Abstract
The Drosophila GeneSwitch system facilitates the spatial and temporal control of gene expression through dietary supplementation of mifepristone (RU486). Because experimental and control groups differ only by treatment with RU486, confounding results from using flies of different genetic backgrounds are eliminated, making GeneSwitch especially useful in studies of aging. However, the effect of RU486 itself on longevity has not been well characterized, particularly in relation to nutritional states known to affect lifespan. Here, we show that RU486 has dose- and diet-dependent effects on longevity in both sexes. On low nutrient diets, RU486 supplementation reduces total food consumption, perhaps exacerbating undernutrition to shorten life. RU486 also inhibits proboscis extension responses to low nutrient diets, suggesting that RU486 has an aversive taste which leads to decreased food consumption and diminished longevity. RU486 is not detrimental to fly lifespan on high nutrient food, correlating with reduced effects of the drug on palatability and total consumption on rich diets. Our results highlight the critical importance of considering how food palatability and nutrient intake might be altered by dietary or drug manipulations in studies of aging and behavior.
Key Words: Aging, Dietary restriction, Longevity, Nutrition, Palatability
Drosophila has long served as an important model in studies of aging due to its relatively short lifespan, the availability of robust genetic techniques, and ease of maintenance and environmental manipulation (1). These attributes have been critical in defining the genetic mechanisms that underlie life-extending interventions such as dietary restriction. Of the many powerful tools available for probing fly genetics, the GAL4/UAS system stands out due to the ease in which it allows the control of gene expression (2). GeneSwitch adds temporal control to the GAL4/UAS system and is one of several methods for achieving inducible expression (3,4). GeneSwitch utilizes a chimeric gene that encodes a GAL4 DNA binding domain, the human progesterone receptor ligand-binding domain, and the activation domain of human p65 protein. In the presence of the synthetic steroid, RU486, the GeneSwitch chimeric protein induces UAS-controlled transgene expression.
One major advantage of the GeneSwitch system is the elimination of potential confounds arising from varied genetic backgrounds, because cohorts differ only by the addition of RU486 to fly food. This has been especially valuable in studies of aging, where genetic background can profoundly impact lifespan (5). However, the impact of RU486 itself on fly health and longevity is not always tested. By including studies on heterozygous controls harboring only the GAL4- or UAS-containing elements, the effect of RU486 can be assessed without the influence of induced gene expression. Such studies have shown mixed results, with the majority observing no effect (e.g. 6–10). However, both positive and negative effects on lifespan have also been reported with RU486 treatment on nonexpressing controls (11–16). One potential explanation for the inconsistent effects on longevity could be the range of different diets used across studies. Importantly, the fly can robustly modify its food consumption in response to changes in diet composition (17–21) or added drugs (22). Such variability in nutrient intake is especially relevant when considering the extensive use of Drosophila for studying the interactions between nutrition and longevity (23,24).
Here, we show that dietary supplementation of RU486 can influence food palatability. On low nutrient diets where RU486 inhibits proboscis extension response (PER), Drosophila food intake and lifespan are also altered. Given the importance of feeding behavior and nutrition on physiology, metabolism, and health, our observations suggest that studies using the GeneSwitch system should carefully consider potential influences of RU486 on nutrient intake, especially when used in combination with dietary manipulations.
Methods
Fly Stocks
Lines were maintained on a standard stock food at ~23 °C under a 12/12-hour light/dark cycle. Canton-S and Dahomey were selected as control strains commonly used in aging studies. These stocks have been maintained in our laboratory for more than 5 years. Act-GS-255B (Act5C-GS) carrying the RU486-inducible GAL4 driver (6) was assessed by driving GFP expression. To generate Act5C-GS/UAS-GFP or Act5C-GS/+, UAS-mCD8-GFP or w1118 males were crossed to female Act5C-GS. For germ-free studies, axenic flies were generated by bleach treatment of embryos and the elimination of microbes was verified periodically by plating swabs from the interior of spent enclosures, as described previously (25).
Food Preparation
Drosophila enclosures (8 oz. round bottom bottles or 25×95mm vials, polypropylene) and plugs (Droso-Plugs) were from Genesee Scientific. Bacto agar and yeast extract (YE) were from BD Diagnostic Systems, and dry active yeast and cornmeal were from LabScientific. All other reagents were from Fisher Scientific or VWR International. Standard stock food contained 1.5% dry active yeast, 5% sucrose, 5% cornmeal, and 1.5% agar (all w/v), supplemented with 0.4% propionic acid and 0.035% phosphoric acid (both v/v). For lifespan studies, YE food contained varying concentrations of YE (0.1%, 0.5%, or 5%, all w/v) in a base medium of 5% sucrose, 8.6% cornmeal, 0.5% agar, 0.4% propionic acid, and 0.035% phosphoric acid. All foods were autoclaved at 121 °C for 30 minutes, with RU486 (100× in 80% ethanol, typically 200 µM final concentration) and acids added after cooling food to less than 65 °C. RU486 (>98%) was obtained from TCI America, except where noted (Sigma-Aldrich). RU486 from these two suppliers was confirmed to originate from different sources. Control diets lacking RU486 contained the equivalent volume (1/100) of vehicle (80% ethanol, v/v). Food was dispensed into pre-autoclaved vials (2mL/vial) or bottles (40mL/bottle). Liquid food (for behavioral assays) excluded agar and cornmeal. Where noted, chloroform (CHCl3) or dimethyl sulfoxide (DMSO) was used as the vehicle instead of 80% ethanol.
Lifespan
Fly lifespan was measured as described previously (26). Briefly, adults (0–2 days old) were maintained on fresh stock food for 3 days. Male and female flies (3–5 days old) were then separated under mild CO2 anesthesia and randomly transferred to experimental diets (~20 flies/vial). Flies were maintained at 25 °C under controlled light (12/12-hour light/dark cycle) and humidity (60%). Flies were transferred to fresh food and dead flies were scored every 3–4 days.
Proboscis Extension Response
PER was assessed using nonstarved flies (typically 6–8 days old), as described previously (27,28). Briefly, individual flies were trapped in shortened 200-µL pipette tips with their heads exposed. Flies were assessed with water as a negative control and 5% sucrose as a positive control touched to the labellum; flies responding to water and/or not responding to sucrose were discarded. Each fly was then tested 10 times, alternating 5 times each with food supplemented with 200 µM RU486 or vehicle. The first food tested for each fly (RU486 or vehicle) was also alternated. PER was scored as follows: full extension = 1, half extension = 0.5, no extension = 0. For statistical analysis, average score for each type of food from one fly was considered as one data point.
Feeding
Food intake was measured as described previously (19). Briefly, for the radioisotope-labeling method, flies (~10 per vial) were transferred to food labeled with 1 mCi/mL [α-32P]-dCTP (PerkinElmer). Flies were collected and frozen after 24 hours, and consumption was estimated by measuring label accumulation in flies. Scintillation counts of aliquots of radiolabeled food were used to calculate equivalent food intake. For the capillary feeder assay (CAFE), four flies were presented with liquid food using two 5-µL calibrated capillaries per chamber. Changes in liquid meniscus height were measured over 2–3 days at each capillary change (approximately every 12 hours). Consumption volume was calculated after background subtraction of measurements from control chambers without flies. For the two-choice CAFE assays, a similar setup was used except one capillary of each type of diet (with RU486 or vehicle) was presented. For assessment of water intake, flies were dry starved for 24 hours in the CAFE chamber prior to starting the experiment to increase drinking. Total consumption after introducing capillaries with water, supplemented with RU486 or vehicle, was measured for only 7 hours to avoid fly death caused by starvation. Preference index was calculated as follows: (RU486 consumption – Vehicle consumption) / Total intake.
Statistical Analysis
Statistically significant differences between survival curves and median lifespan were determined by log-rank test and Fisher’s exact test, respectively (29). Statistical analyses for feeding and proboscis extension assays were performed using SigmaPlot (Systat Software). Student’s t test was used as a parametric test for comparing differences between two groups. Mann–Whitney rank-sum test was performed for nonparametric data or for data with low performance power t tests (30). Differences were considered significant at p less than .05.
Results
RU486 Is Detrimental to Fly Lifespan on Low Nutrient Diets
Increasing evidence points to dietary protein:carbohydrate (P:C) ratio as the primary determinant of fly lifespan (17,20,31). Yeast is the main source of protein in the fly diet, and its concentration is commonly manipulated to modulate P:C ratio and lifespan in dietary restriction studies (17,20,26,32). We examined the effect of RU486 on fly lifespan on diets containing 0.1%, 0.5%, or 5% (w/v) YE in a standard sucrose-cornmeal base medium (see Methods). Studies using GeneSwitch commonly supplement fly food with 20–500 µM RU486 (6,10,16,33,34), and we used 200 µM RU486 in our initial studies. Act5C-GS was selected as a commonly used GeneSwitch GAL4 driver (6,8,35), and inducible gene expression was verified by feeding RU486 to Act5C-GS/UAS-GFP flies (Supplementary Figure 1).
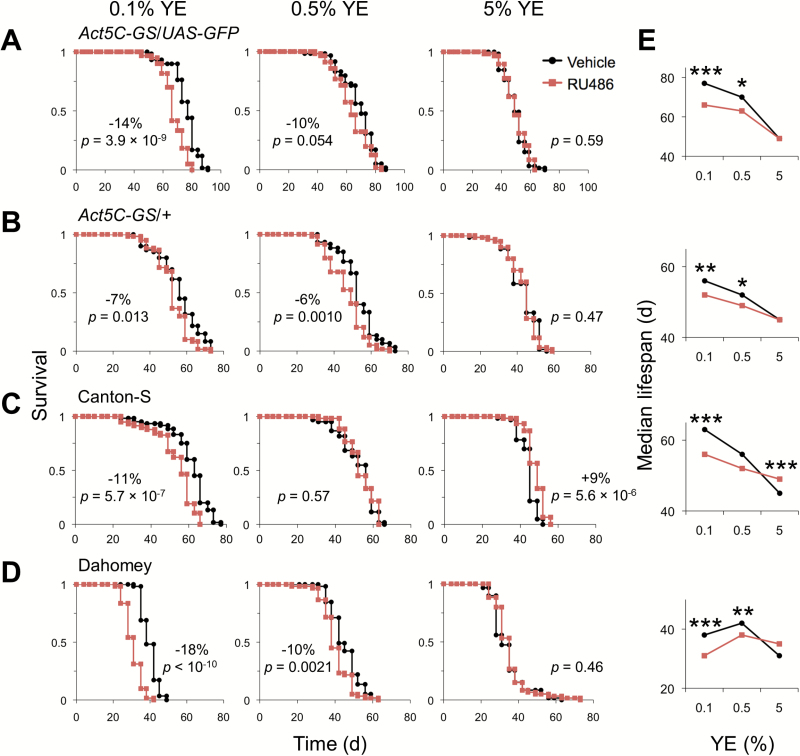
Act5C-GS/UAS-GFP male lifespan was measured on the various diets supplemented with RU486 or vehicle (Figure 1A). On 5% YE medium, RU486 had no effect on lifespan. In contrast, RU486 decreased fly median lifespan on the 0.1% and 0.5% YE diets by 14% and 10%, respectively, compared with the vehicle control. Shortened life was also observed with RU486 supplementation on lower yeast diets in Act5C-GS/+ (Figure 1B), ruling out the influence of transgene expression on longevity. Similar results were obtained with the commonly used control strains, Canton-S and Dahomey (Figure 1C and D), demonstrating that the effects of RU486 on lifespan are independent of the GeneSwitch driver or any other transgenes. For all genotypes, lifespan was decreased on 5% YE diet and RU486 supplementation had either no effect or extended life on this medium (Figure 1E). RU486 was increasingly detrimental to longevity as YE concentration was decreased across all genotypes tested, even though the beneficial effect of dietary restriction on lifespan was maximized on either 0.1% or 0.5% YE medium (Figure 1E).
Figure 1.
Diet-dependent effects of RU486 on male lifespan. Survival is shown for (A) Act5C-GS/UAS-GFP, (B) Act5C-GS/+, (C) Canton-S, and (D) Dahomey on 0.1%, 0.5%, or 5% YE diet supplemented with 200 µM RU486 or vehicle control. The percent change in median lifespan between RU486- and vehicle-fed flies is shown when Fisher’s exact test is statistically significant (p < .05). All log-rank p values between survival curves are shown. (E) Median lifespan of lines tested in A–D. Significant differences between RU486- and vehicle-supplemented control are shown for each diet (Fisher’s exact test: *p < .05; **p < .01; ***p < .001). n = 56–61 flies per condition. See Supplementary Table 1 for statistics. YE = yeast extract.
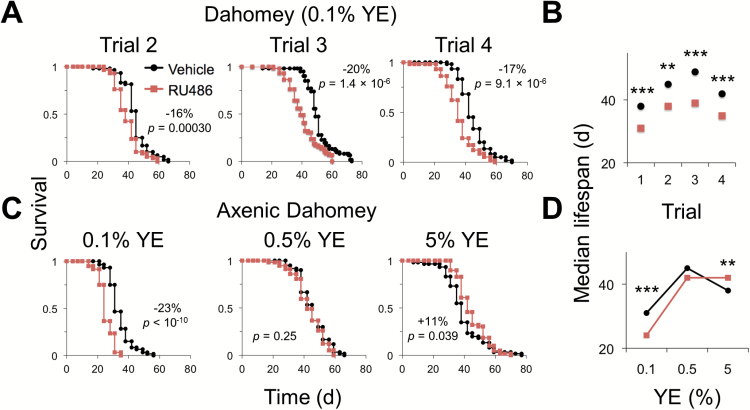
To assess the variability of the effect of RU486 on longevity, we measured Dahomey male lifespan on 0.1% YE diet supplemented with RU486 or vehicle in three additional independent replicates (Figure 2A). In all trials (four in total), median lifespan was significantly decreased with RU486 supplementation (Figure 2B).
Figure 2.
Effect of RU486 on Dahomey male lifespan. (A) Three independent lifespan trials on 0.1% YE diet supplemented with 200 µM RU486 or vehicle control. n = 58–61 flies per condition. (B) Median lifespan from independent trials. Data are from A and Figure 1D. (C) Effect of RU486 on axenic lifespan. Survival is shown of germ-free Dahomey males on 0.1%, 0.5%, or 5% YE diet supplemented with 200 µM RU486 or vehicle control. n = 57–60 flies per condition. (D) Median lifespan values from C. (A, C) The percent change in median lifespan between RU486- and vehicle-fed flies is shown when Fisher’s exact test is statistically significant (p < .05). All log-rank p values between survival curves are shown. (B, D) Significant differences between RU486- and vehicle-supplemented control are shown (Fisher’s exact test: **p < .01; ***p < .001). See Supplementary Table 1 for statistics. YE = yeast extract.
We previously showed that microbes can supplement fly protein intake to rescue lifespan on undernutrition diets (25). To determine whether the effect of RU486 is dependent on microbes, we assessed axenic (germ-free) Dahomey male survival. Consistent with our previous study (25), life was shortened on low nutrient (0.1% YE) diet in axenic compared with conventionally raised flies; however, the detrimental effect of RU486 on longevity was conserved in axenic animals on this diet (Figure 2C and D). These results suggest that the effect of RU486 on longevity is independent of the presence of microbes.
Previous studies and our own results (see following sections) show that flies consume up to twofold more low YE food than the 5% YE medium (17,26). To assess whether reduced absolute drug intake is responsible for the lack of a deleterious effect on high yeast diet, we tested higher RU486 concentration. Increasing the RU486 concentration by twofold (400 µM) did not affect Dahomey male lifespan on the 5% YE diet, suggesting that differences in absolute ingestion of drug are not a confound in our studies (Supplementary Figure 2).
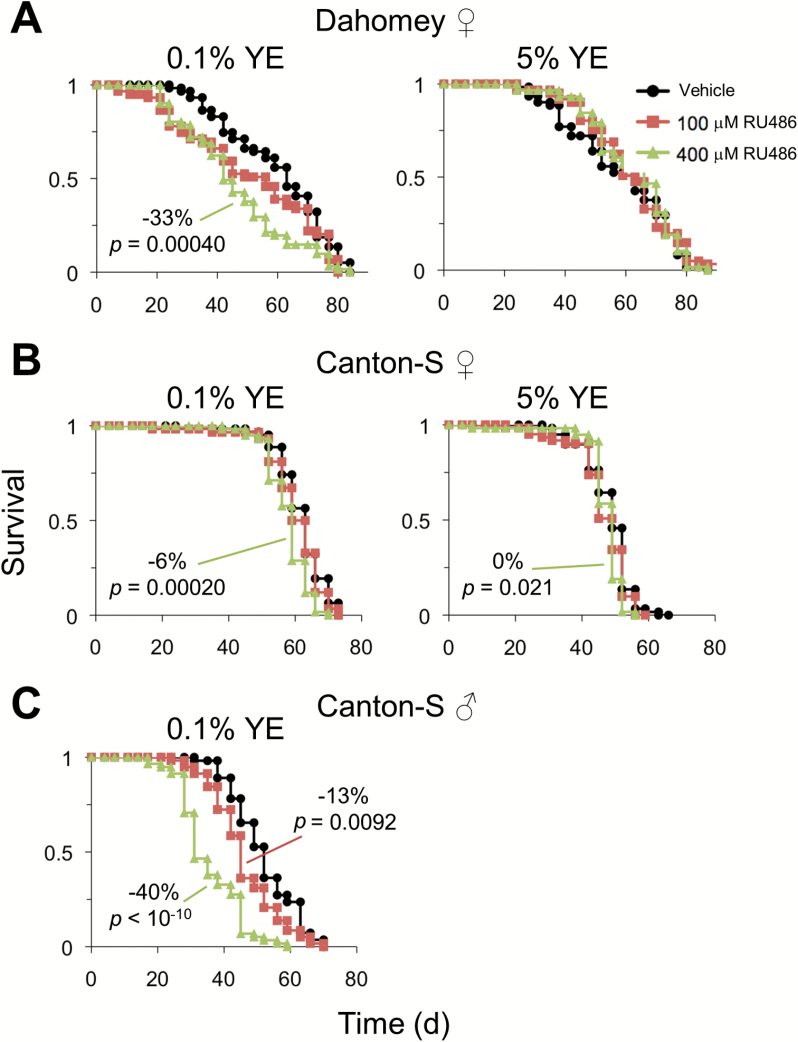
RU486 was also detrimental to female lifespan on 0.1% YE diet, although this was apparent only with the highest RU486 concentration tested (400 µM) for Canton-S (Figure 3A and B). A dose-dependent effect of RU486 on Canton-S male lifespan was also apparent on low yeast diet (Figure 3C). Consistent with our male lifespan results, RU486 (100 or 400 µM) was not detrimental to Canton-S or Dahomey female lifespan on 5% YE medium (Figure 3A and B). Collectively, our results suggest that the impact of RU486 on lifespan is dose dependent, conserved in both sexes, and more prominent on low nutrient diets.
Figure 3.
Effect of RU486 dosage on lifespan. Survival of (A) Dahomey or (B) Canton-S females on 0.1% or 5% YE diet supplemented with 100 or 400 µM RU486 or vehicle control. n = 58–62 flies per condition. (C) Survival of Canton-S males on 0.1% YE diet supplemented with 100 or 400 µM RU486 or vehicle control. n = 55–58 flies per condition. Log-rank p values for survival comparisons to vehicle control are shown for p < .05. The percent change in median lifespan between RU486- and vehicle-fed flies is shown when Fisher’s exact test is statistically significant (p < .05). See Supplementary Table 1 for statistics. YE = yeast extract.
Diet Modulates Aversion to RU486
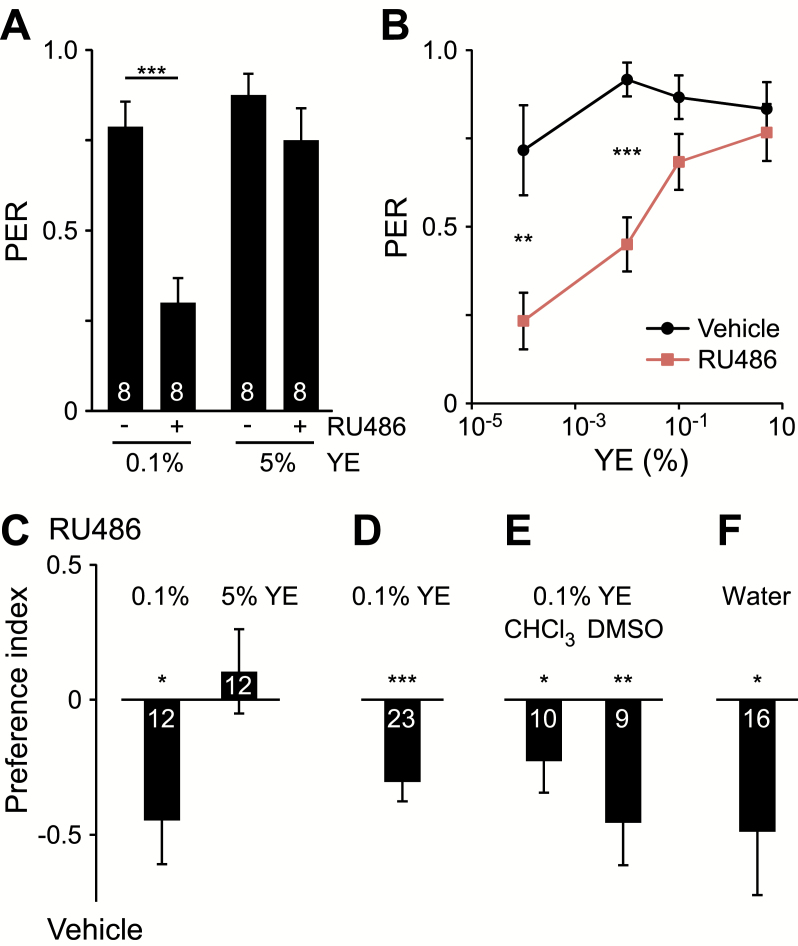
We recently showed that food pH can affect palatability—on more alkaline medium, lower palatability and decreased total consumption can shorten fly life (27). To determine whether RU486 might be an aversive tastant that decreases food palatability, we measured PER to different diets supplemented with 200 µM RU486 or vehicle. Nonstarved flies were used to assess PER under conditions typical of our lifespan studies. Dahomey males showed high PER to 0.1% and 5% YE diets supplemented with only vehicle (Figure 4A). RU486 supplementation significantly reduced PER only to the low nutrient diet. Further decreasing nutrient concentration exacerbated the effect of RU486 on PER (Figure 4B).
Figure 4.
Effect of RU486 on PER and food intake preference in Dahomey males. (A) PER to 0.1% or 5% YE diet supplemented with 200 µM RU486 (+) or vehicle (−). n = number of flies per condition, superimposed on each bar. (B) PER to varying YE concentrations (w/v) supplemented with 200 µM RU486 or vehicle. n = 6 flies for each condition. For (A, B), significant differences between RU486- and vehicle-supplemented diet are shown (Student’s t test or Mann–Whitney rank-sum test: **p < .01; ***p < .001). Preference index for the indicated diet with 200 µM RU486 or vehicle, assessed in a two-choice CAFE consumption assay, to test (C) YE concentration, (D) an alternative source of RU486 (Sigma-Aldrich), (E) alternative solvents for use as drug vehicle, and (F) absence of food ingredients (water only). Preference index was calculated from consumption over (C–E) 24 hours or (F) 7 hours. Except where noted (E), vehicle was 80% ethanol. n = number of CAFE chambers, superimposed on each bar. For (C–F), significant differences between consumption of RU486- and vehicle-supplemented diet are shown above preference index plots (Student’s t test: *p < .05; **p < .01; ***p < .001). Data shown are means ± SEM. CHCl3 = chloroform; DMSO = dimethyl sulfoxide; PER = proboscis extension response; YE = yeast extract.
We next compared total food intake in a two-choice CAFE assay where food containing RU486 or vehicle were presented simultaneously. On 0.1% YE diet, Dahomey males showed a significant preference for food lacking RU486, whereas no preference was observed on the high nutrient medium (Figure 4C). RU486 from another distributor (Sigma-Aldrich) was also effective in influencing food choice on 0.1% YE diet, suggesting that our results are not specific to a particular source (Figure 4D). To test whether the effects of RU486 are dependent on the presence of the ethanol vehicle, we repeated the two-choice CAFE assay using RU486 dissolved in different solvents. Regardless of the vehicle used (CHCl3 or DMSO), flies showed significant preference for consuming food lacking RU486 (Figure 4E).
To assess whether food ingredients are required for the aversive behavior induced by RU486, we repeated the two-choice CAFE assay with only water. Dahomey males preferred water supplemented with vehicle over RU486 (Figure 4F). These results suggest that RU486 is directly aversive or bitter, but do not rule out the possibility that the molecule additionally inhibits positive responses to food (36).
RU486 Reduces Total Consumption
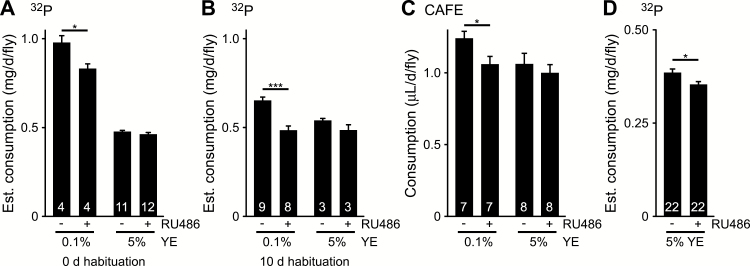
To determine whether the effects of RU486 on food palatability correlate with changes in consumption in no-choice situations, we measured ingestion in Dahomey males over 24 hours using the radioisotope-labeling method, which quantifies fly accumulation of a 32P-labeled tracer to estimate food intake (18,19,26). As expected from previous studies showing compensatory feeding (18,26), estimated consumption on the low nutrient diet was greater than that on the high nutrient medium (Figure 5A). RU486 supplementation reduced fly 32P accumulation on the 0.1% YE diet, whereas there was no statistically significant effect on 5% YE medium (Figure 5A). These results were consistent in flies habituated on the test diet for 10 days, although extended habituation resulted in an overall decrease in estimated food intake on the low nutrient diet (Figure 5B).
Figure 5.
Effect of RU486 on total food intake in Dahomey males. Estimated consumption over 24 hours, assessed by radioisotope labeling, after (A) 0 or (B) 10 days of habituation on the indicated diet. (C) Total consumption over 24 hours of the indicated diet, assessed by CAFE assay. (D) Estimated consumption over 24 hours of 5% YE diet, assessed by radioisotope labeling. Diets were supplemented with 200 µM RU486 (+) or vehicle (−). Data shown are means ± SEM. n = number of vials or CAFE chambers, superimposed on each bar. Significant differences between RU486- and vehicle-supplemented diet are shown (Student’s t test or Mann–Whitney rank-sum test: *p < .05; ***p < .001). CAFE = capillary feeder; YE = yeast extract.
Previous studies have shown that accumulation of radioactive tracer over 24 hours is reflective of consumption (19). However, we also directly measured food intake volume using the CAFE assay to rule out caveats of radiolabel dynamics or impaired tracer absorption in the fly (22). Flies were fed a liquid diet identical to the solid medium except for the exclusion of cornmeal and agar. Consistent with our radiotracer studies, Dahomey males on 0.1% YE diet showed less total consumption of RU486- than vehicle-supplemented medium (Figure 5C). RU486 had no statistically significant effect on consumption of 5% YE food (Figure 5C).
Because our behavioral studies were consistent with the idea that high nutrient concentrations mask aversion to RU486, we hypothesized that differences in total consumption might be difficult to resolve on 5% YE medium. Of the assays assessed, the radioisotope accumulation method was previously shown to have the greatest resolving power (19). With a sample size appropriate for distinguishing effect sizes of ~5% (19), we observed significantly decreased 32P accumulation in flies fed 5% YE food supplemented with 200 µM RU486 (Figure 5D).
Discussion
We show that dietary RU486 supplementation can be detrimental to fly lifespan on low nutrient diets, including those typically associated with dietary restriction–mediated longevity. We observed a broad effect of RU486 across multiple genotypes, although the extent of lifespan-shortening may be especially variable in females. This variability might be caused by the recently reported genotype-specific lifespan extension induced by RU486 in mated females (15).
Similar to the conclusions of our recent study on the effect of food pH on fly feeding and longevity (27), decreased lifespan on low nutrient diets containing RU486 is correlated with reduced food palatability (assessed by PER) and consumption. Although we hypothesize that it is the bitter or aversive taste of RU486 that directly leads to reduced food intake and shortened life, additional postingestive mechanisms such as RU486-induced toxicity might also affect consumption and longevity—independent of the effect on taste. Further studies will be needed to identify feeding-dependent and feeding-independent effects of RU486 on fly physiology and health. However, it will be challenging to resolve these factors. Although sweeteners are commonly used to reduce the perception of bitterness (37) or to overcome aversion to bitter compounds (38), PER does not always predict food intake—perhaps due to the presence of internal (pharyngeal) taste-sensing organs (39) and other nutrient-sensing mechanisms (40–44). Additionally, assessment of toxicity or general ill health, and their contribution to feeding behavior, is not trivial in flies. Ultimately, the identification of gustatory and any postingestive mechanisms for recognizing sterols will be of interest because they may be important in limiting the ingestion of other nonmetabolizable or toxic steroidal compounds (45–47).
Because food composition profoundly affects palatability, increased nutrient concentration likely mitigates the aversive taste of RU486, and this correlates with reduced or eliminated effects on consumption and lifespan on higher YE diets. We hypothesized that reduced consumption of nutrient-rich medium supplemented with RU486 might be slight and difficult to resolve. Indeed, a statistically significant difference was only observed in a larger-scale experiment, with the required sample size correctly predicted from a previous analysis of Drosophila feeding assays (19). Slightly reduced daily consumption—over a lifetime—may have profound consequences on physiology and health, and an aversive food additive that reduces total consumption could be detrimental to flies when nutrients are limiting but beneficial when nutrients are in excess. Interestingly, in Canton-S males and one out of two Dahomey male trials, lifespan was extended with 200 µM RU486 supplementation on nutrient-rich diet, where longevity is normally diminished. Future work using RU486 and other bitter tastants over a range of concentrations and diets might help establish the relationship between small changes in feeding behavior and lifespan. The use of high resolution feeding assays will be instrumental, as suggested in efforts on identifying potential anti-aging compounds using Drosophila (48,49).
Various laboratory diets will likely have different influences on the taste of food; thus, how RU486 modulates palatability under different conditions will require further study. Notably, previous studies using whole yeast as the source of protein (instead of YE) have also reported significant changes in the lifespan of nonexpressing controls with RU486 treatment (11,14,16), suggesting that our results might be generalized to other diets and that care should be taken in the design and interpretation of GeneSwitch studies. RU486-specific effects can be estimated by using heterozygous GAL4- and UAS-only controls with the appropriate genetic backgrounds, ultimately allowing proper inferences to be made about the induced transgene. We note, however, that these controls are not always included in published studies. Where these controls are lacking, alternative approaches to GeneSwitch could be presented to support conclusions. Our results also more broadly suggest that studies using drugs or other additives, combined with nutritional manipulations, may be especially susceptible to consequences arising from influences on feeding. Therefore, food intake analysis in both control and experimental cohorts will be desirable, particularly in studies where slight changes in feeding behavior might have a marked impact. At a minimum, future studies should avoid the use of low resolution assays to rule out differences in feeding or consider reporting the smallest effect size that can be resolved from their methods—this is often as large as 50%–100% when using the popular dye labeling and proboscis extension assays (19).
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by grants R21DK092735 and R01AG045036 from the National Institutes of Health; a New Scholar in Aging award from The Ellison Medical Foundation; and a Glenn Award for Research in Biological Mechanisms of Aging from the Glenn Foundation for Medical Research awarded to W.W.J.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Supplementary Material
Acknowledgments
We thank Subhash Katewa, Pankaj Kapahi, and Linda Partridge for fly lines; and Kim Bruce and Frédéric Marion-Poll for comments on the manuscript.
References
- 1. Brandt A, Vilcinskas A. The fruit fly Drosophila melanogaster as a model for aging research. Adv Biochem Eng Biotechnol. 2013;135:63–77. doi:10.1007/10_2013_193 [DOI] [PubMed] [Google Scholar]
- 2. Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. [DOI] [PubMed] [Google Scholar]
- 3. Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci USA. 2001;98:12596–12601. doi:10.1073/pnas.221303298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci USA. 2001;98:12602–12607. doi:10.1073/pnas.221303998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Partridge L, Gems D. Benchmarks for ageing studies. Nature. 2007;450:165–167. doi:10.1038/450165a [DOI] [PubMed] [Google Scholar]
- 6. Ford D, Hoe N, Landis GN, et al. Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. Exp Gerontol. 2007;42:483–497. doi:10.1016/j.exger.2007.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alic N, Giannakou ME, Papatheodorou I, et al. Interplay of dFOXO and two ETS-family transcription factors determines lifespan in Drosophila melanogaster. PLoS Genet. 2014;10:e1004619. doi:10.1371/journal.pgen.1004619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katewa SD, Demontis F, Kolipinski M, et al. Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab. 2012;16:97–103. doi:10.1016/j.cmet.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tricoire H, Battisti V, Trannoy S, Lasbleiz C, Pret AM, Monnier V. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech Ageing Dev. 2009;130:547–552. doi:10.1016/j.mad.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 10. Zid BM, Rogers AN, Katewa SD, et al. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi:10.1016/j.cell.2009.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen J, Curtis C, Tavaré S, Tower J. A screen of apoptosis and senescence regulatory genes for life span effects when over-expressed in Drosophila. Aging. 2009;1:191–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shaposhnikov M, Proshkina E, Shilova L, Zhavoronkov A, Moskalev A. Lifespan and stress resistance in Drosophila with overexpressed DNA repair genes. Sci Rep. 2015;5:15299. doi:10.1038/srep15299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ren Y, Hughes KA. Vitellogenin family gene expression does not increase Drosophila lifespan or fecundity. F1000Res. 2014;3:125. doi:10.12688/f1000research.3975.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ulgherait M, Rana A, Rera M, Graniel J, Walker DW. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014;8:1767–1780. doi:10.1016/j.celrep.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landis GN, Salomon MP, Keroles D, Brookes N, Sekimura T, Tower J. The progesterone antagonist mifepristone/RU486 blocks the negative effect on life span caused by mating in female Drosophila. Aging. 2015;7:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bahadorani S, Hur JH, Lo T, Jr, Vu K, Walker DW. Perturbation of mitochondrial complex V alters the response to dietary restriction in Drosophila. Aging Cell. 2010;9:100–103. doi:10.1111/j.1474-9726.2009.00537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruce KD, Hoxha S, Carvalho GB, et al. High carbohydrate-low protein consumption maximizes Drosophila lifespan. Exp Gerontol. 2013;48:1129–1135. doi:10.1016/j.exger.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat Methods. 2005;2:813–815. doi:10.1038/nmeth798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deshpande SA, Carvalho GB, Amador A, et al. Quantifying Drosophila food intake: comparative analysis of current methodology. Nat Methods. 2014;11:535–540. doi:10.1038/nmeth.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee KP, Simpson SJ, Clissold FJ, et al. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105:2498–2503. doi:10.1073/pnas.0710787105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng C, Du Y, Alberico T, Seeberger J, Sun X, Zou S. Gender-specific prandial response to dietary restriction and oxidative stress in Drosophila melanogaster. Fly. 2011;5:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ja WW, Carvalho GB, Mak EM, et al. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA. 2007;104:8253–8256. doi:10.1073/pnas.0702726104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Partridge L, Piper MD, Mair W. Dietary restriction in Drosophila. Mech Ageing Dev. 2005;126:938–950. doi:10.1016/j.mad.2005.03.023 [DOI] [PubMed] [Google Scholar]
- 24. Simpson SJ, Raubenheimer D. The nature of nutrition: a unifying framework. Aust J Zool. 2011;59:350–368. [Google Scholar]
- 25. Yamada R, Deshpande SA, Bruce KD, Mak EM, Ja WW. Microbes promote amino acid harvest to rescue undernutrition in Drosophila. Cell Rep. 2015;10:865–872. doi:10.1016/j.celrep.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ja WW, Carvalho GB, Zid BM, Mak EM, Brummel T, Benzer S. Water- and nutrient-dependent effects of dietary restriction on Drosophila lifespan. Proc Natl Acad Sci USA. 2009;106:18633–18637. doi:10.1073/pnas.0908016106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deshpande SA, Yamada R, Mak CM, et al. Acidic food pH increases palatability and consumption and extends Drosophila lifespan. J Nutr. 2015. doi:10.3945/jn.115.222380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shiraiwa T, Carlson JR. Proboscis extension response (PER) assay in Drosophila. J Vis Exp. 2007:193. doi:10.3791/193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang JS, Nam HJ, Seo M, et al. OASIS: online application for the survival analysis of lifespan assays performed in aging research. PLoS One. 2011;6:e23525. doi:10.1371/journal.pone.0023525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Winter JCF. Using the Student’s t-test with extremely small sample sizes. Pract Assess Res Eval. 2013;18 Retrieved from http://pareonline.net/getvn.asp?v=18&n=10 [Google Scholar]
- 31. Lee KP. Dietary protein:carbohydrate balance is a critical modulator of lifespan and reproduction in Drosophila melanogaster: a test using a chemically defined diet. J Insect Physiol. 2015;75:12–19. doi:10.1016/j.jinsphys.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 32. Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi:10.1371/journal.pbio.0030223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6:e1001159. doi:10.1371/journal.pgen.1001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Copeland JM, Cho J, Lo T, Jr, et al. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol. 2009;19:1591–1598. doi:10.1016/j.cub.2009.08.016 [DOI] [PubMed] [Google Scholar]
- 35. Waskar M, Landis GN, Shen J, et al. Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy. Aging. 2009;1:903–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. French AS, Sellier MJ, Ali Agha M, et al. Dual mechanism for bitter avoidance in Drosophila. J Neurosci. 2015;35:3990–4004. doi:10.1523/JNEUROSCI.1312-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mennella JA, Reed DR, Mathew PS, Roberts KM, Mansfield CJ. “A spoonful of sugar helps the medicine go down”: bitter masking by sucrose among children and adults. Chem Senses. 2015;40:17–25. doi:10.1093/chemse/bju053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeong YT, Shim J, Oh SR, et al. An odorant-binding protein required for suppression of sweet taste by bitter chemicals. Neuron. 2013;79:725–737. doi:10.1016/j.neuron.2013.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. LeDue EE, Chen YC, Jung AY, Dahanukar A, Gordon MD. Pharyngeal sense organs drive robust sugar consumption in Drosophila. Nat Commun. 2015;6:6667. doi:10.1038/ncomms7667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi:10.1016/j.cell.2012.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dus M, Min S, Keene AC, Lee GY, Suh GS. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci USA. 2011;108:11644–11649. doi:10.1073/pnas.1017096108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fujita M, Tanimura T. Drosophila evaluates and learns the nutritional value of sugars. Curr Biol. 2011;21:751–755. doi:10.1016/j.cub.2011.03.058 [DOI] [PubMed] [Google Scholar]
- 43. Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr Biol. 2011;21:746–750. doi:10.1016/j.cub.2011.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stafford JW, Lynd KM, Jung AY, Gordon MD. Integration of taste and calorie sensing in Drosophila. J Neurosci. 2012;32:14767–14774. doi:10.1523/JNEUROSCI.1887-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Behmer ST, Elias DO, Bernays EA. Post-ingestive feedbacks and associative learning regulate the intake of unsuitable sterols in a generalist grasshopper. J Exp Biol. 1999;202:739–748. [DOI] [PubMed] [Google Scholar]
- 46. Schmelz EA, Grebenok RJ, Ohnmeiss TE, Bowers WS. Interactions between Spinacia oleracea and Bradysia impatiens: a role for phytoecdysteroids. Arch Insect Biochem Physiol. 2002;51:204–221. doi:10.1002/arch.10062 [DOI] [PubMed] [Google Scholar]
- 47. Marion-Poll F, Calas D, Darazy-Choubaya D, Faucher C, Descoins C. Tasting toxicants as bitter: phytoecdysteroids. SEB Exp Biol Ser. 2009;63:127–138. [PubMed] [Google Scholar]
- 48. Dong Y, Guha S, Sun X, Cao M, Wang X, Zou S. Nutraceutical interventions for promoting healthy aging in invertebrate models. Oxid Med Cell Longev. 2012;2012:718491. doi:10.1155/2012/718491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jafari M. Drosophila melanogaster as a model system for the evaluation of anti-aging compounds. Fly. 2010;4:253–257. doi:10.4161/fly.4.3.11997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.