaus K, Boller T, Hirt H (2000) Differential activation of four specific MAPK pathways by distinct elicitors. J Biol Chem 275: 36734–36740 [DOI] [PubMed] [Google Scholar]
- Cardinale F, Meskiene I, Ouaked F, Hirt H (2002) Convergence and divergence of stress-induced mitogen-activated protein kinase signaling pathways at the level of two distinct mitogen-activated protein kinase kinases. Plant Cell 14: 703–711 [PMC free article] [PubMed] [Google Scholar]
- Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212: 475–486 [DOI] [PubMed] [Google Scholar]
- Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53: 159–182 [DOI] [PubMed] [Google Scholar]
- Degols G, Russell P (1997) Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol Cell Biol 17: 3356–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P (1996) Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol 16: 2870–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Ichimura K, Shinozaki K, Neill SJ (2001) Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiol 126: 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189: 147–163 [DOI] [PubMed] [Google Scholar]
- Goyer RA (1997) Toxic and essential metal interactions. Annu Rev Nutr 17: 37–50 [DOI] [PubMed] [Google Scholar]
- Gustin MC, Albertyn J, Alexander M, Davenport K (1998) MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev 62: 1264–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JL, Williams LE (2003) Transition metal transporters in plants. J Exp Bot 54: 2601–2613 [DOI] [PubMed] [Google Scholar]
- Ichimura K, Tena G, Henry Y, Zhang Z, Hirt H, Wilson C, Morris P, Mundy J, Innes R, Ecker J, et al (2002) Mitogen-activated protein kinase cascade in plants: a new nomenclature. Trends Plant Sci 7: 301–308 [DOI] [PubMed] [Google Scholar]
- Jonak C, Kiegerl S, Ligterink W, Barker PJ, Huskisson NS, Hirt H (1996) Stress signaling in plants: a mitogen-activated protein kinase pathway is activated by cold and drought. Proc Natl Acad Sci USA 93: 11274–11279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak C, Ökresz L, Bögre L, Hirt H (2002) Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol 5: 415–424 [DOI] [PubMed] [Google Scholar]
- Kiegerl S, Cardinale F, Siligan C, Gross A, Baudouin E, Liwosz A, Eklof S, Till S, Bögre L, Hirt H, et al (2000) SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell 12: 2247–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga M, Zwaal R, Guan KL, Avery L, Ohshima Y (2000) A Caenorhabditis elegans MAP kinase kinase, MEK-1, is involved in stress responses. EMBO J 19: 5148–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97: 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie M, Kondor N, DeWitt JG (2003) Gene expression in cadmium-tolerant Datura innoxia: detection and characterization of cDNAs induced in response to Cd2+. Plant Mol Biol 52: 81–89 [DOI] [PubMed] [Google Scholar]
- Meagher RB (2000) Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol 3: 153–162 [DOI] [PubMed] [Google Scholar]
- Munnik T, Ligterink W, Meskiene I, Calderini O, Beyerly J, Musgrave A, Hirt H (1999) Distinct osmo-sensing protein kinase pathways are involved in signaling moderate and severe hyper-osmotic stress. Plant J 20: 381–388 [DOI] [PubMed] [Google Scholar]
- Nakagami H, Kiegerl S, Hirt H (2004) OMTK1, a novel MAPKKK, channels oxidative stress signaling through direct MAPK interaction. J Biol Chem 279: 26959–26966 [DOI] [PubMed] [Google Scholar]
- Ouaked F, Rozhon W, Lecourieux D, Hirt H (2003) A MAPK pathway mediates ethylene signaling in plants. EMBO J 22: 1282–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22: 153–183 [DOI] [PubMed] [Google Scholar]
- Polle A, Schützendübel A (2003) Heavy metal signalling in plants: linking cellular and oganismic responses. In H Hirt, K Shinozaki, eds, Plant Responses to Abiotic Stress, Vol 4. Springer-Verlag, Berlin, pp 187–215
- Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, et al (2004) OXI1 kinase is necessary for oxidative burst-mediated signaling in Arabidopsis. Nature 427: 858–861 [DOI] [PubMed] [Google Scholar]
- Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49: 643–668 [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Weber MJ (1999) Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol 19: 2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53: 1351–1365 [PubMed] [Google Scholar]
- Smith DA, Toone WM, Chen D, Bahler J, Jones N, Morgan BA, Quinn J (2002) The Srk1 protein kinase is a target for the Sty1 stress-activated MAPK in fission yeast. J Biol Chem 277: 33411–33421 [DOI] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18: 321–336 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Koizumi N, Sano H (2001) Screening of cadmium-responsive genes in Arabidopsis thaliana reveals protein denaturation and oxidative stresses to be critical components of cadmium toxicity. Plant Cell Environ 24: 1177–1188 [Google Scholar]
- Tena G, Asai T, Chiu WL, Sheen J (2001) Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol 4: 392–400 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang H, Wei Y, Collinge D (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Usami S, Banno H, Ito Y, Nishihama R, Machida Y (1995) Cutting activates a 46-kilodalton protein kinase in plants. Proc Natl Acad Sci USA 92: 8660–8664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL (1999) Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 79: 143–180 [DOI] [PubMed] [Google Scholar]
- Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10: 1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JR (2000) Map kinases in fungal pathogens. Fungal Genet Biol 31: 137–152 [DOI] [PubMed] [Google Scholar]
- Yuasa T, Ichimura K, Mizoguchi T, Shinozaki K (2001) Oxidative stress activates ATMPK6, an Arabidopsis homologue of MAP kinase. Plant Cell Physiol 42: 1012–1016 [DOI] [PubMed] [Google Scholar]