Abstract
The study aims to describe a three-dimensional printed (3DP) posterior fixation implant used for C1/C2 fusion in a 65-year-old female. Spinal fusion remains a common intervention for a range of spinal pathologies including degenerative disc and facet disease when conservative methods are unsuccessful. However, fusion devices are not always entirely efficacious in providing the desired fixation, and surgeons rely on ‘off the shelf’ implants which may not provide an anatomical fit to address the particular pathology. 3DP refers to a process where three-dimensional objects are created through successive layering of material, so called ‘additive manufacturing’. Although this technology enables accurate fabrication of patient-specific orthopaedic and spinal implants, literature on its utilization in this regard is rare. A 65-year-old female, with severe facet arthropathy at the C1/C2 level, osteophyte formation and impingement of the exiting C2 nerve root underwent a C1/C2 posterior fusion and rhizolysis of the C2 nerve roots. A custom posterior fixation implant was designed and on-laid over the C2 spinous process and lamina, with screw holes made to a depth and angulation that was pre-calculated based on the preoperative CT based 3D modelling. The patient had an uneventful recovery and reported a significant reduction in occipital neuralgia and sub-occipital pain and 2-month follow-up. We report the first case of a customized 3DP spinal prosthesis for posterior C1/C2 fusion. The implant added significant value reducing the overall time of the procedure, and safety with a reduced risk of neurovascular compromise.
Keywords: 3D printed spine implant, degenerative cervical spine disease, spine surgery, patient specific implant, arthrodesis
Introduction
Three-dimensional printing (3DP) refers to a process wherein a 3D object is created from a digital design through successive layering of material under computer control, so called ‘additive manufacturing’. Having gained much interest in recent years, 3DP has found applications in many surgical and medical fields, with variations in printing solutions permitting various options in the architecture of the final structure across a wide range orthopaedic and facial or plastics procedures (1,2). The application of 3DP in orthopaedics is only recent, with positive early results demonstrating the potential to alter future orthopaedic practice (3,4). Custom 3DP of prosthesis for spinal surgery has a potential significant impact due to customisation of complex shapes and materials to manage complex pathologies (5-7).
Currently, little information is available in the literature regarding the use of 3DP implants specific to spine surgery, with evidence limited to low quality studies (8). Moreover, there are no reports of customised models to assist with C1/C2 posterior fixation. Herein, we report on a unique design (Dr. P D’Urso) incorporating a 3D printed prosthesis that spans the posterior elements of C1 and C2, and provides pre-angled screw holes to assist with placement of trans-articular C1/C2 screws. This report adds to the rapidly expanding field of additive manufacturing with spinal prostheses.
Case presentation
A 65-year-old female presented with severe sub occipital pain and progressive loss of rotation of the cervical spine. Computerised tomography (CT) and bone scan demonstrated facet arthropathy at the level of C1/C2 with osteophyte formation and impingement of the exiting C2 nerve root. Preoperatively, her occipital neuralgia pain was 8/10 on the VAS and post operatively 3/10. Following a discussion on options, the patient consented to proceed with a C1/C2 posterior fusion and rhizolysis of the C2 nerve roots. A custom posterior fixation implant was designed based on the unique posterior anatomy of the patient through the integration of her high-resolution CT with a computer aided design (CAD) model (anatomics/Australia). Two separate biomodels were also 3D printed. One had been ‘realigned’ to simulate surgery and incorporated the intended screw holes and their pre-determined trajectories for fixation to avoid inadvertent vertebral artery injury. A second ‘reference’ biomodel was also 3D printed. A drill guide was also manufactured to contour match to the C2 lamina and lateral mass to guide a drills trajectories through the pars interarticularis and across the C12 lateral articulations. The corrected biomodel was translated to a 3D titanium printer (CSIRO, Australia), as defined by previous reviews (1,8). The final implant was printed as a porous titanium (Ti) patient specific prosthesis to assist with osseointegration around the device to encourage C1 and C2 arthrodesis (9).
Following surgical exposure of the posterior elements at C1/C2 using a standardised posterior approach technique and subperiosteal muscle dissection, the custom 3DP drill guide was used to place 44 mm trans articular screws through the fixation device which was on-laid over the C2 spinous process and lamina (see Figure 1) (10). The implant assisted with the accurate placement of trans-articular screws at C1/C2, making this aspect of the procedure rapid and low-risk. Lateral image intensification was necessary to verify the trajectories and ensure ‘real time safety’. The screw length and trajectory was predetermined for the patients’ specific anatomy. Furthermore, the implant had additional C1 arch screw holes pre-planned, with the placement of ×2 arch screws (Figures 2,3). Additional autograft was used between the posterior elements of C1 and C2 to contribute to fusion. There were no adverse consequences from a surgical perspective. At 7 months follow-up the patient reported significant reduction in occipital neuralgia and sub-occipital pain. Post operative X-ray imaging 1 month, 3 months and a CT scan at 6 months were all satisfactory.
Figure 1.
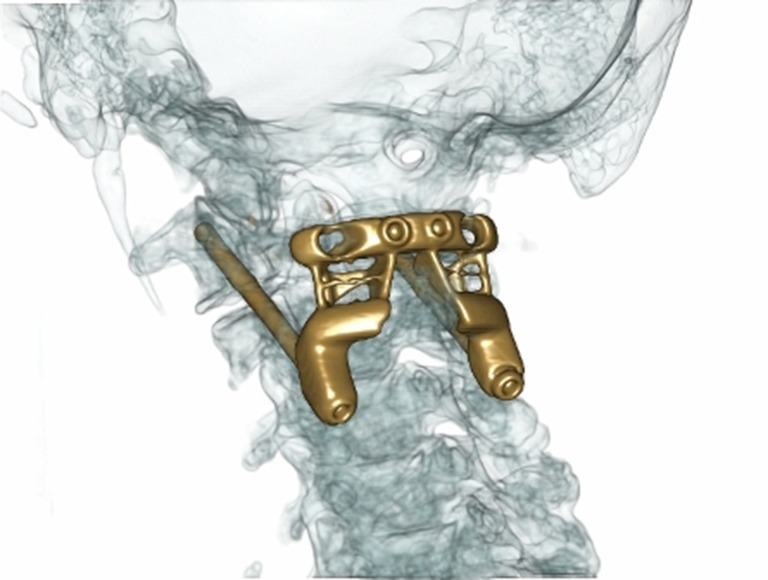
Computer modelled augmented sagittal view of high-resolution contrast CT with proposed titanium implant overlaid. CT, computerised tomography.
Figure 2.
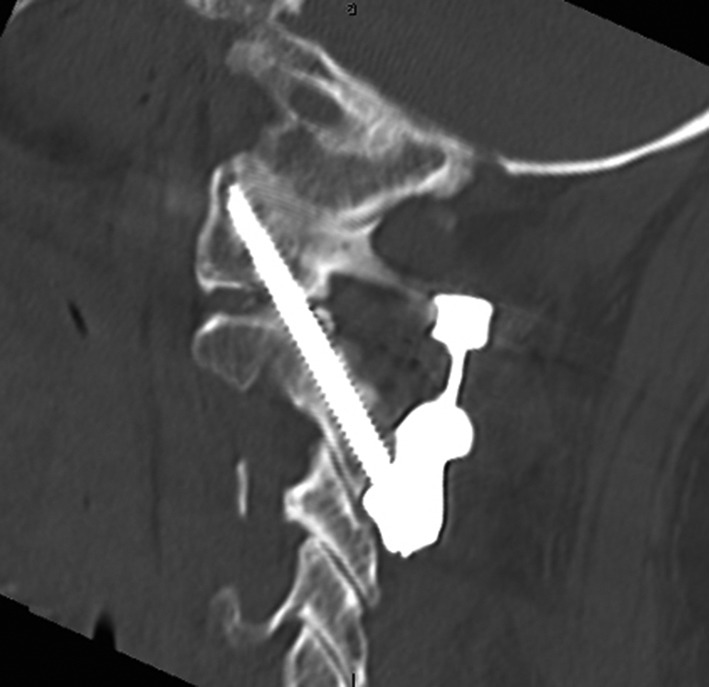
Post-operative cervical spine CT scan demonstrating accurate implant positioning. CT, computerised tomography.
Figure 3.
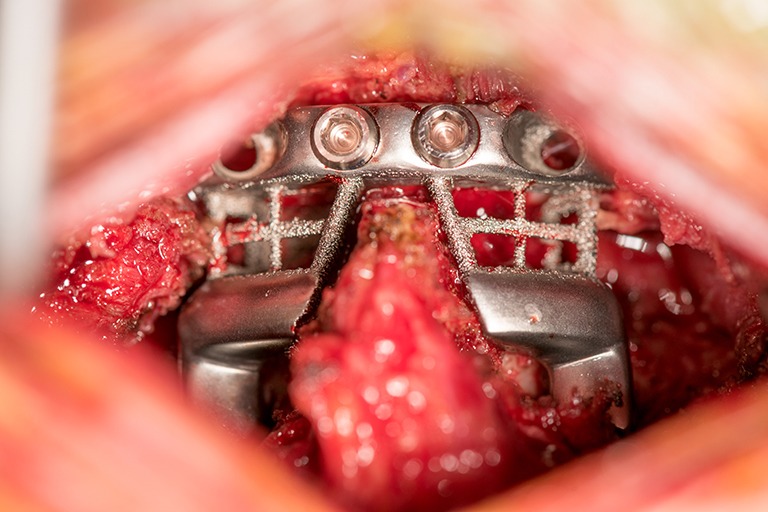
Intra-operative window demonstrating 3DP device implantation. 3DP, three-dimensional printed.
Discussion
3D printing has gained much interest in recent years and it has found applications in many surgical and medical fields. Medical applications may include surgical planning, printing of models to assist with intraoperative guidance and anatomy definition, prosthesis development, patient specific implant production, medical education, and numerous other applications (11). Due to its ability to produce custom-made items, 3DP may bring great benefits to patients as well as surgeons. The use of 3DP allows the production of implants that can be customised according to the anatomy and requirements of the patient. At the same time, they can potentially reduce the operating time, as they significantly improve surgical planning and graft from another site of the patient’s body may not be required (12).
Currently, 3DP is widely available in surgical planning for a variety of orthopaedic, neurosurgical and other procedures, being extremely useful in complex trauma and tumour cases and patients with congenital deformity (4). A 3D model can help the surgeon visualise fracture patterns in far more detail than sequential CT slices, anticipate intra-operative difficulties, and select the optimal surgical approach and specific equipment required for the procedure (13). Custom-made 3DP implants have been used to repair a range of bone structures and in the setting of joint arthroplasty when the patient requires specific implant characteristics (3). Other applications of 3DP in orthopaedics include the use of customized external fixators to assist with fracture reduction (14) and 3D printed casts. 3DP has also been described in musculoskeletal tumour excision with Chinese surgeons recently replacing a segment of cancerous cervical vertebrae in a 12-year-old patient with a C2 Ewing sarcoma with a 3DP titanium customised vertebral body (15). Similar to the presented case, this case study utilised a titanium alloy powder as the main component within the final implanted device. The use of porous titanium in screws has previously been demonstrated to support and facilitate osteo-integration and eventual arthrodesis through optimising the bone-metal interface (16), commonly utilised in a range of FDA approved total disc replacement devices, fusion cages, and pedicle screws (17). In our case, additional autograft was utilised due to its availability to aid with arthrodesis across the C1/C2 segment, although it is hypothesised that with an increase in the quality of implant design and improvements in biomaterials, the requirement of further grafting may soon be unnecessary (15). The current authors believe that the use of autograft and bone graft substitutes may eventually be unnecessary with use for arthrodesis and implant integration, as developments in biomaterials and 3DP continues to advance.
Initial translation of 3DP knowledge into the field of spinal surgery has been demonstrating promising results. Early studies by Berry et al. [2005], demonstrated the use of 3DP guides derived from cadaveric CT data to be efficacious in aiding pedicle screw insertion accuracy, with 20/20 screws successfully inserted without any error across all spinal regions. Across clinical studies by Chen et al. [2015], the implementation of 3DP guide plate for lumbar fusion (n=43) demonstrated a marginal improvement in peri-operative outcomes and overall screw placement in comparison to those not using the guides (18). Including surgical guides, current applications of 3DP in spine care comprises mainly four areas of interest: surgical preplanning and training (models of the vertebral segments), surgical instrumentation (screw guide templates), implantable devices (interbody fusion cages, vertebral body replacement cages, disc implants for total disc replacement) and tissue engineering (scaffolds for cartilage regeneration) (19). These latter domains remain an area of interest with numerous studies into different synthesis techniques for implants, and differences in both 3DP material composition and architectural characteristics ongoing. Recent developments into 3DP scaffolds as a total disc replacement substitute are limited to lab-based testing or animal studies (20,21).
In the setting of cervical spine pathology, spinal fusion and total disc replacement remain the two most common surgical options when conservative therapies have been unsuccessful. However, spinal fusion cages and disc implants are not always entirely successful in providing the desired level of fixation (22), and standard ‘off the shelf’ implants may not be suitable in some cases due to patients’ particular characteristics. Moreover, in the case of spinal reconstruction, the artificial implant should ideally be manufactured at a specific size depending on the defect shape. On this basis, the steady introduction of custom-made individualised patient specific implants is currently a priority in spinal surgery and 3DP seems a valuable solution.
Despite its acknowledged potential advantages, the use of 3D implants in surgery has several current limitations. The introduction in orthopaedics, and more specifically in spinal surgery, is only recent and hence there is a lack of long-term follow-up in comparison to traditional techniques. As recently noted by Provaggi et al. [2016], the pool of data specific to the spine continues to rapidly increase, though the quality of data is limited to case reports or cadaveric study (19). Additionally, there is no regulatory framework established that guides surgeons or companies as to how best have these implants registered or approved for production and implantation. There is an urgent need for this regulatory void to be addressed.
Currently, 3DP for spine surgery presents a number of challenges: high cost due to its customised ‘single use’ profile, regulatory aspects requiring an appropriate framework for health authorities, hospitals and insurers, the potential for low quality implants, although the cost is expected to decrease over the coming years, while the quality of products should improve with advances in 3D printing devices and biomaterials. Standardisation within the manufacturing process and the characteristics of similar devices established using different methods remains a question of ongoing interest. Interestingly, despite the high cost our research indicates, 3DP surgical implants are being investigated across numerous regions across the globe including Australia, Canada, China, India, the United Kingdom, and the United States.
Finally, cost is not the only barrier to the expanded implementation of the technology in hospitals, as 3D software and implant modelling requires specific skills that most surgeons do not have (8). Developments in ease-of-use software will likely address the issue of rapid implant design, that in the future will likely take place in the surgeons’ office at the time of consultation.
Conclusions
Herein, we reported the first case of a customized 3DP spinal prosthesis for posterior C1/C2 fusion. The patient specific implant added significant value reducing the overall time of the procedure, and safety with a reduced risk of neurovascular compromise. The patient demonstrated a rapid recovery, advocating for the future implementation of similar individualised 3DP devices in the setting of cervical spine disease should resources permit.
Acknowledgements
None.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: Dr. Paul D’Urso is the founder and executive chairman of Anatomics Pty Ltd.
References
- 1.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng 2015;9:4. 10.1186/s13036-015-0001-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chae MP, Rozen WM, McMenamin PG, et al. Emerging Applications of Bedside 3D Printing in Plastic Surgery. Front Surg 2015;2:25. 10.3389/fsurg.2015.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eltorai AE, Nguyen E, Daniels AH. Three-Dimensional Printing in Orthopedic Surgery. Orthopedics 2015;38:684-7. 10.3928/01477447-20151016-05 [DOI] [PubMed] [Google Scholar]
- 4.Mobbs RJ, Coughlan M, Thompson C, et al. The utility of 3D printing for surgical planning and patient-specific implant design for complex spinal pathologies: case report. JNS Spine 2016. doi: . 10.3171/2016.9.SPINE16371 [DOI] [PubMed] [Google Scholar]
- 5.D'Urso PS, Askin G, Earwaker JS, et al. Spinal biomodeling. Spine (Phila Pa 1976) 1999;24:1247-51. 10.1097/00007632-199906150-00013 [DOI] [PubMed] [Google Scholar]
- 6.D'Urso PS, Williamson OD, Thompson RG. Biomodeling as an aid to spinal instrumentation. Spine (Phila Pa 1976) 2005;30:2841-5. 10.1097/01.brs.0000190886.56895.3d [DOI] [PubMed] [Google Scholar]
- 7.Izatt MT, Thorpe PL, Thompson RG, et al. The use of physical biomodelling in complex spinal surgery. Eur Spine J 2007;16:1507-18. 10.1007/s00586-006-0289-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martelli N, Serrano C, van den Brink H, et al. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016;159:1485-500. 10.1016/j.surg.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 9.Rao PJ, Pelletier MH, Walsh WR, et al. Spine Interbody Implants: Material Selection and Modification, Functionalization and Bioactivation of Surfaces to Improve Osseointegration. Orthop Surg 2014;6:81-9. 10.1111/os.12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérez-Mañanes R, Burró JA, Manaute JR, et al. 3D Surgical Printing Cutting Guides for Open-Wedge High Tibial Osteotomy: Do It Yourself. J Knee Surg 2016;29:690-5. 10.1055/s-0036-1572412 [DOI] [PubMed] [Google Scholar]
- 11.Tack P, Victor J, Gemmel P, et al. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online 2016;15:115. 10.1186/s12938-016-0236-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AlAli AB, Griffin MF, Butler PE. Three-Dimensional Printing Surgical Applications. Eplasty 2015;15:e37. [PMC free article] [PubMed] [Google Scholar]
- 13.Mulford JS, Babazadeh S, Mackay N. Three-dimensional printing in orthopaedic surgery: review of current and future applications. ANZ J Surg 2016;86:648-53. 10.1111/ans.13533 [DOI] [PubMed] [Google Scholar]
- 14.Qiao F, Li D, Jin Z, et al. Application of 3D printed customized external fixator in fracture reduction. Injury 2015;46:1150-5. 10.1016/j.injury.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 15.Xu N, Wei F, Liu X, et al. Reconstruction of the Upper Cervical Spine Using a Personalized 3D-Printed Vertebral Body in an Adolescent With Ewing Sarcoma. Spine (Phila Pa 1976) 2016;41:E50-4. 10.1097/BRS.0000000000001179 [DOI] [PubMed] [Google Scholar]
- 16.Palmquist A, Snis A, Emanuelsson L, et al. Long-term biocompatibility and osseointegration of electron beam melted, free-form-fabricated solid and porous titanium alloy: experimental studies in sheep. J Biomater Appl 2013;27:1003-16. 10.1177/0885328211431857 [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Cai H, Lv J, et al. Biomechanical and histological evaluation of roughened surface titanium screws fabricated by electron beam melting. PLoS One 2014;9:e96179. 10.1371/journal.pone.0096179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Wu D, Yang H, et al. Clinical Use of 3D Printing Guide Plate in Posterior Lumbar Pedicle Screw Fixation. Med Sci Monit 2015;21:3948-54. 10.12659/MSM.895597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provaggi E, Leong JJ, Kalaskar DM. Applications of 3D printing in the management of severe spinal conditions. Proc Inst Mech Eng H 2016. [Epub ahead of print]. 10.1177/0954411916667761 [DOI] [PubMed] [Google Scholar]
- 20.Rosenzweig DH, Carelli E, Steffen T, et al. 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. Int J Mol Sci 2015;16:15118-35. 10.3390/ijms160715118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Uden S, Silva-Correia J, Correlo VM, et al. Custom-tailored tissue engineered polycaprolactone scaffolds for total disc replacement. Biofabrication 2015;7:015008. 10.1088/1758-5090/7/1/015008 [DOI] [PubMed] [Google Scholar]
- 22.Spetzger U, Frasca M, König SA. Surgical planning, manufacturing and implantation of an individualized cervical fusion titanium cage using patient-specific data. Eur Spine J 2016;25:2239-46. 10.1007/s00586-016-4473-9 [DOI] [PubMed] [Google Scholar]


