Abstract
In recent years, significant advances have been made in cancer immunotherapy. Here, we present the first report of a patient with lymphoepithelioma-like carcinoma (LELC) of the lung, an Epstein-Barr virus (EBV)-associated lung cancer, who was treated with nivolumab, a fully human IgG4 anti-PD-1 monoclonal antibody. We also carry out a focused review to identify and examine studies of LELC of the lung in the literature. This case report highlights the need to further assess the role of immune checkpoint inhibitors in LELC of the lung.
Keywords: Lymphoepithelioma-like carcinoma of the lung (LELC of the lung), immunotherapy, nivolumab
Case presentation
Ms. Y is a 37-year-old Chinese female with a history of lymphoepithelioma-like carcinoma (LELC) of the lung and hepatitis B virus infection who has never smoked. She was initially diagnosed in 2012 with an 8.6 cm mass in the left upper lobe and underwent two cycles of neoadjuvant chemotherapy with cisplatin and docetaxel followed by left upper lobectomy and mediastinal lymph node dissection. The pathologic stage of disease was stage IIIA (T3N2M0). After being lost to follow-up for 2 years, she presented to the Georgetown Lombardi Comprehensive Cancer Center in 2014 with a three-month history of cough, shortness of breath, and upper back pain. A positron emission tomography-computed tomography (PET-CT) scan showed multiple, bilateral [18F]-fluoro-2-deoxy-D-glucose (FDG)-avid pulmonary nodules, extensive hilar and mediastinal lymphadenopathy, and multiple skeletal metastases. A magnetic resonance imaging (MRI) of the spine confirmed the presence of osseous metastatic disease throughout the vertebral column. A brain MRI showed no evidence of intra-cranial metastasis.
Pathologic evaluation of the resected tumor confirmed the diagnosis of LELC of the lung (Figure 1). Molecular analyses of the original tumor showed no EGFR, ALK or KRAS genetic alterations. Initial treatment consisted of decompression spinal corpectomy (C7–T3) and palliative radiation therapy (10 fractions) to L2–L5 metastatic lesions followed by systemic chemotherapy with 6 cycles of carboplatin and pemetrexed. The tumor was refractory to chemotherapy with disease progression in the bones and lungs and development of hepatic metastases (Figure 2A). The next line of treatment consisted of nivolumab 3 mg/kg administered intravenously every 2 weeks. Ten days after the first dose of nivolumab, the patient developed tachypnea and cough that were concerning for treatment-induced pneumonitis. CT chest showed an increase in pulmonary and hepatic metastatic disease and a large right pleural effusion (Figure 2B). Due to the possibility of pseudoprogression and lack of other effective therapy, the patient underwent a therapeutic thoracentesis and continued treatment with nivolumab. Within the next 2 weeks the patient developed abdominal discomfort, diarrhea, and leukocytosis, suggestive of immune-related colitis. She was admitted to the hospital for further management. Imaging studies showed new splenic and adrenal metastases, a pericardial effusion, and deep venous thrombosis in the left subclavian vein. Due to rapidly progressive lung cancer, comfort care measures were initiated and the patient passed away, 3 years after her initial diagnosis and 1 year after recurrence.
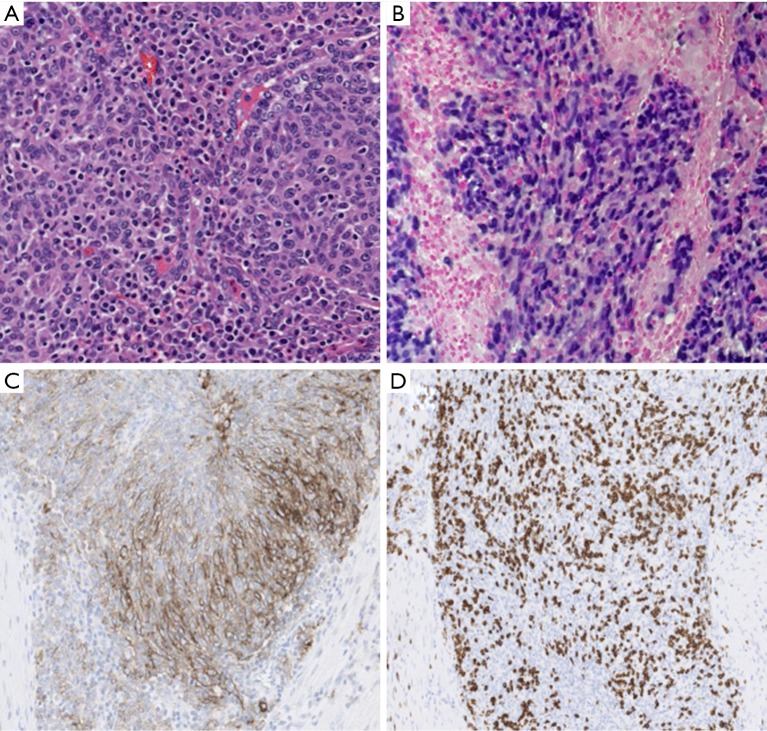
Figure 1.
Microscopic findings of the resected tumor. Undifferentiated carcinoma with poorly defined nests of neoplastic cells within a stroma exhibiting prominent lymphoplasmacytic infiltrate (A, hematoxylin and eosin, 20×). EBV-encoded RNA (EBER) in situ hybridization shows intense signal in most of the neoplastic cells (B, 20×). Moderate to strong PD-L1 expression by the majority of neoplastic cells (C, 20×). Intratumoral CD8+ positive lymphoid cells are also present (D, 20×).
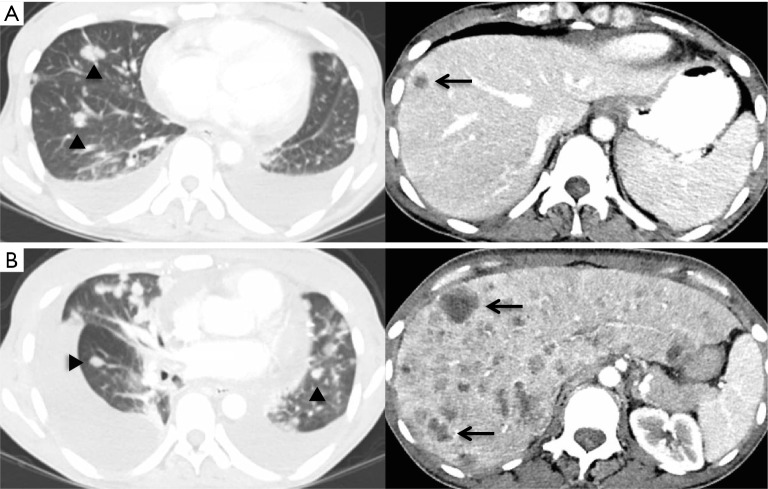
Figure 2.
Computed tomography (CT) findings. (A) A restaging CT scan obtained after 6 cycles of systemic chemotherapy shows new and enlarging pulmonary metastases (arrowheads) and hepatic metastases (arrows); (B) a restaging scan obtained after one dose of nivolumab shows increased pulmonary (arrowheads) and hepatic metastatic lesions (arrows).
Review of literature and results
We performed a search of the literature with PubMed using the search term (lymphoepithelioma[tiab]) and (pulmonary[tiab] or lung[tiab]) on February 12, 2016. A total of 139 articles were retrieved and reviewed. Case series reported in English with more than 10 cases were read in full and summarized. A manual search of references cited in case series and systematic reviews was performed.
Between 1987 and 2016, 12 case series were identified that described a total of 501 cases of LELC of the lung (Table 1). The mean age at diagnosis was 53.6 years and 46.7% individuals were males. Among 11 studies that reported the percentages of ever smokers, 24.5% of patients were smokers. Two hundred and fifty eight (51.5%) patients were diagnosed with early stage disease (stage I or II). The majority of patients received multimodality therapy consisting of surgery in combination with chemotherapy, radiation, or both. In advanced cases, palliative chemotherapy and/or radiation were used. The most commonly used chemotherapy regimens included cisplatin or carboplatin in combination with 5-fluorouracil, paclitaxel, docetaxel, or gemcitabine (14-17). Although there was heterogeneity in reporting prognosis, patients with early stage disease had a longer overall survival (OS).
Table 1. Case series of lymphoepithelioma-like carcinoma of the lung published between 1987 and 2016.
| Author [publication year] | No. cases | Male (%) | Mean age [range] | Asian [%] | No. smokers* (%) | No. tumor stage I/II/III/IV | Treatment [No.] | Prognosis |
|---|---|---|---|---|---|---|---|---|
| Chan et al. [1995] (1) | 11 | 5 (54.5) | 55.8 [38–78] | 11 [100] | 2 (18.2) | 7/1/2/1 | Surgery [8] Surgery/palliative RT [1] |
Stage I: 5 alive at 5–24 months Stage II: 1 alive at 6 months Stage III: 1 died at 4 months (not treated), 1 alive at 18 months |
| Han et al. [2001] (2,3) | 32 | 22 (68.8) | 54.4 [39–72] | 32 [100] | NA | 12/8/11/1 | Surgery [14] Surgery/RT [14] Surgery/CTX [1] Surgery/CTX/RT [3] |
2- and 5-year OS rates (%) Stage I: 75.0 and 53.5 Stage II: 100 and 62.5 Stage III and IV: 80.8 and 60.6 |
| Chang et al. [2002] (4) | 23 | 7 (30.4) | 57 [42–80] | 23 [100] | 6 (26.1) | 8/3/8/4 | Surgery [17] CTX alone [3] CTX then surgery [1] CTX/RT [2] |
Stage I: 6 alive at 11–74 months, 1 died of asthma, 1 lost to follow-up Stage II: 2 alive at 26–66 months, 1 died of disease at 17 months Stage III: 7 alive at 4–55 months, 1 died of disease at 18 months Stage IV: 3 alive at 4–27 months, 1 lost to follow-up |
| Ngan et al. [2004] (5) | 19 | 9 (47.3) | 52.7 [NA] | 19 [100] | 8 (42.1) | 4/2/8/5 | Surgery [4] CTX alone [6] CTX/RT [3] RT [2] |
Median OS: 24 months |
| Liang et al. [2012] (6) | 52 | 29 (55.8) | 51 [9–74] | 52 [100] | 13 (25.0) | 16/9/24/3 | Surgery [18] Surgery/CTX ± RT [25] CTX [8] CTX/RT [1] |
2- and 5-year OS rates (%) in all patients: 88 and 62 Median survival of 12 patients with advanced disease received treatment containing CTX: 39.1 months |
| Huang et al. [2012] (7) | 21 | 5 (23.8) | 55.6 [37–75] | 21 [100] | 6 (28.6) | 2/2/13/4 | Surgery [2] Surgery/CTX [6] Surgery/CTX/RT [5] CTX/RT [6] CTX [2] |
Median OS Stage I and II: not reached Stage III and IV: 3.4 years |
| Liu et al. [2014] (8) | 32 | 11 (34.4) | 50.9 [25–71] | 32 [100] | 7 (21.9) | 9/6/12/5 | Surgery [9] Surgery/CTX [18] CTX/RT [1] CTX [4] |
NA |
| Mo et al. [2014] (9)** | 35 | 20 (57.1) | 54.7 [35–74] | 37 [100] | 7 (20.0) | 11/9/13/2 | Surgery [22] Surgery/CTX [10] CTX/RT [1] CTX [1] |
Median OS for all patients: not reached 2- and 5-year OS rates (%) in all patients: 81 and 51 |
| Sun et al. [2014] (10) | 18 | 11 (61.1) | 57.3 [NA] | 18 [100] | 4 (22.2) | 8/4/6/0 | NA | Median OS survival for all patients: not reached |
| Chang et al. [2015] (11) | 66 | 25 (37.9) | 57.5 [39–85] | 66 [100] | 8 (12.1) | 26/14/26 (stage III/IV) | Surgery [33] Surgery/CTX [15] Surgery/RT [1] Surgery/CTX/RT [7] CTX/RT [5] |
5-year OS rate (%) Stage I: 100 Stage II: 75 Stage III and IV: 69.2 |
| Jiang et al. [2015] (12)** | 79 | 39 (49.4) | 52 [11–74] | 79 [100] | 22 (27.8) | 44 (stage I/II)/35 (stage III/IV) | Surgery [38] Surgery/CTX or RT [35] CTX [6] |
3- and 5-year OS rate (%): 88 and 79 |
| Fang et al. [2015] (13)** | 113 | 51 (45.1) | 52 [28–74] | 113 [100] | 32 (28.3) | 29/24/45 (stage IIIA)/15 (stage IIIB/IV) | Surgery [36] Surgery/CTX [77] |
5-year OS rate (%) Stage I: 87.5 Stage II: 92.9 Stage IIIA: 64.2 Stage IIIB/IV: 36.6 |
*, Including active and past smokers; **, study populations are from the same hospital in China. CTX, chemotherapy; NA, not available; OS, overall survival; RT, radiation therapy.
Discussion
LELC, an Epstein-Barr virus (EBV)-driven cancer, is more prevalent in the nasopharynx, but can originate from other organs such as the lung and gastrointestinal tract (14). LELC of the lung is a rare subtype of lung cancer that was first described in 1987 (18). Since then, several case series, most of which are from Asia, have been published (14); cases series in the western population are rarer and smaller. Based on our literature review, LELC of the lung occurs more often in younger patients with no history of smoking, and is associated with a better prognosis than adenocarcinoma, squamous cell carcinoma, or large cell carcinoma of the lung (10,19). Due to the rarity of LELC of the lung, there is a lack of prospective studies comparing various treatment strategies and therefore treatment guidelines are similar to those used for other forms lung cancer.
Immune checkpoint inhibitors, targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) are a recent addition to the treatment arsenal against different types of advanced cancers, including lung cancer (20). Two anti-PD-1 antibodies, nivolumab and pembrolizumab, have shown survival benefits over standard second-line chemotherapy for advanced non-small cell lung cancer (NSCLC) (21,22), and are approved for treatment of unresectable NSCLC after progression on platinum-containing chemotherapy, or appropriate molecular-targeted therapy in patients with EGFR-sensitizing mutations or ALK translocations. The role of immune checkpoint blockade in LELC of the lung remains unknown, but activity of immune checkpoint inhibitors observed in other virus-associated cancers warrants further evaluation of this new class of cancer therapeutics in patients with LELC of the lung. For example, the objective response rate (ORR) in patients with virus-associated hepatocellular carcinoma (HCC) treated with nivolumab was 32%, which was higher than the ORR (15%) in uninfected patients with HCC (23). Similarly, the use of pembrolizumab for the treatment of recurrent or metastatic squamous cell carcinoma led to a higher ORR in patients with human papillomavirus (HPV)-positive tumor (25%), compared to those with HPV-negative tumor (14%) (24). In patients with Merkel-cell carcinoma, a skin cancer linked to the Merkel-cell polyomavirus, the ORR was 56% after treatment with pembrolizumab (25). In these studies, patients who responded to anti-PD-1 blockade experienced durable responses.
To our knowledge, this is the first report of the use of nivolumab in a patient with LELC of the lung. Several studies have shown that tumors expressing PD-1 ligand (PD-L1) are more likely to respond to PD-1/PD-L1 blockade (22,26-28). In patients with previously treated advanced NSCLC who were treated with pembrolizumab, the ORR was 19.4% in unselected cases, but the ORR was much higher at 45.2% in those with intratumoral PD-L1 expression of 50% or greater (27). In two phase III trials of nivolumab in NSCLC, PD-L1 expression on tumor cells was predictive of treatment outcomes including ORR and OS in patients with non-squamous NSCLC, but not in those with squamous NSCLC (21,29). More research is needed to determine the optimal assay and cutoff for determining PD-L1 positivity. Three retrospective series analyzed PD-L1 expression in a total of 258 cases of LELC of the lung (11-13). The threshold for a positive result was membranous expression in >5% tumor cells. Of a total of 359 samples analyzed, 210 (59%) were PD-L1 positive. It has been suggested that EBV-induced latent membrane protein 1 (LMP1) and interferon-gamma pathways may be implicated in upregulation of PD-L1 expression (30). Taken together, these findings provide a rationale to treat patients with LELC of the lung with immune checkpoint therapy.
Unfortunately, treatment with nivolumab did not result in an objective response in our patient with rapidly progressing disease. Several factors might explain the lack of response in this case, despite tumor cell expression of PD-L1. First, tumors with a high mutational burden appear to be more likely to respond to immune checkpoint inhibitors (31). The patient was a never-smoker and therefore it is possible that her tumor had a low mutational burden. Second, other factors appear to influence the ability of tumors to respond to anti-PD-1 therapy including the presence of a T-cell-inflamed tumor microenvironment and changes in other biologic pathways such as DNA mismatch repair (32,33). Intratumoral infiltration of CD8+ T cells, which is associated with responses to anti-PD-1 therapy (34), was present in this case. The lack of response to nivolumab despite the presence of intratumoral CD8+ T cells suggests that other immunological factors are also playing a role in innate resistance to nivolumab. Recently, a transcriptional signature called innate anti-PD-1 resistance (IPRES) was found to be predictive of treatment outcomes after anti-PD-1 treatment in patients with melanoma (35). Validation of the predictive value of IPRES in other tumor types including lung cancer may shed further light on the mechanisms of resistance to immune checkpoint inhibitors. Although the tumor sample in this case was not checked for the presence of microsatellite instability (the phenotypic feature of mismatch repair deficiency), analyses of gastric carcinomas have shown low levels of microsatellite instability in EBV-positive cases (36). Third, the interaction between immune cells and cancer is dynamic in nature. Immunological biomarkers including PD-L1 expression and intratumoral infiltration of CD8+ T cells can change over time as well as in response to treatment such as chemotherapy (37,38). Given that her tumor sample was collected from surgery and the patient underwent chemotherapy prior to treatment with nivolumab, the immunological characterization of her tumor at the time of nivolumab therapy might be different from that of the baseline tumor tissue. Various strategies to improve responses to immune checkpoint inhibitors are being explored to overcome resistance to immune checkpoint inhibitors. These include combination of immune checkpoint blockade with chemotherapy, radiation therapy, targeted therapy, and other immunostimulatory treatments, and methods to generate a T-cell-inflamed tumor microenvironment (39).
Lastly, the patient did not have any targetable genetic aberrations, which is not surprising because several studies have reported that driver mutations were infrequent in LELC of the lung. For example, in a study from Taiwan, EGFR mutations were detected only in 12.1% of patients and there were no ALK or ROS1 genetic aberrations (11). Another study of 42 patients with LELC of the lung showed that there was only one patient (2.4%) with EGFR activating mutation (L858R) and none of the patients had ALK rearrangement (40).
In summary, we report here a case of LELC of the lung, which is an uncommon subtype of lung cancer with lack of response to immune checkpoint blockade with nivolumab. Since a large fraction of LELC of the lung express PD-L1, a potential biomarker of response to PD-1/PD-L1 therapy, further prospective studies are needed to define the clinical activity and safety of immune checkpoint blockade in these patients. In patients with a lack of response to single-agent immune checkpoint inhibitor therapy, additional strategies need to be explored to overcome resistance including combination with other anticancer treatments.
Acknowledgements
None.
Informed Consent: Our effort to reach the patient’s family members to obtain consent was unsuccessful. All the patients’ information in the manuscript is de-identified and strict care was taken to prevent the identification of the patient and any other relevant family members.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chan JK, Hui PK, Tsang WY, et al. Primary lymphoepithelioma-like carcinoma of the lung. A clinicopathologic study of 11 cases. Cancer 1995;76:413-22. [DOI] [PubMed] [Google Scholar]
- 2.Han AJ, Xiong M, Zong YS. Association of Epstein-Barr virus with lymphoepithelioma-like carcinoma of the lung in southern China. Am J Clin Pathol 2000;114:220-6. 10.1309/148K-ND54-6NJX-NA61 [DOI] [PubMed] [Google Scholar]
- 3.Han AJ, Xiong M, Gu YY, et al. Lymphoepithelioma-like carcinoma of the lung with a better prognosis. A clinicopathologic study of 32 cases. Am J Clin Pathol 2001;115:841-50. 10.1309/BUAN-BGFW-69U9-C3H8 [DOI] [PubMed] [Google Scholar]
- 4.Chang YL, Wu CT, Shih JY, et al. New aspects in clinicopathologic and oncogene studies of 23 pulmonary lymphoepithelioma-like carcinomas. Am J Surg Pathol 2002;26:715-23. 10.1097/00000478-200206000-00004 [DOI] [PubMed] [Google Scholar]
- 5.Ngan RK, Yip TT, Cheng WW, et al. Clinical role of circulating Epstein-Barr virus DNA as a tumor marker in lymphoepithelioma-like carcinoma of the lung. Ann N Y Acad Sci 2004;1022:263-70. 10.1196/annals.1318.041 [DOI] [PubMed] [Google Scholar]
- 6.Liang Y, Wang L, Zhu Y, et al. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer 2012;118:4748-58. 10.1002/cncr.27452 [DOI] [PubMed] [Google Scholar]
- 7.Huang CJ, Feng AC, Fang YF, et al. Multimodality treatment and long-term follow-up of the primary pulmonary lymphoepithelioma-like carcinoma. Clin Lung Cancer 2012;13:359-62. 10.1016/j.cllc.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 8.Liu Q, Ma G, Yang H, et al. Lack of epidermal growth factor receptor gene mutations in exons 19 and 21 in primary lymphoepithelioma-like carcinoma of the lung. Thorac Cancer 2014;5:63-7. 10.1111/1759-7714.12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mo Y, Shen J, Zhang Y, et al. Primary lymphoepithelioma-like carcinoma of the lung: distinct computed tomography features and associated clinical outcomes. J Thorac Imaging 2014;29:246-51. 10.1097/RTI.0000000000000070 [DOI] [PubMed] [Google Scholar]
- 10.Sun YH, Lin SW, Hsieh CC, et al. Treatment outcomes of patients with different subtypes of large cell carcinoma of the lung. Ann Thorac Surg 2014;98:1013-9. 10.1016/j.athoracsur.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 11.Chang YL, Yang CY, Lin MW, et al. PD-L1 is highly expressed in lung lymphoepithelioma-like carcinoma: A potential rationale for immunotherapy. Lung Cancer 2015;88:254-9. 10.1016/j.lungcan.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 12.Jiang L, Wang L, Li PF, et al. Positive expression of programmed death ligand-1 correlates with superior outcomes and might be a therapeutic target in primary pulmonary lymphoepithelioma-like carcinoma. Onco Targets Ther 2015;8:1451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang W, Hong S, Chen N, et al. PD-L1 is remarkably over-expressed in EBV-associated pulmonary lymphoepithelioma-like carcinoma and related to poor disease-free survival. Oncotarget 2015;6:33019-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho JC, Wong MP, Lam WK. Lymphoepithelioma-like carcinoma of the lung. Respirology 2006;11:539-45. 10.1111/j.1440-1843.2006.00910.x [DOI] [PubMed] [Google Scholar]
- 15.Ho JC, Lam WK, Ooi GC, et al. Chemoradiotherapy for advanced lymphoepithelioma-like carcinoma of the lung. Respir Med 2000;94:943-7. 10.1053/rmed.2000.0856 [DOI] [PubMed] [Google Scholar]
- 16.Chan AT, Teo PM, Lam KC, et al. Multimodality treatment of primary lymphoepithelioma-like carcinoma of the lung. Cancer 1998;83:925-9. [DOI] [PubMed] [Google Scholar]
- 17.Ho JC, Lam DC, Wong MK, et al. Capecitabine as salvage treatment for lymphoepithelioma-like carcinoma of lung. J Thorac Oncol 2009;4:1174-7. 10.1097/JTO.0b013e3181b28f15 [DOI] [PubMed] [Google Scholar]
- 18.Bégin LR, Eskandari J, Joncas J, et al. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol 1987;36:280-3. 10.1002/jso.2930360413 [DOI] [PubMed] [Google Scholar]
- 19.He J, Shen J, Pan H, et al. Pulmonary lymphoepithelioma-like carcinoma: a Surveillance, Epidemiology, and End Results database analysis. J Thorac Dis 2015;7:2330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 23.El-Khoueiry AB, Melero I, Crocenzi TS, et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J Clin Oncol 2015;33:abstr LBA101.
- 24.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956-65. 10.1016/S1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
- 25.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med 2016;374:2542-52. 10.1056/NEJMoa1603702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin J, Han G, Schalper KA, et al. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:46-54. 10.1001/jamaoncol.2015.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang W, Zhang J, Hong S, et al. EBV-driven LMP1 and IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget 2014;5:12189-202. 10.18632/oncotarget.2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin Oncol 2015;42:663-71. 10.1053/j.seminoncol.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016;165:35-44. 10.1016/j.cell.2016.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung SY, Yuen ST, Chung LP, et al. Microsatellite instability, Epstein-Barr virus, mutation of type II transforming growth factor beta receptor and BAX in gastric carcinomas in Hong Kong Chinese. Br J Cancer 1999;79:582-8. 10.1038/sj.bjc.6690092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016;16:275-87. 10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 2015;3:436-43. 10.1158/2326-6066.CIR-15-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajan A, Kim C, Heery CR, et al. Nivolumab, anti-programmed death-1 (PD-1) monoclonal antibody immunotherapy: Role in advanced cancers. Hum Vaccin Immunother 2016;12:2219-31. 10.1080/21645515.2016.1175694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Lin Y, Cai Q, et al. Detection of rearrangement of anaplastic lymphoma kinase (ALK) and mutation of epidermal growth factor receptor (EGFR) in primary pulmonary lymphoepithelioma-like carcinoma. J Thorac Dis 2015;7:1556-62. [DOI] [PMC free article] [PubMed] [Google Scholar]