Abstract
Children who suffer with cerebral palsy (CP) have a significant chance of developing scoliosis during their early years and adolescence. The behavior of this scoliosis is closely associated with the severity of the CP disability and unlike idiopathic scoliosis, it continues to progress beyond skeletal maturity. Conservative measures may slow the progression of the curve, however, surgery remains the only definitive management option. Advances in surgical technique over the last 50 years have provided methods to effectively treat the deformity while also reducing complication rates. The increased risk of surgical complications with these complex patients make decisions about treatment challenging, however with careful pre-operative optimization and post-operative care, surgery can offer a significant improvement in quality of life. This review discusses the development of scoliosis in CP patient, evaluates conservative and surgical treatment options and assesses post-operative outcome.
Keywords: Cerebral palsy (CP), scoliosis, child, spine, treatment outcome
Introduction
Cerebral palsy (CP) is defined as a permanent, non-progressive abnormality of motor function that is a result of injury to the developing brain (1). It can occur pre, peri or post-natally from a variety of causes. The term encompasses a heterogeneous group of conditions characterized by abnormal muscle tone, movement and posture. The incidence of CP is estimated at 2.0 per 1,000 live births in the UK (2). There is a strong link between CP and the development of scoliosis. It is estimated scoliosis occurs in between 21% and 64% of patients with CP (3-5). Spinal deformity is thought to occur before 10 years of age (5-7) and unlike in idiopathic scoliosis, has been shown to progress beyond skeletal maturity (8).
Risk factors for development of scoliosis
The development of scoliosis is strongly linked to the level of global disability caused by the CP. There are classification systems that describe the spectrum of disability that can occur under the umbrella term of CP. The most widely used of these is the Gross Motor Function Classification System (GMFCS), which splits children into 5 categories depending on their functional capacity (Table 1) (9). A large study conducted by Persson-Bunke et al. (5) highlights the statistically significant relationship between GMFCS level and development of scoliosis with 50% of children GMFCS IV–V developing a severe scoliosis. A Cobb angle of greater than 40o at an early age has been found to predict significant progression of a CP scoliosis (6,7). Furthermore an inverse relationship between development of scoliosis and ambulation has been suggested, with the least mobile patients at greatest risk (4,6). Gu et al. (7) suggested age was the most important risk factor and found no relationship between height and weight of children and curve progression.
Table 1. Summary of the GMFCS classification (9).
| GMFCS Level | Description |
|---|---|
| I | Walks without limitations. Limitations in more advanced motor skills |
| II | Walks without assistive devices. Limitations walking outdoors and in the community |
| III | Walks with assistive mobility devices. Limitations walking outdoors and in the community |
| IV | Self-mobility with limitations. Children are transported or use power mobility outdoors or in the community |
| V | Self-mobility severely limited even with the use of assistive technology |
The rate of progression of the scoliotic curves is variable, which in the adult, largely non ambulatory population, can range between 3.0o and 4.4o per year (10). Rate of progression was found to vary according to size of curve; larger curves (>50o) have been shown to progress almost twice as fast than smaller curves (<50o) (8). Curves were most likely to progress in non-ambulatory, quadriplegic patients (GMFCS IV and V) (8).
Etiology
The etiology of scoliosis in CP has yet to be well defined. Spinal deformity is thought to be associated with muscular imbalance around the spinal axis from either spastic or flaccid muscular weakness (11). In studies focusing on CP, the factors contributing to the development of spinal deformity have been suggested to include spasticity, muscle weakness and poor muscle control (12). In a meta-analysis of the risk factors for the development of scoliosis in CP the authors were unable to draw firm conclusions (13). The authors highlighted the lack of evidence and the poor methodological quality of the research.
Pattern of deformity
Two distinct patterns of scoliotic curves have been described in patients with CP (14). Group-I curves can be considered double curves with a thoracic and lumbar component and occurred most often in ambulatory patients, with minimal pelvic obliquity (14). Group-II curves are single curves in either the thoracic or lumbar spines and were of greater magnitude. They occur more frequently in quadriplegic patients and almost all display significant pelvic obliquity (14) (Figure 1). Pelvic obliquity may be defined as an angulation of the pelvis to the horizontal plane (15). Spinal deformity, hip contractures, leg length discrepancy or a combination of these factors will contribute to the cause of pelvic obliquity (16). Pelvic obliquity can be usefully categorized as supra-pelvic (spine/trunk disorder), pelvic or infra-pelvic (hip joint and lower limb) (17) aiding both diagnosis and management. CP has long been associated with the formation of pelvic obliquity (16,18) with large thoracolumbar curves contributing a supra-pelvic component and asymmetrical lower limb contractures recognized as an infra-pelvic cause. Consequences of untreated pelvic obliquity include the development of pressure points and decubitus ulceration, impaired sitting balance and significant hip joint deformity (17,19), hence making its diagnosis and consideration in treatment planning extremely important.
Figure 1.

Preoperative radiograph of a child with cerebral palsy and scoliosis.
Non-surgical management
The aim of non-surgical management of scoliosis in CP is to improve sitting control and reduce or modify curve progression without the need for surgical intervention. Historical reports suggest the use of supportive bracing in children with CP was poorly tolerated and ineffective (3). There is a paucity of evidence for the use of modern bracing techniques. However, more recent studies suggest bracing improves sitting balance and trunk support, which provides better control of the head and neck as well as enhanced use of the upper limbs (20,21) as they are not required to support the trunk in the sitting position. Evidence for the use of orthoses to prevent scoliotic curve progression is mixed. Some authors have suggested bracing may slow curve progression (20), especially in younger patients with curves less than 40 degrees (20,22). Other groups have reported less success (21,22) and suggest braces may be beneficial as an interim measure before definitive surgical correction.
In non-ambulatory patients, methods of optimizing seating position have been shown to provide increased support and improve functional outcomes (23). Few studies have focused on the effect of seating systems on correction of spinal deformity. The placement of a 3-point system of lateral support pads was shown to offer a more symmetrical trunk posture and correct curve angles by 35% in non-ambulatory CP patients with scoliosis (24). Botulinum toxin injection has been used as an effective method for reducing spasticity in the limbs of patients with CP (25,26). Nuzzo et al. (27) administered botulinum toxin to a small patient population as a supplement to planned surgical therapy for CP patients with scoliosis. Reportedly, it did not worsen scoliosis and provided some reduction in magnitude of the curve in all patients.
Intrathecal baclofen (ITB) has been used for the treatment of global spasticity in a number of neuromuscular conditions and has been proven to be efficacious in patients with CP (28). The use of ITB pumps, which deliver a continuous infusion, offers improvement in spasticity, ease of care and reduction in pain (29). There is conflicting evidence as to whether ITB pumps can cause progression of a scoliosis. In a number of case series, a significant increase in Cobb angle was observed following ITB pump insertion (30,31). On the contrary, cohort studies using matched patients have shown there to be no difference in progression of scoliotic curves (32,33). The effect of an ITB pump at the time of scoliosis surgery is also controversial. There is an increase in the risk of infection, re-operation and re-hospitalization when compared to matched controls undergoing the same procedure (34). However, in another study by Borowski et al. (35) the insertion of ITB pumps before, during or after posterior spinal fusion had no significant effect on outcome. An economic analysis of the use of ITB has demonstrated it to be a cost effective method of reducing spasticity in CP patients in both the UK (36) and US (37,38) healthcare systems. The use of ITB remains controversial for the reasons outlined above but may be beneficial in patients with severe spasticity in which non-invasive treatments have failed.
Surgical management
Surgery remains the only option for the definitive management of scoliosis in CP. The aims of surgical correction include achieving a balanced spine, prevention of curve progression and improvement in functional quality of life. The timing of surgery should be considered on an individual case basis. Nonetheless surgery should be considered in those patients with large curves (>50o), in those continuing to progress beyond skeletal maturity and in significant curves resulting in functional or physiological disturbance.
Pre and peri-operative considerations
Pre-operative planning is an important consideration before embarking on scoliosis surgery. The complex nature of CP will often cause the child to have concurrent multi-system pathology that requires optimization. A comprehensive preoperative assessment is required including history, physical examination, laboratory and imaging investigations as well as discussion amongst a multi-disciplinary team (MDT). In our center the MDT comprises a scoliosis surgeon, general pediatrician, pediatric respiratory physician and physiotherapist, anesthetist and cardiologist.
Neurological
Pre-existing intra-cerebral lesions may cause seizure disorders in children with CP. The child therefore may be taking a combination of anti-seizure medication, which can have side effects and interactions with both anesthetic and analgesic agents that must be considered. Phenytoin, phenobarbital and sodium valproate have been shown to alter calcium absorption, leading to a decrease in bone mineral density (39), which may be significant when selecting placement and type of spinal instrumentation and increase the risk of failure through implant pull out. Furthermore sodium valproate is known to cause abnormalities in clotting (40). In a study of 114 CP patients undergoing surgery a 26% increase in blood loss was observed in patients taking sodium valproate (41). Considering the major nature of corrective spinal surgery, abnormalities in clotting may risk serious adverse events. There have been reports of coagulopathies including disseminated intravascular coagulation (DIC) developing during major spinal surgery (42) therefore the clotting profile must be closely monitored before, during and after the surgery.
Respiratory
Post-operative complications involving the respiratory system occur frequently in children with CP (43). Abnormalities in pulmonary function secondary to factors such as poor upper airway tone, recurrent aspiration and thoracic cage deformity (44) add to this risk. A comprehensive evaluation of a child’s respiratory system including thorough history and examination, laboratory testing and formal pulmonary function tests, are imperative to guide both pre-operative planning and peri-operative management. The use of pre-operative non-invasive ventilation (NIV) training to strengthen respiratory muscles has shown promise in improving outcomes in patients with neuromuscular disease following spinal surgery (45). Current practice demonstrates a movement away from long-term post-operative mechanical ventilation (46) owing to the advances of peri-operative medicine. NIV has been gaining popularity in the management of respiratory disease in pediatric patients with CP (47) and represents a safe and effective option to help mitigate against and manage respiratory complications.
Gastrointestinal
Gastrointestinal disorders in children with CP are common. Gastro-esophageal reflux disease (GORD) is present in up to 70% (48) of CP patients leading to a greatly enhanced risk of bronchopulmonary-aspiration (49), which is often significant on a background of poor respiratory reserve. Optimization of GORD in children with CP using surgical techniques such as fundoplication has been shown to be an effective way of controlling symptoms (50,51). However, the high risk of postoperative complications, morbidity and mortality from anti-reflux procedures must be considered (52-54). Children with CP often experience problems with feeding, leading to an inadequate oral intake and malnutrition (55). A low preoperative serum albumin has been shown to correlate with increased rates of postoperative complications and overall longer hospital stay (56). Thorough nutritional assessment is therefore required with consideration for the use of nutritional supplements or alternative feeding regimens. Intensive nutritional support has been shown to have a significant influence on nutritional status and body composition when administered over a 4 week period (57). Additionally, nasogastric tube feeding may be beneficial in those with swallowing difficulties however should only be used as a short-term measure (58).
Spinal
The past decades have seen major changes in surgery for spinal deformity leading to the development of modern pedicle screw fixation technique; a method that has now been widely adopted. The Harrington rod, initially created for the treatment of spinal deformity secondary to polio (59), represents one of the early methods of spinal fusion in neuromuscular spinal deformity (3). Although this method provided some correction of the spinal curve, complications were both frequent and significant (60,61). This technique was quickly superseded by segmental spinal instrumentation pioneered by Luque et al. (62) which made use of sublaminar wires inserted at each vertebral level to give enhanced immobilization and correction of the curve. The concurrent use of the Galveston method of fixing intramedullary rods into the iliac diaphysis was shown to enhance pelvic fixation and created a hybrid Luque-Galveston instrumentation technique. The initial results reported by Luque demonstrated an average correction of 72% over an 18-month follow up (62). A number of authors have since reported good results with similar instrumentation with a reduction of Cobb angle of between 46–65%, an improvement in pelvic obliquity and reduced rates of pseudarthrosis formation (63-67).
Modification of a single rod into a single U-shaped structure, used alongside Galveston pelvic fixation and sublaminar wires gave rise to the Unit-rod method of fixation. This procedure was first described in a mixed population with neuromuscular scoliosis and achieved an average curve correction of 54.6% (68). Further data has shown curve correction between 62-76% and a reduction in pelvic obliquity of 86-88% (60,64,69) however these studies are limited by relatively small numbers. A large study by Tsirikos et al. (70) demonstrated a 68% reduction in scoliotic curve and a 71% reduction in pelvic obliquity in a series of 287 patients.
Other segmental systems including the Cotrel-Dubousset instrumentation have a limited evidence base for use in CP patients. A number of small studies have reported good outcomes, Piazzolla et al. (71) showed an improvement in Cobb angle of 57.2% with a reduction in pelvic obliquity of 58.9% which is comparable to Luque and unit rod systems. Furthermore, in a study of 60 patients followed up over 7 years, the authors demonstrated the Cotrel-Dubousset technique to offer effective, sustained scoliosis correction (72).
Advances in methods of spinal instrumentation produced the pedicle screw technique, offering enhanced correction of three-dimensional deformity (73) (Figure 2). Despite being widely accepted as a safe and reliable method of correcting adolescent idiopathic scoliosis, there have been few studies on the use of pedicle screws in neuromuscular scoliosis. Modi et al. (74) reported a satisfactory decrease in the magnitude of coronal and sagittal curves with a reduced rate of major complications over 3 years follow up. In a retrospective analysis of 45 patients on which pedicle screw instrumentation was performed, a favorable correction of scoliotic curve and pelvic obliquity was reported with a high carer/parent satisfaction rate (75). Although this data is promising, more work is required to assess the long-term efficacy of pedicle screw constructs.
Figure 2.
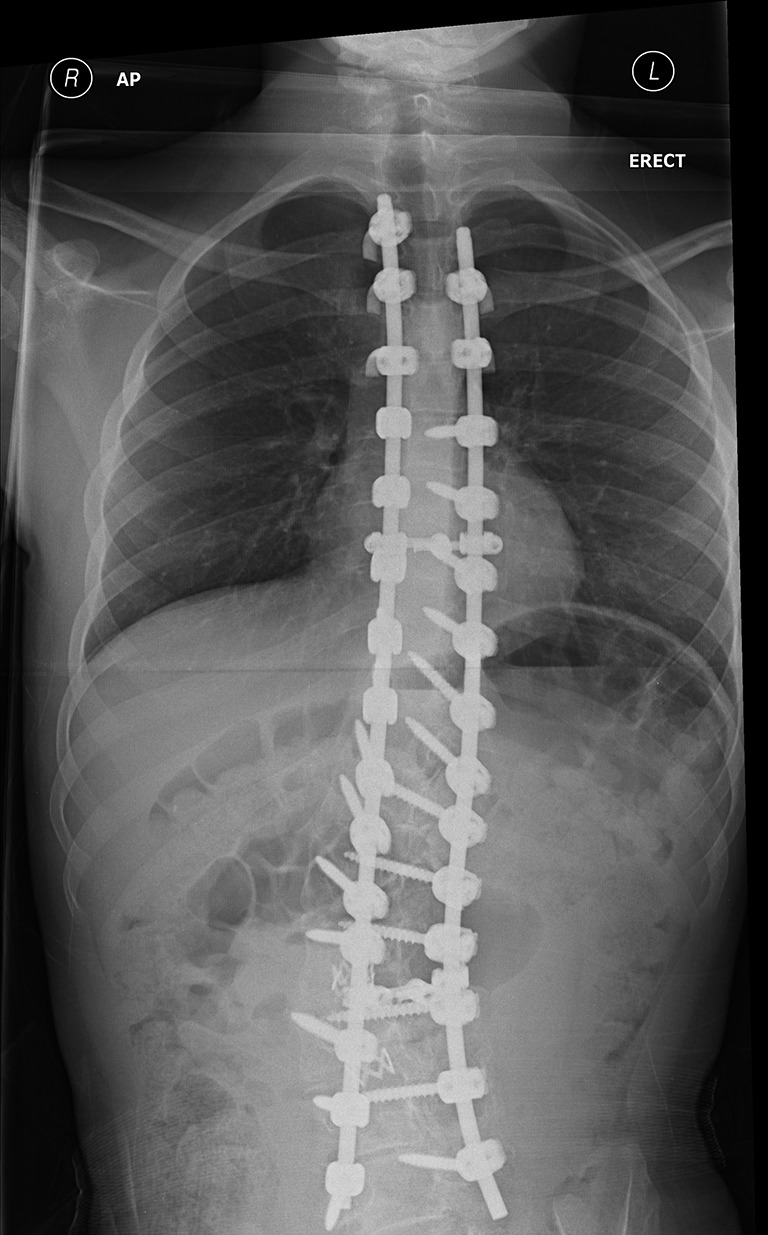
Postoperative radiograph following pedicle screw instrumentation.
Although the majority of patients with CP develop spinal deformity from the age of 10 onwards, there is a population of patients in which significant deformity occurs at an early age. Complete spinal fusion using the above techniques can cause problems when performed on skeletally immature patients, impairing the growth of the spine and thorax with effects on respiratory development and function. More recently technology has been developed to allow for the use of ‘growing rods’ to provide spinal support and correct deformity whilst allowing for growth. A study by McElroy et al. (76) represents the only published use of growing rods in patients with CP. Reports included a sustained improvement in Cobb angle and pelvic obliquity, however a significant complication rate, particularly from infection.
Anterior release and fusion procedures have been traditionally used for large, stiff curves and may, therefore, be considered for use in patients with CP. A number of indications for use of the anterior approach have been suggested including an inability to correct pelvic obliquity on forward flexion (77) and stiff thoracolumbar curves that exceed 70o on radiographic imaging (78,79). Furthermore, the disruption of the growth plate and subsequent inter body fusion achieved using the anterior approach, in skeletally immature patients, has been shown to prevent further growth of the anterior column and subsequent ‘crankshaft phenomenon’ (80). Access to the anterior spinal column can require opening of body cavities (thorax and abdomen) exposing patients to a greater risk of complications. Interestingly, in a small patient series, Auerbach et al. (78) demonstrated there to be no significant difference in the rate of postoperative complication between posterior only and anterior and posterior surgical techniques. Contemporary instrumentation techniques using pedicle screws have challenged the requirement for an anterior approach. The use of posterior-only pedicle screw constructs has been shown to offer excellent curve correction with a minimal complication rate (74). In fact, the increasing popularity of this instrumentation technique is diminishing the need for anterior release procedures. Moreover, alternative, posterior only techniques such as vertebral osteotomy (81) and vertebral column resection (82) have been reported in an attempt to manage large, stiff scoliotic curves
There are currently no studies directly comparing the current methods of spinal instrumentation in the CP population. Second generation techniques as well as pedicle screw instrumentation have all been shown to provide effective correction of deformity in terms of Cobb angle correction and reduction in pelvic obliquity. Current practice in this center involves the use of all pedicle screw constructs with anterior release procedures reserved for large, stiff thoracolumbar curves aiming to prevent the need for pelvic fixation.
Complications
Postoperative complications from spinal surgery in patients with CP are common. The reported overall complication rate in the literature is variable, ranging between 17-68% (67,83-86). High risk of complication is associated with non-ambulatory status and greater angle of scoliosis curve (85,87). These patients often suffer from the greatest physical disability and a number of preoperative medical comorbidities, which may account for this increased risk. Complications have been reported to affect many body systems including respiratory, gastrointestinal and neurological. In a recent meta-analysis of complications following surgery for neuromuscular scoliosis, pulmonary complications were found to be most common (22.7%), followed by implant complications (12.5%) and infections (10.9%) (88). The authors suggested age at the time of surgery (<13 years) was associated with higher rates of neurological (15.1% compared to 3%) and pseudarthrosis (11.6% compared to 1.7%) (88). No explanation of this association was offered, however younger, smaller patients may cause an increased difficulty in placement of instrumentation therefore increasing risk of neural injury and failure of fusion.
Strategies to reduce complication rates in CP patients undergoing spinal fusion are sparsely reported. Contemporary evidence has suggested the use of vancomycin powder may reduce the rate of wound infection in spinal surgery. In a large retrospective review the overall infection rate was reported to be less than 1% (89) when using vancomycin powder. Interestingly, the only randomized control trial has shown there to be no significant difference in the rate of infection when using combination intravenous and intra-wound antibiotics compared to intravenous alone (90). Recent best practice guidelines advocate the use of intra-wound vancomycin in high-risk patients (91) such as those with CP.
Outcome
Measurement of outcome following corrective surgery in CP can be difficult. Assessing the opinion of children who have varying degrees of learning difficulty makes the use of traditional methods of measuring postoperative outcomes problematic. Surveys of patients and parents in the postoperative period suggest a high level of satisfaction following surgery, with a large majority of parents willing to recommend the procedure to others (92). Up to 99% of parents reported being satisfied with the outcome of the procedure with 85–94% willing to consider surgical intervention for their children again (83,93). The retrospective nature of these studies does open them to the influence of bias, with few reporting a pre-operative assessment as a comparator. Furthermore, targeting the opinions of parents who will often have made the ultimate decision about their child’s treatment may not provide an objective measure of outcome. Interestingly, in a comparative study between opinions of parents and caregivers (education professionals, therapists) of children with CP, Tsirkos et al. (92) demonstrated both groups noted significant improvement in both appearance and function following surgery.
On the other hand there are prospective studies that have shown that whilst parents remain satisfied with the postoperative outcome, surgery provides no improvement in function, school attendance or co-morbidities (94,95). Askin et al. (95) prospectively measured functional outcome in patients following scoliosis surgery and found there to be a decline in function over the first 6 months and no overall improvement 12 months postoperatively. Small patient numbers, a short follow up period and heterogeneous patient group may have influenced this data set.
Quality of life is perhaps the most important outcome measure in any postoperative CP patient. In a systematic review, the evidence suggests an improvement in postoperative quality of life in CP patients who underwent scoliosis surgery (96). Nevertheless, the authors commented there are conflicting reports and the literature is currently lacking well-controlled, prospective studies (96). It is therefore imperative that careful consideration of the risks and benefits of surgery takes place on an individual patient basis, with involvement of the patient, family and wider members of the multi-disciplinary team.
Conclusions
Neuromuscular scoliosis is a common manifestation in children with CP. Without timely therapeutic intervention, scoliotic curves will continue to progress and cause impairment in function and increased risk of poor health. Management options are available that include the use of external bracing through to modern surgical techniques using segmental spinal fusion and pedicle screws. Current spinal instrumentation techniques offer a significant decrease in the magnitude of scoliotic curves and pelvic obliquity, which is sustained throughout long term follow up. As CP is a multi-system disease, careful consideration must be given to the preoperative optimization and the postoperative management of the child. A multi-disciplinary approach involving pediatric specialists will allow for this. Nonetheless, these patients remain at high risk of postoperative complications. Outcome following surgery is difficult to assess, however, parents and caregivers report satisfaction with the positional and functional improvements gained. The risks and benefits of all options must be extensively discussed with patients, their families and their caregivers before a decision is made. Surgically, posterior spinal fusion, which in the modern era is based on the pedicle screw construct, should be offered to children with large, progressive curves, which limit function and risk further morbidity. Thorough preoperative assessment should precede surgery to mitigate potential complications with pharmacological changes, respiratory support and anti-infective agents used where appropriate.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 2007;109:8-14. [PubMed] [Google Scholar]
- 2.Surman G, Bonellie S, Chalmers J, et al. UKCP: a collaborative network of cerebral palsy registers in the United Kingdom. J Public Health (Oxf) 2006;28:148-56. 10.1093/pubmed/fdi087 [DOI] [PubMed] [Google Scholar]
- 3.Balmer GA, MacEwen GD. The incidence and treatment of scoliosis in cerebral palsy. J Bone Joint Surg Br 1970;52:134-7. [PubMed] [Google Scholar]
- 4.Madigan RR, Wallace SL. Scoliosis in the institutionalized cerebral palsy population. Spine (Phila Pa 1976) 1981;6:583-90. 10.1097/00007632-198111000-00009 [DOI] [PubMed] [Google Scholar]
- 5.Persson-Bunke M, Hägglund G, Lauge-Pedersen H, et al. Scoliosis in a total population of children with cerebral palsy. Spine (Phila Pa 1976) 2012;37:E708-13. 10.1097/BRS.0b013e318246a962 [DOI] [PubMed] [Google Scholar]
- 6.Saito N, Ebara S, Ohotsuka K, et al. Natural history of scoliosis in spastic cerebral palsy. Lancet 1998;351:1687-92. 10.1016/S0140-6736(98)01302-6 [DOI] [PubMed] [Google Scholar]
- 7.Gu Y, Shelton JE, Ketchum JM, et al. Natural history of scoliosis in nonambulatory spastic tetraplegic cerebral palsy. PM R 2011;3:27-32. 10.1016/j.pmrj.2010.09.015 [DOI] [PubMed] [Google Scholar]
- 8.Thometz JG, Simon SR. Progression of scoliosis after skeletal maturity in institutionalized adults who have cerebral palsy. J Bone Joint Surg Am 1988;70:1290-6. 10.2106/00004623-198870090-00002 [DOI] [PubMed] [Google Scholar]
- 9.Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214-23. 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 10.Majd ME, Muldowny DS, Holt RT. Natural history of scoliosis in the institutionalized adult cerebral palsy population. Spine (Phila Pa 1976) 1997;22:1461-6. 10.1097/00007632-199707010-00007 [DOI] [PubMed] [Google Scholar]
- 11.Halawi MJ, Lark RK, Fitch RD. Neuromuscular Scoliosis: Current Concepts. Orthopedics 2015;38:e452-6. 10.3928/01477447-20150603-50 [DOI] [PubMed] [Google Scholar]
- 12.I Tsirikos A. Development and treatment of spinal deformity in patients with cerebral palsy. Indian J Orthop 2010;44:148-58. 10.4103/0019-5413.62052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loeters MJ, Maathuis CG, Hadders-Algra M. Risk factors for emergence and progression of scoliosis in children with severe cerebral palsy: a systematic review. Dev Med Child Neurol 2010;52:605-11. 10.1111/j.1469-8749.2010.03617.x [DOI] [PubMed] [Google Scholar]
- 14.Lonstein JE, Akbarnia A. Operative treatment of spinal deformities in patients with cerebral palsy or mental retardation. An analysis of one hundred and seven cases. J Bone Joint Surg Am 1983;65:43-55. 10.2106/00004623-198365010-00007 [DOI] [PubMed] [Google Scholar]
- 15.SRS Terminology Committee and Working Group on Spinal Classification Revised Glossary of Terms. Available online: https://www.srs.org/professionals/online-education-and-resources/glossary/revised-glossary-of-terms
- 16.Winter RB, Pinto WC. Pelvic obliquity. Its causes and its treatment. Spine (Phila Pa 1976) 1986;11:225-34. 10.1097/00007632-198604000-00008 [DOI] [PubMed] [Google Scholar]
- 17.Dubousset J. Pelvic obliquity: a review. Orthopedics 1991;14:479-81. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths GJ, Evans KT, Roberts GM, et al. The radiology of the hip joints and pelvis in cerebral palsy. Clin Radiol 1977;28:187-91. 10.1016/S0009-9260(77)80099-8 [DOI] [PubMed] [Google Scholar]
- 19.Kalen V, Conklin MM, Sherman FC. Untreated scoliosis in severe cerebral palsy. J Pediatr Orthop 1992;12:337-40. 10.1097/01241398-199205000-00010 [DOI] [PubMed] [Google Scholar]
- 20.Terjesen T, Lange JE, Steen H. Treatment of scoliosis with spinal bracing in quadriplegic cerebral palsy. Dev Med Child Neurol 2000;42:448-54. 10.1017/S0012162200000840 [DOI] [PubMed] [Google Scholar]
- 21.Miller A, Temple T, Miller F. Impact of orthoses on the rate of scoliosis progression in children with cerebral palsy. J Pediatr Orthop 1996;16:332-5. 10.1097/01241398-199605000-00007 [DOI] [PubMed] [Google Scholar]
- 22.Olafsson Y, Saraste H, Al-Dabbagh Z. Brace treatment in neuromuscular spine deformity. J Pediatr Orthop 1999;19:376-9. 10.1097/01241398-199905000-00017 [DOI] [PubMed] [Google Scholar]
- 23.Angsupaisal M, Maathuis CG, Hadders-Algra M. Adaptive seating systems in children with severe cerebral palsy across International Classification of Functioning, Disability and Health for Children and Youth version domains: a systematic review. Dev Med Child Neurol 2015;57:919-30. 10.1111/dmcn.12762 [DOI] [PubMed] [Google Scholar]
- 24.Holmes KJ, Michael SM, Thorpe SL, et al. Management of scoliosis with special seating for the non-ambulant spastic cerebral palsy population--a biomechanical study. Clin Biomech (Bristol, Avon) 2003;18:480-7. 10.1016/S0268-0033(03)00075-5 [DOI] [PubMed] [Google Scholar]
- 25.Hoare BJ, Wallen MA, Imms C, et al. Botulinum toxin A as an adjunct to treatment in the management of the upper limb in children with spastic cerebral palsy (UPDATE). Cochrane Database Syst Rev 2010;(1):CD003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molenaers G, Fagard K, Van Campenhout A, et al. Botulinum toxin A treatment of the lower extremities in children with cerebral palsy. J Child Orthop 2013;7:383-7. 10.1007/s11832-013-0511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuzzo RM, Walsh S, Boucherit T, et al. Counterparalysis for treatment of paralytic scoliosis with botulinum toxin type A. Am J Orthop (Belle Mead NJ) 1997;26:201-7. [PubMed] [Google Scholar]
- 28.Albright AL, Cervi A, Singletary J. Intrathecal baclofen for spasticity in cerebral palsy. JAMA 1991;265:1418-22. 10.1001/jama.1991.03460110084029 [DOI] [PubMed] [Google Scholar]
- 29.Gooch JL, Oberg WA, Grams B, et al. Care provider assessment of intrathecal baclofen in children. Dev Med Child Neurol 2004;46:548-52. 10.1111/j.1469-8749.2004.tb01013.x [DOI] [PubMed] [Google Scholar]
- 30.Segal LS, Wallach DM, Kanev PM. Potential complications of posterior spine fusion and instrumentation in patients with cerebral palsy treated with intrathecal baclofen infusion. Spine (Phila Pa 1976) 2005;30:E219-24. 10.1097/01.brs.0000158869.90908.f8 [DOI] [PubMed] [Google Scholar]
- 31.Sansone JM, Mann D, Noonan K, et al. Rapid progression of scoliosis following insertion of intrathecal baclofen pump. J Pediatr Orthop 2006;26:125-8. 10.1097/01.bpo.0000191555.11326.bd [DOI] [PubMed] [Google Scholar]
- 32.Shilt JS, Lai LP, Cabrera MN, et al. The impact of intrathecal baclofen on the natural history of scoliosis in cerebral palsy. J Pediatr Orthop 2008;28:684-7. 10.1097/BPO.0b013e318183d591 [DOI] [PubMed] [Google Scholar]
- 33.Senaran H, Shah SA, Presedo A, et al. The risk of progression of scoliosis in cerebral palsy patients after intrathecal baclofen therapy. Spine (Phila Pa 1976) 2007;32:2348-54. 10.1097/BRS.0b013e3181557252 [DOI] [PubMed] [Google Scholar]
- 34.Caird MS, Palanca AA, Garton H, et al. Outcomes of posterior spinal fusion and instrumentation in patients with continuous intrathecal baclofen infusion pumps. Spine (Phila Pa 1976) 2008;33:E94-9. 10.1097/BRS.0b013e3181642aae [DOI] [PubMed] [Google Scholar]
- 35.Borowski A, Shah SA, Littleton AG, et al. Baclofen pump implantation and spinal fusion in children: techniques and complications. Spine (Phila Pa 1976) 2008;33:1995-2000. 10.1097/BRS.0b013e31817bab42 [DOI] [PubMed] [Google Scholar]
- 36.Sampson FC, Hayward A, Evans G, et al. Functional benefits and cost/benefit analysis of continuous intrathecal baclofen infusion for the management of severe spasticity. J Neurosurg 2002;96:1052-7. 10.3171/jns.2002.96.6.1052 [DOI] [PubMed] [Google Scholar]
- 37.de Lissovoy G, Matza LS, Green H, et al. Cost-effectiveness of intrathecal baclofen therapy for the treatment of severe spasticity associated with cerebral palsy. J Child Neurol 2007;22:49-59. 10.1177/0883073807299976 [DOI] [PubMed] [Google Scholar]
- 38.Saulino M, Guillemette S, Leier J, et al. Medical cost impact of intrathecal baclofen therapy for severe spasticity. Neuromodulation 2015;18:141-9; discussion 149. 10.1111/ner.12220 [DOI] [PubMed] [Google Scholar]
- 39.Farhat G, Yamout B, Mikati MA, et al. Effect of antiepileptic drugs on bone density in ambulatory patients. Neurology 2002;58:1348-53. 10.1212/WNL.58.9.1348 [DOI] [PubMed] [Google Scholar]
- 40.Allarakhia IN, Garofalo EA, Komarynski MA, et al. Valproic acid and thrombocytopenia in children: a case-controlled retrospective study. Pediatr Neurol 1996;14:303-7. 10.1016/0887-8994(96)00052-5 [DOI] [PubMed] [Google Scholar]
- 41.Chambers HG, Weinstein CH, Mubarak SJ, et al. The effect of valproic acid on blood loss in patients with cerebral palsy. J Pediatr Orthop 1999;19:792-5. 10.1097/01241398-199911000-00018 [DOI] [PubMed] [Google Scholar]
- 42.Mayer PJ, Gehlsen JA. Coagulopathies associated with major spinal surgery. Clin Orthop Relat Res 1989;(245):83-8. [PubMed] [Google Scholar]
- 43.Anderson PR, Puno MR, Lovell SL, et al. Postoperative respiratory complications in non-idiopathic scoliosis. Acta Anaesthesiol Scand 1985;29:186-92. 10.1111/j.1399-6576.1985.tb02183.x [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald DA, Follett J, Van Asperen PP. Assessing and managing lung disease and sleep disordered breathing in children with cerebral palsy. Paediatr Respir Rev 2009;10:18-24. 10.1016/j.prrv.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 45.Khirani S, Bersanini C, Aubertin G, et al. Non-invasive positive pressure ventilation to facilitate the post-operative respiratory outcome of spine surgery in neuromuscular children. Eur Spine J 2014;23 Suppl 4:S406-11. 10.1007/s00586-014-3335-6 [DOI] [PubMed] [Google Scholar]
- 46.Almenrader N, Patel D. Spinal fusion surgery in children with non-idiopathic scoliosis: is there a need for routine postoperative ventilation? Br J Anaesth 2006;97:851-7. 10.1093/bja/ael273 [DOI] [PubMed] [Google Scholar]
- 47.Young HK, Lowe A, Fitzgerald DA, et al. Outcome of noninvasive ventilation in children with neuromuscular disease. Neurology 2007;68:198-201. 10.1212/01.wnl.0000251299.54608.13 [DOI] [PubMed] [Google Scholar]
- 48.Reyes AL, Cash AJ, Green SH, et al. Gastrooesophageal reflux in children with cerebral palsy. Child Care Health Dev 1993;19:109-18. 10.1111/j.1365-2214.1993.tb00718.x [DOI] [PubMed] [Google Scholar]
- 49.Orenstein SR, Orenstein DM. Gastroesophageal reflux and respiratory disease in children. J Pediatr 1988;112:847-58. 10.1016/S0022-3476(88)80204-X [DOI] [PubMed] [Google Scholar]
- 50.Capito C, Leclair MD, Piloquet H, et al. Long-term outcome of laparoscopic Nissen-Rossetti fundoplication for neurologically impaired and normal children. Surg Endosc 2008;22:875-80. 10.1007/s00464-007-9603-3 [DOI] [PubMed] [Google Scholar]
- 51.Mathei J, Coosemans W, Nafteux P, et al. Laparoscopic Nissen fundoplication in infants and children: analysis of 106 consecutive patients with special emphasis in neurologically impaired vs. neurologically normal patients. Surg Endosc 2008;22:1054-9. 10.1007/s00464-007-9578-0 [DOI] [PubMed] [Google Scholar]
- 52.Smith CD, Othersen HB, Jr, Gogan NJ, et al. Nissen fundoplication in children with profound neurologic disability. High risks and unmet goals. Ann Surg 1992;215:654-8; discussion 658-9. 10.1097/00000658-199206000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez DA, Ginn-Pease ME, Caniano DA. Sequelae of antireflux surgery in profoundly disabled children. J Pediatr Surg 1992;27:267-71; discussion 271-3. 10.1016/0022-3468(92)90324-Z [DOI] [PubMed] [Google Scholar]
- 54.Pearl RH, Robie DK, Ein SH, et al. Complications of gastroesophageal antireflux surgery in neurologically impaired versus neurologically normal children. J Pediatr Surg 1990;25:1169-73. 10.1016/0022-3468(90)90756-Y [DOI] [PubMed] [Google Scholar]
- 55.Sullivan PB, Lambert B, Rose M, et al. Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study. Dev Med Child Neurol 2000;42:674-80. 10.1017/S0012162200001249 [DOI] [PubMed] [Google Scholar]
- 56.Jevsevar DS, Karlin LI. The relationship between preoperative nutritional status and complications after an operation for scoliosis in patients who have cerebral palsy. J Bone Joint Surg Am 1993;75:880-4. 10.2106/00004623-199306000-00008 [DOI] [PubMed] [Google Scholar]
- 57.García-Contreras AA, Vásquez-Garibay EM, Romero-Velarde E, et al. Intensive nutritional support improves the nutritional status and body composition in severely malnourished children with cerebral palsy. Nutr Hosp 2014;29:838-43. [DOI] [PubMed] [Google Scholar]
- 58.Nutrition in neurologically impaired children. Paediatr Child Health 2009;14:395-401. [PMC free article] [PubMed] [Google Scholar]
- 59.HARRINGTON PR . Treatment of scoliosis. Correction and internal fixation by spine instrumentation. J Bone Joint Surg Am 1962;44-A:591-610. [PubMed] [Google Scholar]
- 60.Rinsky LA. Surgery of spinal deformity in cerebral palsy. Twelve years in the evolution of scoliosis management. Clin Orthop Relat Res 1990;(253):100-9. [PubMed] [Google Scholar]
- 61.Sullivan JA, Conner SB. Comparison of Harrington instrumentation and segmental spinal instrumentation in the management of neuromuscular spinal deformity. Spine (Phila Pa 1976) 1982;7:299-304. 10.1097/00007632-198205000-00016 [DOI] [PubMed] [Google Scholar]
- 62.Luque ER. Segmental spinal instrumentation for correction of scoliosis. Clin Orthop Relat Res 1982;(163):192-8. [PubMed] [Google Scholar]
- 63.Gersoff WK, Renshaw TS. The treatment of scoliosis in cerebral palsy by posterior spinal fusion with Luque-rod segmental instrumentation. J Bone Joint Surg Am 1988;70:41-4. 10.2106/00004623-198870010-00007 [DOI] [PubMed] [Google Scholar]
- 64.Bulman WA, Dormans JP, Ecker ML, et al. Posterior spinal fusion for scoliosis in patients with cerebral palsy: a comparison of Luque rod and Unit Rod instrumentation. J Pediatr Orthop 1996;16:314-23. 10.1097/01241398-199605000-00005 [DOI] [PubMed] [Google Scholar]
- 65.Gau YL, Lonstein JE, Winter RB, et al. Luque-Galveston procedure for correction and stabilization of neuromuscular scoliosis and pelvic obliquity: a review of 68 patients. J Spinal Disord 1991;4:399-410. 10.1097/00002517-199112000-00001 [DOI] [PubMed] [Google Scholar]
- 66.Sussman MD, Little D, Alley RM, et al. Posterior instrumentation and fusion of the thoracolumbar spine for treatment of neuromuscular scoliosis. J Pediatr Orthop 1996;16:304-13. 10.1097/01241398-199605000-00004 [DOI] [PubMed] [Google Scholar]
- 67.Benson ER, Thomson JD, Smith BG, et al. Results and morbidity in a consecutive series of patients undergoing spinal fusion for neuromuscular scoliosis. Spine (Phila Pa 1976) 1998;23:2308-17; discussion 2318. 10.1097/00007632-199811010-00012 [DOI] [PubMed] [Google Scholar]
- 68.Bell DF, Moseley CF, Koreska J. Unit rod segmental spinal instrumentation in the management of patients with progressive neuromuscular spinal deformity. Spine (Phila Pa 1976) 1989;14:1301-7. 10.1097/00007632-198912000-00006 [DOI] [PubMed] [Google Scholar]
- 69.Dias RC, Miller F, Dabney K, et al. Surgical correction of spinal deformity using a unit rod in children with cerebral palsy. J Pediatr Orthop 1996;16:734-40. 10.1097/01241398-199611000-00007 [DOI] [PubMed] [Google Scholar]
- 70.Tsirikos AI, Lipton G, Chang WN, et al. Surgical correction of scoliosis in pediatric patients with cerebral palsy using the unit rod instrumentation. Spine (Phila Pa 1976) 2008;33:1133-40. 10.1097/BRS.0b013e31816f63cf [DOI] [PubMed] [Google Scholar]
- 71.Piazzolla A, Solarino G, De Giorgi S, et al. Cotrel-Dubousset instrumentation in neuromuscular scoliosis. Eur Spine J 2011;20 Suppl 1:S75-84. 10.1007/s00586-011-1758-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teli MG, Cinnella P, Vincitorio F, et al. Spinal fusion with Cotrel-Dubousset instrumentation for neuropathic scoliosis in patients with cerebral palsy. Spine (Phila Pa 1976) 2006;31:E441-7. 10.1097/01.brs.0000221986.07992.fb [DOI] [PubMed] [Google Scholar]
- 73.Suk SI, Lee CK, Kim WJ, et al. Segmental pedicle screw fixation in the treatment of thoracic idiopathic scoliosis. Spine (Phila Pa 1976) 1995;20:1399-405. 10.1097/00007632-199506020-00012 [DOI] [PubMed] [Google Scholar]
- 74.Modi HN, Hong JY, Mehta SS, et al. Surgical correction and fusion using posterior-only pedicle screw construct for neuropathic scoliosis in patients with cerebral palsy: a three-year follow-up study. Spine (Phila Pa 1976) 2009;34:1167-75. 10.1097/BRS.0b013e31819c38b7 [DOI] [PubMed] [Google Scholar]
- 75.Tsirikos AI, Mains E. Surgical correction of spinal deformity in patients with cerebral palsy using pedicle screw instrumentation. J Spinal Disord Tech 2012;25:401-8. 10.1097/BSD.0b013e318227728c [DOI] [PubMed] [Google Scholar]
- 76.McElroy MJ, Sponseller PD, Dattilo JR, et al. Growing rods for the treatment of scoliosis in children with cerebral palsy: a critical assessment. Spine (Phila Pa 1976) 2012;37:E1504-10. 10.1097/BRS.0b013e31826fabd3 [DOI] [PubMed] [Google Scholar]
- 77.Vialle R, Delecourt C, Morin C. Surgical treatment of scoliosis with pelvic obliquity in cerebral palsy: the influence of intraoperative traction. Spine (Phila Pa 1976) 2006;31:1461-6. 10.1097/01.brs.0000219874.46680.87 [DOI] [PubMed] [Google Scholar]
- 78.Auerbach JD, Spiegel DA, Zgonis MH, et al. The correction of pelvic obliquity in patients with cerebral palsy and neuromuscular scoliosis: is there a benefit of anterior release prior to posterior spinal arthrodesis? Spine (Phila Pa 1976) 2009;34:E766-74. 10.1097/BRS.0b013e3181b4d558 [DOI] [PubMed] [Google Scholar]
- 79.Swank SM, Cohen DS, Brown JC. Spine fusion in cerebral palsy with L-rod segmental spinal instrumentation. A comparison of single and two-stage combined approach with Zielke instrumentation. Spine (Phila Pa 1976) 1989;14:750-9. 10.1097/00007632-198907000-00018 [DOI] [PubMed] [Google Scholar]
- 80.Dohin B, Dubousset JF. Prevention of the crankshaft phenomenon with anterior spinal epiphysiodesis in surgical treatment of severe scoliosis of the younger patient. Eur Spine J 1994;3:165-8. 10.1007/BF02190580 [DOI] [PubMed] [Google Scholar]
- 81.Suh SW, Modi HN, Yang J, et al. Posterior multilevel vertebral osteotomy for correction of severe and rigid neuromuscular scoliosis: a preliminary study. Spine (Phila Pa 1976) 2009;34:1315-20. 10.1097/BRS.0b013e3181a028bc [DOI] [PubMed] [Google Scholar]
- 82.Suk SI, Chung ER, Kim JH, et al. Posterior vertebral column resection for severe rigid scoliosis. Spine (Phila Pa 1976) 2005;30:1682-7. 10.1097/01.brs.0000170590.21071.c1 [DOI] [PubMed] [Google Scholar]
- 83.Comstock CP, Leach J, Wenger DR. Scoliosis in total-body-involvement cerebral palsy. Analysis of surgical treatment and patient and caregiver satisfaction. Spine (Phila Pa 1976) 1998;23:1412-24; discussion 1424-5. 10.1097/00007632-199806150-00022 [DOI] [PubMed] [Google Scholar]
- 84.Lonstein JE, Koop SE, Novachek TF, et al. Results and complications after spinal fusion for neuromuscular scoliosis in cerebral palsy and static encephalopathy using luque galveston instrumentation: experience in 93 patients. Spine (Phila Pa 1976) 2012;37:583-91. 10.1097/BRS.0b013e318225ebd5 [DOI] [PubMed] [Google Scholar]
- 85.Master DL, Son-Hing JP, Poe-Kochert C, et al. Risk factors for major complications after surgery for neuromuscular scoliosis. Spine (Phila Pa 1976) 2011;36:564-71. 10.1097/BRS.0b013e3181e193e9 [DOI] [PubMed] [Google Scholar]
- 86.Mohamad F, Parent S, Pawelek J, et al. Perioperative complications after surgical correction in neuromuscular scoliosis. J Pediatr Orthop 2007;27:392-7. 10.1097/01.bpb.0000271321.10869.98 [DOI] [PubMed] [Google Scholar]
- 87.Lipton GE, Miller F, Dabney KW, et al. Factors predicting postoperative complications following spinal fusions in children with cerebral palsy. J Spinal Disord 1999;12:197-205. [PubMed] [Google Scholar]
- 88.Sharma S, Wu C, Andersen T, et al. Prevalence of complications in neuromuscular scoliosis surgery: a literature meta-analysis from the past 15 years. Eur Spine J 2013;22:1230-49. 10.1007/s00586-012-2542-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Molinari RW, Khera OA, Molinari WJ, 3rd. Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J 2012;21 Suppl 4:S476-82. 10.1007/s00586-011-2104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tubaki VR, Rajasekaran S, Shetty AP. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine (Phila Pa 1976) 2013;38:2149-55. 10.1097/BRS.0000000000000015 [DOI] [PubMed] [Google Scholar]
- 91.Vitale MG, Riedel MD, Glotzbecker MP, et al. Building consensus: development of a Best Practice Guideline (BPG) for surgical site infection (SSI) prevention in high-risk pediatric spine surgery. J Pediatr Orthop 2013;33:471-8. 10.1097/BPO.0b013e3182840de2 [DOI] [PubMed] [Google Scholar]
- 92.Tsirikos AI, Chang WN, Dabney KW, et al. Comparison of parents' and caregivers' satisfaction after spinal fusion in children with cerebral palsy. J Pediatr Orthop 2004;24:54-8. 10.1097/01241398-200401000-00010 [DOI] [PubMed] [Google Scholar]
- 93.Watanabe K, Lenke LG, Daubs MD, et al. Is spine deformity surgery in patients with spastic cerebral palsy truly beneficial?: a patient/parent evaluation. Spine (Phila Pa 1976) 2009;34:2222-32. 10.1097/BRS.0b013e3181948c8f [DOI] [PubMed] [Google Scholar]
- 94.Jones KB, Sponseller PD, Shindle MK, et al. Longitudinal parental perceptions of spinal fusion for neuromuscular spine deformity in patients with totally involved cerebral palsy. J Pediatr Orthop 2003;23:143-9. 10.1097/01241398-200303000-00002 [DOI] [PubMed] [Google Scholar]
- 95.Askin GN, Hallett R, Hare N, et al. The outcome of scoliosis surgery in the severely physically handicapped child. An objective and subjective assessment. Spine (Phila Pa 1976) 1997;22:44-50. 10.1097/00007632-199701010-00008 [DOI] [PubMed] [Google Scholar]
- 96.Mercado E, Alman B, Wright JG. Does spinal fusion influence quality of life in neuromuscular scoliosis? Spine (Phila Pa 1976) 2007;32:S120-5. 10.1097/BRS.0b013e318134eabe [DOI] [PubMed] [Google Scholar]


