Abstract
Age-associated changes in cardiac structure and function, together with estrogen loss, contribute to the progression of heart failure with preserved ejection fraction in older women. To investigate the effects of aging and estrogen loss on the development of its precursor, asymptomatic left ventricular diastolic dysfunction, echocardiograms were performed in 10 middle-aged (20 months) and 30 old-aged (30 months) female Fischer344×Brown-Norway rats, 4 and 8 weeks after ovariectomy (OVX) and sham procedures (gonads left intact). The cardioprotective potential of administering chronic G1, the selective agonist to the new G-protein-coupled estrogen receptor (GPER), was further evaluated in old rats (Old-OVX+G1) versus age-matched, vehicle-treated OVX and gonadal intact rats. Advanced age and estrogen loss led to decreases in myocardial relaxation and elevations in filling pressure, in part, due to reductions in phosphorylated phospholamban and increases in cardiac collagen deposition. Eight weeks of G-protein-coupled estrogen receptor activation in Old-OVX+G1 rats reversed the adverse effects of age and estrogen loss on myocardial relaxation through increases in sarcoplasmic reticulum Ca2+ ATPase expression and reductions in interstitial fibrosis. These findings may explain the preponderance of heart failure with preserved ejection fraction in older postmenopausal women and provide a promising, late-life therapeutic target to reverse or halt the progression of left ventricular diastolic dysfunction.
Key Words: Diastolic dysfunction, Menopause, Ovariectomy, Fibrosis, SERCA2, Phospholamban
The incidence of heart failure with preserved ejection fraction (HFpEF) among older women is considerable and is forecasted to increase in the United States as the population ages and because women have a longer life expectancy than men (1). At least 50% of patients with HFpEF are women (2). The consequences of this emerging epidemic with regard to health care resource utilization and cost is already apparent; women account for more than half of all heart failure (HF) hospitalizations (3), and patients 75 years of age and older account for more than two-thirds of hospitalized HF patients (4). Because no effective pharmacotherapies are available to reduce mortality, morbidity, or hospitalizations for HFpEF, expanding our knowledge of the pathophysiology of HFpEF will provide important insights into the management of this disease.
Since asymptomatic left ventricular diastolic dysfunction (LVDD), or Stage B HF, antedates overt HF, small animal models of LVDD can be used to gain a fundamental understanding of the mechanisms that underlie the development of HFpEF (5). Although rodents do not experience a natural menopause, surgical ovariectomy (OVX) can be used to mimic clinical menopause and to evaluate the role of estrogen in the maintenance of cardiovascular structure and function (6). In the renin-overexpressing mRen2.Lewis rat, we showed that early estrogen loss following OVX at 5 weeks of age exacerbated the development of hypertension, left ventricular (LV) remodeling, and LVDD, whereas estrogen replacement or activation of the new membrane G-protein-coupled estrogen receptor (GPER) with its agonist G1 attenuated the adverse effects of estrogen deficiency (7,8). Although these and other models of female sex-specific diastolic dysfunction support the cardioprotective actions of estrogen and/or GPER activation (9–11), the cardiac structural and functional implications of estrogen status in the context of aging are less clear.
Both postmenopausal LVDD and cardiac aging are characterized by increased LV stiffness from hypertrophy and interstitial fibrosis, and impairment in LV relaxation due to abnormal cardiac calcium cycling (12). Thus, a more clinically relevant animal model for understanding the relationship between cardiac aging and estrogen loss on the progression of diastolic dysfunction might be the middle-aged and old female Fischer344×Brown-Norway (F344BN) rat (13). When compared to other rodent models, this hybrid model has fewer age-related pathologies (14), and its cardiovascular phenotype is not confounded by overt hypertension, obesity, or renal dysfunction with aging (15). However, unlike menopausal women, old F344BN rats have estradiol levels near that of their younger counterparts in diestrus, even past 20 months of age (16,17). Thus, OVX procedures are necessary even in senescent F344BN rats in order to determine how estrogen participates in the modulation of cardiac aging and the progression of LVDD late in life.
In this study, we compared the effects of age and estrogen deficiency on cardiac structure and diastolic function in sham-operated and ovariectomized middle-aged (from 17 to 20 months of age) and old (from 26 to 30 months of age) F344BN female rats and determined the cardioprotective potential of late-life GPER activation by its selective agonist G1. We hypothesized that estrogen loss at middle-age would result in premature cardiac aging and that chronic G1 treatment administered late in life would reverse the cardiac phenotype of the old, estrogen-deficient female rat to that of its middle-aged, intact counterpart. Although the benefits of GPER activation parallel that of estradiol in various tissues (18,19), its agonist G1 lacks significant binding or biologic functions at estrogen receptor (ER)α or ERβ (20,21). Moreover, unlike estradiol’s effects via the classic ERs, G1/GPER actions are nongenomic and nonnuclear (22), making it an appealing target for therapeutic interventions against female sex-specific HFpEF.
Methods
Animals
Middle-aged (17 months; n = 10) and old (26 months; n = 30) female F344BN rats were obtained from the National Institute on Aging through Harlan Industries (Indianapolis, IN). Animals were maintained on a 12-hour light/dark cycle with ad libitum access to standard rat chow (Nestle Purina, St. Louis, MO) and tap water, and housed in pairs. Animal care was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Care and Use Committee of Wake Forest School of Medicine approved the protocol. Body weights of all rats were recorded weekly throughout the study.
Experimental Protocol
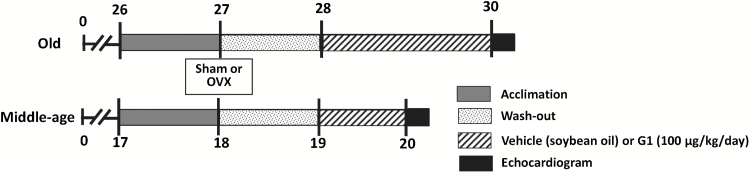
Figure 1 shows the experimental timeline that was used to characterize the effects of age, estrogen status, and late-life GPER activation on cardiac structure and function of female F344BN rats. Following an acclimation period of one month, the “middle-aged” (Mid-age) and the “Old” rats underwent either bilateral oophorectomy (OVX) or sham surgery (gonads left intact), as previously described (23). The surgery was followed by a 1-month washout period. At the age of 28 months, Old-OVX rats were divided equally to receive daily subcutaneous injections of either G1 (Old-OVX+G1, n = 10) (100 µg/kg; Cayman Chemical Company, Ann Arbor, MI) or vehicle (Old-OVX, n = 10; pharmaceutical grade soybean oil; Xcelience Inc., Tampa, FL), whereas gonadal intact rats received vehicle only (Old-intact, n = 10), for 8 weeks. At the age of 19 months, Mid-aged OVX and gonadal intact rats received daily injections of vehicle, for 4 weeks. The dose of G1 used in Old OVX rats was based on our previous studies showing that 50–100 µg/kg/d of G1 preserved diastolic function and cardiac structure without overtly affecting blood pressure in the hypertensive OVX-mRen2.Lewis rat (5). Systolic blood pressure (SBP) was measured at bimonthly intervals in rats using the tail-cuff method (NIBP-LE5001, Panlab, Barcelona, Spain). At the end of the study, when Mid-aged and Old rats were 20 and 30 months of age, respectively, transthoracic echocardiograms were performed with anesthesia (ketamine/xylazine: 50/4.5mg/kg, intramuscular) using a Philips 5500 echocardiograph (Philips Medical Systems, Andover, MA) and a 12-MHz-phased array probe, as previously described (24–26). Immediately following the sonogram, deeply anesthetized animals were euthanized via exsanguination by cardiac puncture and hearts were rapidly removed, weighed, and dissected into left and right ventricles and atrial tissue sections. Left ventricles were further cut horizontally into thirds and stored in −80°C for RNA and immunoblot hybridization studies, or fixed in 4% paraformaldehyde for 24 hours prior to histopathologic analyses. Tibial length was measured in all animals with a micrometer. The cardiac weight index was expressed as heart weight (mg)/tibial length (mm) (27). All animals were examined for the presence or absence of ovaries and uteri were dissected and weighed.
Figure 1.
Experimental timeline in months for middle (Mid)-aged (17–20 months) and Old (26–30 months) female F344BN rats. Rats acclimated to their environment and were trained for tail-cuff blood pressure measures during the month prior to undergoing bilateral oophorectomy (OVX) or sham procedures (indicated by the gray bar). A 1-month washout period followed the surgery (indicated by the stippled bar). At the age of 28 months, Old-OVX rats were subdivided to receive either daily subcutaneous injections of the GPER agonist, G1 (100 μg/kg; Old-OVX+G1) or vehicle (soybean oil; Old-OVX), whereas sham-operated, gonadal intact rats (Old-intact) received vehicle only, for 8 weeks (indicated by hashed bar). At 19 months of age, sham-operated, gonadal intact and OVX middle-aged rats received daily injections of vehicle only, for 4 weeks. At the end of the study, when Mid-aged and Old rats were at 20 and 30 months, respectively, transthoracic echocardiograms were performed with anesthesia. Body weights were recorded weekly and conscious systolic blood pressure measures were obtained bimonthly throughout the study protocol.
Histopathology
Paraformaldehyde-fixed cardiac cross-sections, 2-mm thick, were dehydrated in graded ethanols before being embedded into paraffin blocks. Slices (4 μm) were mounted onto Superfrost Plus (Fisher Scientific, Pittsburgh, PA) slides and stained with picrosirius red to evaluate interstitial collagen deposition within the tissue, as previously described (23,26). Images were captured using an Axiovert 200 microscope (Carl Zeiss Microscopy, Thornwood, NY). The ratio of collagen-stained pixels to unstained pixels was quantified using NIH ImageJ software (http://rsbweb.nigh.gov/ij/).
Immunoblot Hybridization
Cardiac microsomes were prepared and protein concentrations were determined as previously reported (24). Fifty micrograms of homogenate protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on a 7.5% acrylamide gel and transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). Immunocomplexes were detected with anti-sarcoplasmic-endoplasmic reticulum calcium ATPase 2 (SERCA2; 1:1000; Abcam, Cambridge, MA) and anti-phospho-phospholamban Ser16 (p-PLB; 1:5000; Millipore, Temecula, CA). To normalize the variability of protein loading, blots were reprobed with the antibody to glyceraldehyde-3-dehydrogenase (GAPDH; 1:5000 dilution; Cell Signaling, Danvers, MA). The bound antibodies were resolved with a chemiluminescent/enhancer kit (Pierce ECL Western Blotting Substrate; Pierce, Rockford, IL), exposed to Amersham Hyperfilm (Amersham Biosciences, Piscataway, NJ), and analyzed using densitometry. The resulting densitometric measures for both SERCA2 and p-PLB were normalized to their respective GAPDH intensities and expressed in arbitrary units.
Ex vivo Ca2+ Uptake Assay
To further evaluate the potential impact of G1 versus vehicle on the rate of Ca2+ uptake into the cardiac sarcoplasmic reticulum (SR), an affiliated study was performed using skinned (sarcolemmal-free) cardiac fascicles rendered from five readily available mature adult, normotensive Wistar females obtained from the breeding colony at the Biomedical Sciences Institute at Federal University of Rio de Janeiro (Rio de Janeiro, Brazil). Detailed methods of the ex vivo Ca2+ kinetic studies, implemented at the Biomedical Sciences Institute in Brazil, are outlined in Supplementary Methods. Although the Wistar strain is commonly used in aging research, its lifespan is shorter than the F344BN strain (28). However, given this fact and the recent discovery of ETS2, a specific cardiac transcription factor that links cardiomyocyte loss to reduced longevity, particularly among aging Wistar rats (28), we chose to use cardiac fascicles from 8-month as opposed to 20-month-old Wistars in order to limit any factors that might interfere with G1’s influence on Ca2+ kinetics and to mimic the presumed physiology of an older F344BN rat.
Statistical Analyses
All values are expressed as mean ± SEM. Two-way ANOVA was used to determine the effect of age (Mid-age vs Old) and estrogen status (OVX vs intact). Because G1 treatment applied to only the old rats, its effect was examined in a separate one-way ANOVA (Old-Intact; Old-OVX; Old-OVX+G1), followed by Tukey’s multiple comparisons test. A two-way repeated measures ANOVA was used to determine the effect of loading time and treatment on caffeine-induced contracture of skinned fibers and post hoc comparisons were evaluated using Bonferroni’s test of multiple comparisons. Data were analyzed using the software GraphPad Prism Version 6 (GraphPad Software, Inc., La Jolla, CA). A p value < .05 was considered statistically significant.
Results
Animal Characteristics
Before reaching the end of the protocol, 6 of 30 Old rats (Old-intact n = 3; Old-OVX n = 2; Old-OVX+G1 n = 1) died from natural causes or required early euthanasia for being encumbered by an expanding mammary tumor, ataxia, or failure to thrive. The final sample size of the old rats ranged from 7 to 9 per group, whereas the sample size of the middle-aged rats remained at 5 per group. Physical characteristics of the animals and SBP changes in all experimental groups are presented in Table 1. As expected, body [age effect: F(1,21) = 8.550, p = .008] and postmortem heart weights [age effect: F(1,21) = 8.690, p = 0.008] were significantly higher in Old versus Mid-aged rats, independent of estrogen status (assigned by the presence or absence of ovaries). The heart weight-to-tibial length ratio was significantly higher in Old rats compared with their Mid-aged counterparts [age effect: F(1,21) = 14.00, p = .001], irrespective of estrogen. Among the Old only cohort, heart weight and height weight-to-tibial length ratios were similar between rats, even though body weights were different [F(2,21) = 4.298, p = .0273]; specifically, Old-intact rats were heavier than Old-OVX+G1 rats (p < .05).
Table 1.
Physical Characteristics
| Physical Characteristics and Groups | Intact | OVX | OVX + G1 | Age | E2 | Age × E2 |
|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | p Value | p Value | p Value | |
| Body weight (g) | .008 | .345 | .142 | |||
| Old-aged | 321±9 | 293±14 | 275* ± 11 | |||
| Mid-aged | 270±11 | 277±5 | ||||
| Heart weight (g) | .008 | .644 | .55 | |||
| Old-aged | 0.915±0.036 | 0.869±0.057 | 0.828±0.025 | |||
| Mid-aged | 0.762±0.026 | 0.768±0.021 | ||||
| Tibial length (mm) | <.001 | .729 | .083 | |||
| Old-aged | 46.89±0.22 | 46.33±0.24 | 45.69±0.46 | |||
| Mid-aged | 48.29±0.76 | 49.12±0.28 | ||||
| HW/TL (mg/mm) | .001 | .617 | .755 | |||
| Old-aged | 19.49±0.71 | 18.75±1.21 | 18.11±0.48 | |||
| Mid-aged | 15.81±0.69 | 15.64±0.44 | ||||
| Uterine weight (g) | .003 | .001 | .050 | |||
| Old-aged | 1.50±0.09 | 0.75† ± 0.06 | 0.78† ± 0.07 | |||
| Mid-aged | 0.84±0.25 | 0.61±0.02 | ||||
| SBP (%Δ baseline) | .005 | .075 | .017 | |||
| Old-aged | 24±5 | 20±5 | 9±4 | |||
| Mid-aged | −8±5 | 17±6 | ||||
Notes: %Δ baseline = percent change from baseline; E2 = estrogen status, as per the presence or absence of gonads; g = gram; HW = heart weight; INTACT = sham-surgery (gonads left in place); Mid-aged = middle-aged; mm = millimeter; OVX = ovariectomy; SBP = systolic blood pressure; TL = tibial length. Physical characteristics were performed on Mid-aged, intact (N = 5) and Mid-aged, OVX (N = 5) rats at 20 months of age, following 4 weeks of subcutaneous daily injections of vehicle (soybean oil). Physical characteristics of Old-aged, intact (Old-intact, N = 7), Old-aged, OVX (Old-OVX, N = 8), and Old-aged, OVX+G1 (Old-OVX+G1, N = 9) rats were performed at 30 months of age, following 8 weeks of subcutaneous daily injections of G1 (100 μg/kg) or vehicle (soybean oil). Using a two-way analysis of variance (ANOVA), significant differences in the physical characteristics, with respect to age, estrogen status (intact vs OVX), and age × estrogen status, were determined. p-values are presented on the right side of the table. Among the Old-only rats (Old-intact, Old-OVX, Old-OVX+G1), a separate one-way ANOVA followed by Tukey’s multiple comparisons test was used to determine the influence of gonadal status, G1, or vehicle on the physical characteristics. For convenience, significant differences are indicated in bold.
*p < .05, Old-OVX+G1 versus Old-intact.
†p < .001, Old-OVX, Old-OVX+G1 versus Old-intact.
Although the nonappearance of ovaries confirms the efficacy of gonadectomy, increases in uterine weight signify an estrogenic response (29). Accordingly, we used uterine weights in lieu of plasma estradiol levels to determine the effect of age on estrogenicity, since estradiol levels among Old-aged rats fell below the limits of detection, irrespective of their gonadal status. As expected, uterine weights were significantly affected by age [age effect: F(1,21) = 10.93, p = .003] and estrogen status [estrogen effect: F(1,21) = 16.37, p = 0.001], with heavier postmortem uteri rendered from Old and gonadal intact rats compared with the uteri from their Mid-aged and OVX counterparts. Late-life estrogen loss appears to have had a greater influence on uterine regression than midlife estrogen loss [Age × E2 effect: F(1,21) = 4.340, p = .050], which substantiates the estrogenic potential of senescent ovaries, and our reasoning for performing OVX in old rats at the age of 27 months. In the Old only cohort, uterine weights from OVX rats, regardless of G1 treatment, were significantly lower than uteri from age-matched, gonadal intact rats [F(2,21) = 29.52, p < .001; Table 1]. Importantly, unlike E2’s effects on the classic estrogen receptors, ERα and ERβ, GPER activation by G1 is not known to have specific uterotrophic actions (30).
Baseline SBPs, prior to surgery, were similar among groups, ranging from 113 mmHg to 126 mmHg (Mid-aged, intact = 115±7 mmHg; Mid-aged, OVX = 126±8; Old, intact = 114±3; Old, OVX = 113±2; and Old, OVX + G1 = 118±6). Over the course of the protocol, there was a significant age-related increase in SBP [age effect: F(1,19) = 9.79, p = .005], whereas E2 loss accounted for SBP increases among Mid-aged rats only [age × E2 effect: F(1,19) = 6.761, p = 0.017]. The delta increase from baseline SBP ranged from 20% to 24% in Old-intact and Old-OVX rats, respectively, whereas SBP changes over the duration of the study were negligible in the Mid-aged cohort. Amid the Old only rats, the percent changes in SBP ranged from 9% to 24% (Table 1). Even though no differences in this variable occurred among Old-intact, Old-OVX and Old-OVX+G1 rats, it is important to note that G1-treated OVX rats showed the least variation in SBP over the course of the experiment. Indeed, G1’s negligible effect on SBP in Old OVX rats, at the dose of 100 μg/kg/d, is consistent with previous findings in hypertensive, OVX-mRen2.Lewis females receiving 2 weeks of low-dose subcutaneous G1 treatment (8).
Cardiac Structure and Function
The effect of age, estrogen loss, and chronic G1 administration on M-mode measurements of LV dimensions, wall thicknesses, and systolic and diastolic functional parameters are summarized in Table 2. The LV end-diastolic dimension (LVEDD) exhibited a marked increase in Old compared with Mid-aged rats, independent of estrogen [age effect: F(1,21) = 5.581; p = 0.028]. No differences in the LV end-systolic dimension were observed with respect to age or estrogen status. Despite an age-related increase in anterior wall thickness at end diastole [age effect: F(1,21) = 9.465; p = .006], posterior wall thickness and relative wall thickness were not influenced by age or estrogen. Corresponding to the gravimetric findings, LV mass was greater in Old versus Mid-aged rats [age effect: F(1,21) = 18.21; p = .0003], irrespective of estrogen status. Percent fractional shortening, or global systolic function, was not significantly affected by age or estrogen status. In the Old only cohort, overall differences in LV end-systolic dimension, anterior wall thickness, and percent fractional shortening were observed; specifically, Old OVX rats exhibited smaller LV end-systolic dimension, thicker anterior walls, and modestly higher percent fractional shortening compared with their G1-treated OVX counterparts. All the same, LVEDD and relative wall thickness among the Old animals were not affected by G1 or gonadal status.
Table 2.
Cardiac Geometry, Left Ventricular Systolic and Diastolic Functional Parameters, and Heart Rate
| Echocardiographic Indices and Groups | Intact | OVX | OVX + G1 | Age | E2 | Age × E2 |
|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | p Value | p Value | p Value | |
| LV Structure | ||||||
| LVESD (cm) | .080 | .654 | .056 | |||
| Old-aged | 0.327±0.019 | 0.299±0.019 | 0.358* ± 0.008 | |||
| Mid-aged | 0.258±0.015 | 0.302±0.005 | ||||
| LVEDD (cm) | .028 | .658 | .648 | |||
| Old-aged | 0.675±0.024 | 0.675±0.031 | 0.731±0.018 | |||
| Mid-aged | 0.593±0.037 | 0.62±0.015 | ||||
| AWT (cm) | .006 | .432 | .192 | |||
| Old-aged | 0.167† ± 0.006 | 0.185±0.004 | 0.158‡ ± 0.003 | |||
| Mid-aged | 0.152±0.006 | 0.148±0.015 | ||||
| PWT (cm) | .695 | .933 | .955 | |||
| Old-aged | 0.183±0.015 | 0.183±0.009 | 0.187±0.004 | |||
| Mid-aged | 0.176±0.017 | 0.178±0.01 | ||||
| RWT | .593 | .634 | .634 | |||
| Old-aged | 0.554±0.059 | 0.554±0.046 | 0.502±0.018 | |||
| Mid-aged | 0.614±0.09 | 0.557±0.028 | ||||
| LV MASS (g) | .0003 | .98 | .775 | |||
| Old-aged | 0.737±0.024 | 0.748±0.018 | 0.699±0.014 | |||
| Mid-aged | 0.602±0.055 | 0.593±0.044 | ||||
| Systolic function | ||||||
| FS (%) | .804 | .93 | .057 | |||
| Old-aged | 52±1.8 | 55±1.0 | 51* ± 1.0 | |||
| Mid-aged | 56±2.1 | 52±3.0 | ||||
| s′ (cm/s) | .013 | .175 | .270 | |||
| Old-aged | 2.76§ ± 0.10 | 2.70±0.21 | 3.43* ± 0.16 | |||
| Mid-aged | 3.53±0.33 | 3.02±0.15 | ||||
| Diastolic function | ||||||
| E max, cm/s | .627 | .241 | .623 | |||
| Old-aged | 57±1.8 | 57±1.6 | 62±4.0 | |||
| Mid-aged | 53±3.5 | 58±2.0 | ||||
| A max, cm/s | .672 | .178 | .411 | |||
| Old-aged | 38±3.5 | 40±2.4 | 36±1.7 | |||
| Mid-aged | 37±2.2 | 44±2.0 | ||||
| E/A | .905 | .908 | .437 | |||
| Old-aged | 1.54±0.10 | 1.46±0.12 | 1.69±0.12 | |||
| Mid-aged | 1.44±0.07 | 1.54±0.14 | ||||
| E dec time, s | .0001 | .694 | .37 | |||
| Old-aged | 0.069±0.004 | 0.067±0.002 | 0.057±0.004 | |||
| Mid-aged | 0.046±0.003 | 0.05±0.002 | ||||
| Heart rate, beats/min | .002 | .518 | .718 | |||
| Old-aged | 254±6 | 262±6 | 268±7 | |||
| Mid-aged | 283±10 | 286±10 | ||||
Notes: %FS = percent fractional shortening; Amax = maximum late transmitral filling velocity; AWT = anterior wall thickness end diastole; cm = centimeter; cm/s = centimeter/second; E2 = estrogen status, as per the presence or absence of gonads; E/A = early-to-late transmitral filling velocity ratio; E dec time = early-filling deceleration time; Emax = maximum early transmitral filling velocity; g = gram; INTACT = sham-surgery (gonads left in place); LV = left ventricle; LVEDD = left ventricular end-diastolic dimension; LVESD = left ventricular end-systolic dimension; LV mass = left ventricular mass; Mid-aged = middle-aged; min = minute; OVX = ovariectomy; PWT = posterior wall thickness, end diastole; RWT = relative wall thickness; s′ = systolic wall motion; sec = second. Echocardiograms for cardiac characteristics were performed on Mid-aged, intact (N = 5) and Mid-aged, OVX (N = 5) rats at 20 months of age, following 4 weeks of subcutaneous daily injections of vehicle (soybean oil). Echocardiograms on Old-aged, intact (Old-intact, N = 7), Old-aged, OVX (Old-OVX, N = 8), and Old-aged, OVX+G1 (Old-OVX+G1, N = 9) rats were performed at 30 months of age, following 8 weeks of subcutaneous daily injections of G1 (100 μg/kg) or vehicle (soybean oil). Using a two-way ANOVA, significant differences in the cardiac characteristics, with respect to age, estrogen status (intact vs OVX), and age x estrogen status, were determined. p Values are presented on the right side of the table. Among the Old-only rats (Old-intact, Old-OVX, Old-OVX+G1), a separate one-way ANOVA followed by Tukey’s multiple comparisons test was used to determine the influence of gonadal status, G1, or vehicle on the cardiac characteristics. For convenience, significant differences are indicated in bold.
*p < .05 Old-OVX+G1 versus Old-OVX.
† p < .01 Old-intact versus Old-OVX.
‡ p < .01, Old-OVX+G1 versus Old-OVX.
§ p < .05 Old-intact versus Old-OVX+G1.
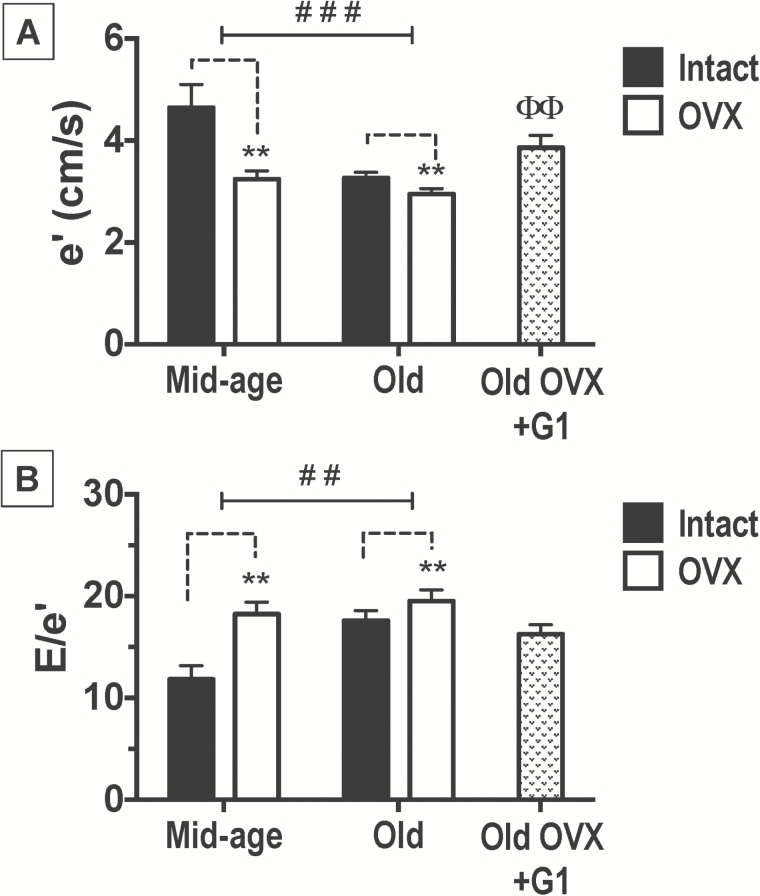
Transmitral-pulsed wave and myocardial tissue Doppler indices of diastolic and systolic function are shown in Table 2 and Figure 2. The velocities of early (Emax) and late (Amax) transmitral filling and the ratio of early-to-late filling were not influenced by age, estrogen, or G1 treatment. Even so, aged hearts exhibited a prolongation in early deceleration time (Edec time) compared to their mid-aged counterparts [age effect: F(1,21) = 45.91, p < 0.0001], whereas among Old-only rats, G1 nor gonadal status overtly affected this Doppler-derived time interval. The tissue Doppler measure of myocardial relaxation, mitral annular descent (e′), was affected by both age and estrogen status, as e′ was significantly lower in Old versus Mid-aged rats [age effect: F(1,21) = 15.08, p < 0.001] and in OVX versus intact animals [estrogen effect: F(1,21) = 15.90, p < 0.001]. The adverse effect of estrogen loss on e′ was more profound among Mid-aged versus Old rats [Age × E2: F(1,21) = 6.42, p = .019], suggesting that endogenous estrogens at mid-age are still capable of exerting myocardial relaxant actions in this model. Among only the Old rats, differences in e′ were detected [F(2,21) = 6.786, p = .005]; specifically, G1 treatment in Old OVX rats led to significant increases in e′ compared with age-matched, OVX counterparts receiving vehicle (p < 0.01; Figure 2). Similarly, echo-derived filling pressures, or the ratio of early filling to mitral annular descent (E/e′), were higher in Old rats [age effect: F(1,21) = 9.3, p = .0061] and in those animals that underwent OVX [estrogen effect: F(1,21) = 13.07, p = 0.002] compared with respective Mid-aged and gonadal intact rats. Among the Old-only rats, E/e′ was not affected by treatment (G1 or vehicle) or gonadal status. Comparable to the age-related reductions in myocardial motion during diastole, or e′, there was also a significant negative effect of age on myocardial tissue velocity during systole, or s′, [age effect: F(1,21) = 7.38, p = .013]. Among the Old cohort of rats, 8 weeks of G1 treatment significantly improved this systolic septal wall motion abnormality [F(2,21) = 6.914, p = .005; p < .05 Old-OVX+G1 versus Old-OVX]. As expected, heart rates under isoflurane anesthesia were significantly lower in Old animals compared with their Mid-aged counterparts, irrespective of estrogen status [age effect: F(1,21) = 7.978, p = 0.010], or G1.
Figure 2.
Early mitral annular velocity (e′) and early transmitral filling velocity-to-mitral annular velocity ratio (E/e′) in middle (Mid)-aged and Old F344BN female rats. Data indicate differences in (A) septal e′ and (B) E/e′ with respect to age and estrogen status (intact versus OVX). The effect of G1 treatment among Old only rats is also presented. Values are reported as mean ± SEM; Mid-aged rats, N = 5 per group. Old rats, N = 7–9 per group. Black bars represent gonadal intact rats; open bars represent OVX rats; and stippled bars represent Old-OVX+G1 rats. Old rats had reduced ventricular lusitropy (e′) and higher filling pressures (E/e′) compared with Mid-aged rats and estrogen loss also adversely affected these diastolic indices (2-way ANOVA). Among the Old only, e′ was higher in OVX+G1 rats compared with vehicle-treated, age-matched OVX rats (1-way ANOVA). ###p < .001; ##p < .01 (age effect); **p < 0.01 (estrogen effect or “intact versus OVX”); ΘΘp < .01, Old-OVX+G1 versus Old-OVX. ANOVA = analysis of variance; SEM = standard error of the mean.
Cardiac Fibrosis
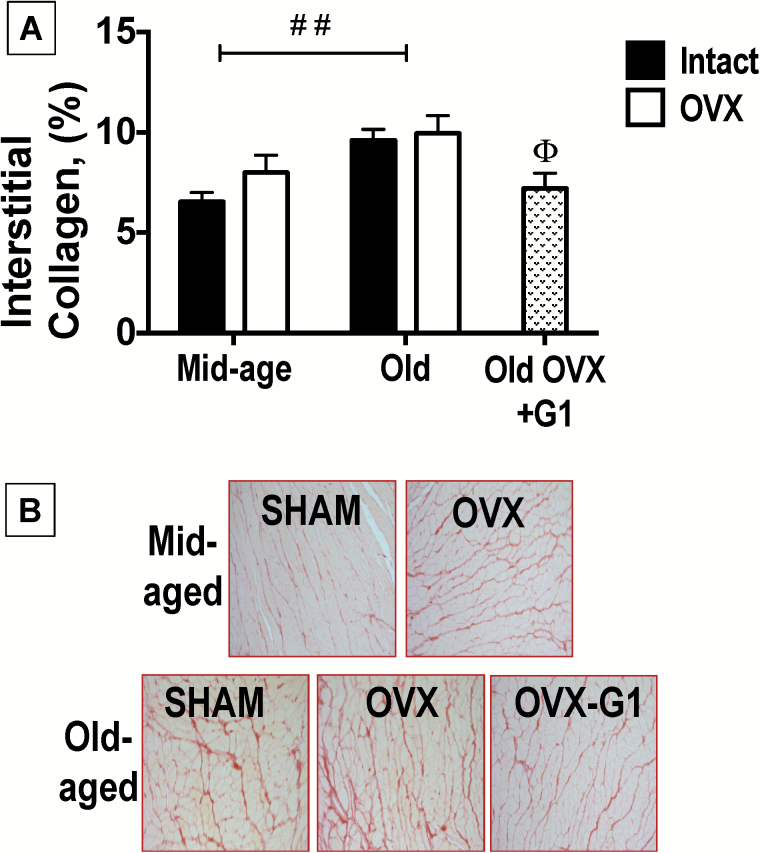
Quantitative histological analysis of interstitial collagen deposition in the LVs of Mid-aged and Old, intact and OVX, plus Old-OVX+G1 rats are presented in Figure 3A, with representative images shown in Figure 3B. As reported for LVs of senescent male F344BN rats (31), there was a modest but significant age-related increase in interstitial collagen in heart tissue from Old compared with Mid-aged female rats [age effect: F(1,21) = 11.52, p = .003], irrespective of estrogen status. Importantly, among only Old rats, interstitial collagen content was different between rats [F(2,19) = 26.44, p = .001; p < .001], with a lesser amount of fibrosis noted in hearts from Old-OVX+G1 rats compared with hearts from Old-OVX rats receiving vehicle (p < .05).
Figure 3.
Cardiac interstitial collagen in middle (Mid)-aged and Old F344BN female rats. (A) Quantification of cardiac interstitial collagen from histological analysis. Data indicate differences in %interstitial collagen deposition with respect to age and estrogen status (intact versus OVX). The effect of G1 treatment among Old only rats is also presented. Values are reported as means ± SEM; Mid-aged rats, N = 5 per group. Old rats, N = 7–9 per group. Black bars represent gonadal intact rats; open bars represent OVX rats; and stippled bars represent Old-OVX+G1 rats. Old-aged hearts exhibited more collagen deposition than Mid-aged hearts, irrespective of estrogen status (2-way ANOVA). Amid the Old cohort alone, OVX+G1 hearts exhibited a lesser amount of collagen than hearts rendered from Old-OVX rats receiving vehicle (1-way ANOVA). ##p < .01 (age effect); Θp < 0.05, Old-OVX+G1 versus Old-OVX. (B) Representative images of LV free wall interstitial collagen deposition were taken at 20X magnification. The picrosirius-red stained slides show an age-related increase in collagen deposition and chronic G1 treatment limited this effect in Old-OVX rats. ANOVA = analysis of variance; SEM = standard error of the mean.
LV SERCA2 and p-PLB Expression
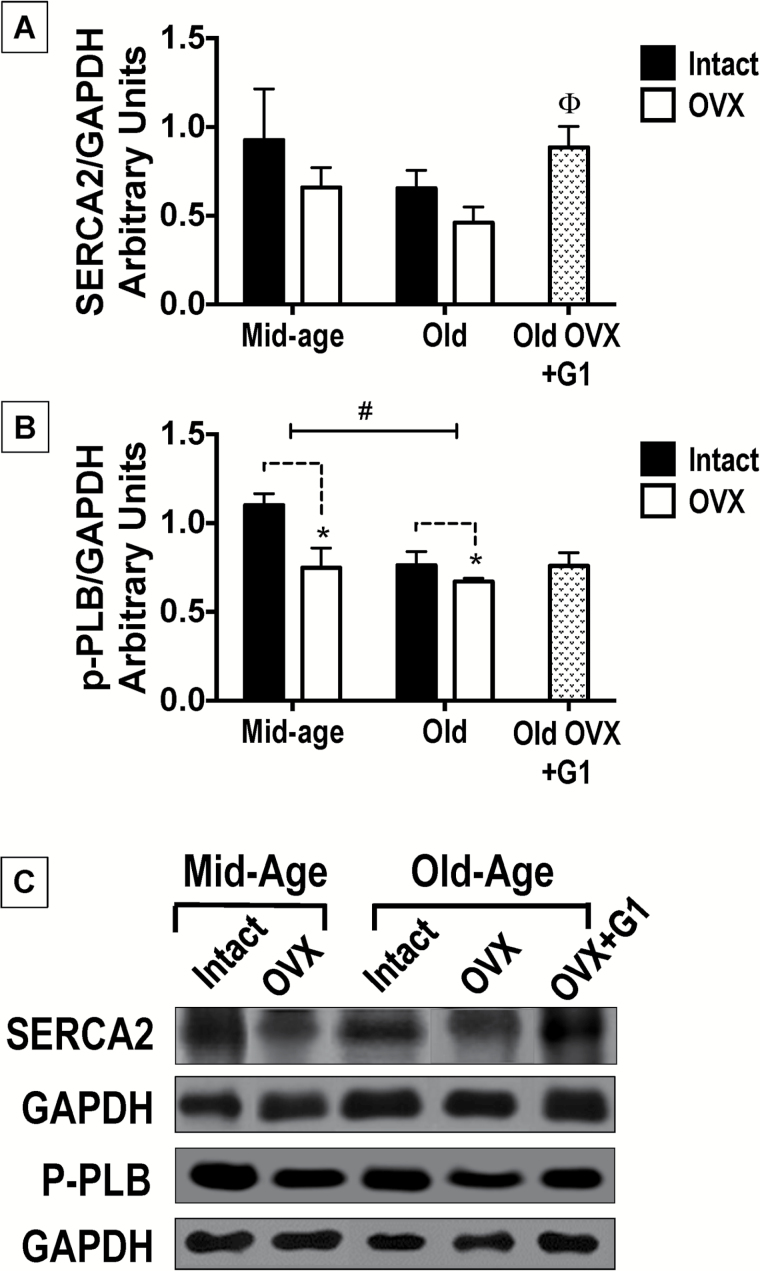
SERCA2 expression tended to be lower in Old rats compared with their Mid-aged counterparts [age effect: F(1,17) = 3.013, p = .103], with age accounting for approximately 15% of the total variance in the sample (Figure 4A). Notably, among only Old animals, SERCA2 expression was different [F(2,12) = 4.281, p = .03], particularly when hearts from Old-OVX+G1 rats were compared with hearts from their vehicle-treated OVX counterparts (p < .05; Figure 4A). As expected, cardiac expression of the protein that “turns on” SERCA2, phosphorylated-PLB (p-PLB), was lower in Old- versus Mid-aged rats [age effect: F(1,17) = 6.024, p = .025; Figure 4B] and p-PLB expression was also reduced with the loss of estrogen [estrogen status: F(1,17) = 6.927, p = .018]. Among Old rats, neither gonadal status nor treatment with G1 influenced p-PLB expression. Although we cannot exclude the possibility that small age-related changes in the housekeeping protein, GAPDH, could interfere with SERCA2 and p-PLB interpretations (32), the quantity of GAPDH protein expressed in our cardiac samples was not statistically different across age groups (data not shown). Moreover, GAPDH is commonly used as the loading control in cardiac aging studies (33,34).
Figure 4.
Expression of sarcoplasmic-endoplasmic Ca2+ adenosine triphosphatase (SERCA2) and phosphorylated phospholamban (p-PLB) in the hearts of middle (Mid)-aged and Old F344BN female rats. (A) Quantification of cardiac SERCA2 expression normalized to respective GAPDH expression. Data indicate differences in SERCA2 with respect to age and estrogen status (intact versus OVX). The effect of G1 treatment among Old only rats is also presented. Neither advanced age nor gonadal status had substantial affects on the expression of this calcium regulatory protein (2-way ANOVA). Among the Old only cohort, 8 weeks of G1 treatment in OVX rats significantly increased SERCA2 expression compared with OVX counterparts receiving vehicle (1-way ANOVA). (B) Quantification of cardiac p-PLB expression normalized to respective GAPDH expression. Data indicate differences in p-PLB with respect to age and estrogen status (intact versus OVX). The effect of G1 treatment among Old-only rats is also presented. Both advanced age and estrogen loss associated with significant reductions in cardiac p-PLB expression. Among the Old-only group, the levels of p-PLB expression were not different. For graphic depictions of both SERCA2 and p-PLB data, values are reported as means ± SEM; Mid-aged rats, N = 5 per group. Old rats, N = 7–9 per group. Black bars represent gonadal intact rats; open bars represent OVX rats; and stippled bars represent Old-OVX+G1 rats. #p < .05 (age effect); *p < .05 (estrogen effect or “intact versus OVX”); Θp < .05 Old-OVX+G1 versus Old-OVX. (C) Representative immunoblots of SERCA2, p-PLB and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in heart tissues from each age and estrogen status group, as well as from G1-treated Old-OVX rats. ANOVA = analysis of variance; SEM = standard error of the mean.
Ca2+ Loading From SR
Using the amplitude of the isometric tension (% Po) of the skinned, sarcolemma-free ventricular fascicles as a measure of Ca2+ uptake into the SR, the contractile response to caffeine exposure (20 mmol/L) in the presence of vehicle or G1 (reported as loading times with pCa 6.8 solution [(0.126 μmole/L Ca2+]) increased from 5 seconds to 8 minutes (Figure 5 insert). Compared with vehicle, G1 significantly increased Ca2+accumulation within the SR in a concentration- and SR-Ca2+ loading time-dependent manner. Caffeine-induced contraction with low concentrations of G1 (10 μM) increased after 30 seconds of exposure to pCa 6.8, whereas tensions with high G1 (30 μM) increased by 5 seconds of pCa 6.8 exposure and plateaued at 60 seconds [loading-time effect: F(3,12) = 22.40, p < .0001; Figure 5].
Figure 5.
Sarcoplasmic reticulum-Ca2+ (SR-Ca2+) uptake on saponin-skinned cardiac muscle fascicles from 8-month-old female Wistar rats (N = 5 rats). The effects of G1 (10 μM and 30 μM) and vehicle (VEH) on the rate of Ca2+ uptake into the SR, defined by isometric tension, were determined as loading times with the pCa 6.8 solution (0.126 μmol/L Ca2+) increased from 5 seconds to 8 minutes (insert). Caffeine (20 mmol/L)-induced isometric tension was measured after each loading time and this value was presented as a percentage of Po (%Po). Caffeine-induced increases in force were loading-time dependent. Compared with vehicle, treatment with G1 increased Ca2+ accumulation within the SR in a concentration and loading-time manner. Caffeine-induced contraction with low G1 (10 µM) increased within 30 seconds, whereas effects with higher G1 (30 µM) occurred between 5 and 60 seconds [loading time effect: F(3,12) = 22.40, p < .0001]. *p < .0001: G1 (30 µM) compared to vehicle; ∞p < .001: G1 (10 µM) compared with vehicle.
Discussion
Since LVDD precedes the development of HFpEF and is also a common feature of mammalian aging (35), reverse translational female rodent models of healthy aging, in the presence and absence of ovarian estrogens, aid our understanding of the onset of HFpEF in the context of both intrinsic aging and female sex. Moreover, healthy or normal aging models can be used to provide insight into potential interventions that might halt or reverse the age-specific progression of this common geriatric syndrome. Herein, we show that estrogen loss at midlife in the “healthy” aging F344BN female rat led to reductions in myocardial relaxation (e′) and increases in filling pressure (E/e′), mirroring that of its old-aged counterpart. This “accelerated” cardiac aging associated with a reduction in p-PLB, the form of phospholamban that facilitates the action of SERCA2, and a modest increase in interstitial collagen deposition. Second, late-life G1 treatment in Old OVX rats reversed some of these unfavorable effects of age and estrogen loss that contributed to LVDD in this model, likely by improving intracellular Ca2+ handling and lessening the extent of cardiac interstitial collagen deposition.
Similar to healthy aging community dwellers (36,37), distinct age-related changes in cardiac structure and function were observed between middle-aged and old F344BN female rats. LV mass increased with aging in adult rats, which has also been seen in normotensive and hypertensive patients (38). Although relative wall thickness was not different between mid-age and old rats, modest increases in SBP in the old age group that were observed over the course of the study might have partially contributed to the differences in heart weight. Indeed, increases in blood pressure within the normal range have been related to increases in LV mass in the clinical setting (39). However, given that modest elevations in SBP among estrogen-deficient, mid-aged rats did not translate to differences in LV mass compared with gonadal intact, mid-aged rats suggests that estrogen status may have a less profound effect than aging on LV remodeling in this rat strain.
Slowing of myocardial relaxation and decreased LV chamber compliance are two functional hallmarks of sedentary aging (40,41). These characteristics were manifested by lower early diastolic tissue Doppler velocities (e′), prolonged early deceleration times, and increased Doppler-derived filling pressures (E/e′) in old F344BN rats compared with their mid-aged counterparts. Similar to aging humans (42), diastolic dysfunction in old female F344BNs was independent of overt differences in global systolic function compared with mid-aged rats. Although the modest reduction in systolic mitral annular displacement (s′) found in the old versus mid-aged rats cannot be ignored, there is general agreement in the clinical literature that abnormalities in regional systolic function, defined by an abnormal extent and velocity of longitudinal shortening, is not responsible for the clinical syndrome of diastolic HF or HFpEF (43,44). The changes in tissue Doppler variables observed in this study highlight an important aspect of the senescent mammalian heart, namely the loss of vigorous diastolic suction (45). Both in preclinical and clinical settings, decreased suction could impair filling of a stiff noncompliant heart during conditions of physical stress such as rapid heart rate changes during exercise. In rats, this effect manifests as reduced exercise tolerance on the treadmill (46), whereas in patients, symptoms of fatigue and shortness of breath, in addition to exercise intolerance, are often revealed (47).
The passive elastic properties of the ventricle are important determinants of diastolic function. Abnormal passive elastic properties are caused mainly by an increased myocardial mass and alterations in extra myocardial collagen deposition (48). As reported previously in male F344BNs (31,49), interstitial collagen was increased in the hearts from old females compared with their mid-aged counterparts, independent of estrogen status. Aging has noticeable effects on the balance between myocyte volume and the extracellular matrix. The aged rat heart has substantially fewer myocytes than the younger heart, and this is accompanied by prominent increases in the volume fraction of collagen (50,51). Also, in healthy human hearts, focal areas of interstitial fibrosis, mainly in the subendocardium of the LV, have been shown to increase with age, presumably due to the loss of myocyte volume, also termed “replacement fibrosis” (52).
Of the variety of well-described changes that occur within the aging cardiac myocyte (53), changes in Ca2+ handling are central to understanding the mechanical processes that underlie relaxation. Early diastole is extremely dependent on reuptake of Ca2+ from the cytosol back into the SR. This is an energy-dependent process requiring the activity of SERCA2. Regulation of SERCA2 is modulated by the inhibitory protein PLB. Phosphorylation of PLB removes its inhibition on SERCA2 activity and increases SR Ca2+ uptake (54). Phosphorylation of PLB via protein kinase A is known to accelerate relaxation of ventricular myocytes (54). Normal aging results in various changes in this calcium regulatory system in the heart, including reduced activity of SERCA2, reduced protein levels of SERCA2, decreased SERCA2-to-PLB ratio, and reduced phosphorylation of PLB or the SERCA2-PLB complex (55–58). In the hearts of F344BN females, we did not observe significant age- or estrogen-related differences in the immunoreactive SR Ca2+-ATPase content using SERCA2-specific antibodies. However, the reduced cardiac expression of phosphorylated PLB in old and estrogen-deficient rats likely contributed to the slowing of early myocardial relaxation, indirectly by inhibiting SERCA2 (59).
In addition to comparing the effects of age and estrogen status on cardiac structure and function in a healthy aging rodent model, we determined that activation of the new membrane estrogen receptor GPER with its specific agonist G1 could reverse the senescent cardiac phenotype of the F344BN female to that of its mid-aged, gonadal intact counterpart. GPER is a recently discovered membrane-bound receptor whose localization and signaling differs from the classical estrogen receptors ERα and ERβ (60,61). GPER does not mediate estrogen’s classical actions on body weight or uterotrophy, a receptor profile that makes GPER a good candidate for selectively eliciting estrogen’s cardiovascular benefits. Moreover, its agonist G1 does not interact with nuclear estrogen receptors, nor has it been shown to increase estradiol or progesterone levels (30). Specifically, in our study, 8 weeks of low-dose G1 treatment improved regional wall motion elongation (e′) and shortening (s′) in part through improvements in cardiac Ca2+ dynamics. However, in contrast to the benefits of a younger age and intact gonads on disinhibiting SERCA2, presumably via phosphorylated PLB, the beneficial actions of GPER on cardiac Ca2+ regulation and lusitropic and contractile function appear to be related to increases in SERCA2 protein expression. In a female OVX-rat model of isoproterenol-induced HF, chronic G1 treatment improved contractility at the whole heart and single myocyte levels by increasing the expression of β2-adrenergic receptors and normalizing the expression of β1-adrenergic receptors, which in turn enhanced cardiac Ca2+ dynamics in a protective manner (62). Specifically, if G1 increases cAMP, protein kinase A activity, or other components of this pathway, then Ca2+ release and contraction size could increase, as well as the rate of Ca2+ transient decay. Further investigation of these pathways in cardiomyocytes from mice with GPER intact and GPER knockout cardiomyocytes are underway.
To further determine whether differences in SR Ca2+ uptake by G1 could account for improvements in myocardial relaxation, SR Ca2+ loading was performed in the absence (vehicle) and in the presence of G1 (10 and 30 μM) in sarcolemmal-free ventricular fibers from 8-month old female Wistar rats. SR Ca2+ content evaluated by caffeine-induced tension after different loading conditions showed that compared with vehicle, treatment with G1 increased Ca2+ accumulation in a concentration and loading-time manner. Although increased diastolic Ca2+ levels have been observed in cardiomyocytes isolated from OVX animals compared with sham controls (63,64), it is not known whether this effect is due to impairments in Ca2+ extrusion mechanisms or re-sequestration. Our observations suggest that chronic GPER activation may increase cardiac calcium mobilization by augmenting SERCA activity or the number of calcium pump sites (65), which, in turn, could improve relaxation in aging female hearts exposed to low levels of endogenous estrogens. Indeed, further studies focusing on GPER modulation of Ca2+ handling and signaling pathways that regulate SR Ca2+ uptake and release in the healthy aging F344BN strain are warranted in order to fully understand the contribution of these pathways to age-related LVDD.
Another role for GPER activation in the reversal of age-related diastolic dysfunction might be related to its effects on interstitial fibrosis. Although there appeared to be no effect of estrogen status on age-related collagen deposition in old F344BN rats, chronic G1 treatment in OVX rats reduced fibrosis compared with vehicle treatment. A similar benefit of GPER activation was observed after 2 weeks of G1 treatment in the estrogen-deficient, hypertensive mRen2.Lewis rat (8). As GPER is expressed in cardio fibroblasts, activation of this membrane estrogen receptor might favorably affect extracellular remodeling by limiting cardio fibroblast proliferation via suppression of cell cycle proteins (66). Cyclin family proteins have been implicated in the fibrogenesis that occurs in HF (67). There is also accumulating evidence that cardiac chymase contributes to fibrosis in hypertensive heart disease and HF through the generation of angiotensin II (Ang II) from angiotensin I or angiotensin-(1–12), (68–70). Although cardiac Ang II is a local promoter of cell growth and proliferation and collagen deposition (71,72), chymase can independently promote LV remodeling by affecting collagen metabolism via activation of transforming growth factor-β (73), attraction of inflammatory cells (74), and activation of metalloproteinase-9 (75). Whether GPER activation has a role in mitigating the production of intracellular chymase and/or activation of the local renin–angiotensin system in cardio fibroblasts would be an important area of investigation.
Conclusion
In conclusion, advanced age and estrogen loss led to decreases in myocardial relaxation and elevations in filling pressure in the F344BN strain, in part, due to reductions in phosphorylated phospholamban and increases in cardiac collagen deposition. Eight weeks of GPER activation by G1 in Old-OVX rats reversed the adverse effects of age and E2 loss on myocardial relaxation by enhancing SERCA2 expression and limiting interstitial fibrosis. These findings may explain the preponderance of HFpEF in older women after the menopause and provide a promising, late-life therapeutic target to reverse or halt the progression of LVDD, without the possibility of introducing any of the unfavorable side effects of classic estrogen receptor activation, such as cell proliferation, on aging breast or reproductive tissue (76).
Clinical Perspective
Impaired HFpEF is seen more often in women, and older women develop HFpEF twice as often as men of the same age (77). Although hospitalization rates and readmission rates for HF are similar between sexes (78), women with HF tend to have a worse quality of life than men, more physical limitations, longer hospital lengths of stay, and higher rates of depression (78). Currently, there is a significant gap in our knowledge regarding HFpEF and how to treat it (79). Taken together with the fact that preclinical LVDD antedates HFpEF, strategies that address the aging continuum and the cumulative impact of long-term exposure to such cardiovascular risk factors as estrogen deficiency, and its association with the pathobiology of aging, should be considered for optimal impact. Our study supports the notion that advanced age and estrogen deficiency are risk factors for diastolic dysfunction in the aged female heart, in part through effects on interstitial fibrosis and SR Ca2+ handling, and it emphasizes the potential benefit of late-in-life interventions involving GPER activation that could attenuate progression of this disease process.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by the National Institute on Aging (R01 AG-033727 to L.G.), the National Heart, Lung, and Blood Institute (P01 HL-051952 to C.M.F.) at the National Institutes of Health, and Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES to A.A.), (CAPES Proc. 001529/2014-05 to G.Z.S.), and (CAPES Proc. 007002/2014-09 to R.T.S.).
Supplementary Material
References
- 1. Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. 2011;26:562–568. doi:10.1097/HCO.0b013e32834b7faf [DOI] [PubMed] [Google Scholar]
- 2. Bhuiyan T, Maurer MS. Heart failure with preserved ejection fraction: persistent diagnosis, therapeutic enigma. Curr Cardiovasc Risk Rep. 2011;5:440–449. doi:10.1007/s12170-011-0184-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein L, Grau-Sepulveda MV, Bonow RO, et al. Quality of care and outcomes in women hospitalized for heart failure. Circ Heart Fail. 2011;4:589–598. doi:10.1161/CIRHEARTFAILURE.110.960484 [DOI] [PubMed] [Google Scholar]
- 4. Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000–2010. NCHS Data Brief. 2012;(108):1–8. [PubMed]
- 5. Zhao Z, Wang H, Jessup JA, Lindsey SH, Chappell MC, Groban L. Role of estrogen in diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H628–H640. doi:10.1152/ajpheart.00859.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maffucci JA, Gore AC. Age-related changes in hormones and their receptors in animal models of female reproductive senescence. In: Conn PM, ed. Handbook of Models for Human Aging. Burlington, MA: Elsevier; 2006:533–552. [Google Scholar]
- 7. Jessup JA, Wang H, MacNamara LM, et al. Estrogen therapy, independent of timing, improves cardiac structure and function in oophorectomized mRen2.Lewis rats. Menopause. 2013;20:860–868. doi:10.1097/GME.0b013e31820589a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L. Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2.Lewis rats. Cardiovasc Res. 2012;94:96–104. doi:10.1093/cvr/cvs090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhuiyan MS, Shioda N, Fukunaga K. Ovariectomy augments pressure overload-induced hypertrophy associated with changes in Akt and nitric oxide synthase signaling pathways in female rats. Am J Physiol Endocrinol Metab. 2007;293:E1606–E1614. doi:10.1152/ajpendo.00246.2007 [DOI] [PubMed] [Google Scholar]
- 10. Tagashira H, Bhuiyan S, Shioda N, Fukunaga K. Distinct cardioprotective effects of 17β-estradiol and dehydroepiandrosterone on pressure overload-induced hypertrophy in ovariectomized female rats. Menopause. 2011;18:1317–1326. doi:10.1097/gme.0b013e31821f915b [DOI] [PubMed] [Google Scholar]
- 11. Li J, Jubair S, Janicki JS. Estrogen inhibits mast cell chymase release to prevent pressure overload-induced adverse cardiac remodeling. Hypertension. 2015;65:328–334. doi:10.1161/HYPERTENSIONAHA.114.04238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walton RD, Jones SA, Rostron KA, et al. Interactions of short-term and chronic treadmill training with aging of the left ventricle of the heart. J Gerontol A Biol Sci Med Sci. 2015. In press. doi:10.1093/gerona/glv093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fannin J, Rice KM, Thulluri S, et al. Age-associated alterations of cardiac structure and function in the female F344xBN rat heart. Age (Dordr). 2014;36:9684 doi:10.1007/s11357-014-9684-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway x Fischer 344, and Fischer 344 x brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:B54–B59. doi:10.1093/gerona/51A.1.B54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell SJ, Scheibye-Knudsen M, Longo DL, de Cabo R. Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci. 2015;3:283–303. doi:10.1146/annurev-animal-022114-110829 [DOI] [PubMed] [Google Scholar]
- 16. Rice KM, Fannin JC, Gillette C, Blough ER. Efficacy of female rat models in translational cardiovascular aging research. J Aging Res. 2014;2014:153127 doi:10.1155/2014/153127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pei W, Bellows CG, Jia Y, Heersche JN. Effect of age on progesterone receptor expression, and osteoprogenitor proliferation and differentiation in female rat vertebral cell populations. J Endocrinol. 2006;190:261–270. [DOI] [PubMed] [Google Scholar]
- 18. Han G, White RE. G-protein-coupled estrogen receptor as a new therapeutic target for treating coronary artery disease. World J Cardiol. 2014;6:367–375. doi:10.4330/wjc.v6.i6.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer MR, Prossnitz ER, Barton M. GPER/GPR30 and regulation of vascular tone and blood pressure. Immunol Endocr Metab Agents Med Chem. 2011;11:255–261. doi:10.2174/1871522211108040255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bologa CG, Revankar CM, Young SM, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. [DOI] [PubMed] [Google Scholar]
- 21. Dennis MK, Field AS, Burai R, et al. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol. 2011;127:358–366. doi:10.1016/j.jsbmb.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prossnitz ER, Barton M. Estrogen biology: new insights into GPER function and clinical opportunities. Mol Cell Endocrinol. 2014;389:71–83. doi:10.1016/j.mce.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jessup JA, Zhang L, Chen AF, et al. Neuronal nitric oxide synthase inhibition improves diastolic function and reduces oxidative stress in ovariectomized mRen2.Lewis rats. Menopause. 2011;18:698–708. doi:10.1097/gme.0b013e31820390a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Groban L, Yamaleyeva LM, Westwood BM, et al. Progressive diastolic dysfunction in the female mRen(2). Lewis rat: influence of salt and ovarian hormones. J Gerontol A Biol Sci Med Sci. 2008;63:3–11. [DOI] [PubMed] [Google Scholar]
- 25. Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PLoS One. 2010;5:e15433 doi:1371/journal.pone.0015433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jessup JA, Zhang L, Presley TD, et al. Tetrahydrobiopterin restores diastolic function and attenuates superoxide production in ovariectomized mRen2.Lewis rats. Endocrinology. 2011;152:2428–2436. doi:10.1210/en.2011-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol. 1982;243:H941–H947. [DOI] [PubMed] [Google Scholar]
- 28. Sheydina A, Volkova M, Jiang L, et al. Linkage of cardiac gene expression profiles and ETS2 with lifespan variability in rats. Aging Cell. 2012;11:350–359. doi:10.1111/j.1474-9726.2012.00794.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim HS, Kang TS, Kang IH, et al. Validation study of OECD rodent uterotrophic assay for the assessment of estrogenic activity in Sprague-Dawley immature female rats. J Toxicol Environ Health A. 2005;68:2249–2262. [DOI] [PubMed] [Google Scholar]
- 30. Wang C, Dehghani B, Magrisso IJ, et al. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22:636–648. doi:10.1210/me.2007-0359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sergeant S, McQuail JA, Riddle DR, et al. Dietary fish oil modestly attenuates the effect of age on diastolic function but has no effect on memory or brain inflammation in aged rats. J Gerontol A Biol Sci Med Sci. 2011;66:521–533. doi:10.1093/gerona/glr017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lowe DA, Degens H, Chen KD, Alway SE. Glyceraldehyde-3-phosphate dehydrogenase varies with age in glycolytic muscles of rats. J Gerontol A Biol Sci Med Sci. 2000;55:B160–B164. doi:10.1093/gerona/55.3.B160 [DOI] [PubMed] [Google Scholar]
- 33. Qin F, Siwik DA, Lancel S, et al. Hydrogen peroxide-mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J Am Heart Assoc. 2013;2:e000184 doi:10.1161/JAHA.113.000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Q, Ren J. Influence of cardiac-specific overexpression of insulin-like growth factor 1 on lifespan and aging-associated changes in cardiac intracellular Ca2+ homeostasis, protein damage and apoptotic protein expression. Aging Cell. 2007;6:799–806. doi:10.1111/j.1474-9726.2007.00343.x [DOI] [PubMed] [Google Scholar]
- 35. Wan SH, Vogel MW, Chen HH. Pre-clinical diastolic dysfunction. J Am Coll Cardiol. 2014;63:407–416. doi:10.1016/j.jacc.2013.10.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shub C, Klein AL, Zachariah PK, Bailey KR, Tajik AJ. Determination of left ventricular mass by echocardiography in a normal population: effect of age and sex in addition to body size. Mayo Clin Proc. 1994;69:205–211. [DOI] [PubMed] [Google Scholar]
- 37. Carvalho JC, Farand P, Do HD, Brochu MC, Bonenfant F, Lepage S. Effect of age and sex on echocardiographic left ventricular diastolic function parameters in patients with preserved ejection fraction and normal valvular function. Cardiol J. 2013;20:513–518. doi:10.5603/CJ.2013.0137 [DOI] [PubMed] [Google Scholar]
- 38. de Simone G, Daniels SR, Kimball TR, et al. Evaluation of concentric left ventricular geometry in humans: evidence for age-related systematic underestimation. Hypertension. 2005;45:64–68. doi:10.1161/01.HYP.0000150108.37527.57 [DOI] [PubMed] [Google Scholar]
- 39. Burke GL, Arcilla RA, Culpepper WS, Webber LS, Chiang YK, Berenson GS. Blood pressure and echocardiographic measures in children: the Bogalusa Heart Study. Circulation. 1987;75:106–114. doi:10.1161/01.CIR.75.1.106 [DOI] [PubMed] [Google Scholar]
- 40. Arbab-Zadeh A, Dijk E, Prasad A, et al. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi:10.1161/01.CIR.0000142863.71285.74 [DOI] [PubMed] [Google Scholar]
- 41. Pérez-David E, García-Fernández MA, Ledesma MJ, et al. Age-related intramyocardial patterns in healthy subjects evaluated with Doppler tissue imaging. Eur J Echocardiogr. 2005;6:175–185. doi:10.1016/j.euje.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 42. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi:10.1161/01.CIR.0000048893.62841.F7 [DOI] [PubMed] [Google Scholar]
- 43. Aurigemma GP, Zile MR, Gaasch WH. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation. 2006;113:296–304. doi:10.1161/CIRCULATIONAHA.104.481465 [DOI] [PubMed] [Google Scholar]
- 44. Petrie MC, Caruana L, Berry C, McMurray JJ. “Diastolic heart failure” or heart failure caused by subtle left ventricular systolic dysfunction? Heart. 2002;87:29–31. doi:10.1136/heart.87.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Popović ZB, Prasad A, Garcia MJ, et al. Relationship among diastolic intraventricular pressure gradients, relaxation, and preload: impact of age and fitness. Am J Physiol Heart Circ Physiol. 2006;290:H1454–H1459. doi:10.1152/ajpheart.00902.2005 [DOI] [PubMed] [Google Scholar]
- 46. Groban L, Wang H, Machado FS, et al. Low glial angiotensinogen improves body habitus, diastolic function, and exercise tolerance in aging male rats. Cardiovasc Endocrinol. 2012;1:49–58. doi:10.1097/XCE.0b013e32835a2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kitzman DW, Groban L. Exercise intolerance. Cardiol Clin. 2011;29:461–477. doi:10.1016/j.ccl.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi:10.1056/NEJMoa032566 [DOI] [PubMed] [Google Scholar]
- 49. Groban L, Pailes NA, Bennett CD, et al. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. [DOI] [PubMed] [Google Scholar]
- 50. Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67:871–885. doi:10.1161/01.RES.67.4.871 [DOI] [PubMed] [Google Scholar]
- 51. Eghbali M, Eghbali M, Robinson TF, Seifter S, Blumenfeld OO. Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc Res. 1989;23:723–729. doi:10.1093/cvr/23.8.723 [DOI] [PubMed] [Google Scholar]
- 52. Olivetti G, Giordano G, Corradi D, et al. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26:1068–1079. doi:10.1016/0735-1097(95)00282-8 [DOI] [PubMed] [Google Scholar]
- 53. Janczewski AM, Lakatta EG. Modulation of sarcoplasmic reticulum Ca(2+) cycling in systolic and diastolic heart failure associated with aging. Heart Fail Rev. 2010;15:431–445. doi:10.1007/s10741-010-9167-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li L, Desantiago J, Chu G, Kranias EG, Bers DM. Phosphorylation of phospholamban and troponin I in beta-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol. 2000;278:H769–H779. [DOI] [PubMed] [Google Scholar]
- 55. Taffet GE, Tate CA. CaATPase content is lower in cardiac sarcoplasmic reticulum isolated from old rats. Am J Physiol. 1993;264(Pt 2):H1609–H1614. [DOI] [PubMed] [Google Scholar]
- 56. Schmidt U, del Monte F, Miyamoto MI, et al. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circulation. 2000;101:790–796. doi:10.1161/01.CIR.101.7.790 [DOI] [PubMed] [Google Scholar]
- 57. MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi:10.1038/nrm1151 [DOI] [PubMed] [Google Scholar]
- 58. Xu A, Narayanan N. Effects of aging on sarcoplasmic reticulum Ca2+-cycling proteins and their phosphorylation in rat myocardium. Am J Physiol. 1998;275(Pt 2):H2087–H2094. [DOI] [PubMed] [Google Scholar]
- 59. Sande JB, Sjaastad I, Hoen IB, et al. Reduced level of serine(16) phosphorylated phospholamban in the failing rat myocardium: a major contributor to reduced SERCA2 activity. Cardiovasc Res. 2002;53:382–391. doi:10.1016/S0008-6363(01)00489-8 [DOI] [PubMed] [Google Scholar]
- 60. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi:10.1126/science1106943 [DOI] [PubMed] [Google Scholar]
- 61. Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi:10.1210/en.2004-1064 [DOI] [PubMed] [Google Scholar]
- 62. Kang S, Liu Y, Sun D, et al. Chronic activation of the G protein-coupled receptor 30 with agonist G-1 attenuates heart failure. PLoS One. 2012;7:e48185 doi:10.1371/journal.pone.0048185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fares E, Parks RJ, Macdonald JK, Egar JM, Howlett SE. Ovariectomy enhances SR Ca²+ release and increases Ca²+ spark amplitudes in isolated ventricular myocytes. J Mol Cell Cardiol. 2012;52:32–42. doi:10.1016/j.yjmcc.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 64. Ma Y, Cheng WT, Wu S, Wong TM. Oestrogen confers cardioprotection by suppressing Ca2+/calmodulin-dependent protein kinase II. Br J Pharmacol. 2009;157:705–715. doi:10.1111/j.1476-5381.2009.00212.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mobley BA. Ca2+ capacity and uptake rate in skinned fibers of myodystrophic muscle. Exp Neurol. 1985;87:137–146. doi:10.1016/0014-4886(85)90140-2 [DOI] [PubMed] [Google Scholar]
- 66. Wang H, Zhao Z, Lin M, Groban L. Activation of GPR30 inhibits cardiac fibroblast proliferation. Mol Cell Biochem. 2015;405:135–148. doi:10.1007/s11010-015--2405-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi:10.1161/01.CIR.0000147181.65298.4D [DOI] [PubMed] [Google Scholar]
- 68. Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM. Uptake and metabolism of the novel peptide angiotensin-(1-12) by neonatal cardiac myocytes. PLoS One. 2011;6:e15759 doi:10.1371/journal.pone.0015759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Miyazaki M, Takai S. Local angiotensin II-generating system in vascular tissues: the roles of chymase. Hypertens Res. 2001;24:189–193. doi:10.129/hypres.24.189 [DOI] [PubMed] [Google Scholar]
- 70. Ahmad S, Varagic J, Groban L, et al. Angiotensin-(1-12): a chymase-mediated cellular angiotensin II substrate. Curr Hypertens Rep. 2014;16:429 doi:10.1007/s11906-014-0429-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bouzegrhane F, Thibault G. Is angiotensin II a proliferative factor of cardiac fibroblasts? Cardiovasc Res. 2002;53:304–312. doi:10.1016/S0008-6363(01)00448-5 [DOI] [PubMed] [Google Scholar]
- 72. Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi:10.1152/ajpcell.00287.2006 [DOI] [PubMed] [Google Scholar]
- 73. Zhao XY, Zhao LY, Zheng QS, et al. Chymase induces profibrotic response via transforming growth factor-beta 1/Smad activation in rat cardiac fibroblasts. Mol Cell Biochem. 2008;310:159–166. doi:10.1007/s11010-007-9676-2 [DOI] [PubMed] [Google Scholar]
- 74. Takai S, Jin D, Miyazaki M. Multiple mechanisms for the action of chymase inhibitors. J Pharmacol Sci. 2012;118:311–316. doi:10.1254/jphs.11R11CP [DOI] [PubMed] [Google Scholar]
- 75. Oyamada S, Bianchi C, Takai S, Chu LM, Sellke FW. Chymase inhibition reduces infarction and matrix metalloproteinase-9 activation and attenuates inflammation and fibrosis after acute myocardial ischemia/reperfusion. J Pharmacol Exp Ther. 2011;339:143–151. doi:10.1124/jpet.111.179697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Deschamps AM, Murphy E, Sun J. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends Cardiovasc Med. 2010;20:73–78. doi:10.1016/tcm.2010.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. doi:10.1016/j/jacc.2003.07.046 [DOI] [PubMed] [Google Scholar]
- 78. Shin JJ, Hamad E, Murthy S, Piña IL. Heart failure in women. Clin Cardiol. 2012;35:172–177. doi:10.1002/clc.21973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Upadhya B, Taffet GE, Cheng CP, Kitzman DW. Heart failure with preserved ejection fraction in the elderly: scope of the problem. J Mol Cell Cardiol. 2015;83:73–87. doi:10.1016/j.yjmcc.2105.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.