Abstract
Novel therapies have turned to delivering compounds to the brain using nasal sprays, bypassing the blood brain barrier, and enriching treatment options for brain aging and/or Alzheimer’s disease. We conducted a series of in vivo experiments to test the impact of intranasal Apidra, a zinc-free insulin formulation, on the brain of young and aged F344 rats. Both single acute and repeated daily doses were compared to test the hypothesis that insulin could improve memory recall in aged memory-deficient animals. We quantified insulin signaling in different brain regions and at different times following delivery. We measured cerebral blood flow (CBF) using MRI and also characterized several brain metabolite levels using MR spectroscopy. We show that neither acute nor chronic Apidra improved memory or recall in young or aged animals. Within 2 hours of a single dose, increased insulin signaling was seen in ventral areas of the aged brains only. Although chronic Apidra was able to offset reduced CBF with aging, it also caused significant reductions in markers of neuronal integrity. Our data suggest that this zinc-free insulin formulation may actually hasten cognitive decline with age when used chronically.
Key Words: Cognition, Diabetes, Metabolism, Vascular
Cognitive decline in elderly people whether resulting from medication use or pre-existing pathological conditions like stress, inflammation, or brain injury is clearly an important feature of aging that carries a large socioeconomic burden. Estimated prevalence for mild cognitive decline is near 20% for individuals older than 70 years (1,2), and recent evidence suggests that this may be partially mediated by the presence of diabetes at earlier times (3,4). Several therapies have been suggested and implemented to address symptoms of memory loss with aging and mostly include life style changes associated with increasing exercise, managing cardiovascular risk factors, and improving diet (5–7). To date, however, no effective pharmacological treatment is available, which is particularly bothersome given the expansion of the aging population, its relationship to Alzheimer’s disease (AD), and the tripling in the incidence of dementia predicted by 2050 (8).
It has been shown recently that in young adults, intranasal (IN) delivery of insulin can improve declarative and spatial memory recall (9–12). Similarly, in patients with mild cognitive impairment in the early stages of AD, several clinical studies have noted improvement in word recall, as well as in delayed memory and cognitive abilities (11,13–15). In these studies, improved recall was seen following a single insulin dose (14,15), as well as in response to four repeated daily doses for 8 weeks (9,10) or for 21 days, or two repeated daily doses for 4 months (13). Further, these clinical studies typically conducted cognitive tests as early as 15–60 minutes, and in some cases as late as 12 hours after the last IN insulin dose. Importantly, almost all prior clinical trials (with the exception of a single dose pilot trial (16) and the ongoing Study of Nasal Insulin to Fight Forgetfulness—SNIFF trial) have used a non zinc–containing formulation for the placebo/control group whereas the insulin formulations used for IN delivery contained zinc. Also, mostly short or intermediate-acting insulin formulations have been used in the past. However, a recent study using long-lasting insulin (Levemir) reported on enhanced visuospatial working memory in mild cognitive impairment and AD patients carrying the APOE epsilon 4 (17). Overall, these studies strongly suggest that even with such diversity in experimental designs, the vast majority of clinical reports shows improved memory in response to IN insulin.
However, the mechanisms underlying these results in humans have not been clearly defined, and only a few reports have measured the impact of IN insulin on cognitive function in animal models (18–21). Understanding the mechanism of action, the duration of action, and the cellular targets of IN insulin in animal models is potentially a critical issue as chronic activation of the receptor in the brain could lead to receptor downregulation/desensitization. Indeed, animal studies using intracerebroventricular (ICV) insulin delivery have shown both beneficial and detrimental effects of insulin depending on whether insulin exposure is acute or prolonged (22–24) and whether younger or older animals are used (24). Further, AD mice treated once with IN insulin 24 hours prior to training on the T-maze task show improved learning as well as retention 7 days later (20). Administration of a single dose, however, 7 days prior to training did not improve performance. In the same study, a single dose of IN insulin administered 5 minutes but not 24 hours after training was able to ameliorate retention when tested 7 days later. These results indicate insulin needs to be present during task acquisition and may also participate in strengthening integration or storage during task consolidation. What is less clear, still, is the mechanism of action for IN insulin once it reaches the brain, the duration of the signal, or whether neuronal, glial, or vascular elements respond to that signal.
Given the pressing need to understand the actions of IN insulin on memory or recall function, a series of experiments were initiated to characterize the potential impact of IN zinc-free insulin (insulin glulisine—Apidra) on reversing cognitive decline with age in F344 rats. Based on prior work from our group providing evidence that IN insulin could improve recall without altering learning (21), we tested whether we could reproduce the beneficial impact of repeated insulin application with a single application of Apidra on the day of recall. We used similar techniques, doses, and time of application, as well as similar animals and behavioral outcomes as in our recently published study (21) to measure the actions of Apidra in the brain. Outcome measures include Morris water maze (MWM) learning and 24-hour recall, molecular biology (Western pAkt/Akt signaling), and MRI to measure blood flow as well as to quantify selected brain metabolites with magnetic resonance spectroscopy (MRS).
Methods
All animal experiments were conducted in compliance with the ARRIVE (Animal Research: Reporting in Vivo Experiments) guidelines. The work was performed according to the Revised Guide for the Care and Use of Laboratory Animals (U.S. government) and strictly adhered to our institutional licensing committee for the care and use of animals (Institutional Animal Care and Use Committee—IACUC 000959M2003). Animals were housed in pairs and tail marked for identification. All F344 rats (males) were obtained from the National Institute on Aging colony (Charles River Laboratories) and were barrier raised under specific pathogen-free conditions. All animals were maintained on a 12 hours ON, 12 hours OFF light schedule and were fed the Teklad global 18% protein rodent diet ad libitum (2018; Harlan Laboratories, Madison, WI).
Intranasal Delivery
We used the same methods as previously published (21) to deliver insulin. Because one group of animals only received a single dose of insulin (acute), while all other animals received multiple doses, the study was conducted with experimenters knowing the group allocation during the experiment and during the analysis phase. Briefly, the animals were transiently held supine in a DecapiCone (Braintree Scientific, Braintree, MA) while two 5 µL doses (1 minute apart) of either sterile saline or short-acting Apidra were delivered to the right naris using a P10 Eppendorf pipetter. The Apidra dose (0.0715 IU/ 10 µL) was chosen to approximate levels used in numerous clinical trials (equivalent to 10 IU/day) and because this dose was shown recently to improve memory recall in aged F344 rats using the same application protocol and duration (21). Apidra (insulin glulisine) was made fresh weekly and diluted from a U-100 vial (Sanofi-Aventis, Bridgewater, NJ) using a sterile saline solution. Insulin or saline treatment was initiated 4 days prior to the initiation of training (cue day) and was delivered on average, 2 hours before testing on the MWM. For the acute phase of the study, all animals received one daily saline dose for 8 days, with MWM training (cue day) starting on Day 5. On Day 9 (the memory recall day), animals received either Apidra or saline. For the chronic phase, animals received either daily Apidra or saline for 9 days.
Blood Glucose Levels
To test whether Apidra might cross the brain-to-blood barrier and lower peripheral blood sugar levels, we measured blood glucose (FreeStyle Lite glucometer; Abbott Laboratories, Abbott Park, IL) from dorsal tail veins in three aged animals exposed to IN insulin (0.0715 IU/ 10 µL). Measures were taken prior and 60 as well as 90 minutes after IN delivery.
Spatial Behavior
Young (3 months old) and aged (21 months old) male F344 rats were used for all experiments. Behavior was conducted on 60 animals (n = 10 per group). The groups included young chronic saline, young chronic insulin, young acute insulin, aged chronic saline, aged chronic insulin, and aged acute insulin. Four animals in the aged chronic insulin group were removed from the analysis based on poor performance. One animal was in poor physical health (weak and thin) even prior to the initiation of IN insulin delivery, and the other three displayed typical signs of thigmotactic behavior keeping close to the wall of the pool on almost all trials. It is not clear whether this heightened state of anxiety was related to the insulin delivery. Overall, behavioral data are presented on 56 animals.
Water temperature in the MWM was maintained between 25 °C and 26 °C and was made opaque with black tempura paint to hide a submerged escape platform placed approximately 1.5cm below the surface of the water. The pool is 190cm and the escape platform is 15cm in diameter. Animals were allowed 60 seconds to find the platform, after which they were guided to it. Each animal stayed on the platform for 30 seconds before returning to a heated holding chamber for about 2 minutes. On the first day of training (visual cue day), a white cup hanging above the partially submerged platform helped orient the animals for three consecutive 60-second trials (data are not reported on cue day). Following cue day, animals were subjected to three trials per day (semi-random drop location for each trial) for 3 days. Twenty-four hours after the last training day, a probe trial was initiated with the platform removed (60 seconds of max swim time). Swim speed was derived from the distance travelled over time on the last trial of the third training day and on the first trial of the cue day. No difference in swim speed was seen across aging or treatment in this cohort of animals (data not shown). On all cue and training days, the intertrial interval was approximately 150 seconds. We present data on path length measures and on the numbers of platform crossings on the 24-hour memory recall day (probe). A Videomex-V acquisition and analysis software (version 4.64, Columbus Instruments, Columbus, OH) was used to track and measure animal location.
Western Blotting
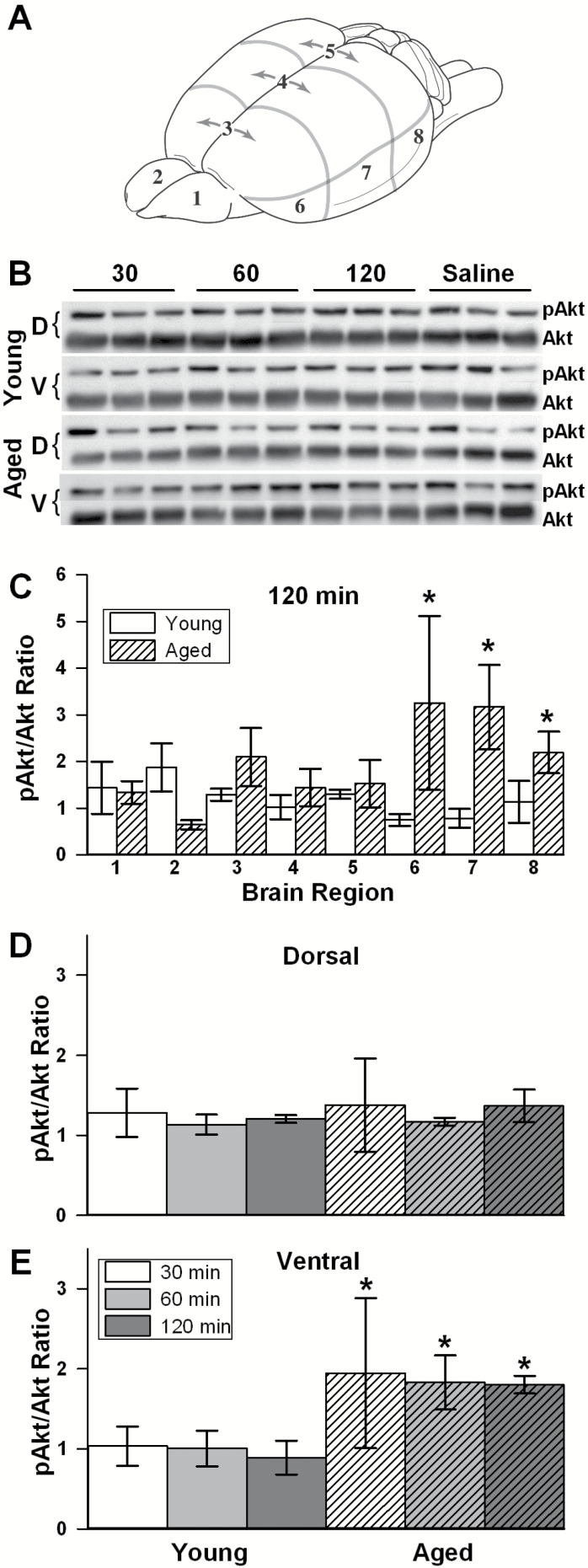
Twenty-four animals (n = 12 young [3–4 months old] and 12 aged [21–24 months of age]) were used for Western blot analyses of acute IN Apidra effects in eight brain regions. Those different brain regions were separated into left and right olfactory bulbs and as well as three dorsal (rostral, medial, and caudal) and three ventral areas (rostral, medial, and caudal; see Figure 2). Brains were cut in ice-cold low-calcium artificial cerebrospinal fluid of the following composition (in mM): 128 NaCl, 1.25 KH2PO4, 10 Glucose, 26 NaHCO3, 3 KCl, 0.1 CaCl2, 2 MgCl2; and regions were placed on ice in cold tubes filled with cold homogenization buffer supplemented with a protease and phosphatase inhibitor cocktail (sucrose base, Triton X buffer with sodium dodecyl sulfate). Homogenization was accomplished within 1 hour of collection (Geno/Grinder 2010, SPEX SamplePrep, Metuchen, NJ).
Figure 2.
Insulin signaling. (A) The brain of each animal was cut into regions. (B) Tissue was harvested 30, 60, or 120 minutes following intranasal (IN) Apidra and was probed with pAkt and Akt antibodies. (C) Data were normalized to the saline condition on each gel and show greater increases in insulin signaling in the three ventral regions of the brain at 120 minutes post IN insulin in aged animals. (D) No increase in insulin signaling was noted in the combined dorsal regions. (E) A significant increase in the ventral regions (combined) was seen in aged compared with young animals (two-way analysis of variance [ANOVA]; p < .05) as early as 30 minutes post delivery (two-way ANOVA; p < .05). Data represent mean ± SEM from 24 animals.
We determined the levels of phosphorylated Akt (Ser473) and total Akt (pan) using primary antibodies (4051 and 4685, respectively) and secondary antibodies (7076 and 7074, respectively) from Cell Signaling Technologies (Danvers, MA). All antibodies were used at 1:1,000 except for the anti-rabbit secondary antibody to pan Akt, which was used at 1:25,000. The ratio of these two proteins represents activation of the insulin signaling cascade with greater numbers indicating elevated signal transduction though the canonical pathway. The pAkt/Akt ratios were derived from tissues harvested at 30, 60, and 120 minutes following IN delivery of either 0.075 IU/10 µL Apidra (n = 3 rats/age/time point/condition) or saline (n = 3 young and 3 aged, with one animal for each time point). For all gel quantification, signals were normalized to the saline condition obtained from the same brain region in IN saline age-matched animals. The G:BOX-XT4 and Genesys software (Syngene, Frederick, MD) were used for image acquisition. Band quantification used the area-under-the-curve method (Image J version 1.47u; NIH).
MRI/MRS Brain Scans
Twenty animals chosen randomly from the acute Apidra and the chronic saline arms of the study (5 young saline, 5 aged saline, 5 young insulin, and 5 aged insulin) were used for MRI measures following a single dose of either saline or insulin approximately 20 days after the last exposure to insulin or saline. Ten animals randomly chosen from the chronic Apidra arm of the study (5 young and 5 aged) were used for MRI measures following approximately 3–4 weeks of continued daily dosing (total of 16–20 Apidra doses). To keep in time with the results of the behavioral analysis, animals were scanned on average 2 hours after IN saline or insulin delivery.
MRI experiments were performed on a 7T Clinscan MR scanner (Siemens, Germany) at the Magnetic Resonance Imaging & Spectroscopy Center of the University of Kentucky. Rats were anesthetized with 4.0% isoflurane for induction and then maintained in a 1%–2.5% isoflurane and air mixture using a nose cone. Heart rate (90–110 bpm), respiration rate (50–80 breaths/min), and rectal temperature (36±1 °C) were continuously monitored. A water bath with circulating water at 50–55 °C was placed outside the room and used to maintain body temperature.
Cerebral blood flow (CBF) was measured using a pseudocontinuous arterial spin labeling technique. Paired images were acquired with field of view (FOV) = 40×30mm2, matrix = 128×128, slice thickness = 1mm, slice = 4, 120 measurements, labeling duration = 2,100ms, repetition time (TR) = 4,000ms, and echo time (TE) = 20ms. Quantitative CBF (mL/g/min) was computed employing codes written in Matlab (Natick, MA). A total of 30 animals were scanned, however, in three aged animals (two saline and one acute Apidra), image resolution was compromised and the CBF variable was not included in the analysis. As such, CBF data are reported on 3–5 animals per group.
Following CBF measurements, we acquired proton (1H) MR spectra (MRS) to determine brain metabolite levels. In vivo 1H-MRS were obtained using a point-resolved spectroscopy sequence. Water-suppressed spectra were acquired with following parameters: TR = 1,500ms, TE = 135ms, spectral width = 60 Hz and average = 400. A voxel of interest of 18.2mm3 (2.0×7.0×1.3mm) covered bilateral hippocampus. An acquisition of nonwater suppressed spectrum with 10 averages was followed (the rest of the parameters were kept the same). Both with- and without-water suppression spectra were then processed using LCModel to determine the concentrations of the metabolites. LCModel uses a linear combination of model spectra of metabolite solutions in vitro to analyze the major resonances of in vivo spectra (25). MRS data are reported on 30 animals, with n = 5 per group. The protocol, including MRI and MRS, took just less than 60 minutes for each rat.
Statistics
Statistical analyses tested for significance (p < .05) on main factors and interactions using two-way analysis of variances and repeated measures analysis of variances. Bonferroni or Tukey’s tests were used for post hoc comparisons. All tests were conducted using StatView statistical package (version 5, Cary, NC) or GraphPad Prism V5 (San Diego, CA).
Results
Acute IN Apidra Does Not Lower Systemic Blood Glucose Levels
As previously shown (21), IN insulin does not lower serum glucose levels, indicating very little insulin, if any, travelled in the brain-to-blood direction. The technique rapidly increases levels of insulin and other substances via a perivascular pathway from the nasal epithelium to the brain (26,27). To test whether insulin glulisine could lower peripheral blood glucose levels, three non-fasted aged F344 rats were given 0.075 IU Apidra in two 5 µL IN doses 1 minute apart as described in the methods section. Blood glucose levels measured before and 60 or 90 minutes after IN delivery did not decrease over time indicating IN Apidra does not lower systemic glucose (preinsulin levels = 55.7±10.5; post insulin 60 minutes = 58.6±10.9 in mg/dL; post insulin 90 minutes = 65.0±15.0; p > .05).
Neither Acute Nor Chronic IN Apidra Improves Learning or Memory on the Spatial MWM
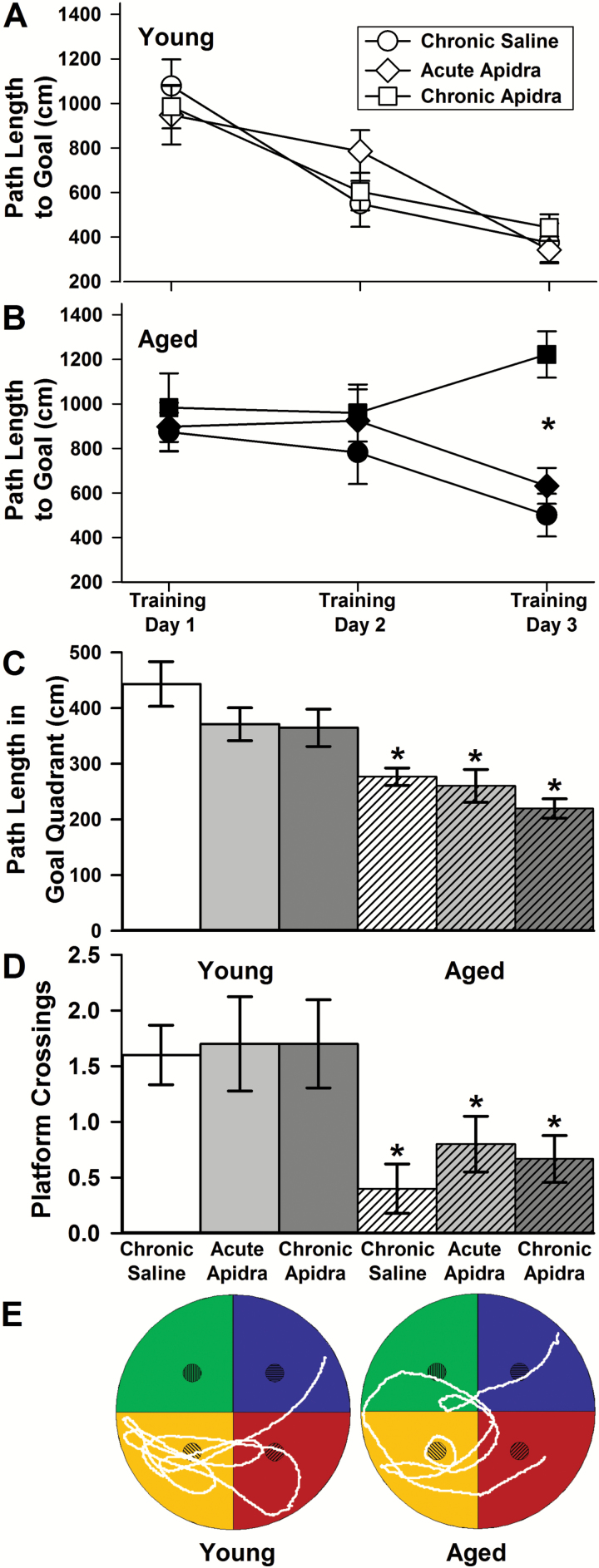
In this series of experiments, we tested whether a single or several repeated daily doses of IN insulin could ameliorate 24-hour recall on the MWM task in aged F344 rats. As shown in Figure 1, neither a single nor repeated Apidra dose improved learning or memory of the platform location. Based on path length measures, young animals learned the task across days of training (Figure 1A; F(2,54) = 32.3; p < .0001), however as a group, aged animals did not (Figure 1B; F(2,46) = 1.1; p = .34). This was mostly mediated by the poor performance of chronically treated animals compared with saline or acutely treated animals on the third day of training (p < .001). Further, upon testing for recall of platform location 24 hours following the last training day, aged animals continued to perform poorly, showing shorter path lengths in the goal quadrant during the initial 30 seconds of the probe trial (Figure 1C; F(1,50) = 30.8; p < .0001) and significant reductions in the number of platform location crossings in that period (Figure 1D; F(1,50) = 15.4; p < .0003). Thus, neither single dose nor repeated doses of IN Apidra could improve memory recall, and more importantly, chronic insulin appeared to have a negative impact on learning in aged animals. For these experiments, insulin was always delivered approximately 2 hours prior to testing on the MWM. This is a surprising result given our prior work showing that repeated daily doses of either Humalog or Levemir, a short and a long-acting insulin formulation (respectively), improved recall on the MWM task (21). Based on this new result and the lack of a peripheral glucose effect, we sought to test whether IN Apidra could increase insulin signaling in the brain of young and aged F344 rats.
Figure 1.
Spatial learning and memory. Path length to platform across three training days is shown in (A) young and (B) aged animals receiving chronic intranasal (IN) saline, acute IN Apidra, or chronic IN Apidra. It is important to note that in the learning phase of the task, the saline groups show similar patterns as the acute insulin groups given that those animals were only exposed to insulin once, approximately 2 hours prior to the probe trial (eg, Day 4). Although all young animals learned to find the platform across days of training (two-way analysis of variance [ANOVA]; p < .0001), as a group, aged animals did not (two-way ANOVA; p = .34). This was due mostly to the poor performance seen on Day 3 in the aged animals treated chronically with IN Apidra (post hoc; p < .001). Twenty-four hours after training, probe trial data yielded changes (C) in path length in the goal quadrant during the first 30 seconds as well as (D) in the number of platform crossings. Both variables indicate poor memory recall in aged animals (path length; two-way ANOVA; p < .0001 and platform crossings; two-way ANOVA; p < .0003). Neither acute nor chronic IN insulin affected recall performance. (E) Representative paths taken by a young and an aged saline-treated animal during the first 30 seconds of the 24-hour recall trial. Data represent mean ± SEM from 6–10 animals per group.
Acute IN Apidra Increases Insulin Signaling Across Different Brain Regions in Aged Animals
To test whether Apidra could penetrate the brain and alter insulin signaling, we completed a series of experiments in young and aged rats quantifying the canonical insulin signaling pathway using phospho Akt (pAkt) and total Akt immunoblotting in eight different brain areas (Figure 2). The pAkt/Akt ratios were derived from tissues harvested 30, 60, and 120 minutes following IN delivery of either 0.075 IU/10 µL Apidra or saline (n = 24 rats). For each blot, the pAkt/Akt ratio derived from animals treated with insulin was normalized to the ratio obtained in the same brain area but in animals treated with saline. The group data at the 2-hour time point show that aged animals responded to Apidra with greater increases in signaling when compared with young animals (F(1,32) = 5.9; p < .05; Figure 2C). No significant differences were noted between age groups at the 30- and the 60-minute time points (data not shown). Whereas no change in insulin signaling was detected in the dorsal regions of the brain irrespective of age or treatment (Figure 2D), greater insulin signaling was seen in the ventral regions of the brain (F(1,12) = 6.0; p < .05; Figure 2E).
Chronic IN Apidra Increases CBF and Alters Metabolism
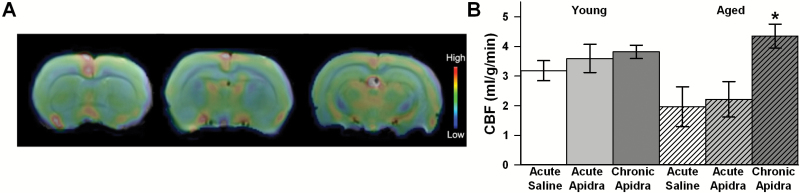
With evidence of Apidra permeation and increased signaling centered around a 2-hour window following IN delivery, we tested whether Apidra could alter CBF and/or cellular metabolism using MRI and MRS, respectively. Experiments were conducted to test the impact of Apidra following a single dose (acute) or following 16–20 repeated doses (chronic). In this cohort of animals, an age-dependent trend for reduced CBF (F(1,21) = 3.6; p = .07) was noted and was significantly reversed by chronic IN Apidra animals (F(2,21) = 6.6; p < .006; Figure 3B).
Figure 3.
MRI data on cerebral blood flow (CBF). (A) Representative CBF scans (pseudocolored) from aged animals. (B) Quantification reveals a trend for a decrease in CBF with aged animals compared with young animals (two-way analysis of variance [ANOVA]; p = .07) together with a significant increase in CBF in response to chronic intranasal Apidra in aged animals only (two-way ANOVA; p < .006). Data represent mean ± SEM from 5 animals per group.
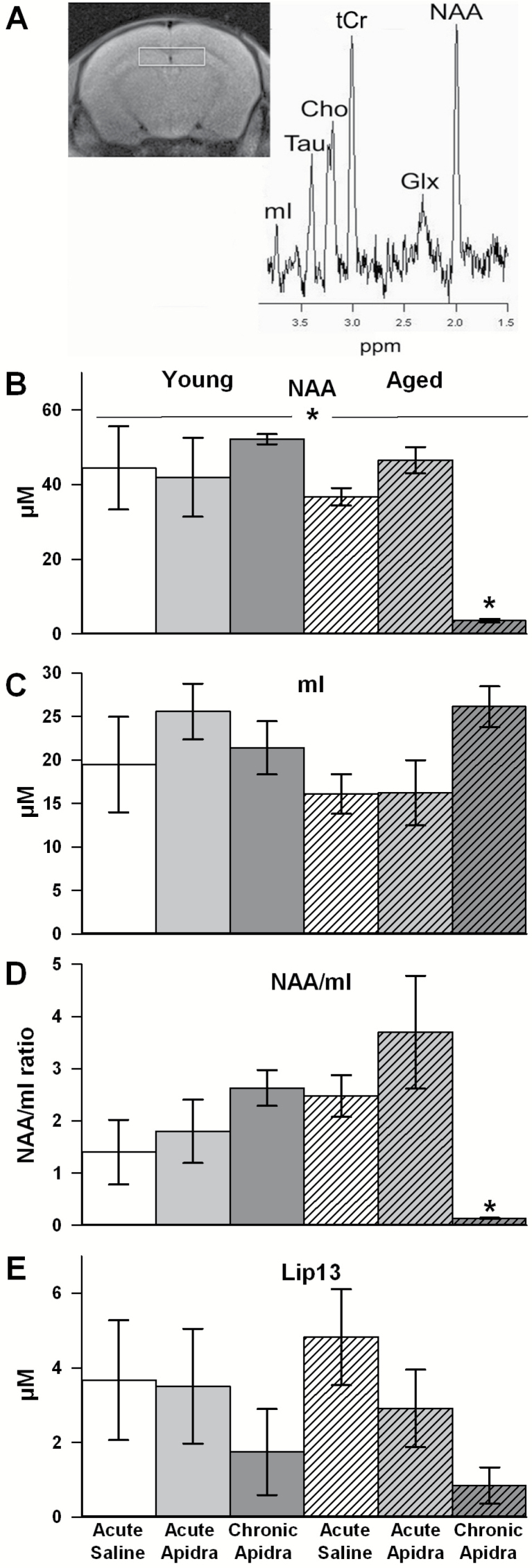
As previously reported (28), proton MR spectroscopy analyses of hippocampal regions revealed a significant main effect of age with reductions in N-acetylaspartate (NAA, a marker of neuronal viability signals (F(1,24) = 10.5; p < .005; Figure 4B). Post hoc analysis reveals that chronic IN Apidra severely intensified this signal decline in aged animals only (p < .01; Figure 4B). No main effect of age or IN insulin was found on measures of the astrocyte reactivity marker myo-Inositol (mI; Figure 4C), however, the ratio of NAA and mI, thought to gauge the extent of neurodegeneration in numerous disease states (28), was found to be significantly changed. Two-way analysis of variance results indicate the presence of a significant interaction term (F(2,24) = 7.5; p < .005; Figure 4D) whereby chronic IN insulin significantly reduced this ratio in aged animals only (p < .05). Of note, in both young and aged animals chronically treated with IN Apidra, a trend for reduced lipid peak signals at 1.3ppm (lip13) was found (F(2,24) = 2.9; p = .07; Figure 4E). Increases in this particular metabolite have been associated with lipid storage disorders and increases in demyelination as well as in glioblastomas and tumor metastasis (29).
Figure 4.
Magnetic resonance spectroscopy data on several brain metabolites. (A) Representative section scan showing the region of interest around the hippocampus (left) and the resulting spectrum used to identify key metabolites (right). (B) A significant main effect of age in n-acetylaspartate (NAA) signal was noted (two-way analysis of variance [ANOVA]; p < .005) and post hoc analysis revealed a dramatic loss in signal in the aged animals treated chronically with intranasal Apidra (p > 0.01). (C) Although myo-Inositol (mI) signals were unaffected by age or treatment, (D) the ratio of NAA to mI signal was significantly reduced in aged animals chronically treated with intranasal Apidra (two-way ANOVA interaction term, p < .005). (E) A robust trend for a drug effect was also noted on the lipid peak signal at 1.3ppm (lip-13; two-way ANOVA; p = .07). Data represent mean ± SEM from 3–5 animals per group.
Discussion
In this study, we investigated whether IN Apidra could alleviate aspects of memory decline seen in aged animals. Here we provide evidence that chronic IN Apidra reduced learning and memory in aged animals. Our test of whether IN insulin could increase insulin signaling in the brain of young and aged animals showed increases in the pAkt/Akt ratio approximately 2 hours following delivery. This signaling was particularly more pronounced in the aged animals and to a greater extent, in the ventral regions of the brain. Finally, using MRS techniques, we were able to identify potential mechanisms by which chronic IN insulin was able to reduce learning in aged animals. Those included a large decrease in NAA signal and a trend for a decrease in mI, markers of neuronal integrity and glial overactivation (inflammation), respectively.
Why Study Apidra?
We chose to study zinc-free insulin (Apidra) because zinc is an important component of most insulin formulations, and almost all prior clinical IN studies have used zinc-containing insulin. In our prior work using IN insulin delivery, we showed that zinc-containing Levemir or Humalog could improve memory recall in two different cohorts of aged animals (21). In that same study, we also showed that both zinc and Apidra could reduce the Ca2+-dependent afterhyperpolarization (AHP) in young and aged hippocampal neurons, highlighting a potential mechanism for insulin’s effects on memory. Still, because zinc is a key nutrient and an integral part of glutamate neurotransmission in the brain (30–32), and because of prior reports that zinc use may be associated with nasal endothelium toxicity (33,34), we sought to test whether a zinc-free insulin formulation could enhance memory in aged animals.
Surprisingly, we show here that although a single IN presentation of Apidra was able to increase insulin signaling (Figure 2), neither the acute nor the chronic delivery of Apidra was able to improve memory recall in aged animals (Figure 1). Importantly, chronic delivery of Apidra reduced performance on Day 3 of training unmasking a potentially negative consequence of insulin on the learning process. A recent report in mild-to-moderate ApoE4 carrier subjects shows that acute single-dose IN Apidra failed to ameliorate cognition (16). This may reflect a cohort-specific effect however, as neither insulin formulations used appears to have increased performance over placebo in the SNIFF trial. Our results show that in an animal model of aging, use of IN Apidra does not seem able to combat cognitive decline in brain aging.
Based on prior work showing that Apidra has slightly faster pharmacokinetics than regular human insulin (reviewed in 35) but similar affinity for the insulin receptor (36,37), we used the same dose of insulin (0.0715 IU/ 10 µL) as used in our prior study (21) and assumed equal uptake and distribution of the zinc-free peptide. Although the pAkt/Akt data presented here support entry of insulin into the brain, at 2 hours post-dose, signaling was found to be limited to the ventral areas of the brain which may have reduced the impact of insulin. Indeed, the entire hippocampus and several adjacent brain areas are critical for spatial mapping of the escape platform during acquisition and memory retrieval (38,39). Thus, perhaps a longer period between delivery and testing is necessary when working with zinc-free insulin formulations. Further signaling experiments using IN Humalog or Apidra should help to clarify whether distribution changes with time following delivery. Finally, it is possible that the cognitive demands imposed on this cohort of aged animals may have required increased doses of insulin. It should be noted, however, that larger doses of insulin did not improve recall behavior when compared with lower doses in aged F344 rats (21) and younger Sprague-Dawley rats (40).
Is the Aged Brain Less or More Sensitive to Insulin?
In the brain, insulin receptors, message, and function have been shown to decline with age (41–43), but see (44). As individuals age, the effects of diabetes and peripheral insulin resistance appear to have a greater impact on cognitive function. However, given that learning induces increases in insulin receptor signaling in the brain (45), the reduction in insulin signaling seen in aging could well reflect poor learning in aged animals. Alternatively, this may also reflect signaling saturation and transport/receptor downregulation due to chronic elevations in peripheral insulin, at least during long prediabetic phases. Indeed, recent evidence from clinical cerebrospinal fluid samples and aged animals reveals that reduction in insulin transport is likely mediating the insulin signaling reduction with aging in the presence of peripheral insulin resistance (46,47). Importantly, this work shows that if insulin can be delivered to the brain (eg, ICV), insulin signaling appears enhanced in aged compared with young animals (46). These results are in line with the evidence presented here suggesting that compared with young animals, insulin sensitivity (eg, pAkt/Akt) appears increased in the aged brain.
These results are also supported by prior work showing that the Ca2+-dependent AHP, a robust electrophysiological marker of brain aging, appears more sensitive to insulin in aged compared with young animals (48). In this and other (49) ex vivo experiments, insulin was added directly to hippocampal brain slices and was shown to acutely reduce the AHP. These results, however, are in stark contrast to a recently published article using ICV chronic delivery of zinc-containing insulin (24). In this study, young but not aged animals responded to insulin with improved learning, and no improvement in memory recall (ie, probe) was noted in either young or aged animals. We believe that this result is different from our results because of the use of a chronic delivery approach (24 hours per day for 4 weeks). Indeed, Kamal and colleagues recently reported that sustained chronic ICV insulin (23), as well as chronic peripheral hyperinsulinemia (50), can weaken synaptic plasticity mechanisms, increase the AHP and therefore, reduce learning. However, all prior clinical trials have used acute, repeated daily doses of IN insulin with success (11,51), and a single dose of ICV insulin was also shown to enhance memory recall (22). To further support this notion, prior work from our lab shows that short-term peripheral hyperinsulinemia has no impact on electrophysiological Ca2+-related markers of brain aging in young animals (49). Thus, it is possible that chronic delivery of insulin may hasten cognitive decline in aged animals by helping to develop insulin resistance (24).
Alternatively, it is possible that a mechanism similar to receptor downregulation is at play in young animals where a sustained level of signaling is present and interferes with further increases in signaling during exogenous applications of insulin. In contrast, with aging, insulin brain signaling has been reported to be significantly lower, which may allow for increases in signaling in response to exogenously applied insulin. This could reconcile the evidence of reduced insulin signaling with aging together with maintained sensitivity to insulin. As such, the greatest hurdle limiting insulin signaling with aging may well only be the ability of insulin to enter the brain as recently suggested (46,47).
What is the Mechanism of IN Apidra?
Potential mechanisms of insulin actions in the brain include metabolic, ionic (eg, gamma-aminobutyric acid [GABA], N-methyl-D-aspartatic acid [NMDA], alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid [AMPA], AHP), and genomic processes that individually or together are able to increase performance on memory tasks (11,52), and it is becoming very clear that insulin plays a central role in memory processing and recall both in humans and in animal models. In particular, effects of insulin on vascular function (ie, vasoreactivity) and CBF have been reported in several clinical studies. These have noted increases in CBF in response to IN insulin in both healthy volunteers and in type 2 diabetes patients ((53,54), reviewed in 55). Compared with the results seen in type 2 diabetes patients however, in control/lean individuals (53,56) and young adults (57), IN insulin did not significantly alter CBF. Here we show that chronic repeated daily doses but not single-dose Apidra increase CBF in aged animals. Enhanced CBF has shown to be associated with improved cognition (58,59). Interestingly, we found that poor memory performance in the aged chronically treated animals correlates with dramatically elevated CBF. In contrast, our behavioral data were in good agreement with the metabolic profile. Of interest here, we report on a large and significant reduction in the NAA signal and the NAA/mI ratio measured in aged animals treated chronically with Apidra. The NAA and mI signatures are attributed almost exclusively to neuronal and glial integrity, respectively (28,60). As a result, the shift in NAA/mI ratio indicates neuronal damage and neuroinflammation induced by glial overactivation (28) which may be driving forces, independently or interactively, for cognitive impairment. The abnormal increase in CBF in the aged rats treated chronically with IN glulisine might be another indicator of glial overactivation and neuroinflammation. Collectively, these physiological signal changes (CBF, NAA, and mI) may highlight a potential mechanism by which IN Apidra reduces learning and memory performance.
Our results also highlight a strong trend in reduction in Lip-13 a lipid biomarker in response to IN Apidra in both age groups. This may reflect the impact of IN insulin on increasing glucose uptake and oxidation, thereby reducing metabolic demands on lipid oxidation. Works from McNay and colleagues have provided evidence that acute hippocampal insulin delivery increases glucose metabolism in vivo (40,61), and insulin has also been shown to induce glucose transporters (GLUT 3 and 4) translocation in hippocampal neurons (62) and primary neurons in culture (63). Further experiments are warranted to determine whether the reduction in Lip-13 metabolites indeed is dependent on a glucocentric shift in metabolism.
Conclusion
Some of the limitations associated with our study clearly reflect that the data are only suggestive and do not provide definitive preclinical information about the difference on the impact of zinc-containing and zinc-free insulin formulations. Although our prior published work using zinc-containing insulin formulations (21) was conducted with similar dosing protocols (eg, time of day, dose, delivery method) and using similar animals, we cannot directly compare these two studies and address whether Apidra has fundamentally different effects compared with Levemir or Humalog. Still, here we provide new evidence that not all insulin formulation have similar impact on learning and memory while increasing insulin signaling. Based on our prior work, we suggest that repeated once-daily exposures to IN insulin with Levemir or Humalog may be more effective than Apidra, however, direct side-by-side comparisons as those ongoing in the SNIFF trial are necessary to definitively establish this relationship in the clinic.
Eighteen years ago, although the idea was controversial, Wickelgren helped raise awareness about the role of insulin in normal brain function (64), and although it is now very clear that insulin once delivered to the brain can improve memory recall in multiple settings and conditions, the specific mechanism(s) for this action or the importance of the pulsatile nature of the delivery still escape us. Clearly, insulin signaling plays a critical role in memory and recall as evidenced by reductions in memory performance and hippocampal plasticity when insulin signaling is reduced (40,65,66). Here, we provide new MRS data that may explain why acute IN Apidra may not be conducive to improved learning. Although the results of our study do not support the hypothesis that zinc-free insulin can redress cognitive decline with aging, other formulations in animal models of aging and AD as well as in the clinic have provided strong evidence of success. Given the sustained growth in the aging population, implementing strategies that can maintain healthy cognitive function will tremendously benefit the aging population, and by extension, reduce the incidence of neurodegenerative diseases that share cellular mechanisms with brain aging (ie, AD).
Funding
The work presented here was supported by grants from the National Institute on Aging AG033649-06 to O.T. and K01AG040164 to A-L.L. The 7T ClinScan small animal MRI scanner of UK was funded by the S10 NIH Shared Instrumentation Program Grant (1S10RR029541-01).
Conflict of Interest
The authors report that there are no competing financial interests in relation to the work presented in this article.
References
- 1. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. 148/6/427 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–897. doi:10.1212/WNL.0b013e3181f11d85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rawlings AM, Sharrett AR, Schneider AL, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–793. doi:10.7326/M14-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crane PK, Walker R, Hubbard RA, et al. Glucose levels and risk of dementia. N Engl J Med. 2013;369:540–548. doi:10.1056/NEJMoa1215740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solfrizzi V, Frisardi V, Seripa D, et al. Mediterranean diet in predementia and dementia syndromes. Curr Alzheimer Res. 2011;8:520–542. BSP/CAR /0177 [pii] [DOI] [PubMed] [Google Scholar]
- 6. Woodside JV, Gallagher NE, Neville CE, McKinley MC. Mediterranean diet interventions to prevent cognitive decline–opportunities and challenges. Eur J Clin Nutr. 2014;68:1241–1244. doi:10.1038/ejcn.2014.178 [DOI] [PubMed] [Google Scholar]
- 7. Lövdén M, Xu W, Wang HX. Lifestyle change and the prevention of cognitive decline and dementia: what is the evidence? Curr Opin Psychiatry. 2013;26:239–243. doi:10.1097/YCO.0b013e32835f4135 [DOI] [PubMed] [Google Scholar]
- 8. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11:718–726. doi:10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 9. Benedict C, Hallschmid M, Hatke A, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi:10.1016/j.psyneuen.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 10. Benedict C, Hallschmid M, Schmitz K, et al. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology. 2007;32:239–243. doi:10.1038/sj.npp.1301193 [DOI] [PubMed] [Google Scholar]
- 11. Freiherr J, Hallschmid M, Frey WH, 2nd, et al. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27:505–514. doi:10.1007/s40263-013-0076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brünner YF, Kofoet A, Benedict C, Freiherr J. Central insulin administration improves odor-cued reactivation of spatial memory in young men. J Clin Endocrinol Metab. 2015;100:212–219. doi:10.1210/jc.2014-3018 [DOI] [PubMed] [Google Scholar]
- 13. Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi:10.1001/archneurol.2011.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reger MA, Watson GS, Frey WH, 2nd, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27:451–458. doi:10.1016/j.neurobiolaging.2005.03.016 [DOI] [PubMed] [Google Scholar]
- 15. Reger MA, Watson GS, Green PS, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenbloom MH, Barclay TR, Pyle M, et al. A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer’s disease. CNS Drugs. 2014;28:1185–1189. doi:10.1007/s40263-014-0214-y [DOI] [PubMed] [Google Scholar]
- 17. Claxton A, Baker LD, Hanson A, et al. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis. 2015;44:897–906. doi:10.3233/JAD-141791 [DOI] [PubMed] [Google Scholar]
- 18. Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci. 2009;29:6734–6751. doi:10.1523/JNEUROSCI.1350-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Apostolatos A, Song S, Acosta S, et al. Insulin promotes neuronal survival via the alternatively spliced protein kinase CdeltaII isoform. J Biol Chem. 2012;287:9299–9310. doi:10.1074/jbc.M111.313080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salameh TS, Bullock KM, Hujoel IA, et al. Central nervous system delivery of intranasal insulin: mechanisms of uptake and effects on cognition. J Alzheimers Dis. 2015;47:715–728. doi:10.3233/JAD-150307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maimaiti S, Anderson KL, DeMoll C, et al. Intranasal insulin improves age-related cognitive deficits and reverses electrophysiological correlates of brain aging. J Gerontol A Biol Sci Med Sci. 2016;71:30–39. doi:10.1093/gerona/glu314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68:509–514. S0031-9384(99)00220-6 [pii] [DOI] [PubMed] [Google Scholar]
- 23. Kamal A, Ramakers GM, Gispen WH, Biessels GJ. Effect of chronic intracerebroventricular insulin administration in rats on the peripheral glucose metabolism and synaptic plasticity of CA1 hippocampal neurons. Brain Res. 2012;1435:99–104. doi:10.1016/j.brainres.2011.11.057 [DOI] [PubMed] [Google Scholar]
- 24. Adzovic L, Lynn AE, D’Angelo HM, et al. Insulin improves memory and reduces chronic neuroinflammation in the hippocampus of young but not aged brains. J Neuroinflammation. 2015;12:63 doi:10.1186/s12974-015-0282-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. [DOI] [PubMed] [Google Scholar]
- 26. Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628. doi:10.1016/j.addr.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 27. Lochhead JJ, Wolak DJ, Pizzo ME, Thorne RG. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab. 2015;35:371–381. doi:10.1038/jcbfm.2014.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin A, Ross BD, Harris K, Wong W. Efficacy of proton magnetic resonance spectroscopy in neurological diagnosis and neurotherapeutic decision making. NeuroRx. 2005;2:197–214. doi:10.1602/neurorx.2.2.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Devos A, Lukas L, Suykens JA, et al. Classification of brain tumours using short echo time 1H MR spectra. J Magn Reson. 2004;170:164–175. doi:10.1016/j.jmr.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 30. Hancock SM, Finkelstein DI, Adlard PA. Glia and zinc in ageing and Alzheimer’s disease: a mechanism for cognitive decline? Front Aging Neurosci. 2014;6:137 doi:10.3389/fnagi.2014.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sensi SL, Paoletti P, Koh JY, Aizenman E, Bush AI, Hershfinkel M. The neurophysiology and pathology of brain zinc. J Neurosci. 2011;31:16076–16085. doi:10.1523/JNEUROSCI.3454-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takeda A, Fujii H, Minamino T, Tamano H. Intracellular Zn(2+) signaling in cognition. J Neurosci Res. 2014;92:819–824. doi:10.1002/jnr.23385 [DOI] [PubMed] [Google Scholar]
- 33. Lim JH, Davis GE, Wang Z, et al. Zicam-induced damage to mouse and human nasal tissue. PLoS One. 2009;4:e7647 doi:10.1371/journal.pone.0007647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamidovic A. Position on zinc delivery to olfactory nerves in intranasal insulin phase I-III clinical trials. Contemp Clin Trials. 2015;45:277–280. doi:10.1016/j.cct.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 35. Home PD. The pharmacokinetics and pharmacodynamics of rapid-acting insulin analogues and their clinical consequences. Diabetes Obes Metab. 2012;14:780–788. doi:10.1111/j.1463-1326.2012.01580.x [DOI] [PubMed] [Google Scholar]
- 36. Stammberger I, Seipke G, Bartels T. Insulin glulisine—a comprehensive preclinical evaluation. Int J Toxicol. 2006;25:25–33. doi:10.1080/10915810500488379 [DOI] [PubMed] [Google Scholar]
- 37. Hennige AM, Strack V, Metzinger E, Seipke G, Häring HU, Kellerer M. Effects of new insulin analogues HMR1964 (insulin glulisine) and HMR1423 on insulin receptors. Diabetologia. 2005;48:1891–1897. doi:10.1007/s00125-005-1870-8 [DOI] [PubMed] [Google Scholar]
- 38. Schmidt B, Hinman JR, Jacobson TK, et al. Dissociation between dorsal and ventral hippocampal theta oscillations during decision-making. J Neurosci. 2013;33:6212–6224. doi:10.1523/JNEUROSCI.2915-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vorhees CV, Williams MT. Assessing spatial learning and memory in rodents. ILAR J. 2014;55:310–332. doi:10.1093/ilar/ilu013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93:546–553. doi:10.1016/j.nlm.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frolich L, Blum-Degen D, Bernstein HG, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105:423–438. [DOI] [PubMed] [Google Scholar]
- 42. Zaia A, Piantanelli L. Insulin receptors in the brain cortex of aging mice. Mech Ageing Dev. 2000;113:227–232. S0047637499001189 [pii] [DOI] [PubMed] [Google Scholar]
- 43. Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol. 2004;490:71–81. doi:10.1016/j.ejphar.2004.02.045 [DOI] [PubMed] [Google Scholar]
- 44. Doré S, Kar S, Rowe W, Quirion R. Distribution and levels of [125I]IGF-I, [125I]IGF-II and [125I]insulin receptor binding sites in the hippocampus of aged memory-unimpaired and -impaired rats. Neuroscience. 1997;80:1033–1040. S0306452297001541 [pii] [DOI] [PubMed] [Google Scholar]
- 45. Zhao W, Chen H, Xu H, et al. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem. 1999;274:34893–34902. [DOI] [PubMed] [Google Scholar]
- 46. Sartorius T, Peter A, Heni M, et al. The brain response to peripheral insulin declines with age: a contribution of the blood-brain barrier? PLoS One. 2015;10:e0126804 doi:10.1371/journal.pone.0126804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heni M, Schopfer P, Peter A, et al. Evidence for altered transport of insulin across the blood-brain barrier in insulin-resistant humans. Acta Diabetol. 2014;51:679–681. doi:10.1007/s00592-013-0546-y [DOI] [PubMed] [Google Scholar]
- 48. Pancani T, Anderson KL, Brewer LD, et al. Effect of high-fat diet on metabolic indices, cognition, and neuronal physiology in aging F344 rats. Neurobiol Aging. 2013;34:1977–1987. doi:10.1016/j.neurobiolaging.2013.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maimaiti S, DeMoll C, Anderson KL, et al. Short-lived diabetes in the young-adult ZDF rat does not exacerbate neuronal Ca biomarkers of aging. Brain Res. 2014;1621:214–221. doi:10.1016/j.brainres.2014.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kamal A, Ramakers GM, Gispen WH, Biessels GJ, Al Ansari A. Hyperinsulinemia in rats causes impairment of spatial memory and learning with defects in hippocampal synaptic plasticity by involvement of postsynaptic mechanisms. Exp Brain Res. 2013;226:45–51. doi:10.1007/s00221-013-3409-4 [DOI] [PubMed] [Google Scholar]
- 51. Schiöth HB, Frey WH, Brooks SJ, Benedict C. Insulin to treat Alzheimer’s disease: just follow your nose? Expert Rev Clin Pharmacol. 2012;5:17–20. doi:10.1586/ecp.11.70 [DOI] [PubMed] [Google Scholar]
- 52. de la Monte SM. Intranasal insulin therapy for cognitive impairment and neurodegeneration: current state of the art. Expert Opin Drug Deliv. 2013;10:1699–1709. doi:10.1517/17425247.2013.856877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Novak V, Milberg W, Hao Y, et al. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care. 2014;37:751–759. doi:10.2337/dc13-1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schilling TM, Ferreira de Sa DS, Westerhausen R, et al. Intranasal insulin increases regional cerebral blood flow in the insular cortex in men independently of cortisol manipulation. Hum Brain Mapp. 2014;35:1944–1956. doi:0.1002/hbm.22304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hughes TM, Craft S. The role of insulin in the vascular contributions to age-related dementia. Biochim Biophys Acta. 2015. doi:10.1016/j.bbadis.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 56. Kullmann S, Heni M, Veit R, et al. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care. 2015;38:1044–1050. doi:10.2337/dc14-2319 [DOI] [PubMed] [Google Scholar]
- 57. Grichisch Y, Cavusoglu M, Preissl H, et al. Differential effects of intranasal insulin and caffeine on cerebral blood flow. Hum Brain Mapp. 2012;33:280–287. doi:10.1002/hbm.21216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ajmani RS, Metter EJ, Jaykumar R, et al. Hemodynamic changes during aging associated with cerebral blood flow and impaired cognitive function. Neurobiol Aging. 2000;21:257–269. S0197-4580(00)00118-4 [pii] [DOI] [PubMed] [Google Scholar]
- 59. Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab. 1991;11:684–689. doi:10.1038/jcbfm.1991.121 [DOI] [PubMed] [Google Scholar]
- 60. Rigotti DJ, Inglese M, Gonen O. Whole-brain N-acetylaspartate as a surrogate marker of neuronal damage in diffuse neurologic disorders. AJNR Am J Neuroradiol. 2007;28:1843–1849. doi:10.3174/ajnr.A0774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McNay EC. Insulin and ghrelin: peripheral hormones modulating memory and hippocampal function. Curr Opin Pharmacol. 2007;7:628–632. doi:10.1016/j.coph.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 62. Reagan LP. Neuronal insulin signal transduction mechanisms in diabetes phenotypes. Neurobiol Aging. 2005;26(suppl 1):56–59. [DOI] [PubMed] [Google Scholar]
- 63. Liu CC, Hu J, Tsai CW, et al. Neuronal LRP1 regulates glucose metabolism and insulin signaling in the brain. J Neurosci. 2015;35:5851–5859. doi:10.1523/JNEUROSCI.5180-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wickelgren I. Tracking insulin to the mind. Science. 1998;280:517–519. [DOI] [PubMed] [Google Scholar]
- 65. Grillo CA, Piroli GG, Lawrence RC, et al. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes. 2015;64:3927–3936. doi:10.2337/db15-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Costello DA, Claret M, Al-Qassab H, et al. Brain deletion of insulin receptor substrate 2 disrupts hippocampal synaptic plasticity and metaplasticity. PLoS One. 2012;7:e31124 doi:10.1371/journal.pone.0031124 [DOI] [PMC free article] [PubMed] [Google Scholar]